Abstract
BACKGROUND
Previous experiments have shown that granulocyte colony-stimulating factor (G-CSF), quantified in the follicular fluid (FF) of individual oocytes, correlates with the potential for an ongoing pregnancy of the corresponding fertilized oocytes among selected transferred embryos. Here we present a proof of concept study aimed at evaluating the impact of including FF G-CSF quantification in the embryo transfer decisions.
METHODS
FF G-CSF was quantified with the Luminex XMap technology in 523 individual FF samples corresponding to 116 fresh transferred embryos, 275 frozen embryos and 131 destroyed embryos from 78 patients undergoing ICSI.
RESULTS
Follicular G-CSF was highly predictive of subsequent implantation. The receiving operator characteristics curve methodology showed its higher discriminatory power to predict ongoing pregnancy in multivariate logistic regression analysis for FF G-CSF compared with embryo morphology [0.77 (0.69–0.83), P < 0.001 versus 0.66 (0.58–0.73), P = 0.01)]. Embryos were classified by their FF G-CSF concentration: Class I over 30 pg/ml (a highest positive predictive value for implantation), Class II from 30 to 18.4 pg/ml and Class III <18.4 pg/ml (a highest negative predictive value). Embryos derived from Class I follicles had a significantly higher implantation rate (IR) than those from Class II and III follicles (36 versus 16.6 and 6%, P < 0.001). Embryos derived from Class I follicles with an optimal morphology reached an IR of 54%. Frozen-thawed embryos transfer derived from Class I follicles had an IR of 37% significantly higher than those from Class II and III follicles, respectively, of 8 and 5% (P < 0.001). Thirty-five per cent of the frozen embryos but also 10% of the destroyed embryos were derived from G-CSF Class I follicles. Non-optimal embryos appear to have been transferred in 28% (22/78) of the women, and their pregnancy rate was significantly lower than that of women who received at least one optimal embryo (18 versus 36%, P = 0.04).
CONCLUSIONS
Monitoring FF G-CSF for the selection of embryos with a better potential for pregnancy might improve the effectiveness of IVF by reducing the time and cost required for obtaining a pregnancy.
Keywords: oocyte quality, G-CSF, in vitro fertilization, pregnancy, follicular fluid
Introduction
Oocyte quality remains one of the main factors limiting the success of assisted reproductive technology (ART) in humans. This is due to the predominant role of maternal factors during early embryo development and to the fragility of oocytes across their lifetime. Oocyte morphology does not discriminate the potential for an ongoing pregnancy well and mainly permits negative selection (Balaban and Urman, 2006; Rienzi et al., 2010). Only ∼5% of fresh oocytes produce a baby. This low rate decreases to 1% in older mothers and reaches its peak, 7%, in oocyte donation programs (Patrizio and Sakkas, 2009). The selection of the optimal embryos for subsequent implantation is traditionally based on morphological observation of the cohort of embryos generated after fertilization, up to the cleavage stage (Days 2–3) or, more recently, until the blastocyst stage (Day 5). At that stage, 20–25% of embryos transferred on Day 2 or 3 produce a baby, and the implantation rate (IR) reaches 30–40% with transfer at the blastocyst stage (Blake et al., 2007). Morphological observation of embryos is effective in predicting subsequent implantation and continues to improve through the development of new non-invasive technology, such as the time-lapse imaging (Meseguer et al., 2011; Hashimoto et al., 2012). Nonetheless, embryos morphology, especially at the early cleavage rate, does not well discriminate potentiality of embryos for ongoing pregnancy (Guerif et al., 2007).
We have shown that granulocyte colony-stimulating factor (G-CSF), quantified in the individual follicular fluid (FF) of the corresponding oocyte, is a non-invasive biomarker of oocyte competence in both stimulated and natural IVF/ICSI cycles (Ledee et al., 2008; Ledee et al., 2010; Ledee et al., 2011). All three studies used a multiplexed immunobead-based assay (Xmap technology) and focused solely on analysis of individual FF corresponding to embryos already selected for fresh transfer. FF G-CSF concentrations significantly predicted the potential for live birth of the corresponding fresh transferred embryo with a discriminatory power ranging from 0.75 to 0.83, according to the receiving operator characteristics (ROC) methodology [(area under the ROC curve (AUCROC)]. In all three assays, FF G-CSF quantification and embryo morphological scoring appeared to be independent predictors of implantation and were not correlated at least until Day 3. Effective tools exist to evaluate the ovarian reserve and have been thoroughly assessed. They can clearly predict the collection of a low number of oocytes, low hormonal response to stimulation, and high cancellation rates for poor response to ovarian stimulation, but they do not provide information about individual oocyte quality (Broer et al., 2010).
