Abstract
STUDY QUESTION
Does the selection of sperm for ICSI based on their ability to bind to hyaluronan improve the clinical pregnancy rates (CPR) (primary end-point), implantation (IR) and pregnancy loss rates (PLR)?
SUMMARY ANSWER
In couples where ≤65% of sperm bound hyaluronan, the selection of hyaluronan-bound (HB) sperm for ICSI led to a statistically significant reduction in PLR.
WHAT IS KNOWN AND WHAT THIS PAPER ADDS
HB sperm demonstrate enhanced developmental parameters which have been associated with successful fertilization and embryogenesis. Sperm selected for ICSI using a liquid source of hyaluronan achieved an improvement in IR. A pilot study by the primary author demonstrated that the use of HB sperm in ICSI was associated with improved CPR. The current study represents the single largest prospective, multicenter, double-blinded and randomized controlled trial to evaluate the use of hyaluronan in the selection of sperm for ICSI.
DESIGN
Using the hyaluronan binding assay, an HB score was determined for the fresh or initial (I-HB) and processed or final semen specimen (F-HB). Patients were classified as >65% or ≤65% I-HB and stratified accordingly. Patients with I-HB scores ≤65% were randomized into control and HB selection (HYAL) groups whereas patients with I-HB >65% were randomized to non-participatory (NP), control or HYAL groups, in a ratio of 2:1:1. The NP group was included in the >65% study arm to balance the higher prevalence of patients with I-HB scores >65%. In the control group, oocytes received sperm selected via the conventional assessment of motility and morphology. In the HYAL group, HB sperm meeting the same visual criteria were selected for injection. Patient participants and clinical care providers were blinded to group assignment.
PARTICIPANTS AND SETTING
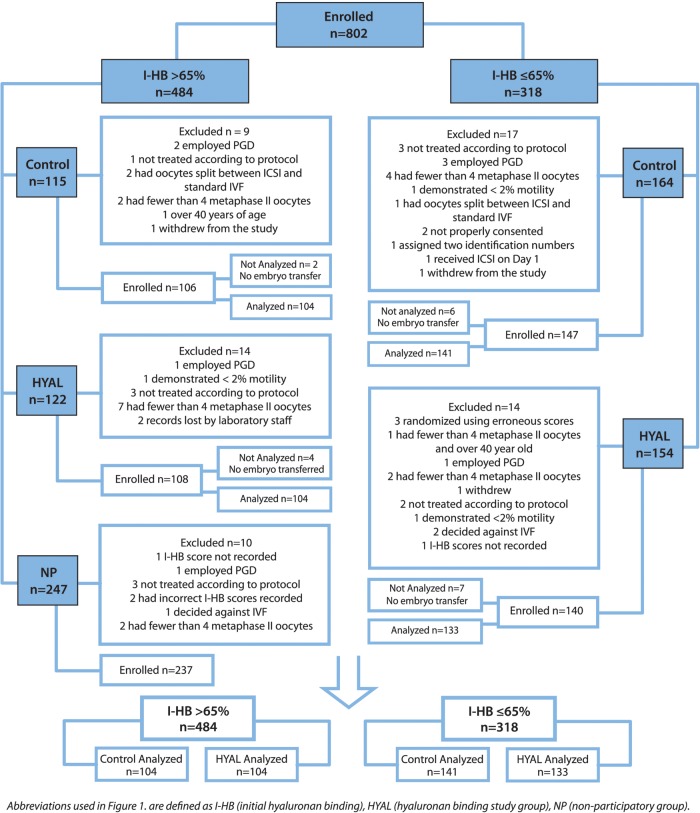
Eight hundred two couples treated with ICSI in 10 private and hospital-based IVF programs were enrolled in this study. Of the 484 patients stratified to the I-HB > 65% arm, 115 participants were randomized to the control group, 122 participants were randomized to the HYAL group and 247 participants were randomized to the NP group. Of the 318 patients stratified to the I-HB ≤ 65% arm, 164 participants were randomized to the control group and 154 participants were randomized to the HYAL group.
MAIN RESULTS AND THE ROLE OF CHANCE
HYAL patients with an F-HB score ≤65% demonstrated an IR of 37.4% compared with 30.7% for control [n = 63, 58, P > 0.05, (95% CI of the difference −7.7 to 21.3)]. In addition, the CPR associated with patients randomized to the HYAL group was 50.8% when compared with 37.9% for those randomized to the control group (n = 63, 58, P > 0.05). The 12.9% difference was associated with a risk ratio (RR) of 1.340 (RR 95% CI 0.89–2.0). HYAL patients with I-HB and F-HB scores ≤65% revealed a statistically significant reduction in their PLR (I-HB: 3.3 versus 15.1%, n = 73, 60, P = 0.021, RR of 0.22 (RR 95% CI 0.05–0.96) (F-HB: 0.0%, 18.5%, n = 27, 32, P = 0.016, RR not applicable due to 0.0% value) over control patients. The study was originally planned to have 200 participants per arm providing 86.1% power to detect an increase in CPR from 35 to 50% at α = 0.05 but was stopped early for financial reasons. As a pilot study had demonstrated that sperm preparation protocols may increase the HB score, the design of the current study incorporated a priori collection and analysis of the data by both the I-HB and the F-HB scores. Analysis by both the I-HB and F-HB score acknowledged the potential impact of sperm preparation protocols.
BIAS, CONFOUNDING AND OTHER REASONS FOR CAUTION
Selection bias was controlled by randomization. Geographic and seasonal bias was controlled by recruiting from 10 geographically unique sites and by sampling over a 2-year period. The potential for population effect was controlled by adjusting for higher prevalence rates of >65% I-HB that naturally occur by adding the NP arm and to concurrently recruit >65% and ≤65% I-HB subjects. Monitoring and site audits occurred regularly to ensure standardization of data collection, adherence to the study protocol and subject recruitment. Subgroup analysis based on the F-HB score was envisaged in the study design.
GENERALIZABILITY TO OTHER POPULATIONS
The study included clinics using different sperm preparation methods, located in different regions of the USA and proceeded in every month of the year. Therefore, the results are widely applicable.
