Abstract
STUDY QUESTION
Do higher leptin levels and lower adiponectin levels predict subsequent development of endometriosis?
SUMMARY ANSWER
Plasma leptin and adiponectin levels were not associated with laparoscopically confirmed endometriosis when collected prior to disease diagnosis.
WHAT IS KNOWN ALREADY
Case–control studies have identified altered levels of the inflammatory adipokines leptin and adiponectin in women with endometriosis, but it remains unclear whether inflammation results in endometriosis or whether the presence of endometriosis creates an inflammatory state.
STUDY DESIGN, SIZE, DURATION
Nested, matched, case–control study within the prospective Nurses' Health Study II (NHS II) cohort. Blood samples were collected between 1996 and 1999 from 29 611 female nurses within the cohort. Women who reported endometriosis before blood collection were excluded.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Plasma leptin and adiponectin levels were assayed by ELISA. Three hundred and fifty cases of laparoscopically confirmed endometriosis were matched 1:2 with 694 controls of comparable race, age, infertility history, menopausal status and time of blood draw. Relative risks (RRs) and 95% confidence intervals (CIs) were calculated using unconditional logistic regression models adjusting for matching factors and BMI.
MAIN RESULTS AND THE ROLE OF CHANCE
After adjusting for BMI, there were no statistically significant associations between endometriosis and leptin [RR = 1.2; 95% CI = 0.7–2.0; P-value, test for linear trend (Ptrend) = 0.72], adiponectin (RR = 0.8; 95% CI = 0.5–1.2; Ptrend = 0.48) or the leptin to adiponectin ratio (RR = 0.8; 95% CI = 0.4–1.4; Ptrend = 0.14) when comparing the upper with the lower quartile. Results were unaltered when analyses were stratified by BMI or restricted to cases diagnosed ≥4 years after blood draw. To evaluate statistical significance and limit the role of chance to the gold standard of 5%, we present 95% CIs about the RRs, and for P-values calculated for linear tests of trend and tests of heterogeneity, we have set the α-level to be 0.05 (i.e. <0.05 is considered to be statistically significant).
LIMITATIONS AND REASONS FOR CAUTION
A limitation of this study is the inability to differentiate the time of endometriosis ‘diagnosis’ from the time of disease ‘onset’ due to the impossibility in identifying a precise time point at which the disease process was first initiated at a molecular or cellular level. Additional limitations include lack of information regarding stage of endometriosis and the possibility of asymptomatic disease in the control population.
WIDER IMPLICATIONS OF THE FINDINGS
The mean age at diagnosis of endometriosis in the study population is 41.7, ∼10 years older than the mean age of diagnosis in the general population. While this may limit the generalizability of the results, there is no reason to suspect that the association between adipokines and endometriosis risk should differ at a younger age of diagnosis in an adult population.
STUDY FUNDING
This study was supported by research grants HD48544, HD52473 and HD57210 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The NHS II is supported by the Public Health Service grant CA50385 from the National Cancer Institute, NIH, U.S. Department of Health and Human Services. H.R.H. is supported by NIH training grant T32 ES007069 and MCHB grant number 5T76MC00001 (formerly MCJ201).
Keywords: leptin, adiponectin, adipokines, endometriosis
Introduction
Endometriosis affects 1 in 10 reproductive aged women in the USA (Gao et al., 2006). Despite the significant morbidity associated with the disease, the precise etiology remains poorly elucidated. The most commonly accepted hypothesis is that retrograde menstruation through the fallopian tubes results in aberrant implantation of endometrial tissue throughout the peritoneal cavity (Sampson, 1927). However, laparoscopy has demonstrated retrograde menstruation in over 90% of women, suggesting that other mechanisms predispose a subset of these women toward developing endometriosis (Halme et al., 1984). Abnormalities in immune response, angiogenesis and inflammation have been identified as factors that may increase a woman's propensity toward implantation and growth of ectopic endometrial tissue.
The adipokines leptin and adiponectin have been implicated in the pathogenesis of endometriosis. Both the 16 kDa leptin and the 30 kDa adiponectin are products of adipose tissue originally identified for their role in regulation of lipid metabolism and energy expenditure (Friedman and Halaas, 1998; Arita et al., 1999). Accumulating evidence, however, suggests that these adipokines are also key modulators of angiogenesis, inflammation and the immune response. Leptin correlates directly with adiposity, is elevated in inflammatory states (Fantuzzi and Faggioni, 2000) and serves as an angiogenic factor in vitro and in vivo (Sierra-Honigmann et al., 1998). In contrast, adiponectin is inversely associated with obesity, exerts anti-inflammatory properties (Goldstein and Scalia, 2004) and has been shown to inhibit angiogenesis (Brakenhielm et al., 2004).