Over the last 20 years, it has been shown convincingly that the family of colony-stimulating factors (CSF) plays a cardinal role in the early cross-talk between mother and conceptus in both human and animal models (Pollard, 1997; Robertson, 2007). Moreover, there is ample evidence that CSF regulates reproductive processes at different times during a woman's reproductive life. The CSF family includes macrophage CSF (M-CSF, CSF-1), granulocyte macrophage CSF (GM-CSF, CSF-2) and granulocyte CSFs (G-CSF, CSF-3). These CSFs are secreted glycoproteins which bind to receptor proteins, thereby activating intracellular signalling pathways associated with cell proliferation and differentiation (Kaushansky, 2006; Marino and Roguin, 2008). M-, GM- and G-CSF, together with their corresponding receptors, have been localized in the ovarian tissue (Zhao et al., 1995; Yanagi et al., 2002; Salmassi et al., 2004; Salmassi et al., 2005a,b). All have been detected in pre-ovulatory follicles and shown to be secreted by granulosa cells with the maximum concentrations at ovulation (Salmassi et al., 2004). G- and GM-CSF have been reported to increase in the serum 10 days after ovulation in successful IVF/ICSI cycles (Salmassi et al., 2005a,b).
This report describes a proof of concept study aiming to evaluate the impact of including FF G-CSF quantification as a new tool for selecting the embryos with the highest potential for pregnancy among the embryo cohorts generated after IVF/ICSI. We quantified FF G-CSF in 523 individual FF samples corresponding to the oocytes for 116 fresh transferred embryos, 275 frozen embryos and 131 destroyed embryos.
Materials and Methods
Patients
The study recruited 78 women undergoing an ICSI attempt between May 2008 and May 2010. Each patient was included only once. Because the protocol aimed to document follicular G-CSF and GM-CSF concentrations of morphologically documented oocytes at the time of the collection, we chose to include ICSI patients only. The Institutional Review Board approved this investigation. Patients were informed that FFs would be preserved for research purposes, in accordance with the Belgian law from December 2008 on residual biological materials. Indications for ICSI were related to pure male infertility for 50 couples, mixed infertility for 14 couples or female infertility (due to age or previous unexplained IVF failure) for 14 couples. Infertility was primary for 61 couples and secondary for 17 couples. The mean age of women in the cohort was 32.4 years old (20–38) and they had undergone a mean of 1.6 previous IVF attempts (range: 1–5).
Treatment
Ovarian hyperstimulation protocols were selected by each treating physician. They included the standard long protocol with a daily GnRH agonist for 56 patients, short protocol with a daily GnRH agonist for 18 patients and an antagonist protocol for 4 patients. The response to stimulation was monitored by serial hormonal blood tests and by ultrasound assessment of follicular and endometrial growth. Ovulation was triggered with hCG when at least four follicles had reached 17 mm. Follicles were retrieved individually by aspiration 35–36 h after ovulation induction under neuroleptic anaesthesia and vaginal ultrasound guidance without flushing of follicles. Cumulus and coronal cells from individual oocytes collected were removed with hyaluronidase type IV S 80 IU/ml (Sigma-Aldrich®, St-Louis, USA). Each mature oocyte was injected under standard magnification with a single sperm in a 5–10-µl droplet of fertilization medium (Irvine Scientific®, Santa Ana, USA or LifeGlobal®, USA), with viscous polyvinylpyrrolidone medium (PVP, Sigma-Aldrich®) to slow down the sperm. Microinjection was performed with fresh ejaculated sperm in 74 cases, autologous cryopreserved sperm in 3 cases and testicular cryopreserved sperm in 1 case. The injected oocytes were individually cultured in a 20-µl microdroplet of cleavage medium (Irvine Scientific® or LifeGlobal®) under oil, at 37°C (±0.5°C) in a 6% (±0.5%) CO2 humid atmosphere.
On Day 3, the embryos were analysed in relation to (a) their fragmentation (0 = no fragmentation, f1: 1–10%, F1: 10–30%, F2: 30–50%, F3: over 50%); (b) blastomere regularity (0 = not different in size, B1: different in size by a factor of <2; B2: different in size by a factor of 2–3; B3: different in size by a factor of >3 times; (c) ooplasmic appearance (homogenous and clear = 0, C1: 1 anomaly such as the vacuole, inclusion, granulation or incorrect coloration; C2: 2 anomalies, C3: 3 combined anomalies) and (d) number of blastomeres (>6 at Day 3, or not). An embryo score was calculated and defined as described in Table I. Category A was defined as optimal and required >6 cells on Day 3, 10% or less of fragmentation and similar sizes of blastomeres.
Table I.
Definition of the embryo score used at the University of Liège – CPMA.
| Day 3 | Excellent = A | Intermediate = B | Poor = C | Discarded = D |
|---|---|---|---|---|
| >6 cells | >6 cells | All others not discarded | <6 cells | |
| E (score 0 for F, B and C) | F1B1 | Absence of cleavage within 24 h | ||
| f1 | F1C1 | >2PN | ||
| F1 | B1C1 | F2 and more | ||
| B1 | F2 | B3 if only one large dominant cell | ||
| C1 | B2 | |||
| f1B1 | C2 | |||
| f1C1 | ||||
| Beginning of compaction |
Fragmentation. 0: no fragmentation, f1: 1–10%, F1: 10–30%, F2: 30–50%, F3: over 50%.
Blastomere regularity. 0: not different in size, B1: different in size by a factor of <2; B2: different in size by a factor of 2–3; B3: different in size by a factor of >3 times.
Ooplasmic appearance. 0: homogenous and clear, C1: 1 anomaly such as vacuole, inclusion, granulation or incorrect coloration; C2: 2 anomalies, C3: 3 combined anomalies.