STUDY FUNDING/COMPETING INTEREST(S)
This study was funded by Biocoat, Inc., Horsham, PA, USA. The statistical analysis plan and subsequent analyses were performed by Sherrine Eid, a biostatistician. The manuscript was prepared by Kathryn C. Worrilow, Ph.D. and the study team members. Biocoat, Inc. was permitted to review the manuscript and suggest changes, but the final decision on content was exclusively retained by the authors. K.C.W is a scientific advisor to Biocoat, Inc. S.E. is a consultant to Biocoat, Inc. D.W. has nothing to disclose. M.P., S.S., J.W., K.I., C.K. and T.E. have nothing to disclose. G.D.B. is a consultant to Cooper Surgical and Unisense. J.L. is on the scientific advisory board of Origio.
TRIAL REGISTRATION NUMBER
Introduction
The in vitro selection of sperm for ICSI is critical and directly influences the paternal contribution to preimplantation embryogenesis. Oligozoospermic men requiring ICSI often carry semen populations, demonstrating increased chromosomal aberrations and compromised DNA integrity. Studies have associated a higher incidence of de novo numerical chromosomal aberrations (Simpson and Lamb, 2001), cytogenetically detectable structural chromosomal aberrations (Bonduelle et al., 2002), chromosomal aneuploidies (Van Steirteghem et al., 2002) and sex chromosome disomies (Palermo et al., 2000) in the embryos resulting from ICSI. Although the primary candidates for ICSI are oligozoospermic men, the use of ICSI is on the rise, thus potentially exposing a broader range of couples to the potential risks inherent to ICSI.
Sperm are most commonly selected for ICSI via the microscopic assessment of motility and morphology. Visual assessment alone can allow the inadvertent selection of sperm with compromised developmental, nuclear and cytoplasmic competence. Chromosomal aberrations readily occur in sperm classified as normal via Strict Kruger morphological assessment (Celik-Ozenci et al., 2004). Disomic and diploid sperm have been identified in all categories of morphological classification (Zavaczki et al., 2006). Such findings question the value of the use of morphology in the selection of sperm for ICSI.
Absent in the process of in vitro sperm selection is the in vivo selection of the functionally most competent sperm afforded by the cumulus cells and the zona pellucida surrounding the oocyte. Within the female reproductive tract, hyaluronan may play a critical role in the selection of functionally competent sperm during in vivo fertilization. Hyaluronan, a high molecular weight glycosaminoglycan, is a major component of the cumulus oophorus matrix surrounding the human oocyte. Similar to the binding observed between sperm and the zona pellucida, developmentally mature sperm bind to cross-linked hyaluronan gels and to hyaluronan chemically attached to a base structure (Huszar, 1999).
The in vitro binding of sperm to hyaluronan is a selective process. Research has demonstrated that not all motile sperm bind to hyaluronan (Huszar et al., 2003). The understanding of the physiology underlying the binding of sperm to hyaluronan has been enhanced by the study of biochemical markers of human sperm development and function. Markers of human sperm development and function (Huszar and Vigue, 1993; Huszar et al., 1994, 1997, 2003; Sakkas et al., 1999; Kovanci et al., 2001; Cayli et al., 2004; Huszar, 2012) are enhanced in hyaluronan-bound (HB) sperm when compared with those parameters found in unbound sperm. HB sperm have completed the spermiogenic process of cytoplasmic extrusion and demonstrate enhanced levels of the testis-expressed HspA2 chaperone protein (Jakab et al., 2005). HB sperm are devoid of DNA fragmentation and the apoptotic marker, caspase-3 (Cayli et al., 2004). Most significantly, sperm bound to hyaluronan display a reduced frequency of chromosomal aneuploidies in comparison to their nonbinding counterparts (Kovanci et al., 2001; Jakab et al., 2005). Each of these biochemical and molecular parameters of developmental maturity play a critical role in the paternal contribution to successful preimplantation embryogenesis. The combined impact of DNA fragmentation and compromised levels of nuclear and cytoplasmic sperm maturation will adversely influence the developing embryo.
A pilot study of 240 patients led to the design of the current randomized study. ROC curve analysis of the resulting data indicated that a 65% binding score was the optimal cutoff point for benefit of in vitro selection of HB sperm in ICSI (Worrilow et al., 2006). Patients with a binding score of ≤65% have a decreased chance of having an HB sperm randomly selected for ICSI. It can be assumed that the likelihood of selecting an HB sperm is high in those specimens with a higher binding score. The design of the current study therefore included patients with >65% binding to confirm the findings of the preliminary study.
The in vitro selection of sperm for ICSI is critical and visual assessment alone may allow the isolation and selection of sperm carrying various levels of pathogenesis. The enhanced levels of developmental maturity and genetic integrity associated with HB sperm suggest that the use of HB sperm in ICSI may serve to improve the paternal contribution to the embryo and thus clinical outcomes. The current randomized study therefore sought to examine and compare the use of HB sperm to those sperm selected via conventional and visual means in the treatment of patients requiring ICSI.
Materials and Methods
Study end-points
The primary end-point of this study was clinical pregnancy rate (CPR) as defined by the presence of at least one positive fetal heartbeat within an intrauterine fetal sac. Secondary end-points included implantation rate (IR) and pregnancy loss rate (PLR).
Clinical trial sites
The study was a prospective, multicenter, double-blinded, randomized Institutional Review Board (IRB)-approved study involving 802 consented couples receiving ICSI as a component of their assisted reproductive therapy (ART). Ten in vitro fertilization (IVF) programs located throughout the USA participated in the study. Each of the clinical trial sites, protocol and patient consent were approved by a governing IRB. The staff at each site were trained in the protocol, patient consenting and data collection process. An audit of each site evaluating the study protocol, data collection, patient confidentiality and consenting process was completed ∼6–9 months after the site-initiated patient enrollment. Patient enrollment occurred over a 2-year period. The study was registered with the Clinical Trial website (www.clinicaltrials.gov) and was assigned the identifier, NCT00741494.
