Abstract
The reasons for differences in vulnerability or resilience to the development of posttraumatic stress disorder (PTSD) are unclear. Here we review key genetic diatheses and molecular targets especially signaling pathways that mediate responses to trauma and severe stress and their potential contribution to the etiology of PTSD. Sensitization of glucocorticoid receptor (GR) signaling and dysregulation of GR modulators FKBP5, STAT5B, Bcl-2, and Bax have been implicated in PTSD pathophysiology. Furthermore, Akt, NFκB, MKP-1, and p11, which are G protein-coupled receptor (GPCR) pathway molecules, can promote or prevent sustained high anxiety and depressive-like behavior following severe stress. Agonist-induced activation of the corticotropin-releasing factor CRF1 receptor is crucial for survival in the context of serious danger or trauma, but persistent CRF1 receptor hypersignaling when a threatening or traumatic situation is no longer present is maladaptive. CRF1 receptor single nucleotide polymorphisms (SNPs) can confer susceptibility or resilience to childhood trauma while a SNP for the PAC1 receptor, another class B1 GPCR, has been linked genetically to PTSD. GRK3 phosphorylation of the CRF1 receptor protein and subsequent binding of βarrestin2 rapidly terminate Gs-coupled CRF1 receptor signaling by homologous desensitization. A deficient GRK-βarrestin2 mechanism would result in excessive CRF1 receptor signaling thereby contributing to PTSD and co-morbid posttraumatic depression. Clinical trials are needed to assess if small molecule CRF1 receptor antagonists are effective prophylactic agents when administered immediately after trauma. βarrestin2-biased agonists for CRF receptors and possibly other GPCRs implicated in PTSD, however, may prove to be novel pharmacotherapy with greater selectivity and therapeutic efficacy.
Keywords: small molecule CRF1 receptor antagonist, biased agonist
1. Introduction
Although activation of stress systems is critical for survival in the context of internal or external threats to homeostasis, rapid counter-regulation of the stress response systems is equally important for re-establishing normal mood, neuroendocrine, autonomic, immune, and metabolic functioning upon threat termination (Bale & Vale 2004; Feder et al 2009; Hauger et al 2006; Juster et al 2010). Abuse or deprivation early in life or exposure to traumatic, uncontrollable stress at any age can permanently increase an individual’s responsiveness to further stress and reduce ability to cope with aversive events (Heim et al 2008). An important conceptualization of “resilience” relates to the threshold at which particular perturbations activate stress systems, as well as the rapidity and degree to which stress responses cease with termination of the aversive stimulus (Feder et al 2009; Gillespie et al 2009; Juster et al 2010). Cell signaling abnormalities may determine vulnerability to the detrimental consequences of trauma and severe stress.
Posttraumatic stress disorder (PTSD) occurs in some but not all who are exposed to trauma or severe stress with the risk for developing PTSD following trauma ranging from 5 to 31% (Kessler et al 1995; Skelton et al 2011), with the most commonly accepted prevalence being in the vicinity of 15%. The reasons for these differences in prevalence are not completely clear, but likely relate to heterogeneity in the populations studied in terms of severity and type of trauma, pre-existing traumatic episodes, and criteria used for diagnosis. For example, diagnostic criteria for PTSD outlined in the Diagnostic and Statistical Manual (APA 2000) are different from those specified in the International Classification of Diseases (World Health Organization 1992). PTSD poses a considerable health and societal burden due to its severity and chronicity, and high rates of comorbidity with major depression and bipolar illness, increased risk of suicide, and marked psychosocial and occupational impairment (Bauer et al 2005; Kessler et al 1995; Nemeroff et al 2006). Furthermore, PTSD patients have an increased incidence of coronary artery disease, chronic inflammation, metabolic syndrome, and early mortality for unknown reasons (Ahmadi et al 2011; Pace & Heim 2011; Rasmusson et al 2010). Recently PTSD patients with a history of childhood trauma were found to have abnormally short telomere length that can accelerate biological aging (O’Donovan et al 2011a). Because preventing PTSD and improving PTSD treatment and outcome are urgent clinical issues, understanding the molecular and cellular mechanisms that confer PTSD susceptibility and chronicity is a high priority.
Earlier studies showed that the intensity and duration of stress exposure determine a significant proportion of an adult’s risk for developing PTSD following a traumatic event (Kessler et al 1995; Nemeroff et al 2006). Accordingly, the incidence of PTSD is particularly high in soldiers traumatized by intense combat and in police, firefighters, and other civilian personnel routinely exposed to violence or life-threatening emergencies (Nemeroff et al 2006). Responsiveness to and recovery from trauma in adulthood can also be modulated by the early developmental environment (Gillespie et al 2009; Heim et al 2008). Individuals subjected to severe abuse and deprivation during their childhood later as adults exhibit dysregulation of the hypothalamic-pituitary-adrenocortical (HPA) axis, persistently severe anxiety, and a high susceptibility to developing PTSD and major depression when exposed to trauma or severe stress (Heim et al 2008; Yehuda et al 2010). Violent assaults occurring before the age of 15 increase the risk of PTSD 5-fold following a traumatic event in adulthood (Breslau et al 1999).
Genetic and molecular abnormalities may determine whether an individual is susceptible to the predisposing effect of severe childhood stress on the development of PTSD or major depression in adulthood. Likewise, genetic and molecular differences may confer vulnerability or resilience to adult trauma (Gillespie et al 2009). The Institute of Medicine (2008) concluded that current medications used to treat PTSD lack a consistent and compelling scientific evidence base. A critical step toward developing PTSD treatments with greater specificity and efficacy is elucidating molecular targets and intracellular signaling pathways that mediate maladaptation or resilience to trauma and severe stress. This article will review candidate genes, novel molecular targets, and regulators of corticotropin releasing factor (CRF) receptor signaling that, when dysregulated, may generate core PTSD endophenotypes. CRF has been proposed to be involved in modulation of the stress response and in emotional memory consolidation, and is therefore a likely candidate for involvement in PTSD in several ways, as will be described next.