Number of blastomeres. >6: more than 6 at Day 3, <6: not more than 6 on Day 3.
Materials collected and analysed
The presence of an oocyte in each FF sample was assessed immediately, and samples without an oocyte were discarded. In 15% of the cases (80/523), two oocytes were collected in the same FF. Quantification of cytokines was then applied equally to both oocytes. We were able to analyse 523 FF samples: 116 associated with fresh transferred embryos, 276 associated with frozen embryos (among which 79 embryos subsequently thawed and transferred) and 131 associated with destroyed embryos. After centrifugation, samples were divided in aliquots and stored, initially at −20°C and then at −80°C until they were assayed. For blinding, samples were identified only by a patient ID number, a stimulation number and an oocyte number within the cohort. Attaching names (and therefore results) to the numbers required a key not available to those performing the test (Medifirst SA, Guyancourt, France).
A Luminex system (Luminex XMap Technology from Bio-Rad) was used to read the concentrations of two cytokines (G-CSF and GM-CSF Laboratories®, Hercules, CA, USA) in the individually collected FF. This technology uses multiplexed microsphere-based immunoassays that apply flow cytometric resolution to measure spectrally distinct microspheres coupled with capture molecules and reporter fluorochromes bound to detection antibodies.
The assays were performed according to the manufacturer's instructions (Bio-Rad). Each FF was diluted by 10 (with the sample diluent provided in the kit) to improve cytokine detection. Each sample was measured in duplicate. A standard curve was traced for each test with the standard provided in the kit, according to the manufacturer's instructions. The same FF sample was measured in each plate for an inter-assay control. Calibration of the Luminex system was verified before each test with the calibration kit from Bio-Rad. The range of detection for FF G-CSF was 5.1–84.24 pg/ml. Its intra-test variation was 3% and the inter-test variation of 12%. The range of detection for FF GM-CSF was 3.4–21.2 pg/ml, its intra-test variation was 3% and its inter-test variation of 24%.
Statistical analysis
To identify predictive factors of implantation or subsequent ongoing pregnancy, we performed multivariable logistic regression analysis, using ROC analysis to determine the performance, sensitivity, specificity and positive and negative predictive values of FF G-CSF and GM-CSF concentrations. Each analyte was first evaluated with covariate factors known to influence outcome, such as age, attempt rank, number of oocytes collected, quantity of gonadotrophins administered during the stimulation and the estradiol level on the day of hCG injection.
We subsequently performed a second run that included the morphological analysis of the embryo according to the Centre de Procréation Médicalement Assistée (CPMA) scoring. Embryos were first classified as either Grades A, B or C, and a corresponding score of 3, 2 or 1 point was attributed to evaluate the performance of the combined FF-GCSF and embryo score. A P-value >0.1 was used as a criterion for exclusion, in accordance with the literature on multivariate prognostic modelling (Steyerberg et al., 2000). Logistic fitting equations combining factors independently and significantly associated with the outcome were constructed from multivariate logistic regression analysis. The following thresholds were used to interpret the AUCROC: 0.9–1: excellent separation; 0.8–0.9: good discrimination; 0.7–0.8: fair discrimination; 0.6–0.7: poor discrimination; 0.5–0.6: no discrimination.
The only outcome considered here was assessed at 12 weeks of amenorrhoea when a gestational sac with cardiac activity was (or was not) viewed by ultrasound. IR was the ratio between the number of gestational sacs with cardiac activity and the total number of embryos transferred. A pregnancy rate in each studied group was the number of patients with at least one gestational sac with cardiac activity. Neither biochemical pregnancies (transient hCG positive test >100 and <1000 IU/ml without any gestational sac) nor early abortions, defined as abortion occurring before 12 weeks of amenorrhoea with hCG >1000 IU or gestational sac without cardiac activity, were counted as pregnancy or implantation or included in these rates. A P-value <0.05 was considered statistically significant.
Results
IVF results
Of the 78 patients with fresh transfers (42 with 1 fresh embryo, 34 with 2 embryos and 2 with 3 embryos), 24 (31%) were pregnant at 12 weeks. In addition, 34 of the 78 (43.5%) had embryos frozen after the IVF attempt. Thawing of frozen embryos allowed 79 further embryos to be transferred resulting in 10 additional ongoing pregnancies. The overall cumulative pregnancy rate was 43.6% (34/78) with 29 singletons and 7 sets of twins.
Samples available to evaluate the power of discrimination of immune biomarkers
To test the discriminatory power of the putative biomarkers (G-CSF and GM-CSF), only samples associated with an IR of either 100% (1 embryo transfer and 1 singleton; 2 embryos transferred and twins) or 0% were included in a multivariate regression logistic analysis. As a single-embryo transfer policy was applied for women younger than 36 years at their first or second ICSI attempt, 13 FF samples were directly traceable to a single pregnancy and 14 corresponded to twins. These 27 positive samples were plotted against 139 FF samples corresponding to embryos that did not implant.