Study design and patient randomization
The study design was a prospective, stratified, randomized, double-blinded, controlled trial comparing clinical outcomes in couples undergoing ICSI using sperm selected based upon the visual examination of morphology and motility alone (control) versus those sperm that underwent an initial hyaluronan binding step followed by final selection using the visual parameters (HYAL). Patients were divided into two cohorts based upon the proportion of HB sperm in their unprocessed or initial semen (I-HB score). The two cohorts were divided based on an I-HB ≤65% versus >65% because ROC curve analysis of data from a pilot study of 240 patients indicated this as the optimal cutoff point (Worrilow et al., 2006). Patients with an I-HB score ≤65% were randomized to control or HYAL groups. However, as pilot studies indicated that more patients demonstrated an I-HB score >65% than ≤65%, patients with an I-HB score >65% were randomized into one of three groups; control, HYAL, or a nonparticipating (NP) group in a 1:1:2 proportion (Fig. 1) in order to balance the numbers of participants with high and low I-HB scores. The addition of the NP group controlled for seasonality and any unexpected variability in the laboratory environment. Without inclusion of the NP group, the data could have been disproportionately affected.
Figure 1.
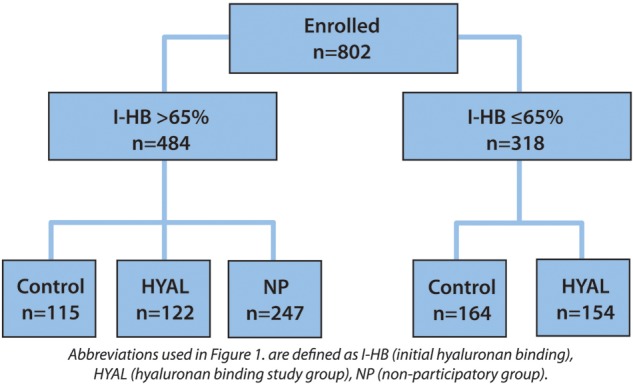
Study design and patient randomization.
Control oocytes received sperm selected via the visual and live assessment of gross morphology and motility. HB sperm meeting the same criteria were used in the ICSI of the HYAL oocytes. The standard ICSI protocols specific to each clinical trial site were followed throughout the duration of the study.
Randomization protocol
Patients were randomly assigned to control, HYAL or NP groups with restrictions using a permuted block design with a computer random number generator. Block randomization and the allocation sequence were provided by a computer generated random number list prepared by an investigator with no clinical involvement in the trial. Each block size was fixed at 200 participants. Stratification was used to define the blocks based on I-HB scores of >65% and ≤65%. The allocation sequence was concealed from the participant, the clinical care providers and the embryologists by retaining the entire sequence with the primary study team (principal investigator and study coordinator). Following patient consent for participation in the clinical trial, the embryologist called the principal investigator or the study coordinator to receive the allocation assignment after providing the I-HB score. The participant identification number was reported to the site embryologist, assigned and recorded on a master log throughout the enrollment period. All participants and the clinical care providers were blinded to the allocated arm. Only embryologists were aware of the group to which the participant was allocated.
Closure of study
During the initial interim analysis, it was noted that the accrual rate was lower than the anticipated rate and the pattern continued to slow during the following year of enrollment. It was decided by the sponsor to close the study to accrual prematurely on 31 December 2010. Calculations based upon the accrual rate at that time indicated that it would take at least an additional 2 years to obtain the sample size and power originally planned. The sponsor could not afford the cost of an additional 2 years and felt that delaying the release of the results of the study could affect patient care in a negative manner. The premature closure of accrual impacted the power of the primary end-point of CPR.
Inclusion and exclusion criteria
Included in the study were IVF patients who received ICSI as part of their ART treatment and whose care was being managed by the staff of the participating IVF programs. Excluded from the study were patients using testicular sperm, donor or cryopreserved gametes, patients receiving preimplantation genetic diagnosis or sperm sorting procedures and patients receiving a partial ICSI where only a proportion of the oocytes received ICSI. Patients whose maternal age was >40 years, who produced fewer than four metaphase II oocytes at the time of their oocyte retrieval, demonstrated an I-HB score < 2%, or a sperm count <10 000 motile sperm/milliliter (ml) were excluded from the study.
Sperm preparation
Semen was collected by masturbation on the day of the oocyte retrieval. The HB score of fresh and processed semen specimens were evaluated using the HBA® Sperm Hyaluronan Binding Assay, a dual chambered slide containing an attached layer of hyaluronan located beneath two individual coverslips (Biocoat, Inc., Horsham, PA). Following the manufacturer's instructions (Origio, Inc., 2011), the HB score was determined by calculating the number of motile HB sperm/the number of total motile sperm. Bound sperm are distinctly different from unbound in that they demonstrate rapidly beating tails with no progressive movement while unbound sperm swim freely. After an initial assessment of volume, the number of motile sperm/ml, % motility and I-HB score, sperm were subjected to centrifugation on a discontinuous gradient and washed with sperm processing media according to the protocol specific for the participating site. The processed or final sperm suspension was scored for the number of motile sperm/ml, % motility and HB score (F-HB). I-HB and F-HB data was collected and analyzed based upon the results of the pilot study (Worrilow et al., 2007), which suggested a possible impact of sperm preparation on the F-HB score. As the sperm used in the injection of oocytes originated from the final sperm preparation, the data were collected.
The final sperm suspension of patients in the control group was placed into standard ICSI dishes for selection. The final sperm suspension of HYAL patients was placed upon microdots of hyaluronan in the PICSI® Sperm Selection Device (Biocoat, Inc., Horsham, PA ) and overlaid with oil. Following a 5-to-10 min incubation period, HB sperm were selected following the manufacturer's instructions (Origio, Inc., 2012). Those sperm demonstrating vigorous tail beating, an absence of progressive motility and clearly bound to the hyaluronan microdots were isolated by aspiration into the ICSI micropipette. HB sperm were placed into media drops for subsequent injection into the oocytes.