2. Potential Genes for PTSD Pathophysiology
Excellent reviews of genetic, epigenetic, and gene expression research in PTSD can be found in this special issue of Neuropharmacology (Mehta & Binder 2011; Skelton et al 2011) and elsewhere (Gillespie et al 2009; Yehuda et al 2011). A major neuroendocrine finding is that PTSD patients exhibit abnormally high glucocorticoid receptor (GR) sensitivity resulting in HPA oversuppression by corticosteroid negative feedback (Yehuda 2009). A recent study suggests that high premorbid GR expression may be a critical vulnerability factor for developing PTSD following combat trauma (van Zuiden et al 2011). The immunophilin FKBP5 is a HSP90 co-chaperone that strongly controls GR sensitivity and signaling. FKBP5 binds to GR in the cytosol thereby decreasing GR ligand affinity and nuclear translocation. FKBP5 single nucleotide polymorphisms (SNPs) have been genetically linked to following: (1) abnormal HPA regulation and brain CRF hypersecretion; (2) dissociative symptoms after trauma (a predictor of PTSD development) in children; (3) adult PTSD risk in individuals subjected to severe childhood abuse; (4) stress-induced onset and recurrence of major depression; and (5) antidepressant response (Binder et al 2008, 2009; Skelton et al 2011). One specific FKPB5 SNP, rs9296158, confers a high risk for developing PTSD in individuals traumatized as children and is associated with excessive GR sensitivity to glucocorticoid negative feedback in PTSD patients (Mehta & Binder 2011; Mehta et al 2011). A recent study found that only risk allele A carriers of rs9296158 exhibited excessive glucocorticoid negative feedback of HPA secretion with adult PTSD following childhood trauma (Mehta et al 2011). Furthermore, gene microarray studies have detected abnormally low FKBP5 expression in blood cells of PTSD patients in carriers of the FKPB5 SNP rs9296158 risk allele A (Yehuda et al 2009; Mehta et al 2011). Therefore, FKBP5 SNPs may dysregulate HPA axis function in PTSD in specific manner by selectively changing cellular expression of FKBP5 and sensitivity of GR signaling (Mehta & Binder 2011).
Gene expression studies have also found a significant reduction in STAT5B mRNA levels in blood cells from PTSD patients, especially those carrying the FKPB5 SNP rs9296158 risk allele A (Mehta et al 2011; Yehuda et al 2009). Since docking of GR at binding site on the STAT5B N-terminus inhibits GR translocation to the nucleus, a STAT5B deficiency could promote excessive GR signal transduction and GR-mediated transcription of target genes.
Since activation of the two CRF receptors expressed in the central nervous system, CRF1 and CRF2, by CRF and the related urocortin peptides mediate behavioral, cognitive, autonomic, neuroendocrine and immune responses during stress (Bale & Vale 2004; Dautzenberg & Hauger 2002; Hauger et al 2009), they have been implicated in PTSD onset and recurrence. As we will discuss later (see section 4), preclinical studies have shown that strong activation of CRF1 receptor signaling can induce severe anxiety and startle hyperreactivity while patients with severe PTSD exhibit overly active brain CRF neurotransmission and abnormal HPA regulation (Feder et al 2009; Hauger et al 2006; Risbrough et al 2004; Risbrough & Stein 2006; Yehuda 2009). SNPs in the CRF1 receptor gene have been shown to modulate emotional consolidation of aversive memories from severe stress in childhood as well as susceptibility or resilience to major depression in adulthood (Gillespie et al 2009). Individuals who are homozygous for alleles TT (SNP rs7209436) or AA (SNP rs242940) in the CRF1 receptor intron 1 are protected against adult major depression after being traumatized as children (Gillespie et al 2009). Other studies have found that the TAT haplotype formed by CRF1 receptor SNPs rs7209436, rs110402, and rs242924 confers resilience against developing depression and HPA dysregulation in adulthood following exposure to severe childhood stress (Polanczyk et al 2009; Tyrka et al 2009). Although the above CRF1 receptor polymorphisms did not alter the risk for adult PTSD after childhood abuse, CRF1 receptor SNP rs12944712 significantly predicted acute onset of PTSD in traumatized pediatric patients (Amstadter et al 2011). A recent preclinical epigenetic study reported that stress could induce CRF hypersecretion by de-methylating the CRF gene promoter (Elliot et al 2010), which may represent another mechanism whereby abnormalities in the CRF system contribute to PTSD.
Rapid agonist-induced activation of brain and anterior pituitary CRF1 receptors generates critical defensive behaviors, HPA hypersecretion, and other physiological responses required to survive trauma and stress but subsequent strong counterregulation of CRF1 receptor signal transduction is necessary to prevent stress pathology (see sections 5-6). Gs-coupled CRF1 receptor signaling is regulated by GPCR kinase 3 (GRK3) phosphorylation and βarrestin2 recruitment (Figure 1). Similarly, the PACAP receptor type 1 (PAC1), a member of the class B1 group of the GPCR superfamily, like both CRF receptors, is homologously desensitized by a GRK3-βarrestin mechanism (Dautzenberg & Hauger 2001). PAC1 receptor expression was found to be upregulated in the amygdala of mice subjected to fear conditioning and in the dorsolateral bed nucleus of the stria terminalis (BNST) of chronically stressed rats (Hammack et al 2010; Ressler et al 2011). With regard to the considerably higher incidence of PTSD in women compared to men, chronic estradiol treatment of female rats increased mRNA levels of PACAP ligand and PAC1 receptors in the BNST, while a SNP in the estrogen response element of the PAC1 receptor has been genetically linked to PTSD (Ressler et al 2011). Interestingly, PACAP and PAC1 receptors are highly expressed in the bed nucleus of the stria terminalis (BNST) and amygdala nuclei that co-express CRF and CRF1 receptors (Hammack et al 2010). In addition, PACAP-expressing neurons synapse directly on CRF-expressing neurons in the BNST and hypothalamic paraventricular nucleus (Hammack et al 2010). While PAC1 and CRF1 receptor signaling pathways may coordinate response and recovery to trauma and severe stress in a synergistic manner, additive effects of these two neuropeptide systems may be an alternative possibility. Furthermore, although speculative, dysregulation in the interaction of CRF and PAC1 receptor signaling pathways may possibly contribute to PTSD pathophysiology.