Prediction of implantation: respective interest of biomarkers
G-CSF was detected in all FF; concentrations ranged from 2 to 194 pg/ml with a median of 25.5 pg/ml. GM-CSF was detectable in 99% of FF, and concentrations ranged from 0 to 669 pg/ml with a median of 189 pg/ml. As previously reported (Ledee et al., 2008), follicular G-CSF was significantly predictive of subsequent implantation. The AUCROC curve in the multivariate analysis evaluating the performance of FF G-CSF in predicting ongoing pregnancy reached 0.77 (0.69–0.83) with a P value of <0.001 indicating a fair discriminatory power. In comparison, the AUCROC related to the embryo morphology was 0.66 (0.58–0.73) (P = 0.01). In contrast, the AUCROC for FF GM-CSF was found to be 0.53 and not significant. No significant differences in the FF concentrations of G-CSF and GM-CSF were observed as a function of the ovarian stimulation protocol applied.
FF G-CSF appeared to be very sensitive (92%) with a high negative predictive value (97%) but its specificity and positive predictive value were low (53 and 28%, respectively). FF G-CSF and embryo morphology on Day 3 appeared to be independent, and combining them increased the specificity of the prediction from 53 to 77%, although the sensitivity was decreased. The ‘immune-morphological' combination allowed the positive predictive value to increase from 28 to 38% with a slight decrease in the negative predictive value (from 97 to 93%).These data are summarized in Table II.
Table II.
Power of discrimination of FF G-CSF, embryo morphology and combined FF-G-CSF and embryo morphology.
| FF G-CSF (multivariate analysis) | Embryo score | Implantation score (Log(P): −4.56 + 0.05 × FF G-CSF + 0.64 × EMBRYO score) | |
|---|---|---|---|
| AuROC (95% CI) | 0.77 (0.69–0.83) | 0.66 (0.58–0.73) | 0.76 (0.69–0.83)* |
| P value | 0.0001 | 0.009 | 0.0001 |
| Sensitivity (FP) (%) | 92.6 | 63 | 70 |
| Specificity (FP) (%) | 53.8 | 64 | 77 |
| Positive predictive value (%) | 28 | 26 | 38 |
| Negative predictive value (%) | 97 | 90 | 93 |
| Cut-off value | >23.83 pg/ml | Type A or B | >0.184 |
The combined morpho G-CSF is the result of logistic regression Log(P): −4.56 + 0.05 × FF G-CSF + 0.64 × EMBRYO score not addition of number.
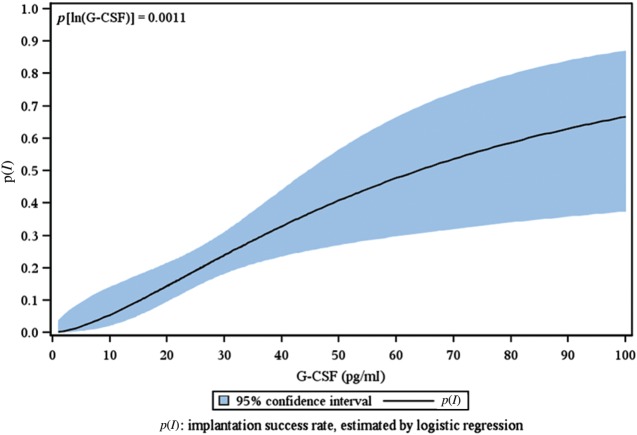
Figure 1 illustrates the logistic regression linking the logarithm of 195 individual FF G-CSF concentrations to the IR of the corresponding transferred embryos.
Figure 1.
The IR is represented as a function of the logarithm of the follicular fluid G-CSF concentration.
We classified FF G-CSF in three categories: Class I: FF G-CSF over 30 pg/ml (a highest positive predictive value for implantation); Class II: from 30 to 18.4 pg/ml and Class III: <18.4 pg/ml (a highest negative predictive value for implantation). Embryo morphology was also classified into three categories: Category A: top quality, category B: medium quality, category C: poor embryo quality (Table I).
Table III summarizes the distribution of 195 fresh and frozen-thawed transferred embryos by immune-morphological category and the corresponding IRs. Embryos derived from oocytes retrieved from follicles with an FF G-CSF concentration >30 pg/ml had an IR of 36.4%, significantly higher than that of embryos derived from oocytes with Classes II (16.8%) and III (6%) (P < 0.001). Embryos morphology categorized as A had an IR of 28%, as B type, of 18% and as C type of 14% (P = 0.01). The IR of embryos classified as IA, that is, FF G-CSF concentration >30 pg/ml with an optimal morphology was 54%, significantly higher than all the other combinations (P < 0.001).
Table III.
Distribution of fresh and frozen-thawed embryos transferred into immuno-morphological categorizations and IRs.
| FF G-CSF (pg/ml) category | No. of embryos | IR (%) | FF G-CSF (A-B-C) and Embryo type (A-B-C) | No. of embryos | IR (%) |
|---|---|---|---|---|---|
| I: >30 pg/ml | 59 | 36.4** | IA | 27 | 53.7** |
| IB | 14 | 28.5 | |||
| IC | 18 | 16.6 | |||
| II: 18–30 pg/ml | 105 | 16.8 | IIA | 36 | 19.4 |
| IIB | 28 | 16 | |||
| IIC | 41 | 14 | |||
| III: <18 pg/ml | 31 | 6 | IIIA | 15 | 6 |
| IIIB | 6 | 8 | |||
| IIIC | 10 | 5 |
The outcome of 116 fresh transferred and 79 frozen and successfully thawed and transferred embryos is considered.