Statistical analysis
Data within and between the clinical trial sites were tested for homogeneity of variance and normalcy. No transformations were necessary. Descriptive statistics of demographic variables were reported as mean + standard deviation. Differences in hCG, presence of an intrauterine fetal sac, fetal cardiac activity, IR, CPR and PLR were evaluated. IR was defined as the number of intrauterine sacs/the number of embryos transferred to the patient and was calculated per patient. A clinical pregnancy was defined as the presence of fetal cardiac activity within an intrauterine gestational sac. PLR was assessed by vaginal ultrasound and was defined in the study as the proportion of patients demonstrating an intrauterine sac at 5–7 weeks of gestation who subsequently demonstrated an absence of fetal cardiac activity at 8–10 weeks of gestation. All data were analyzed and evaluated by both I-HB and F-HB scores. Data were analyzed using independent t-tests, Pearson's chi square and Fisher's exact test in SPSS 15.0 (IBM Stat, SPSS, Chicago, IL). Kruskal–Wallis tests were performed to evaluate differences in the median number of embryos transferred by patient on Days 2, 3, 5 and 6. Median and interquartile ranges are reported. Logistic regression models were run a priori to examine the influence of the I-HB and F-HB scores and collected independent variables on the likelihood of PLR. A P value of <0.05 was considered statistically significant.
Power calculations for the randomized clinical trial were carefully considered and were based upon the results obtained from the pilot study (Worrilow et al., 2007) With the proposed sample size of 200 each for the ≤65% I-HB score control and HYAL groups, the study was powered at 86.1% to detect an increase in CPR from 35 to 50% at α = 0.05. The difference between the control and HYAL populations was estimated and reported with a precision at the 95.0% confidence interval (CI) ∼±0.10 points. Specifically, an observed difference of 15% in the CPR would be reported with a 95.0% CI 0.05–0.25.
Results
Patient demographics and descriptive parameters
Overall, patient demographics and descriptive parameters demonstrated no statistically significant differences when comparing the patients randomized to the control and HYAL groups (Supplementary Table SI). Analysis of patients with an I-HB score >65% demonstrated that there were no statistically significant differences in demographics and descriptive parameters between those patients randomized to the control, HYAL or NP groups [Supplementary Table SI (A and C)]. Additional analysis of demographic and descriptive parameters of those patients within the I-HB ≤ 65% and F-HB ≤ 65% groups also demonstrated no statistically significant differences [Supplementary Table SI (A and B)].
Within the overall study population, the average female and male age was 33 and 36 years, respectively. An average of 11 Metaphase II oocytes and 8.6 2PN zygotes were produced. The average fertilization rate was 76%. Male patients demonstrated an average sperm concentration of 60 million sperm/ml, normal morphology of 15.6% and an I-HB and F-HB of 59 and 74%, respectively. In the≤65% arm, the mean I-HB and F-HB score was 38 and 42%, respectively. In the >65% arm, the mean I-HB and F-HB was 81 and 89%, respectively.
Of the 802 couples consented in the overall study, 484 patients demonstrated I-HB scores of >65%. Of these, 115 were randomized to the control group, 122 to the HYAL group and 247 to the NP group. Of the 802 couples, 318 demonstrated low I-HB scores of ≤65%. Of these, 164 were randomized to the control group and 154 to the HYAL group. Of the 802 couples, 64 were withdrawn from the study. Of the 64 patients withdrawn, 35 did not meet eligibility criteria, 24 patients' procedures deviated from the protocol, 4 were lost to attrition and 1 was recorded twice (Fig. 2).
Figure 2.
Study design and patient randomization.
Relative to those patients dropped from the study, of the 115 patients initially enrolled in the >65% I-HB control group, 9 patients were dropped for not meeting the inclusion criteria. Of the 106 patients accepted and enrolled, 2 did not receive embryo transfers and thus, 104 patients were included in the analysis. Of the 247 patients initially enrolled in the >65% I-HB NP group, 10 patients were dropped for not meeting the inclusion criteria. Of the 122 patients initially enrolled in the >65% I-HB HYAL group, 14 patients were dropped for not meeting the inclusion criteria. Of the 108 patients accepted and enrolled, 4 did not receive embryo transfers and thus, 104 patients were included in the analysis. Of the 164 patients initially enrolled in the ≤65% I-HB control group, 17 patients were dropped for not meeting the inclusion criteria. Of the 147 patients accepted and enrolled, 6 did not receive embryo transfers and consequently, 141 patients were included in the analysis. Of the 154 patients initially enrolled in the ≤ 65% I-HB HYAL group, 14 patients were dropped for not meeting the inclusion criteria. Of the 140 patients accepted and enrolled, 7 did not receive embryo transfers and consequently, 133 patents were included in the analysis (Fig. 2).
Throughout the clinical trial, there were no demonstrated negative effects associated with measures of preimplantation embryogenesis and the use of hyaluronan in sperm selection for ICSI. Use of hyaluronan-facilitated sperm selection did not therefore exert any observed harmful effects to the recipient oocytes or resulting embryos.
Initial (I-HB) and final (F-HB) scores
Patients were placed into ≤65% and >65% HB cohort groups based upon their I-HB score. The F-HB score was determined and collected as the pilot study demonstrated that sperm processing may influence the number of HB sperm within a specimen (Worrilow et al., 2007). The HB score was often increased in the final sperm suspension. Approximately 56% (153/274) of patients with an I-HB score of ≤65% exhibited a F-HB score >65%. As the F-HB score was a more accurate reflection of those sperm used in the injection of all oocytes, the data were evaluated by both I-HB and F-HB scores. Despite the influence of sperm preparation on the HB score, the benefits of using HB sperm in ICSI were evident.