Several case–control studies have demonstrated elevated levels of leptin in serum and peritoneal fluid of women with endometriosis (Matarese et al., 2000; Mahutte et al., 2003; Wu et al., 2010), and disruption of leptin signaling appears to inhibit the establishment of endometriosis in a murine model (Styer et al., 2008). Limited data have also identified lower levels of adiponectin in serum (Takemura et al., 2005a) and peritoneal fluid (Takemura et al., 2005b) of women with endometriosis relative to those without. Others have reported contrasting results; Vigano et al. (2002) found no association between leptin levels drawn at the time of laparoscopy and diagnosis of endometriosis. As there have been no prospective human studies to date, it remains unclear whether inflammation results in endometriosis or whether the presence of endometriosis creates an inflammatory state.
The association between BMI and endometriosis further confounds the role of adipokines in the pathogenesis of the disease. Epidemiologic studies have consistently confirmed an inverse association between BMI and endometriosis (Cramer et al., 1986; Darrow et al., 1993; Signorello et al., 1997; Missmer et al., 2004; Parazzini et al., 2004; Ferrero et al., 2005; Hediger et al., 2005; Matalliotakis et al., 2008; Nagle et al., 2009). As leptin levels are strongly correlated with adiposity (Considine et al., 1996), one may therefore expect lower leptin levels in women with endometriosis—contrary to the results of the case–control observations to date.
The Nurses' Health Study II (NHS II) is an ongoing, prospective cohort of 116 678 women enrolled in 1989. Blood samples were collected from 29 611 participants in the late 1990s, over 300 of whom were subsequently diagnosed with laparoscopically confirmed endometriosis. The design of NHS II provides a unique opportunity to clarify the association between leptin, adiponectin, BMI and endometriosis. We hypothesized that higher leptin levels and lower adiponectin levels may be predictive of subsequent development of endometriosis and that this association may vary with BMI.
Materials and Methods
Data for this study were collected in the NHS II cohort from September 1989 to June 2007. Questionnaires requesting information on incident diseases and demographic, biologic, environmental and lifestyle risk factors are updated and mailed biennially. A total of 116 430 female registered nurses, ranging in age from 25 to 42 and residing in one of 14 states in the USA completed the baseline questionnaire in 1989. Subsequent follow-up of the cohort has been consistently over 90% in each 2-year interval. The study was approved by the Institutional Review Boards of the Harvard School of Public Health and Brigham and Women's Hospital, Boston, MA, USA.
Blood sample collection
Blood samples were collected between 1996 and 1999 from 29 611 members of this cohort who were between the ages of 32 and 54 years at the time of blood draw. The present analysis was limited to those women with intact uteri and no history of malignancy. Women who reported a history of endometriosis before the blood collection were also excluded. Mid-luteal samples were collected 7–9 days before the anticipated start of the next menstrual cycle. Samples were shipped with an ice-pack via overnight courier to the laboratory where they were processed and separated into plasma, red blood cell and white blood cell components. Samples were labeled by number only, and have been frozen at −130°C since the time of initial processing.
All women completed a brief questionnaire at blood collection that recorded the date and time of day the blood sample was drawn, her current weight, current parity, current smoking status, current alcohol use, medication use, hours since last food intake, any recent changes in the character of her menstrual cycle and the first day of the menstrual cycle in which the blood samples were drawn. Each woman was also provided a postcard to indicate the first day of her menstrual cycle following the blood collection. Follow-up of the blood cohort was over 96% in 2005.
Leptin and total adiponectin were measured in the laboratory of Dr Nadir Rafai at Children's Hospital of Boston (Boston, MA, USA) using enzyme-linked immunosorbent assays (R&D Systems, Minneapolis, MN, USA) with detection limits of 7.8 pg/ml for leptin and 4.8 ng/ml for adiponectin. Matched case–control triplets were shipped in the same batch and assayed together in a randomly determined order. Each batch included blinded pooled quality control samples to assess laboratory precision, and the average coefficient of variation from these samples was 10.2% for leptin and 12.5% for adiponectin.
Case selection
Incident cases of endometriosis were restricted to those women who reported laparoscopic confirmation of the diagnosis. Women were first asked if they ‘ever had physician-diagnosed endometriosis’ and if so, whether the diagnosis was confirmed by laparoscopy and when the diagnosis occurred. The same questions were asked in each subsequent questionnaire cycle. Blood samples were collected from 29 611 women between 1996 and 1999 as described above. Of these, 363 women were subsequently diagnosed with laparoscopically confirmed endometriosis. The final analytic data set included 350 cases of endometriosis. Reasons for exclusion included inadequate sample volume or inability to adequately match with a control specimen.