From each group of transferred embryos we may observe the number of resulting implanted embryos (number of gestational sacs).
**P < 0.001 category I versus categories II and III.
FF G-CSF concentration among frozen-thawed embryos
Of the 276 frozen embryos, 44 (16%) were Class III for the FF G-CSF, so theoretically very unlikely to implant, but 97 (35%) were Class I and thus had high potential. Overall, 147 (53%) had not been thawed because the patient became pregnant at the fresh transfer. During the thawing process, 44 embryos lysed: 13 from FF G-CSF Class I, 19 from Class II and 12 from Class III. At the time of freezing, 72% of the lysed embryos had poor morphology, ranked as C. In all, 79 embryos were successfully transferred after the thawing process. Class I frozen-thawed embryos had a significantly higher IR (36%) than Class II (9%) and III (3%) derived frozen-thawed embryos. These data are detailed in Table IV.
Table IV.
IR among frozen-thawed transferred embryos by FF G-CSF concentration
| FF G-CSF among frozen-thawed embryos successfully transferred | Number of embryos analysed | IR (12 weeks) (%) |
|---|---|---|
| Category I: >30 pg/ml | 21 | 35.7*** |
| Category II: 18–30 pg/ml | 44 | 8.6 |
| Category III: <18 pg/ml | 14 | 3.0 |
***P < 0.0001 Category I versus Categories II and III.
FF G-CSF values among destroyed embryos
G-CSF concentrations were assessed for 29 mature oocytes that failed to cleave and be fertilized; in only 1 out of 29, the FF G-CSF concentration was >30 pg/ml. All 102 embryos destroyed on Day 3 had poor morphology, incompatible with freezing. Nonetheless, 26 were from FF G-CSF Class I, 47 were from Class II and 29 were from Class III. For 5 patients, the embryo with the theoretically highest potential calculated from the oocyte evaluation within the available cohort was destroyed. This finding illustrates clearly the high rate of false positivity for a follicular biomarker used alone, since it does not take into account any of the issues related to male factors or culture conditions.
Retrospective analysis of the embryo transfer choices and the combined immune-morphological embryo categories
The priority for effective embryo selection is to discard embryos with a low probability of implantation, to optimize as much as possible the true negative rate and it also seems essential to identify embryos with very high potential for implantation, to increase the pregnancy rate while controlling multiple pregnancy rates. Accordingly, we evaluated the immune-morphological score of each embryo generated from each patient's cohort of oocytes collected after ovarian stimulation. Our objective was to determine if these results would have modified the embryologist's choice. The hypothesis was that the embryo to be transferred fresh should be chosen according to the following rule: IA > IB > IIA > IC > IIB > IIC > IIIA > IIIB > IIIC. As above, the number refers to the classification of the FF G-CSF concentration and the letter that follows it to the embryo morphology. The choice was defined as optimal when embryos chosen for fresh transfer were those with the highest available combination among the cohort of embryos available on Day 3. Overall, 28% (22/78) of the patients did not have an optimal embryo transferred. For patients who did not receive at least one optimal embryo, the pregnancy rate was significantly lower than among the 56 patients who received at least one optimal embryo (18 compared with 36%, P = 0.04).
Of the 42 patients with one fresh transferred embryo, 16 (38%) did not had the optimal embryo transferred, and this decreased subsequent IRs (25 versus 35%). Among these women with an initial non-optimal choice, six subsequently became pregnant by transferring frozen-thawed embryos with a higher immune-morphological score than the fresh-transferred ones. As two embryos were transferred simultaneously after the thawing process, two of these transfers resulted in twin pregnancies. However, 61% of the embryo lysed during thawing and 13% of the destroyed embryos were classified as arising from Class I oocytes. Altogether our data indicate we would have achieved a minimum of 6 additional pregnancies at the fresh transfer stage by documenting FF G-CSF before transfer.
Of 36 patients with two or three embryos transferred, at least one of the fresh transferred embryos was the optimal choice in 82% (30/36) and resulted in a pregnancy rate of 37%. In contrast, no pregnancy resulted in the 6 women for whom both fresh transferred embryos were selected from non-optimal combinations. For these six patients, 23% of the destroyed embryos were classified as Class I and 29% of thawed embryos lysed. In the subgroup with only one optimal embryo transferred over 2, destruction or lysis of FF G-CSF Class I embryos eliminated some potential pregnancies, and two pregnancies resulted from the transfer of frozen thawed embryos. Again, we hypothesize that we would have achieved 3 additional pregnancies in that group receiving two or three embryos at the fresh embryo transfer stage by documenting FF G-CSF before transfer.
Finally, our results suggest that some twin pregnancies could have been prevented. Three of the seven sets of twins occurred in the group with two optimal fresh embryos transferred, and all three of these had two Class IA embryos transferred. This category of embryo has a theoretical implantation probability of 54%, according to our hypothesis. The combinations in the seven twin pregnancies were the following: (i) IIA-IIA, (ii) IC-IIB, (iii) IA-IA, (iv) IA-IA, (v) IIC-IIC, (vi) IA-IA and (vii) IIB-IIC. We may assume that the three twins with double IA combinations could have been prevented and anticipated. This would result in a relative reduction of 55% in the cumulative multiple pregnancy rate (from 9 to 5%) while not affecting the overall pregnancy rate.