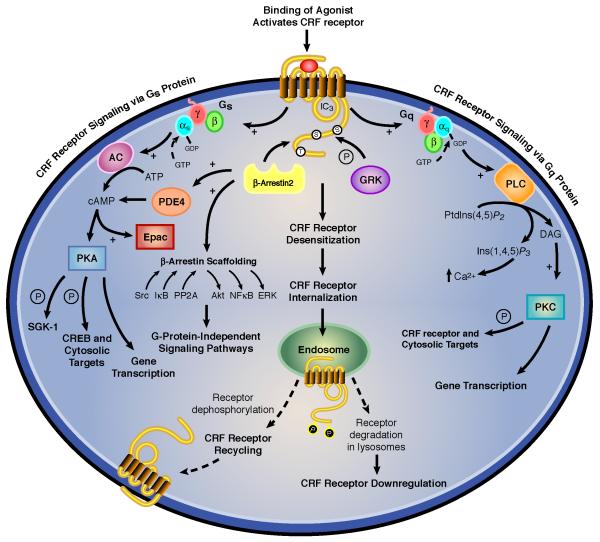
Figure 1. Regulation of intracellular signal transduction pathways for CRF receptors.
The major mode of signaling for both CRF receptors is coupling to Gsα and activating the adenylyl cyclase-protein kinase A cascade although CRF1 and CRF2 receptors can also signal via the phospholipase C-protein kinase C cascade in certain cells and neurons. Gs-coupled CRF1 receptor signaling is stringently regulated by GRK3- and βarrestin2-mediated homologous desensitization. CRF receptors can also activate Akt, NFκB, ERK, STAT, and Bax, which are potential molecular targets for PTSD possibly under regulation by βarrestin2 scaffolding and other upstream signaling proteins.
3. Molecular and Cell Signaling Targets for PTSD and Co-Morbid Depression
Recent receptor molecular biology research has revealed important findings about stress-induced anxiety and depressive disorders. After yeast two-hybrid screens identified p11 as a chaperone of serotonin (5-HT) receptors, mainly 5-HT1b and 5-HT4 receptors, p11 was shown to promote translocation of these serotonin receptors to the cell surface thereby enhance their signaling (Svenningsson et al 2006). A depressive-like phenotype is exhibited by p11 knockout mice exposed to stress, an effect that was reversed by restoring normal p11 expression (Svenningsson et al 2006; Alexander et al 2010). Consistent with this data, forebrain p11 levels are abnormally low in mice exhibiting high learned helplessness following shock stress, and in patients with unipolar depression (Svenningsson et al 2006). Similarly, mice with a siRNA-induced reduction in forebrain p11 expression develop depressive-like behavior when exposed to severe stress (Alexander et al 2010). Prefrontal p11 expression is upregulated, however, in PTSD patients and in an animal model of PTSD (Zhang et al 2008), suggesting that p11 may be differentially involved in stress vs depressive pathophysiology. Further studies are required to clarify these findings, because stress and depressive disorders are often show similarities in pathophysiology, but in this case they may be opposite.
Using whole genome arrays, a transcriptome study discovered upregulation of MAP kinase phosphatase-1 (MKP-1) in the dual specificity phosphatase 1 (DUSP1) pathway and downregulation of downstream signaling proteins MEK2, ERK2 and CREBL1 in postmortem hippocampal samples from patients with major depression (Duric et al 2010). When high hippocampal expression of MKP-1 was induced by uncontrollable stress or a viral MKP-1 transgene, rats exhibited depressive-like behavior (Duric et al 2010). Interestingly, MKP-1 KO mice did not develop anxiety- and depressive-like behavior during stress exposure (Duric et al 2010). In other work, the expression of constitutively active Akt in the ventral tegmentum (VTA) by viral gene transfer also conferred resilience to social defeat stress (Krishnan et al 2008). In contrast, mice in which endogenous Akt activity was blocked by overexpression of an Akt dominant negative protein in the VTA developed stress-induced anxiety- and depressive-like behavior (Krishnan et al 2008). Abnormally prolonged contextual and sensitized fear in response to inescapable stress has been observed, however, in mice with high levels of phosphorylated Akt in the dorsal hippocampus and basolateral amygdala (Dahloff et al 2010). Although further research is required on brain specific actions of these signaling proteins, MKP-1 and PI3K-Akt signaling pathways may be novel molecular targets for PTSD and co-morbid depression.
Cell signaling via the pro-inflammatory NFκB cascade can also mediate anxiety- and depressive-like behavior following stress (Koo et al 2010). In a recent gene microarray study, mRNA levels of NFκB and CREB/ATF were found to be upregulated in monocytes of PTSD patients (O’Donovan et al 2011b). Uncontrollable stress markedly reduces mitochondrial levels of the anti-apoptotic protein B-cell CLL/lymphoma 2 (Bcl-2) in cortical neurons and causes excessive NF-κB signaling, thereby impairing hippocampal neurogenesis (Hunsberger et al 2009; Koo et al 2010). Bag-1, another potential PTSD target, attenuates GR nuclear trafficking and potentiates Bcl-2-mediated cell survival (Hunsberger et al 2009). Severe stress also increases expression of the pro-apoptotic Bax (Bcl-2-associated X protein) and induces neuronal apoptosis in the hippocampus (Li et al 2010). These pro- and anti-apoptotic mediators may contribute to small hippocampal volume and impaired hippocampal function associated with PTSD (Acheson et al 2011).