Control selection
For each incident case of laparoscopically confirmed endometriosis, two controls were randomly selected from the risk set among women of the same race (White, Asian, African-American, Hispanic, other), age (±1 year), infertility history and menopausal status at diagnosis. Cases and controls were also matched on the month (±1 month), time of day (±2 h) and fasting status (<2, 2–4, 5–7, 8–11, >12 h) at blood draw.
Statistical analysis
Analyses were performed using Statistical Analysis Software (SAS®) version 9.1 (SAS Institute, Inc., Cary, NC, USA). Mixed effect models were used to compare absolute adipokine levels between cases and controls. Quartiles were defined based on the distribution of adipokines among the controls. Unconditional logistic regression models were used to calculate relative risks (RRs) with 95% confidence intervals (CIs), adjusting for the matching factors detailed above. Other potential risk factors for endometriosis were considered to be confounders if their inclusion in the model changed the RR by >10% (Greenland, 1989). Based on this criteria, BMI at blood draw (<18.5, 18.5–22.4, 22.5–24.9, 25.0–29.9, 30.0–34.9, 35.0–39.9, ≥40.0) was the only additional factor included in the final models. Other factors considered but not included were: BMI at age 18 years (<18.5, 18.5–22.4, 22.5–24.9, 25–29.9, 30–34.9, 35–39.9, ≥40), birthweight (<5.5, 5.5–6.9, 7–8.4, >8.4 lbs), age at menarche (≤11, 12–13, ≥14), current menstrual cycle length (<21, 21–25, 26–31, 32–39, ≥40 days), parity at blood draw (nulliparous, 1, 2, 3, ≥4), age at first birth (<20, 20–29, 30–39, ≥40 years), time since last birth (≤1, 1–5, 5–10, ≥10 years), smoking status (never, past, or current smoker), alcohol use at blood draw (no alcohol, 1–3 drinks/month, 1–6 drinks/week, ≥1 drink/day), use of oral contraceptives (never, past, or current user) and diagnosis of either diabetes (yes/no) or gestational diabetes (yes/no).
Tests for linear trend for categorical variables were created by setting the participant's value to the median value within their category, and these were included ordinally in the regression models. Two-sided Wald P-values of <0.05 were considered statistically significant. Effect modification by BMI (<25 or ≥ 25 kg/m2) was assessed via the likelihood ratio test comparing the model with main effects only with the model with main effects and interaction terms.
Results
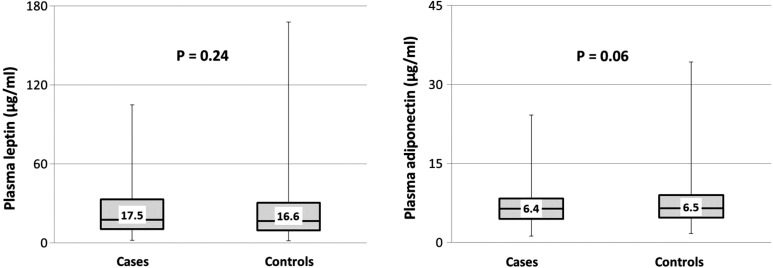
A total of 350 cases and 694 controls comprised the study population. Six controls were excluded due to one of the two factors—either an adipokine level below the detectable limit of the assay or sample hemolyzation that precluded an accurate adipokine value. The median age at blood draw was 41.0 years for cases and 42.0 years for controls, with a range of 32–52 years for both populations. The median time from blood draw to diagnosis of endometriosis was 31 months (inter-quartile range 13–53 months). Demographic characteristics of the study population are shown in Table I. As cases and controls were matched by age, race, menopausal status and infertility history, the distributions of these variables are similar by design. A majority of the study population was Caucasian. Women with endometriosis were more likely to be nulliparous (30.3%) when compared with controls (21.2%). BMI distributions were similar in both groups. Absolute levels of leptin and total adiponectin were also noted to be similar between cases and controls (Fig. 1). The median leptin levels were 17.5 µg/ml in cases (inter-quartile range 10.5–33.0 µg/ml) and 16.6 µg/ml in controls (inter-quartile range 9.7–30.7 µg/ml) (two-sided Wald P-value = 0.24) and the median levels of adiponectin were 6.4 µg/ml in cases (inter-quartile range 4.5–8.4 µg/ml) and 6.5 µg/ml (inter-quartile range 4.7–9.0 µg/ml) in controls (two-sided Wald P-value = 0.06).
Table I.