Based on these observations, we postulate that nine additional pregnancies could have been expected if FF G-CSF had been assessed before transfer, leading to a relative increase of 37.5% in the ongoing pregnancy rate (from 24/78 to 33/78) after the fresh embryo transfer.
Discussion
This proof of concept study suggests that combining the FF G-CSF concentration with the embryo morphology scale might improve the effectiveness of embryo selection. It suggests that a relative increase of 37.5% in the ongoing pregnancy rates at the first fresh transfer is possible by better selection of embryos with a high probability of implantation. This approach would also modify some choices about frozen and destroyed embryos, limiting the number of cryopreserved embryos and restoring the option of fresh transfer for some destroyed embryos. Moreover, it suggests that assessing FF G-CSF can improve the selection of an embryo for single embryo transfer and reduce the number of multiple pregnancies generated.
In contrast, follicular GM-CSF concentrations are not related to corresponding ongoing pregnancy rates in contrast to its described role in embryo supernatants or within the endometrium (Robertson, 2007). CSF-related expression appears crucial for implantation, with each family member playing a very specific predetermined spatial role.
As FF is easily available during oocyte collection, it is the optimal source for non-invasive biochemical predictors (Revelli et al., 2009). Moreover, we have previously shown that follicular G-CSF concentrations are not affected by blood contamination (Ledee et al., 2008). Compared with emerging approaches based on genomic analysis of cumulus cells to evaluate oocyte competence (Assou et al., 2008; Gebhardt et al., 2011), assessment of a protein such as G-CSF in an individual FF sample is a simple and economic method for use in daily practice.
The cost-effectiveness of such a tool to improve embryo selection can only be evaluated in prospective randomized studies that compare embryo selection based on morphology alone to an approach combining morphology and FF G-CSF quantification. The hypothesis would be that this more efficient process would yield more pregnancies in less time and at less expense. The end-points of such a prospective study would therefore be the number of embryos per patient required to obtain a pregnancy, the number of cycles and the time in months required for a pregnancy, the ongoing pregnancy rate per fresh transfer and the cumulative pregnancy rate per oocyte harvested.
Another fundamental issue is to determine if the use of FF G-CSF as a biomarker of oocyte quality might also help patients with documented poor ovarian reserve. This issue was not explored here, for we included only patients younger than 38 years. Multivariate logistic analysis including age, number of oocytes collected and hormonal response to ovarian stimulation did not show that any of these criteria reflecting ovarian reserve influenced FF G-CSF concentrations. As shown for the anti-Mullerian hormone, which also reflects the ovarian reserve (Broer et al., 2010), oocyte quantity and quality seem to be somewhat independent concepts. Further studies must investigate if FF G-CSF might be relevant in situations of low ovarian reserve.
As noted above, demonstration of a relative effective increase of 37.5% in the ongoing pregnancy rates through a more accurate choice of fresh transferred embryos requires prospective randomized studies that use daily FF G-CSF bead-based immunoassay quantification. This assessment should be performed within a day, but would require modification of the oocyte collection procedure. Each individual follicle must be aspirated individually and the corresponding oocyte must be fertilized and cultured individually, to ensure traceability (Wongtra-Ngan et al., 2010). In this study, oocyte retrieval was performed by individual aspiration without flushing. We found that 15% of the FFs collected contained two oocytes. These data show the need for improvement if follicular G-CSF is to be used as a diagnostic tool. Systems of aspiration with double lumen allow the aspirated follicle to be flushed and subsequently re-aspirated (follicular flushing) to increase individual traceability. The duration of the oocyte retrieval procedure would undoubtedly increase, as well as patients’ discomfort during the procedure (Hill and Levens, 2010). This change, however, is a prerequisite for the assessment of individual FF G-CSF concentrations, to increase the subsequent pregnancy rate by a more precise and accurate choice of embryos with the best potential for implantation and live birth.
A current approach for selecting embryos with a high potential for live birth is to select them through a culture until the blastocyst stage (Sills and Palermo, 2010). This policy has been shown to be successful in increasing live birth rates while decreasing the number of cryopreserved embryos when a minimum number of viable embryos are available on Day 2 or 3 (Blake et al., 2007). However, patients with a low number of embryos on Day 2 or 3 might not be good candidates for such a strategy, which might result in the absence of any transfer on Day 5; they would benefit from selection that uses FF G-CSF to document the corresponding oocyte quality.
The selection of embryos through prolonged culture through the blastocyst stage and FF G-CSF quantification both suggest that too many embryos without real potential for pregnancy are cryopreserved. Here, only 35% of cryopreserved embryos were derived from an oocyte retrieved from a high-potential follicle. Better selection would theoretically decrease the time necessary to obtain a live birth and would save both physicians and patients the time and money for unsuccessful cycles of frozen thawed transfers. Another emerging application is clearly in the area of oocyte cryopreservation, as an option to reduce the well-known and documented impact of age and the use of gamete donation (Setti and Bulletti, 2011). In this situation, FF G-CSF appears to be a very promising tool because of its very high sensitivity, compared with the low predictive value of oocyte morphology (Balaban and Urman, 2006; Rienzi et al., 2010).