Implantation rates (IR) and clinical pregnancy rates
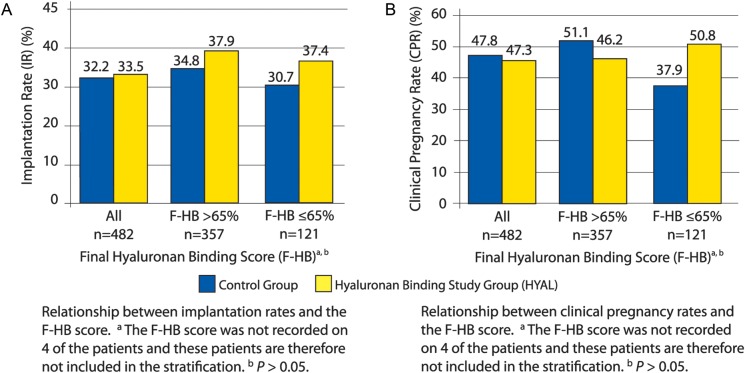
Stratification by HB scores demonstrated that patients with an F-HB score ≤65% randomized to the HYAL group carried an IR of 37.4% when compared with 30.7% in those patients randomized to the control group [n = 63, 58, P > 0.05, 95% CI of the difference (−7.7 to 21.3)] (Fig. 3A). When stratified by F-HB scores, HYAL patients demonstrated a CPR of 50.8% when compared with 37.9% in the control group demonstrating a difference of 12.9% and a relative risk ratio of 1.340 [n = 63, 58, P > 0.05, 95% CI (0.89–2.0)] (Fig. 3B). As the F-HB score decreased, there was a greater difference between the CPR demonstrated by the control and HYAL groups. A Kruskal–Wallis test evaluating the statistical influence of the number of embryos transferred and the day of the embryo transfer between HYAL and control groups within the overall data, I-HB ≤ 65% and F-HB ≤ 65% groups revealed no statistically significant differences. The resulting IR and CPR data generated by the clinical trial were therefore not influenced by the number of embryos transferred or by the day of the embryo transfer (Supplementary Table SII)
Figure 3.
A: Implantation rate. B: Clinical pregnancy rate.
Pregnancy loss rate (PLR)
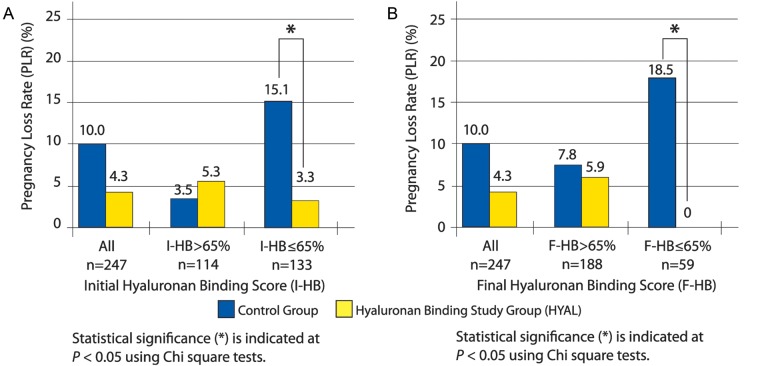
Overall, those patients randomized to the HYAL group demonstrated a PLR of 4.3% compared with those assigned to the control group who were associated with a PLR of 10.0% (n = 130, 117, P = 0.067) (Fig. 4A and B). Stratifying by I-HB scores, HYAL patients with an I-HB score ≤65% demonstrated a statistically significant reduction in PLR over control patients [HYAL: 3.3%, control: 15.1%, n = 73, 60, P = 0.021, risk ratio (RR) 0.22 (RR 95% CI 0.05–0.96)] (Fig. 4A). As the HB score decreased, the statistically significant difference in PLR increased between the control and HYAL groups. There were no pregnancy losses in HYAL patients demonstrating an I-HB score ≤ 50%.
Figure 4.
A: Pregnancy loss rate by I-HB score. B: Pregnancy loss rate by F-HB score.
Stratifying by F-HB scores, HYAL patients with F-HB ≤ 65% demonstrated a statistically significant decrease in PLR when compared with the control group (HYAL: 0%, control: 18.5%, n = 27, 32, P = 0.016, RR not applicable due to 0.0% value) (Fig. 4B). As the F-HB score decreased, the PLRs increased in the control group. There were no pregnancy losses in the HYAL group in F-HB ≤ 65%. Kruskal–Wallis analysis evaluated the statistical influence of the number of embryos transferred and the day of the embryo transfer between HYAL and control groups within the overall data, I-HB ≤ 65% and F-HB ≤ 65% groups and revealed no statistically significant differences. The resulting PLR data generated by the clinical trial was therefore not influenced by the number of embryos transferred or by the day of the embryo transfer (Supplementary Table SII).
Although the study was not initially designed to perform multivariate analysis, a logistic regression model was performed and did not show increased contributions to the likelihood of PLR by either group or strata.
Discussion
This multicenter clinical trial represents the single largest prospective and randomized study evaluating the use of hyaluronan in the selection of sperm for ICSI. In couples with an I-HB and F-HB score of ≤65%, the study demonstrated a statistically significant decrease in the PLR when HB sperm were selected compared with visual selection alone. This was accompanied by improvements in IR and CPR that failed to reach statistical significance. While these results are exciting, it must be pointed out that the PLR is a secondary end-point and this is a weakness of the study. These clinical results support the conclusions offered by previous studies of biochemical and molecular markers of human sperm development and function demonstrating a relationship between HB selected sperm and increased levels of developmental maturity (Huszar et al., 1994, 2003; Cayli et al., 2004), nuclear (Kovanci et al., 2001; Jakab et al., 2005;) and cytoplasmic integrity (Huszar and Vigue, 1993; Allen et al., 1996; Dix et al., 1996; Huszar et al., 1997; Sakkas et al., 1999).The lack of statistical significance for the IR and CPR results may be because the study was initially powered for 86.1% but was closed to accrual before the calculated sample size was reached.
A similar increase in IR and CPR was demonstrated in the pilot study of 240 couples that provided the foundation for the current clinical study (Worrilow et al., 2006, 2007). One other study (Parmegiani et al., 2010) compared conventional sperm selection to the use of sperm selected from a liquid source of hyaluronan and demonstrated an increase in the IR from 10.3 to 17.1%. These studies and our data have demonstrated no negative effects associated with measures of preimplantation embryogenesis and the use of hyaluronan in sperm selection for ICSI. Use of hyaluronan-facilitated sperm selection did not therefore exert any observed harmful effects to the recipient oocytes or resulting embryos.