Characteristics of laparoscopically confirmed endometriosis cases and matched controls within the NHS II blood cohorta.
| Endometriosis cases (n = 350) | Controls (n = 694) | |
|---|---|---|
| Age at blood drawb | 41.7 (4.6) | 42.1 (4.5) |
| Racec | ||
| White | 346 (98.9%) | 689 (99.3%) |
| Black | 1 (0.3%) | 3 (0.4%) |
| Other | 3 (0.9%) | 2 (0.3%) |
| BMI at blood draw (kg/m2)c | ||
| <18.5 | 8 (2.3%) | 14 (2.0%) |
| 18.5–22.4 | 106 (30.5%) | 238 (34.4%) |
| 22.5–24.9 | 72 (20.7%) | 156 (22.5%) |
| 25–29.9 | 88 (25.3%) | 158 (22.8%) |
| 30–34.9 | 37 (10.6%) | 61 (8.8%) |
| 35–39.9 | 23 (6.6%) | 45 (6.5%) |
| 40+ | 14 (4.0%) | 20 (2.9%) |
| BMI at age 18 (kg/m2)c | ||
| <18.5 | 54 (15.6%) | 110 (16.0%) |
| 18.5–22.4 | 212 (61.1%) | 413 (59.9%) |
| 22.5–24.9 | 45 (13.0%) | 105 (15.2%) |
| 25–29.9 | 25 (7.2%) | 47 (6.8%) |
| 30–34.9 | 8 (2.3%) | 7 (1.0%) |
| 35–39.9 | 3 (0.9%) | 6 (0.9%) |
| 40+ | 0 (0.0%) | 1 (0.1%) |
| Parityc | ||
| Nulliparous | 105 (30.3%) | 147 (21.2%) |
| 1 | 45 (13.0%) | 97 (14.0%) |
| 2 | 122 (35.3%) | 249 (36.0%) |
| 3 | 55 (15.9%) | 154 (22.3%) |
| 4+ | 19 (5.5%) | 45 (6.5%) |
| Infertility historyc | ||
| Yes | 97 (27.7%) | 209 (30.1%) |
| No | 253 (72.3%) | 485 (69.9%) |
BMI, body mass index.
aLaparoscopically confirmed cases of endometriosis were matched 1:2 with controls of comparable age, race, infertility history, menopausal status at diagnosis, as well as month, time of day and fasting status at blood draw.
bResults expressed as mean (standard deviation). Age at blood draw ranges between 32 and 52 years.
cResults expressed as n (%). Some categories may not sum to 100 due to rounding. Additionally, four women were missing BMI at blood draw, eight women were missing BMI at age 18 and six women were missing parity.
Figure 1.
Absolute levels of leptin and adiponectin in endometriosis cases and controls. Absolute levels of leptin and adiponectin expressed in µg/ml among 350 case and 694 control subjects in the NHS II cohort. Box denotes median with inter-quartile range (Q1–Q3). Whiskers represent the minimum and maximum values. P-values compare absolute adipokine levels between case and control subjects and were calculated using mixed effect models.
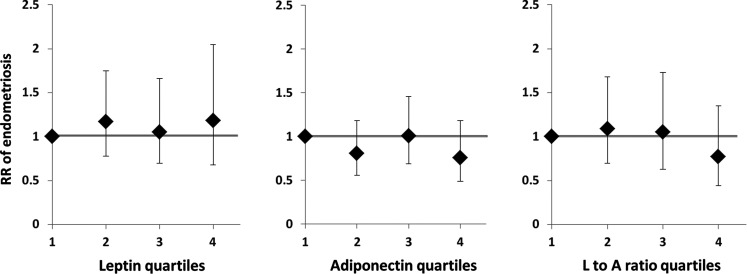
Unadjusted analyses suggested an increased diagnosis of endometriosis among women in the highest quartile of plasma leptin and decreased diagnosis among women in the highest quartile of total plasma adiponectin (data not shown). However, when comparing the highest with the lowest quartiles after adjusting for BMI, there were no significant associations between endometriosis and leptin (RR: 1.2; 95% CI: 0.7–2.0; Ptrend = 0.72), adiponectin (RR: 0.8; 95% CI: 0.5–1.2; Ptrend = 0.48) or the leptin to adiponectin ratio (RR: 0.8; 95% CI: 0.4–1.4; Ptrend = 0.14) (Fig. 2). In addition, the lowest quartile of leptin, total adiponectin and leptin to adiponectin ratio were compared with the upper three quartiles to assess for the presence of a ‘threshold effect’ beyond which the risk of endometriosis changed significantly, but no such thresholds were identified (data not shown).
Figure 2.
RR of endometriosis by the quartile of leptin, adiponectin and leptin to adiponectin ratio. Unconditional regression model of the RR of endometriosis based on increasing quartiles of leptin, adiponectin and leptin to adiponectin ratio among 1044 women in the NHS II cohort, adjusting for age, race, infertility status and BMI. Diamonds represent RRs; error bars denote 95% CIs; bold line illustrates the null effect.