4. CRF Receptor Signaling Regulation and PTSD Pathophysiology
Activation of the two cloned CRF receptor subtypes, CRF1 and CRF2, by CRF and urocortins mediate behavioral, cognitive, HPA, and autonomic responses to stress. Compelling evidence indicates that CRF1 receptor activation is necessary, and in many cases sufficient, to initiate anxiety-like defensive and HPA responses to stress (Bale & Vale 2004; Hauger et al 2006, 2009; Liapakis et al 2011). While CRF1 receptor activation is crucial for survival in the context of threat, persistent CRF1 receptor hypersignaling when danger is no longer present is maladaptive. Over the past decade, “hypersecretion of neuronal CRF” has been an important hypothesis for PTSD pathophysiology based on measurement of abnormally high CRF levels in the cerebrospinal fluid of PTSD patients, with the highest CRF concentrations being associated with greatest illness severity, suicide and psychosis (Baker et al 1999; Bremner et al 1997; Sautter et al 2003). PTSD patients with evidence of brain CRF hypersecretion and HPA dysregulation also exhibit startle hyperreactivity in stressful contexts (Risbrough & Stein 2004). Abnormally high CSF levels of CRF, CRF hyperexpression in forebrain neurons, and aberrant HPA functioning are also associated with major depression with depressed suicide victims having the highest CRF concentrations (Hauger et al 2006, 2009). Thus, excessive brain CRF neurotransmission may contribute to both PTSD and co-morbid depression.
Startle hyperreactivity is a cardinal manifestation of hyperarousal in PTSD. Activating CRF1 receptor signaling by CRF injection, stress, or forebrain CRF overexpression in rodents strongly potentiates startle reactivity and induces sustained anxiety-like defensive behavior (Risbrough et al 2004, 2009; Keen-Rhinehart et al 2008). Conversely, stress- and CRF-induced startle hyperreactivity are inhibited by CRF1 receptor gene knockout or selective CRF1 receptor antagonist treatment (Risbrough et al 2004, 2009). Furthermore, CRF receptor signal transduction can amplify immediate and enduring fear and anxiety responses to threatening and traumatic stimuli (Risbrough et al 2009; Adamec et al 2010). The dominant mode of CRF receptor signal transduction involves coupling of the receptor’s third intracellular loop to Gsα to activate adenylyl cyclase and generate cyclic AMP which, in turn, stimulates protein kinase A (PKA) to phosphorylate cytosolic and nuclear targets (Figure 1) (Hauger et al 2006; Liapakis et al 2011; Perrin & Vale 2002). Since pharmacological inhibition of PKA or PKC activity blocks CRF-induced startle hyperreactivity (Hauger et al 2010), excessive Gs- and Gq-coupled CRF1 receptor signaling may mediate the hypersensitive startle reflex and the severe sustained anxiety, which are cardinal PTSD symptoms.
One interesting downstream target of CRF1 receptor signaling (via the cyclic AMP-PKA cascade) is SGK-1 (Figure 1), a member of the AGC serine/threonine protein kinase family that promotes survival during cellular stress and regulates synaptic plasticity. SGK-1 expression is abnormally low both in the prefrontal cortex of rodents exhibiting learned helplessness after exposure to inescapable stress and in PTSD patients (Licznerski et al 2010). In addition, transgenic mice overexpressing a SGK-1 dominant negative mutant fail to develop stress-induced learned helplessness (Licznerski et al 2010). In hippocampal neurons, prolonged CRF1 receptor signaling via the cyclic AMP-PKA pathway likewise increases mRNA and protein levels of SGK-1 (Sheng et al 2008). Therefore, regulation of SGK-1 function by Gs-coupled CRF1 receptor signaling may be involved in central responses to severe stress and trauma.
CRF1 receptor signaling via the cyclic AMP-PKA-CREB cascade can upregulate central brain-derived neurotrophic factor (BDNF) expression (Bayatti et al 2005). Additionally, independent of PKA, Gs-coupled CRF1 receptor signaling in limbic neurons can activate Epac (exchange protein directly activated by cyclic AMP) which, in turn, potentiates BDNF-stimulated TrkB signaling by trafficking TrkB receptors to neuronal membranes (Traver et al 2006). This CRF-R1 Epac cascade promotes BDNF-induced proliferation of LC noradrenergic neurons, which may alter behavioral adaptation to trauma and severe stress. “Resilient” rats that do not develop excessive anxiety after stressful exposure to a cat (predator) exhibit retracted dendritic arbors with increased branch packing in CRF1 receptor-expressing basolateral amygdala (BLA) neurons (Mitra et al 2009). In contrast, rats developing a persistently high level of anxiety defensive behavior following severe stress have hyperarborization of BLA dendrites (Mitra et al 2009). In addition, dendritic branching of locus coeruleus neurons has been found to be mediated by phosphorylation of RhoA GTPase induced by CRF1 receptor-mediated PKA activation (Swinny & Valentino 2006).