The individual sperm selected for ICSI will determine the degree of paternal pathogenesis shared with the resulting embryo. The integrity of the DNA in selected sperm cannot be assessed visually and can be a major determinant in the overall success of the ICSI procedure. Defects in sperm chromatin have been linked to natural and assisted reproductive failures (Bungum et al., 2007; Carrell et al., 2007). Additional studies have demonstrated that pregnancy outcome is inversely related to the DNA fragmentation index (DFI). Although the DFI value did not negatively affect fertilization and early measures of embryogenesis (Tesarik et al., 1986; Braude et al., 1988), it was associated with late paternal effects causing a decreased blastocyst conversion and IRs (Benchaib et al., 2003; Virro et al., 2004; Seli and Sakkas, 2005). In a systematic review of 11 studies involving 1549 IVF and ICSI cycles, sperm DNA damage was statistically and positively correlated with pregnancy loss (Zini et al., 2008). Finally, diminished HspA2 chaperone activity as seen in developmentally immature sperm has been associated with diminished delivery of DNA repair enzymes and thus increased DNA chain breaks and fragmentation (Allen et al., 1996; Dix et al., 1996; Eddy, 1999; Huszar et al., 2000). There is significant evidence that suggests that the decreased levels of the expression of the HspA2 chaperone protein correlate with both sperm cellular development and IVF clinical outcomes (Huszar et al., 1992, 2000; Ergur et al., 2002). The inadvertent selection of sperm with compromised DNA will exert negative effects on preimplantation embryogenesis and on clinical outcomes.
There is substantial evidence suggesting that HB sperm demonstrate enhanced DNA and chromosomal integrity. Acridine orange fluorescent analysis of HB sperm demonstrated an absence of DNA fragmentation (Yagci et al., 2010). Moreover, FISH analysis demonstrated the effectiveness of HB sperm selection in eliminating aneuploid and diploid sperm in normospermic and oligospermic populations. Despite the level of chromosomal aberration found within the fresh semen population, the HB sperm within the same population demonstrated dramatically reduced levels of aneuploidy and diploidy and were within the range of normospermic men. The frequency of chromosomal disomy, diploidy and sex chromosome disomy was significantly reduced (Jakab et al., 2005). Selection of HB sperm, therefore, provided a substantial improvement to the semen population. Sperm selection using hyaluronan binding provides, for the first time, a means to select individual sperm of enhanced genetic and developmental integrity for use in ICSI. Consequently, the use of HB sperm in ICSI may directly influence the genetic integrity of the paternal contribution to the conceptus, minimizing the potential risks inherent to ICSI (Jakab et al., 2005; Yagci et al., 2010).
Visual selection of sperm for ICSI can allow the inadvertent isolation of sperm with compromised nuclear and developmental parameters. The degree of paternal pathogenesis that can be conveyed to the embryo is substantial. The use of the binding phenomenon between sperm and hyaluronan facilitates the selection of individual sperm with increased developmental and cytoplasmic maturity, nuclear integrity and functional competence thereby reducing the potentially adverse effects exerted by the pathogenesis inherent in the paternal genome. This study demonstrated that where the proportion of HB binding sperm is ≤65%, sperm selection by hyaluronan binding resulted in a statistically significant decrease in PLR and promising although not significant improvements in IR and CPR. Some of the noted improvements were more pronounced if subjects were stratified by the F-HB rather than I-HB score. These results indicate that sperm selection by hyaluronan binding is both promising and significant to improved patient care in patients with a low (≤65%) F-HB score. The statistically significant reduction in PLR, however, was observed in patients with a low (≤65%) I-HB and low (≤65%) F-HB score suggesting that HB sperm selection should be used in patients with an I-HB score of ≤65%. Use of the I-HB score as the determination for the use of HB sperm in ICSI would provide the best balance between providing unnecessary treatment versus denying patients treatment that may improve their clinical outcome. Additional studies are encouraged which incorporate a larger sample size and PLR as a primary end-point such that the beneficial impact of the use of HB sperm in ICSI can be further evaluated.
Supplementary data
Supplementary data are available at http://humrep.oxfordjournals.org/.
Authors' roles
K.C.W.: conception and design, analysis and interpretation, manuscript preparation and critical discussion, S.E.: study design, data collection, analysis and interpretation, manuscript preparation, S.S., J.W., K.I., C.K., T.E. and J.L.: execution of study and acquisition of data, D.W., M.P., G.D.B.: execution of study, acquisition of data and critical discussion.
Funding
Biocoat, Inc. provided necessary HBA and PICSI products, all administrative costs and a small stipend to each participating IVF program. Funding to pay the Open Access publication charges for this article was provided by Biocoat, Inc.
Conflict of interest
K.C.W. is a scientific advisor to Biocoat, Inc., Horsham, PA, USA. S.E. is a consultant to Biocoat, Inc., Horsham, PA, USA. D.W., M.P., S.S., J.W., K.I., C.K. and T.E. have nothing to disclose. G.D.B. is a consultant to Cooper Surgical and Unisense. J.L. is on the scientific advisory board of Origio.
Supplementary Material
Acknowledgements
We thank each of the patients whom participated in our study, our colleagues at each of the clinical trial sites and Marsha Timmerman, M.S. and James Johnston, Ph.D. for all of their work toward the study, data collection and review.