In conclusion, this proof of concept study suggests that quantification of follicular G-CSF may increase the ongoing pregnancy rate by enabling a better choice of embryos, limit multiple pregnancies, reduce embryo cryostorage and use some embryos which are currently destroyed. FF G-CSF monitoring, by enabling better selection of embryos with real potential for implantation, should improve the efficiency of the procedure by reducing the time and expense required for a pregnancy to be achieved. Prospective randomized studies should be conducted to determine whether this tool significantly decreases the number of embryos and the number of cycles required to obtain a pregnancy while improving cumulative delivery rates per oocyte harvested.
Authors’ roles
N.L., S.P.H., C.M. and J.M.F. conceived the study. N.H., M.D. and S.P.H. collected the individual follicular fluid samples. S.R., C.J., O.G., F.W. and F.T. took care of the embryos and clinical data. V.G. organized the anonymous follicular fluid bank and took care of the samples. V.G. and C.M. carried out the experiments on the follicular fluids. N.L. conducted the statistical analysis and wrote the manuscript. S.P. and C.M. revised the manuscript. All authors gave final approval of the submitted version.
Funding
This work was supported by the Agence de la Biomédecine and Uteron SA. Funding to pay the Open Access publication charges for this article was provided by Uteron, our financial support.
Conflict of interest
N. L. is inventor of a PCT patent of application (PCT/EP2007/057430) applied July, 2007.
References
- Assou S, Haouzi D, Mahmoud K, Aouacheria A, Guillemin Y, Pantesco V, Reme T, Dechaud H, De Vos J, Hamamah S. A non-invasive test for assessing embryo potential by gene expression profiles of human cumulus cells: a proof of concept study. Mol Hum Reprod. 2008;14:711–719. doi: 10.1093/molehr/gan067. [DOI] [PubMed] [Google Scholar]
- Balaban B, Urman B. Effect of oocyte morphology on embryo development and implantation. Reprod Biomed Online. 2006;12:608–615. doi: 10.1016/s1472-6483(10)61187-x. [DOI] [PubMed] [Google Scholar]
- Blake DA, Farquhar CM, Johnson N, Proctor M. Cleavage stage versus blastocyst stage embryo transfer in assisted conception. Cochrane Database Syst Rev. 2007:CD002118. doi: 10.1002/14651858.CD002118.pub3. [DOI] [PubMed] [Google Scholar]
- Broer SL, Mol B, Dolleman M, Fauser BC, Broekmans FJ. The role of anti-Mullerian hormone assessment in assisted reproductive technology outcome. Curr Opin Obstet Gynecol. 2010;22:193–201. doi: 10.1097/GCO.0b013e3283384911. [DOI] [PubMed] [Google Scholar]
- Gebhardt KM, Feil DK, Dunning KR, Lane M, Russell DL. Human cumulus cell gene expression as a biomarker of pregnancy outcome after single embryo transfer. Fertil Steril. 2011;96:47–52. doi: 10.1016/j.fertnstert.2011.04.033. e42. [DOI] [PubMed] [Google Scholar]
- Guerif F, Le Gouge A, Giraudeau B, Poindron J, Bidault R, Gasnier O, Royere D. Limited value of morphological assessment at days 1 and 2 to predict blastocyst development potential: a prospective study based on 4042 embryos. Hum Reprod. 2007;22:1973–1981. doi: 10.1093/humrep/dem100. [DOI] [PubMed] [Google Scholar]
- Hashimoto S, Kato N, Saeki K, Morimoto Y. Selection of high-potential embryos by culture in poly(dimethylsiloxane) microwells and time-lapse imaging. Fertil Steril. 2012;97:332–337. doi: 10.1016/j.fertnstert.2011.11.042. [DOI] [PubMed] [Google Scholar]
- Hill MJ, Levens ED. Is there a benefit in follicular flushing in assisted reproductive technology? Curr Opin Obstet Gynecol. 2010;22:208–212. doi: 10.1097/GCO.0b013e3283373bfe. [DOI] [PubMed] [Google Scholar]
- Kaushansky K. Lineage-specific hematopoietic growth factors. N Engl J Med. 2006;354:2034–2045. doi: 10.1056/NEJMra052706. [DOI] [PubMed] [Google Scholar]
- Ledee N, Lombroso R, Lombardelli L, Selva J, Dubanchet S, Chaouat G, Frankenne F, Foidart JM, Maggi E, Romagnani S, et al. Cytokines and chemokines in follicular fluids and potential of the corresponding embryo: the role of granulocyte colony-stimulating factor. Hum Reprod. 2008;23:2001–2009. doi: 10.1093/humrep/den192. [DOI] [PubMed] [Google Scholar]