CRF1 receptors can also signal through other cellular pathways that may be involved in PTSD pathophysiology (Figure 1). CRF1 receptors activated by CRF can stimulate a rapid phosphorylation of Akt at Ser473 that is mediated by upstream Src and PI-3 kinase (Olivares-Reyes & Hauger, unpublished data). Preclinical research has shown that activated Akt in the ventral tegmentum promotes resilience to anxiety- and depressive-like responses to stress (Krishnan et al 2008), while high levels of phosphorylated Akt in the dorsal hippocampus and basolateral amygdala prolongs contextual and sensitized fear induced by inescapable stress (Dahloff et al 2010). Therefore, the consequences of CRF1 receptor Akt signaling during trauma and severe stress may differ depending on the brain region.
CRF1 receptors can also activate the pro-inflammatory regulator NFκB and the pro-apoptotic proteins Bax and Bad (Smith et al 2006; Tsatsanis et al 2005). Uncontrollable stress markedly reduces expression of the anti-apoptotic protein Bcl-2 and increases levels of Bax in the hippocampus, thereby favoring apoptosis (Hunsberger et al 2009), while strong NF-κB signaling has been implicated in severe anxiety and stress-induced depression (Koo et al 2010). Scaffolding of IκB by βarrestin2 can inhibit translocation of NFκB into the nucleus while βArrestin2 promotes Akt signaling by dopamine D2 receptors (Figure 1) (Shenoy & Lefkowitz 2011; Whalen et al 2011). The complexity of CRF1 receptor signaling via these pro-inflammatory and apoptotic pathways requires further investigation to determine the molecular mechanisms regulating their processes and their importance in PTSD pathophysiology.
Interestingly, transgenic mice overexpressing CRF develop high limbic expression of FKBP5, a chaperone regulating GR action and genetically linked to PTSD and stress-induced depression (Peeters et al 2004) (see the earlier discussion of FKBP5 in section 2). CRF1 receptors can also interact via a PKA-CREB mechanism with STAT3, which associates with ligand-bound GR to form a transactivating signaling complex (Mynard et al 2004). Thus, potential cross-talk between CRF receptor and GR signaling pathways may play an important role in vulnerability or resilience to trauma and severe stress.
5. GRK- and βarrestin-Mediation of Homologous GPCR Desensitization
Stringent regulation of cellular signaling by GPCRs is critical for preventing the detrimental and illness-inducing effects of unrestrained receptor signaling. Coincident with the rapid generation of cellular signals by GPCRs following agonist binding is the development of an equally rapid process referred to as homologous desensitization, a process that terminates G protein-mediated signal transduction (Figure 1). Homologous desensitization requires agonist-activated receptors to selectively recruit a specific GRK that phosphorylates targeted serines and threonines in the receptor’s intracellular loops or C-terminus (Shenoy & Lefkowitz 2011; Whalen et al 2011). Immediately afterward, phosphorylated receptors induce translocation of cytoplasmic βarrestin1 and βarrestin2 to the cell surface where arrestin proteins bind to specific intracellular motifs on the GPCR and thereby uncouple the receptor from its cognate Gα subunit (Figure 1) (Shenoy & Lefkowitz 2011; Whalen et al 2011). βArrestins then target GPCRs to clathrin-coated pits, resulting in internalization of receptors into cytosolic endosomes where they are either sorted for dephosphorylation and recycling back to the plasma membrane or trafficked into lysosomes for degradation (Figure 1) (Oakley et al 2007; Shenoy & Lefkowitz 2011; Whalen et al 2011). GPCR-arrestin interactions are divided into two types: (i) Class A GPCRs which dissociate from βarrestin at or near the plasma membrane after forming a transient complex and then the phosphorylated receptor internalizes without the arrestin protein. (ii) Class B GPCRs which form a stable complex with βarrestins and then internalize as a unit into cytoplasmic endocytic vesicles (Oakley et al 2007). In addition, βarrestins can transduce cell signals by forming a scaffold between upstream molecules that activate Akt, NFκB, ERK, or other pathways independent of G protein coupling (Figure 1) (Shenoy & Lefkowitz 2011).
6. GRK Regulation of CRF1 receptor cyclic AMP signaling
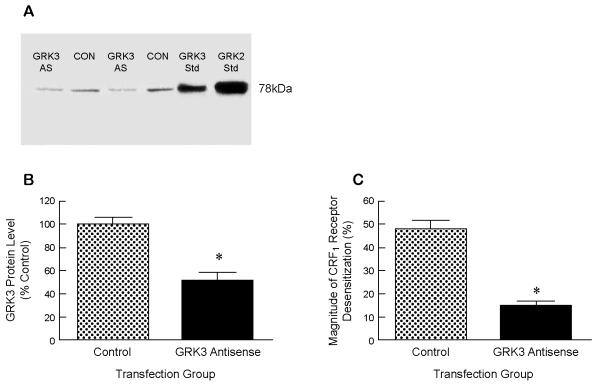
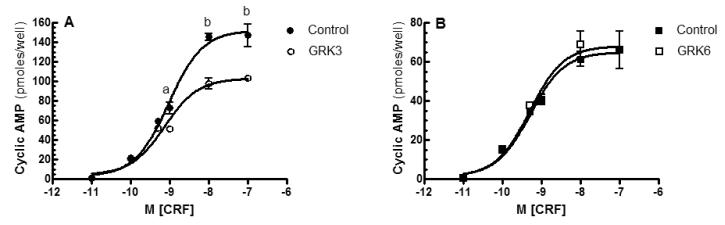
CRF hypersecretion alone cannot be sufficient, however, to cause enhanced brain CRF receptor signaling in PTSD and co-morbid depression considering that GPCRs exposed to high agonist concentrations are rapidly counterregulated by GRK-βarrestin desensitization and internalization (Dautzenberg et al 2001, 2002; Hauger et al 1997, 2006; Perry et al 2005; Teli et al 2005). Accordingly, in the presence of high saturating concentrations of endogenous ligands CRF or UCN1, CRF1 receptors undergo rapid hierarchical phosphorylation, which first occurs in the C-terminus and then proceeds through the third intracellular loop’s STTSET, a motif favored by acidotropic GRKs (Hauger et al 2000; Oakley et al 2007). When HEK293 cells recombinantly expressing CRF1 receptors are acutely stimulated with CRF, GRK3 