Data were then stratified by BMI to assess whether the relation between adipokines and endometriosis differed between normal (BMI <25 kg/m2) when compared with overweight and obese (BMI ≥25 kg/m2) women (Table II). No difference was seen in the RR of endometriosis with increasing leptin, adiponectin or leptin to adiponectin ratio between normal weight and overweight women (P-value, test for heterogeneity ≥0.11 for all). Tests for trend within each BMI group were also not significant (P-value, test for trend ≥0.08 for all).
Table II.
Leptin, total adiponectin, leptin to adiponectin ratio and risk of laparoscopically confirmed endometriosis in the NHS II blood cohort, stratified by BMI (kg/m2)a.
| BMI < 25 (n = 594) | BMI ≥ 25 (n = 446) | |
|---|---|---|
| Leptin (µg/ml) | ||
| Q1 (<9.7) | 1.0 (referent) | 1.0 (referent) |
| Q2 (9.7–16.5) | 1.2 (0.8–1.7) | 0.4 (0.1–2.2) |
| Q3 (16.6–30.6) | 1.2 (0.8–2.0) | 0.2 (0.1–1.4) |
| Q4 (≥30.7) | 1.3 (0.5–3.4) | 0.3 (0.1–1.7) |
| P-value, test for trend | 0.40 | 0.98 |
| P-value, test for heterogeneity | 0.47 | |
| Total adiponectin (µg/ml) | ||
| Q1 (<4.7) | 1.0 (referent) | 1.0 (referent) |
| Q2 (4.7–6.4) | 0.7 (0.4–1.2) | 0.9 (0.6–1.5) |
| Q3 (6.5–8.9) | 0.9 (0.5–1.5) | 1.3 (0.7–2.2) |
| Q4 (≥9.0) | 0.8 (0.5–1.4) | 0.6 (0.3–1.2) |
| P-value test for trend | 0.83 | 0.37 |
| P-value, test for heterogeneity | 0.62 | |
| Leptin to adiponectin ratio | ||
| Q1 (<0.17) | 1.0 (referent) | 1.0 (referent) |
| Q2 (0.17–0.41) | 2.0 (0.7–5.9) | 0.9 (0.6–1.4) |
| Q3 (0.42–0.86) | 1.6 (0.6–4.6) | 1.0 (0.5–1.9) |
| Q4 (≥0.87) | 1.2 (0.4–3.5) | 1.3 (0.4–3.8) |
| P-value test for trend | 0.08 | 0.75 |
| P-value, test for heterogeneity | 0.36 | |
aResults expressed as RRs and 95% CIs calculated using unconditional logistic regression models adjusting for age, race, infertility history, menopausal status at diagnosis, month and time of day of blood draw. Test for heterogeneity was assessed using the likelihood ratio test comparing the model with main effects only with the model with main effects and interaction terms.
To account for the probability that endometriosis had been present prior to surgical diagnosis, subanalyses were conducted restricting to women who were diagnosed with endometriosis more than 2 years after blood draw and, separately, restricting to women who were diagnosed with endometriosis at least 4 years after blood draw. There remained no observed association between the RR of endometriosis and increasing leptin, total adiponectin or leptin to adiponectin ratio, regardless of the time from blood collection to diagnosis (Ptrend≥ 0.17 for all comparisons, data not shown).
Discussion
The principal finding of this study was that plasma leptin and total adiponectin levels were not predictive of endometriosis when collected prior to disease diagnosis. The results were unchanged when data were adjusted for or stratified by BMI, or when restricted to women who were diagnosed at least 4 years after blood collection.
Case–control studies have consistently demonstrated higher levels of leptin (Matarese et al., 2000; Mahutte et al., 2003; Wu et al., 2010) and lower levels of adiponectin (Takemura et al., 2005a,b) in the serum and peritoneal fluid of women with endometriosis. Recent studies have begun to explore the mechanisms by which adipokines exert inflammatory or immunomodulatory actions in endometriosis. Peritoneal fluid leptin concentrations have been positively correlated with the percentage of CD4+ T helper cells in women with endometriosis (Milewski et al., 2008). Additionally, functional leptin receptors identified on peritoneal macrophages have been shown to promote COX-2 expression and production of prostaglandin F2α in response to leptin stimulation (Wu et al., 2010). Using a murine model of endometriosis, Styer et al. (2008) demonstrated that disruption of leptin signaling impaired establishment and proliferation of endometriotic lesions and restricted associated microvascularization, suggesting that leptin may be a necessary factor in the early development of endometriosis. In the present study, adipokine levels did not differ between women with and without endometriosis when collected an average of 2.6 years in advance of disease diagnosis—suggesting that these previously identified associations between adipokines and endometriosis may be indicative of the process at the time of surgical diagnosis or after rather than a causative factor.