References
- Allen JW, Dix DJ, Collins BW, Merrick BA, He C, Selkirk JK, Poorman-Allen P, Dresser M, Eddy E. Hsp70-2 is part of the synaptonemal complex in mouse and hamster spermatocytes. Chromosoma. 1996;104:414–421. doi: 10.1007/BF00352265. doi:10.1007/BF00352265. [DOI] [PubMed] [Google Scholar]
- Benchaib M, Braun V, Lornage J, Hadj S, Salle B, Lejeune H, Guerin J. Sperm DNA fragmentation decreases the pregnancy rate in an assisted reproductive technique. Hum Reprod. 2003;18:1023–1028. doi: 10.1093/humrep/deg228. doi:10.1093/humrep/deg228. [DOI] [PubMed] [Google Scholar]
- Bonduelle M, Van Assche E, Joris H, Keymolen K, Devroey P, Van Steirteghem A, Liebaers I. Prenatal testing in ICSI pregnancies: incidence of chromosomal anomalies in 1586 karyotypes and relation to sperm parameters. Hum Reprod. 2002;17:2600–2614. doi: 10.1093/humrep/17.10.2600. doi:10.1093/humrep/17.10.2600. [DOI] [PubMed] [Google Scholar]
- Braude P, Bolton V, Moore S. Human gene expression first occurs between the four and eight-cell stages of preimplantation development. Nature. 1988;332:459–461. doi: 10.1038/332459a0. doi:10.1038/332459a0. [DOI] [PubMed] [Google Scholar]
- Bungum M, Humaidan P, Axmon A, Spano M, Bungum L, Erenpreiss J, Giwercman A. Sperm DNA integrity assessment in prediction of assisted reproduction technology outcome. Hum Reprod. 2007;22:174–179. doi: 10.1093/humrep/del326. doi:10.1093/humrep/del326. [DOI] [PubMed] [Google Scholar]
- Carrell DT, Emery BR, Hammoud S. Altered protamine expression and diminished spermatogenesis: what is the link? Hum Reprod Update. 2007;13:313–327. doi: 10.1093/humupd/dml057. doi:10.1093/humupd/dml057. [DOI] [PubMed] [Google Scholar]
- Cayli S, Sakkas D, Vigue L, Demir R, Huszar G. Cellular maturity and apoptosis in human sperm: creatine kinase, caspase-3 and Bclx expression in mature and diminished maturity spermatozoa. Mol Hum Reprod. 2004;10:365–372. doi: 10.1093/molehr/gah050. doi:10.1093/molehr/gah050. [DOI] [PubMed] [Google Scholar]
- Celik-Ozenci C, Jakab A, Kovacs T, Catalanotti J, Demir R, Bray-Ward P, Ward D, Huszar G. Sperm selection for ICSI: shape properties do not predict the absence or presence of numerical chromosomal aberrations. Hum Reprod. 2004;19:2052–2059. doi: 10.1093/humrep/deh361. doi:10.1093/humrep/deh361. [DOI] [PubMed] [Google Scholar]
- Dix DJ, Allen JW, Collins BW, Mori C, Nakamura N, Poorman-Allen P. Targeted gene disruption of Hsp70–2 results in failed meiosis, germ cell apoptosis, and male infertility. Proc Natl Acad Sci USA. 1996;93:3264–3268. doi: 10.1073/pnas.93.8.3264. doi:10.1073/pnas.93.8.3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy EM. Role of heat shock protein Hsp70–2 in spermatogenesis. Rev Reprod. 1999;4:23–30. doi: 10.1530/ror.0.0040023. [DOI] [PubMed] [Google Scholar]
- Ergur A, Dokras A, Giraldo J, Habana A, Kovanci E, Huszar G. Sperm maturity and treatment choice of in vitro fertilization (IVF) or intracytoplasmic sperm injection: diminished sperm HspA2 chaperone levels predict IVF failure. Fertil Steril. 2002;77:910–918. doi: 10.1016/s0015-0282(02)03073-x. doi:10.1016/S0015-0282(02)03073-X. [DOI] [PubMed] [Google Scholar]
- Huszar G. Process and system for selection of mature sperm by surface membrane determinants for assisted reproduction. 1999. U.S. Patent 5,897,988.
- Huszar G. Sperm testing and ICSI selection by hyaluronic acid binding: the hyaluronic acid-coated glass slide and petri dish in the andrology and IVF laboratories. In: Nagy ZP, Varghese AC, Agarwal A, editors. Practical Manual of In Vitro Fertilization: Advanced Methods and Novel Devices. New York: Springer; 2012. pp. 241–257. [Google Scholar]
- Huszar G, Vigue L. Incomplete development of human spermatozoa is associated with increased creatine phosphokinase concentration and abnormal head morphology. Mol Reprod Dev. 1993;34:292–298. doi: 10.1002/mrd.1080340309. doi:10.1002/mrd.1080340309. [DOI] [PubMed] [Google Scholar]
- Huszar G, Vigue L, Morphed M. Sperm creatine phosphokinase M-isoform ratios and fertilizing potential of men: a blinded study of 84 couples treated with in vitro fertilization. Fertil Steril. 1992;57:882–888. [PubMed] [Google Scholar]
- Huszar G, Vique L, Oehninger S. Creatine kinase immunocytochemistry of human sperm-hemizona complexes: selective binding of sperm with mature creatine kinase-staining pattern. Fertil Steril. 1994;61:136–142. doi: 10.1016/s0015-0282(16)56466-8. [DOI] [PubMed] [Google Scholar]
- Huszar G, Sbracia M, Vigue L, Miller DJ, Shur BD. Sperm plasma membrane remodeling during spermiogenetic maturation in men: relationship among plasma membrane beta 1,4-galactosyltransferase, cytoplasmic creatine phosphokinase, and creatine phosphokinase isoform ratios. Biol Reprod. 1997;56:1020–1024. doi: 10.1095/biolreprod56.4.1020. doi:10.1095/biolreprod56.4.1020. [DOI] [PubMed] [Google Scholar]
- Huszar G, Stone K, Dix D, Vigue L. Putative creatine kinase M-isoform in human sperm is identified as the 70-kilodalton heat shock protein HspA2. Biol Reprod. 2000;63:925–932. doi: 10.1095/biolreprod63.3.925. doi:10.1095/biolreprod63.3.925. [DOI] [PubMed] [Google Scholar]