rapidly translocates from cytosol to cell membrane (Teli et al 2005). Reducing cellular levels of GRK3 protein by >50% - by transfection of human retinoblastoma Y79 cells endogenously expressing CRF1 receptors with a GRK3 antisense cDNA - inhibited homologous CRF1 receptor desensitization ~70% (Figure 2) (Dautzenberg et al 2001). In agreement with this finding, pretreatment of HEK293 cells with an antibody that blocks endogenous GRK3 action suppressed homologous CRF1 receptor desensitization (Teli et al 2005). During prolonged exposure to high CRF, expression of GRK3 (but not GRK2) markedly upregulated within Y79 cells, presumably to maximize phosphorylation and desensitization of CRF1 receptors (Dautzenberg et al 2002). When the level of GRK3 protein was increased 5.0 ± 0.2-fold above the endogenous level - by transfecting HEK293 cells stably expressing CRF1 receptors with GRK3 cDNA - the maximum CRF-stimulated cyclic AMP accumulation was decreased 33.0 ± 3.2% compared to the cyclic AMP response maximum in control cells (Figure 3). Whereas GRK3 overexpression was observed to desensitize Gs-coupled CRF1 receptor signaling, GRK6 overexpression did not alter CRF-stimulated cyclic AMP accumulation (Figure 3). Specialization of GRK isoform action has previously been established. Overexpression of GRK2 (but not GRK5) strongly promotes homologous desensitization of endothelin receptor signaling although both GRKs phosphorylate the receptor protein (Freedman et al 1997). Agonist-induced phosphorylation of vasopressin V2 receptors and desensitization of Gs-coupled V2 receptor signaling are significantly inhibited by siRNA-induced knockdown of GRK3 but not GRK5 or GRK6 (Ren et al 2005). Emerging evidence indicates GRK specificity creates a “phosphorylation barcode” that imparts a distinct GPCR conformation that governs desensitizing and signaling functions of βarrestins (Shenoy & Lefkowitz 2011).
Figure 2. Effect of GRK3 deficiency on Gs-coupled CRF1 receptor signaling.
A: In a representative experiment, GRK3 protein levels were decreased by 64.2% (lane 1) and 50.6% (lane 3), respectively, in the two different groups of human retinoblastoma Y79 cells transfected with a GRK3 antisense construct 60 h earlier compared to control vector-transfected cells (lanes 3 & 4). B: In six independent experiments, a significant GRK3 deficiency was induced by GRK3 antisense cDNA transfection (>50% decrease) compared to GRK3 protein levels following control vector transfection. C: In six independent experiments, the magnitude of homologous CRF1 receptor desensitization was decreased 69.0 ± 2.1% in GRK3 antisense-transfected cells compared to control cells (Dautzenberg et al 2001). *p<0.0001 vs Control.
Figure 3. Effect of GRK3 or GRK6 overexpression on Gs-coupled CRF1 receptor signaling.
A: After HEK293 cells stably expressing CRF1 receptors were transfected with empty vector (Control) or GRK3, dose-response curves for stimulation of intracellular cyclic AMP accumulation by CRF (0-1 μ M for 15 min) were completed 48 hours later. In this representative experiment, the maximum was significantly decreased in GRK3 overexpressing cells (103 ± 3 pmoles/well) compared to control cells (152 ± 5 pmoles/well). By ANOVA, there was a significant stimulation by CRF in each cell group (p<0.001) and a significant difference across CRF concentrations between CRF1 receptor expressing-HEK293 cells with and without GRK3 overexpression. By planned post-hoc comparisons, the following differences were found to be statistically significant: ap<0.05 vs Control; bp<0.0001 vs Control. In four independent experiments, GRK3 overexpression consistently desensitized CRF1 receptors inducing a 33.0 ± 3.2% decrease in CRF-stimulated cyclic AMP accumulation. B: In a representative experiment, GRK6 overexpression did not desensitize Gs-coupled CRF1 receptor signaling. This result was replicated in two independent experiments in which the levels of GRK6 overexpression were similar to the levels of GRK3 overexpression.
7. βArrestin Regulation of CRF1 receptor signaling
GRK-mediated phosphorylation of C-terminal and/or third intracellular loop serines and threonines of a receptor protein increased the GPCR’s affinity for βarrestins up to 30-fold, thereby triggering the translocation of one or both arrestin proteins from the cytoplasm to the agonist-activated membrane receptors (Shenoy & Lefkowitz 2011). Upon exposure of CRF1-expressing HEK293 cells to 100nM CRF, βarrestin2-GFP rapidly (1-2 min) and robustly translocated from the cytoplasm to the CRF1 receptors at the plasma membrane (Oakley et al 2007; Hauger et al 2009). Importantly, CRF1 receptors appear to preferentially recruit βarrestin2 over βarrestin1 from the cytosol to the cell surface (Hauger et al 2009; Holmes et al 2006; Oakley et al 2007). This selective recruitment and binding of βarrestin2 by the agonist-activated CRF1 receptor is mediated by two distinct domains: (1) a phosphorylation-dependent motif in the C-terminus including a TPST sequence that may be transformed into an active βarrestin2 binding site by GRK3 phosphorylation; (2) a phosphorylation-independent motif in one or more of the intracellular loops (Oakley et al 2007).