The relation between BMI and endometriosis further complicates the role of adipokines in the pathogenesis of the disease. Epidemiologic studies have demonstrated a consistent inverse correlation between BMI and endometriosis (Missmer et al., 2004; Ferrero et al., 2005; Hediger et al., 2005). Our own analysis of the NHS II cohort reveals that a woman's current BMI as well as her BMI at age 18 are both significantly inversely related to her risk of developing endometriosis (Missmer et al., 2004). Meanwhile, circulating leptin levels are well known to be proportional to body fat mass (Halaas et al., 1995; Friedman and Halaas, 1998). Given these relations, one would therefore anticipate leptin to be inversely correlated to the risk of endometriosis—contrary to the findings of the aforementioned case–control studies. Our data did not demonstrate an association between plasma adipokine levels and endometriosis risk in a multivariate model adjusting for BMI. We were similarly unable to identify a differential association of adipokines and endometriosis risk when compared between BMI strata. Taken together, these results suggest that the epidemiologic association between low BMI and increased risk of endometriosis may be mediated independent of adipokine concentrations.
A key strength of this study is that blood samples were collected an average of 2.6 years prior to diagnosis of endometriosis. The prospective study design uniquely permits evaluation of the temporality between inflammation and endometriosis, and the study results suggest that the presence of inflammatory adipokines in the serum and peritoneal fluid of women with endometriosis may be secondary to the disease process rather than a causative factor. This observation is further strengthened by our ability to evaluate the relation by the time from blood collection to surgical diagnosis. In addition, the ability to select population-based controls minimizes the bias introduced by many studies that restrict controls to women with no evidence of endometriosis on laparoscopy. Such a design biases associations in an unpredictable direction, driven by the unknown relation between adipokines and the pathology that prompted the need for laparoscopy among the control population. Finally, the large sample size affords the ability to conduct multivariate analyses adjusting for key confounders such as BMI.
This study has several limitations. The mean age at diagnosis of endometriosis in the study population is 41.7, ∼10 years older than the mean age of diagnosis in the general population and in this cohort as a whole. This is attributable to the design of the NHS II blood collection, which among the women originally enrolled into the cohort between the ages of 25 and 42, did not collect blood until a minimum of 7 years after enrollment. While this may limit the generalizability of the results, there is no reason to suspect that the association between adipokines and endometriosis risk should differ at a younger age of diagnosis in an adult population.
A second limitation is the inability to differentiate the time of endometriosis ‘diagnosis’ from the time of disease ‘onset’ due to the impossibility in identifying a precise time point at which the disease process was first initiated at a molecular or cellular level. Although the study results allow one to reasonably conclude that adipokine levels drawn prior to diagnosis of endometriosis do not predict subsequent development of disease, it is harder to conclusively assess whether adipokine levels were truly altered prior to the onset of disease. The null association between adipokine levels and risk of endometriosis identified in the main analysis persisted when data were analyzed separately in women whose blood draw occurred >2 years and >4 years before diagnosis of endometriosis, suggesting that undiagnosed endometriosis at the time of blood draw, if present, had little impact on the results.
The NHS II cohort does not have detailed information regarding the stage of endometriosis for all case women. As disease stage has not been shown to correlate with symptoms (Porpora et al., 1999) or prognosis, there is little reason to believe that it is significantly correlated with body size. Although it is possible that the requisite laparoscopic confirmation preferentially selects more severe cases of endometriosis by overlooking women with asymptomatic or medically controlled disease, studies have failed to demonstrate increased severity of endometriosis among women with laparoscopically confirmed disease (Sangi-Haghpeykar and Poindexter, 1995). Indeed, the prevalence of stage I/II (minimal/mild) disease within the NHS II has been estimated to be 61% based on surgical record abstraction.
A final concern may be that controls were not restricted to those who had undergone laparoscopy to exclude the presence of endometriosis, and it is therefore likely that some proportion of the control group had asymptomatic disease. As mentioned above, restricting controls to those women with other surgical diagnoses introduces intractable bias, thus invalidating the ‘true’ association with endometriosis relative to healthy women. Although the inclusion of undiagnosed cases among the controls may bias our results toward the null, this misclassification has been minimized by design; as each case was matched with two controls on the basis of infertility history, it is unlikely that both controls had undiagnosed endometriosis. The community prevalence of endometriosis in populations free of infertility or pelvic pain is thought to be <2% (Zondervan, et al., 2002), and the small influence of this proportion is further minimized as sample size is increased.