- Ledee N, Munaut C, Serazin V, Perrier d'Hauterive S, Lombardelli L, Logiodice F, Wainer R, Gridelet V, Chaouat G, Frankenne F, et al. Performance evaluation of microbead and ELISA assays for follicular G-CSF: a non-invasive biomarker of oocyte developmental competence for embryo implantation. J Reprod Immunol. 2010;86:126–132. doi: 10.1016/j.jri.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Ledee N, Frydman R, Osipova A, Taieb J, Gallot V, Lombardelli L, Logiodice F, Petitbarat M, Fanchin R, Chaouat G, et al. Levels of follicular G-CSF and interleukin-15 appear as noninvasive biomarkers of subsequent successful birth in modified natural in vitro fertilization/intracytoplasmic sperm injection cycles. Fertil Steril. 2011;95:94–98. doi: 10.1016/j.fertnstert.2010.03.010. [DOI] [PubMed] [Google Scholar]
- Marino VJ, Roguin LP. The granulocyte colony stimulating factor (G-CSF) activates Jak/STAT and MAPK pathways in a trophoblastic cell line. J Cell Biochem. 2008;103:1512–1523. doi: 10.1002/jcb.21542. [DOI] [PubMed] [Google Scholar]
- Meseguer M, Herrero J, Tejera A, Hilligsoe KM, Ramsing NB, Remohi J. The use of morphokinetics as a predictor of embryo implantation. Hum Reprod. 2011;26:2658–2671. doi: 10.1093/humrep/der256. [DOI] [PubMed] [Google Scholar]
- Patrizio P, Sakkas D. From oocyte to baby: a clinical evaluation of the biological efficiency of in vitro fertilization. Fertil Steril. 2009;91:1061–1066. doi: 10.1016/j.fertnstert.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Pollard JW. Role of colony-stimulating factor-1 in reproduction and development. Mol Reprod Dev. 1997;46:54–60. doi: 10.1002/(SICI)1098-2795(199701)46:1<54::AID-MRD9>3.0.CO;2-Q. discussion 60–51. [DOI] [PubMed] [Google Scholar]
- Revelli A, Delle Piane L, Casano S, Molinari E, Massobrio M, Rinaudo P. Follicular fluid content and oocyte quality: from single biochemical markers to metabolomics. Reprod Biol Endocrinol. 2009;7:40. doi: 10.1186/1477-7827-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rienzi L, Vajta G, Ubaldi F. Predictive value of oocyte morphology in human IVF: a systematic review of the literature. Hum Reprod Update. 2010;17:34–45. doi: 10.1093/humupd/dmq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson SA. GM-CSF regulation of embryo development and pregnancy. Cytokine Growth Factor Rev. 2007;18:287–298. doi: 10.1016/j.cytogfr.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Salmassi A, Schmutzler AG, Huang L, Hedderich J, Jonat W, Mettler L. Detection of granulocyte colony-stimulating factor and its receptor in human follicular luteinized granulosa cells. Fertil Steril. 2004;81(Suppl. 1):786–791. doi: 10.1016/j.fertnstert.2003.09.039. [DOI] [PubMed] [Google Scholar]
- Salmassi A, Schmutzler AG, Schaefer S, Koch K, Hedderich J, Jonat W, Mettler L. Is granulocyte colony-stimulating factor level predictive for human IVF outcome? Hum Reprod. 2005a;20:2434–2440. doi: 10.1093/humrep/dei071. [DOI] [PubMed] [Google Scholar]
- Salmassi A, Zhang Z, Schmutzler AG, Koch K, Buck S, Jonat W, Mettler L. Expression of mRNA and protein of macrophage colony-stimulating factor and its receptor in human follicular luteinized granulosa cells. Fertil Steril. 2005b;83:419–425. doi: 10.1016/j.fertnstert.2004.06.072. [DOI] [PubMed] [Google Scholar]
- Setti PE, Bulletti C. Strategies to improve embryo implantation to supraphysiological rates. Ann N Y Acad Sci. 2011;1221:75–79. doi: 10.1111/j.1749-6632.2011.05950.x. [DOI] [PubMed] [Google Scholar]
- Sills ES, Palermo GD. Human blastocyst culture in IVF: current laboratory applications in reproductive medicine practice. Rom J Morphol Embryol. 2010;51:441–445. [PubMed] [Google Scholar]
- Steyerberg EW, Eijkemans MJ, Harrell FE, Jr, Habbema JD. Prognostic modelling with logistic regression analysis: a comparison of selection and estimation methods in small data sets. Stat Med. 2000;19:1059–1079. doi: 10.1002/(sici)1097-0258(20000430)19:8<1059::aid-sim412>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Wongtra-Ngan S, Vutyavanich T, Brown J. Follicular flushing during oocyte retrieval in assisted reproductive techniques. Cochrane Database Syst Rev. 2010:CD004634. doi: 10.1002/14651858.CD004634.pub2. [DOI] [PubMed] [Google Scholar]
- Yanagi K, Makinoda S, Fujii R, Miyazaki S, Fujita S, Tomizawa H, Yoshida K, Iura T, Takegami T, Nojima T. Cyclic changes of granulocyte colony-stimulating factor (G-CSF) mRNA in the human follicle during the normal menstrual cycle and immunolocalization of G-CSF protein. Hum Reprod. 2002;17:3046–3052. doi: 10.1093/humrep/17.12.3046. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Rong H, Chegini N. Expression and selective cellular localization of granulocyte-macrophage colony-stimulating factor (GM-CSF) and GM-CSF alpha and beta receptor messenger ribonucleic acid and protein in human ovarian tissue. Biol Reprod. 1995;53:923–930. doi: 10.1095/biolreprod53.4.923. [DOI] [PubMed] [Google Scholar]