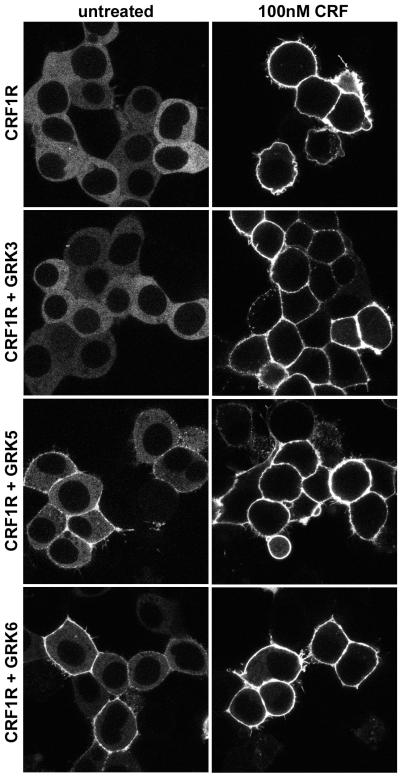
With longer CRF agonist exposure, a punctate pattern of fluorescence appears at the plasma membrane indicating localization of βarrestin2-GFP with the CRF1 receptor in clathrin-coated pits (Figure 4). Although CRF1 receptors internalize in response to agonist via βarrestin- and dynamin-dependent mechanisms (Holmes et al 2006; Oakley et al 2007), βarrestin2-GFP remains at the cell surface and does not traffic with the CRF1 receptor into endocytic vesicles even after prolonged CRF exposure (Figure 4). Because neither of the arrestin isoforms traffics with the receptor into cytosolic endosomes, the CRF1 receptor exhibits a “class A” interaction with βarrestins (Holmes et al 2006; Oakley et al 2007). Overexpressing GRK3, GRK5, or GRK6 in CRF1 receptor-expressing HEK293 cells did not alter the class A pattern or the magnitude of CRF-induced βarrestin2 recruitment (Figure 4). Interestingly, overexpression of GRK5 and GRK6, but not GRK3, promoted a small level of basal translocation of βarrestin2-GFP to CRF1 receptors in the absence of agonist (Figure 4), suggesting that these two membrane-bound GRKs can phosphorylate the CRF1 receptor in an inactive conformation prior to agonist binding. A portion of phosphorylated GPCRs appear to remain at the membrane where they can rapidly bind again to βarrestin2 if they are re-stimulated by agonist (Krasel et al 2005).
Figure 4. Recruitment of βarrestin2 by agonist-activated CRF1 receptors.
Confocal microscopy was used to evaluate the interaction of βarrestin2-GFP with CRF1 receptors (CRF1R) in real time and in live HEK293 cells co-transfected 48 h earlier with either empty vector (top panel), GRK3, GRK5 or GRK6. This representative experiment shows the distribution of βarrestin2-GFP in cells before (untreated) and after stimulation with CRF (100 nM) for 40 min. Note that both in the absence and presence of overexpressed GRKs, βarrestin2-GFP remains localized at the plasma membrane in clathrin-coated pits after translocation to cell surface CRF-activated CRF1 receptors, and does not traffic inside the cell into endocytic vesicles with internalized CRF1 receptors.
8. Conclusions
Pharmacotherapy currently available to treat PTSD is lacking a robust evidence base. One reason is that the molecular processes, and especially cell signaling, which can confer vulnerability or resilience to severe stress and underlying PTSD pathophysiology are not fully understood. Increasing evidence implicates dysregulated GR signaling in the pathogenesis of PTSD. Recently, cellular expression of FKBP5 or STAT5B, both of which regulate GR function, has been shown to be decreased in carriers of the FKPB5 SNP rs9296158 risk allele A that confers a high rate of PTSD following trauma in adults who experienced severe childhood stress (Yehuda et al 2011; Mehta & Binder 2011). PTSD pathophysiology may also involve anti-apoptotic proteins Bcl-2 and possibly Bag-1 that can attenuate GR nuclear trafficking and promote hippocampal neuron survival, and Bax, which is a downstream GR mediator of neuronal apoptosis in the hippocampus during stress-induced glucocorticoid hypersecretion (Hunsberger et al 2009).
Seven-transmembrane G protein-coupled receptors (GPCRs) represent the largest superfamily of cell surface receptors, comprising fully 1% of the human genome and transducing signals from a diverse array of extracellular ligands, some of which are critical mediators of the stress response. Targeting GPCRs has generated some of the most successful pharmacotherapy in modern medicine (Whalen et al 2011). In recent animal model research, signaling proteins in GPCR pathways including Akt, NFκB, MKP-1, and p11 can trigger anxiety- or depressive-like behavior during severe inescapable stress. CRF1 receptor signaling can activate these Akt, NFκB, and STAT3 pathways that may contribute to PTSD pathophysiology. Moreover, preclinical studies have shown that strong Gs-coupled CRF1 receptor signaling - activated by stress, transgenic CRF overexpression, or high exogenous CRF - can induce persistently high anxiety and startle hyperreactivity that are cardinal signs of PTSD, whereas CRF1 receptor gene knockout or selective CRF1 receptor antagonist treatment can block these stress responses (Risbrough et al 2004, 2009; Keen-Rhinehart et al 2008).