In summary, plasma leptin and adiponectin levels were not predictive of endometriosis when collected an average of 31 months prior to disease diagnosis. Adjusting for or stratifying by BMI further attenuated the results. Previously observed altered levels of adipokines in women with endometriosis may therefore be a result of the endometriosis disease process rather than an etiologic factor.
Authors' roles
All authors have had substantial contributions to the conception and design of these data, as well as its analysis and interpretation. D.K.S. has drafted the article and all other authors have revised it critically for intellectual content. All authors have had approved the final version for publication.
Funding
This study was supported by research grants HD48544, HD52473 and HD57210 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The NHS II is supported by the Public Health Service grant CA50385 from the National Cancer Institute, NIH, U.S. Department of Health and Human Services. H.R.H. is supported by NIH training grant T32 ES007069 and MCHB grant number 5T76MC00001 (formerly MCJ201).
Conflict of interest
None declared.
References
- Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- Brakenhielm E, Veitonmaki N, Cao R, Kihara S, Matsuzawa Y, Zhivotovsky B, Funahashi T, Cao Y. Adiponectin-induced antiangiogenesis and antitumor activity involve caspase-mediated endothelial cell apoptosis. Proc Natl Acad Sci USA. 2004;101:2476–2481. doi: 10.1073/pnas.0308671100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- Cramer DW, Wilson E, Stillman RJ, Berger MJ, Belisle S, Schiff I, Albrecht B, Gibson M, Stadel BV, Schoenbaum SC. The relation of endometriosis to menstrual characteristics, smoking, and exercise. JAMA. 1986;255:1904–1908. [PubMed] [Google Scholar]
- Darrow SL, Vena JE, Batt RE, Zielezny MA, Michalek AM, Selman S. Menstrual cycle characteristics and the risk of endometriosis. Epidemiology. 1993;4:135–142. doi: 10.1097/00001648-199303000-00009. [DOI] [PubMed] [Google Scholar]
- Fantuzzi G, Faggioni R. Leptin in the regulation of immunity, inflammation, and hematopoiesis. J Leukoc Biol. 2000;68:437–446. [PubMed] [Google Scholar]
- Ferrero S, Anserini P, Remorgida V, Ragni N. Body mass index in endometriosis. Eur J Obstet Gynecol Reprod Biol. 2005;121:94–98. doi: 10.1016/j.ejogrb.2004.11.019. [DOI] [PubMed] [Google Scholar]
- Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- Gao X, Outley J, Botteman M, Spalding J, Simon JA, Pashos CL. Economic burden of endometriosis. Fertil Steril. 2006;86:1561–1572. doi: 10.1016/j.fertnstert.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Goldstein BJ, Scalia R. Adiponectin: a novel adipokine linking adipocytes and vascular function. J Clin Endocrinol Metab. 2004;89:2563–2568. doi: 10.1210/jc.2004-0518. [DOI] [PubMed] [Google Scholar]
- Greenland S. Modeling and variable selection in epidemiologic analysis. Am J Public Health. 1989;79:340–349. doi: 10.2105/ajph.79.3.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- Halme J, Hammond MG, Hulka JF, Raj SG, Talbert LM. Retrograde menstruation in healthy women and in patients with endometriosis. Obstet Gynecol. 1984;64:151–154. [PubMed] [Google Scholar]
- Hediger ML, Hartnett HJ, Louis GM. Association of endometriosis with body size and figure. Fertil Steril. 2005;84:1366–1374. doi: 10.1016/j.fertnstert.2005.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahutte NG, Matalliotakis IM, Goumenou AG, Vassiliadis S, Koumantakis GE, Arici A. Inverse correlation between peritoneal fluid leptin concentrations and the extent of endometriosis. Hum Reprod. 2003;18:1205–1209. doi: 10.1093/humrep/deg233. [DOI] [PubMed] [Google Scholar]
- Matalliotakis IM, Arici A, Cakmak H, Goumenou AG, Koumantakis G, Mahutte NG. Familial aggregation of endometriosis in the Yale Series. Arch Gynecol Obstet. 2008;278:507–511. doi: 10.1007/s00404-008-0644-1. [DOI] [PubMed] [Google Scholar]