- Huszar G, Ozenci C, Cayli S, Zavaczki Z, Hansch E, Vique L. Hyaluronic acid binding by human sperm indicates cellular maturity, viability and unreacted acrosomal status. Fertil Steril. 2003;79(Suppl. 3):1616–1624. doi: 10.1016/s0015-0282(03)00402-3. doi:10.1016/S0015-0282(03)00402-3. [DOI] [PubMed] [Google Scholar]
- Jakab A, Sakkas D, Delpiano E, Cayli S, Kovanci E, Ward D, Ravelli A, Huszar G. Intracytoplasmic sperm injection: a novel selection method for sperm with normal frequency of chromosomal aneuploidies. Fertil Steril. 2005;84:1665–1673. doi: 10.1016/j.fertnstert.2005.05.068. doi:10.1016/j.fertnstert.2005.05.068. [DOI] [PubMed] [Google Scholar]
- Kovanci E, Kovacs T, Moretti E, Bray-Ward P, Ward DC, Huszar G. FISH assessment of aneuploidy frequencies in mature and immature human spermatozoa classified by the absence or presence of cytoplasmic retention. Hum Reprod. 2001;16:1209–1217. doi: 10.1093/humrep/16.6.1209. doi:10.1093/humrep/16.6.1209. [DOI] [PubMed] [Google Scholar]
- Origio, Inc. 2011. Instructions for Use: HBA® Sperm Hyaluronan Binding Assay http://www.origio.com/download%20center/quality%20and%20regulatory%20documents/instructions%20for%20use/~/media/files_to_download/IFU/MAD/HBA%20IFU%20For%20US%20 Distribution%20Only%202352%20Rev%20A%20LM%20201108.19.ashx. (21 November 2012, date last accessed)
- Origio, Inc. 2012. PICSI® Sperm Selection Device: Instructions for Use http://www.origio.com/download%20center/quality%20and%20regulatory%20documents/instructions%20for%20use/~/media/files_to_download/IFU/MAD/PICSI%20US%20ONLY%20IFU%20283200%20Rev%20A%20LM%2020110819.ashx. (21 November 2012, date last accessed)
- Palermo GD, Neri QV, Hariprashad JJ, Davis OK, Veeck LL, Rosenwaks Z. ICSI and its outcome. Semin Reprod Med. 2000;18:161–169. doi: 10.1055/s-2000-12555. doi:10.1055/s-2000-12555. [DOI] [PubMed] [Google Scholar]
- Parmegiani L, Cognigni GE, Ciampaglia W, Bernardi S, Troilo E, Filicori M. ‘Physiologic ICSI:’ Hyaluronic acid (HA) favors selection of sperm without DNA fragmentation and with normal nucleus, resulting in improvement of embryo quality. Fertil Steril. 2010;93:598–604. doi: 10.1016/j.fertnstert.2009.03.033. doi:10.1016/j.fertnstert.2009.03.033. [DOI] [PubMed] [Google Scholar]
- Sakkas D, Mariethoz E, St John JC. Abnormal sperm parameters in humans are indicative of an abortive apoptotic mechanism linked to the Fas mediated pathway. Exp Cell Res. 1999;251:350–351. doi: 10.1006/excr.1999.4586. doi:10.1006/excr.1999.4586. [DOI] [PubMed] [Google Scholar]
- Seli E, Sakkas D. Spermatozoal nuclear determinants of reproductive outcome: implications for ART. Hum Reprod Update. 2005;11:337–349. doi: 10.1093/humupd/dmi011. doi:10.1093/humupd/dmi011. [DOI] [PubMed] [Google Scholar]
- Simpson JL, Lamb DJ. Genetic effects of intracytoplasmic sperm injection. Semin Reprod Med. 2001;19:239–249. doi: 10.1055/s-2001-18043. doi:10.1055/s-2001-18043. [DOI] [PubMed] [Google Scholar]
- Tesarik J, Kopecny V, Plachot M, Mandelbaum J. Activation of nucleolar and extranucleolar RNA synthesis and changes in ribosomal content of human embryos developing in vitro. J Reprod Fertil. 1986;78:463–470. doi: 10.1530/jrf.0.0780463. doi:10.1530/jrf.0.0780463. [DOI] [PubMed] [Google Scholar]
- Van Steirteghem A, Bonduelle M, Devroey P, Liebaers I. Follow-up of children born after ICSI. Hum Reprod Update. 2002;8:111–116. doi: 10.1093/humupd/8.2.111. doi:10.1093/humupd/8.2.111. [DOI] [PubMed] [Google Scholar]
- Virro MR, Larson-Cook KL, Evenson DP. Sperm chromatin structure assay (SCSA) parameters are related to fertilization, blastocyst development, and ongoing pregnancy in in vitro fertilization and intracytoplasmic sperm injection cycles. Fertil Steril. 2004;81:1289–1295. doi: 10.1016/j.fertnstert.2003.09.063. doi:10.1016/j.fertnstert.2003.09.063. [DOI] [PubMed] [Google Scholar]
- Worrilow KC, Huynh HT, Bower J, Peters AJ, Johnston JB. The clinical impact associated with the use of PICSI-derived embryos. Fertil Steril. 2006;86(Suppl. 3):S62. doi:10.1016/j.fertnstert.2006.07.169. [Google Scholar]
- Worrilow KC, Huynh HT, Bowers JB, Anderson A, Schillings W, Crain J. PICSI versus ICSI: Statistically significant improvement in clinical outcomes in 240 in vitro fertilization (IVF) patients. Fertil Steril. 2007;88(Suppl. 1):S37. doi:10.1016/j.fertnstert.2007.07.133. [Google Scholar]
- Yagci A, Murk W, Stronk J, Huszar G. Spermatozoa bound to solid state hyaluronic acid show chromatin structure with high DNA chain integrity: An acridine orange fluorescence study. J Androl. 2010;31:566–572. doi: 10.2164/jandrol.109.008912. doi:10.2164/jandrol.109.008912. [DOI] [PubMed] [Google Scholar]
- Zavaczki Z, Celik-Ozenci C, Ovari L, Jakab A, Sati G, Ward D, Huszar G. Dimensional assessment of X-bearing and Y-bearing haploid and disomic human sperm with the use of fluorescence in situ hybridization and objective morphometry. Fertil Steril. 2006;85:121–127. doi: 10.1016/j.fertnstert.2005.07.1295. doi:10.1016/j.fertnstert.2005.07.1295. [DOI] [PubMed] [Google Scholar]
- Zini A, Borman JM, Belzile E, Ciampi A. Sperm DNA damage is associated with an increased risk of pregnancy loss after IVF and ICSI: Systematic review and meta-analysis. Hum Reprod. 2008;23:2663–2668. doi: 10.1093/humrep/den321. doi:10.1093/humrep/den321. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.