- Matarese G, Alviggi C, Sanna V, Howard JK, Lord GM, Carravetta C, Fontana S, Lechler RI, Bloom SR, De Placido G. Increased leptin levels in serum and peritoneal fluid of patients with pelvic endometriosis. J Clin Endocrinol Metab. 2000;85:2483–2487. doi: 10.1210/jcem.85.7.6703. [DOI] [PubMed] [Google Scholar]
- Milewski L, Barcz E, Dziunycz P, Radomski D, Kaminski P, Roszkowski PI, Korczak-Kowalska G, Malejczyk J. Association of leptin with inflammatory cytokines and lymphocyte subpopulations in peritoneal fluid of patients with endometriosis. J Reprod Immunol. 2008;79:111–117. doi: 10.1016/j.jri.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Missmer SA, Hankinson SE, Spiegelman D, Barbieri RL, Marshall LM, Hunter DJ. Incidence of laparoscopically confirmed endometriosis by demographic, anthropometric, and lifestyle factors. Am J Epidemiol. 2004;160:784–796. doi: 10.1093/aje/kwh275. [DOI] [PubMed] [Google Scholar]
- Nagle CM, Bell TA, Purdie DM, Treloar SA, Olsen CM, Grover S, Green AC. Relative weight at ages 10 and 16 years and risk of endometriosis: a case–control analysis. Hum Reprod. 2009;24:1501–1506. doi: 10.1093/humrep/dep048. [DOI] [PubMed] [Google Scholar]
- Parazzini F, Chiaffarino F, Surace M, Chatenoud L, Cipriani S, Chiantera V, Benzi G, Fedele L. Selected food intake and risk of endometriosis. Hum Reprod. 2004;19:1755–1759. doi: 10.1093/humrep/deh395. [DOI] [PubMed] [Google Scholar]
- Porpora MG, Koninckx PR, Piazze J, Natili M, Colagrande S, Cosmi EV. Correlation between endometriosis and pelvic pain. J Am Assoc Gynecol Laparosc. 1999;6:429–434. doi: 10.1016/s1074-3804(99)80006-1. [DOI] [PubMed] [Google Scholar]
- Sampson JA. Metastatic or embolic endometriosis, due to the menstrual dissemination of endometrial tissue into the venous circulation. Am J Pathol. 1927;3:93–110.43. [PMC free article] [PubMed] [Google Scholar]
- Sangi-Haghpeykar H, Poindexter AN., III Epidemiology of endometriosis among parous women. Obstet Gynecol. 1995;85:983–992. doi: 10.1016/0029-7844(95)00074-2. [DOI] [PubMed] [Google Scholar]
- Sierra-Honigmann MR, Nath AK, Murakami C, Garcia-Cardena G, Papapetropoulos A, Sessa WC, Madge LA, Schechner JS, Schwabb MB, Polverini PJ, et al. Biological action of leptin as an angiogenic factor. Science. 1998;281:1683–1686. doi: 10.1126/science.281.5383.1683. [DOI] [PubMed] [Google Scholar]
- Signorello LB, Harlow BL, Cramer DW, Spiegelman D, Hill JA. Epidemiologic determinants of endometriosis: a hospital-based case-control study. Ann Epidemiol. 1997;7:267–741. doi: 10.1016/s1047-2797(97)00017-3. [DOI] [PubMed] [Google Scholar]
- Styer AK, Sullivan BT, Puder M, Arsenault D, Petrozza JC, Serikawa T, Chang S, Hasan T, Gonzalez RR, Rueda BR. Ablation of leptin signaling disrupts the establishment, development, and maintenance of endometriosis-like lesions in a murine model. Endocrinology. 2008;149:506–514. doi: 10.1210/en.2007-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura Y, Osuga Y, Harada M, Hirata T, Koga K, Morimoto C, Hirota Y, Yoshino O, Yano T, Taketani Y. Serum adiponectin concentrations are decreased in women with endometriosis. Hum Reprod. 2005a;20:3510–3513. doi: 10.1093/humrep/dei233. [DOI] [PubMed] [Google Scholar]
- Takemura Y, Osuga Y, Harada M, Hirata T, Koga K, Yoshino O, Hirota Y, Morimoto C, Yano T, Taketani Y. Concentration of adiponectin in peritoneal fluid is decreased in women with endometriosis. Am J Reprod Immunol. 2005b;54:217–221. doi: 10.1111/j.1600-0897.2005.00300.x. [DOI] [PubMed] [Google Scholar]
- Vigano P, Somigliana E, Matrone R, Dubini A, Barron C, Vignali M, di Blasio AM. Serum leptin concentrations in endometriosis. J Clin Endocrinol Metab. 2002;87:1085–1087. doi: 10.1210/jcem.87.3.8286. [DOI] [PubMed] [Google Scholar]
- Wu MH, Huang MF, Chang FM, Tsai SJ. Leptin on peritoneal macrophages of patients with endometriosis. Am J Reprod Immunol. 2010;63:214–221. doi: 10.1111/j.1600-0897.2009.00779.x. [DOI] [PubMed] [Google Scholar]
- Zondervan KT, Cardon LR, Kennedy SH. What makes a good case–control study? Design issues for complex traits such as endometriosis. Hum Reprod. 2002;17:1415–1423. doi: 10.1093/humrep/17.6.1415. [DOI] [PubMed] [Google Scholar]