While CRF1 receptor activation is crucial for survival in the context of severe danger or trauma, persistent CRF1 receptor hypersignaling when threat or severe stress is no longer present is maladaptive. GRK3 and βarrestin2 appear to have critical roles in terminating Gs-coupled CRF1 receptor signaling (Hauger et al 2006, 2009; Holmes et al 2006; Teli et al 2005). Current evidence indicates that a large deficiency in GRK3 results in CRF1 receptor supersensitivity, while high cellular levels of GRK3 maximize CRF1 receptor desensitization (Dautzenberg et al 2001; Figures 2-3). Interestingly, GRK3 protein levels in the amygdala and locus coeruleus are significantly reduced in rats developing learned helplessness after unpredictable, inescapable stress, while resilience to this severe stressor is associated with normal GRK3 expression and function (Taneja et al 2011). CRF-induced activation of CRF1 receptors promotes one of the strongest translocation responses of βarrestin2 from cytosol to cell surface observed for studied class A GPCRs (Hauger et al 2009). Additionally, Gs-coupled CRF1 receptor signaling becomes dramatically upregulated be five-fold in cells with a deletion of the βarrestin2 gene (Hauger & Oakley, unpublished data). The swift return of βarrestin2 to phosphorylated CRF1 receptors in response to repeated trauma may prevent excessive CRF1 receptor signaling and, if this regulatory mechanism fails, would be expected to markedly impair stress recovery and increase the magnitude and duration of anxiety responses. Therefore, the loss of GRK-βarrestin2-mediated homologous desensitization resulting in unrestrained CRF1 receptor signaling via the adenylyl cyclase-protein kinase A cascade, combined with brain CRF hypersecretion, may represent a critical pathophysiological mechanism for PTSD (Figure 5).
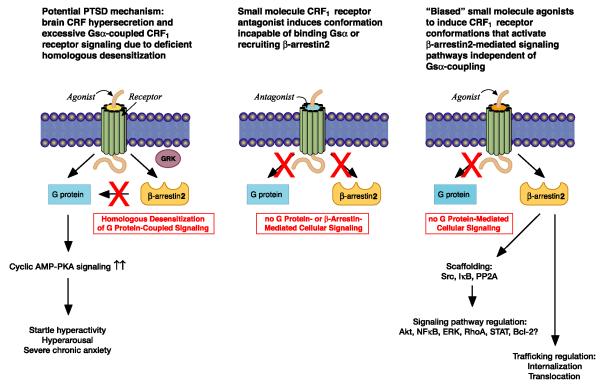
Figure 5.
Potential PTSD pathophysiology and pharmacotherapy
Small molecule antagonists that bind to CRF1 receptor transmembrane spanning J-domain and allosterically inhibit Gsαcoupling to CRF-R1 remain untested in PTSD patients, although they have equivocal antidepressant effects (Hauger et al 2009; Liapakis et al 2011). CRF1 receptor antagonism has the strongest anxiolytic action in preclinical models with high “trait” anxiety, or in animals exposed to severe stress that “sensitizes” subsequent stress responses, both of which are presumably mediated by abnormally high CRF levels and excessive CRF1 receptor signaling (Hauger et al 2006). Thus, a CRF1 receptor antagonist would be expected to normalize pathological anxiety states resulting from hyperactive CRF1 receptors without altering normal CRF1 receptor-mediated physiology (Steckler 2010). Accordingly, small molecule CRF1 receptor antagonists may be effective in treating PTSD, especially as acute prophylactic agents administered immediately after trauma in order to block stress-induced CRF1 receptor signal transduction in vulnerable individuals (Figure 5).
CRF1 receptor antagonists inhibit, however, βarrestin2 recruitment and HPA responses to trauma, which may be critical for homeostatic stress adaptation. An important new GPCR ligand class is the “biased agonist”, which induces a stable receptor conformation incapable of coupling to Gα but strongly recruiting βarrestin to shift signaling to βarrestin-mediated G protein-independent pathways (Figure 5). βArrestin-biased agonists targeting angiotensin II type 1 receptors have already entered clinical trials in patients with acute heart failure (DeWire & Violin 2011). Future research will be required, however, to determine if biased agonists directing CRF1 receptors to selectively activate specific βarrestin2 pathway molecules without triggering Gs-coupled signaling will prove to be effective PTSD pharmacotherapy.
Highlights.
Dysregulated FKBP5, STAT5B, and Bcl-2 interaction with GR may be involved in PTSD.
Akt, NFκB, MKP-1, and p11 may mediate anxiety and depressive responses to stress.
CRF1 and PAC1 receptor pathway interactions may contribute to PTSD pathophysiology.
High CRF release and excessive CRF1 signaling induces severe anxiety after stress.
Deficient GRK-βarrestin2 desensitization of CRF1 receptors may contribute to PTSD.
Acknowledgments
Dr. Hauger was supported by a Merit Review grant from the Department of Veterans Affairs (DVA); the VA Center of Excellence for Stress and Mental Health (CESAMH) and Mental Illness Research, Education and Clinical Center (MIRECC) of VISN22; and NIH/NIA (AG018386) and NIH/NIMH (MH074697) RO1 grants. Dr. Oakley received support from the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences. Dr. Lohr was supported by the VA Center of Excellence for Stress and Mental Health (CESAMH). Dr. Olivares-Reyes was supported by CINVESTAV-IPN, a UC MEXUS-CONACYT grant for collaborative projects, and a Grant for Research on Health from Fundacion Miguel Aleman 2010.
Keywords/Abbreviations
- CRF receptor
corticotropin releasing factor receptor
- GRK
G protein-coupled receptor (GPCR) kinase
- GR
βarrestin; glucocorticoid receptor
- FKBP5
FK506 binding protein 51
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement The authors declare no conflict of interest.
REFERENCES
- Acheson DT, Gresack JE, Risbrough VB. Hippocampal dysfunction effects on contextual memory: possible etiology for post-traumatic stress disorder. Neuropharmacology. 2011 doi: 10.1016/j.neuropharm.2011.04.029. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamec R, Fougere D, Risbrough V. CRF receptor blockade prevents initiation and consolidation of stress effects on affect in the predator stress model of PTSD. Int J Neuropsychopharmacol. 2010;13:747–757. doi: 10.1017/S1461145709990496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadi N, Hajsadeghi F, Mirshkarlo HB, Budoff M, Yehuda R, Ebrahimi R. Post-traumatic stress disorder, coronary arteriosclerosis, and mortality. Am J Cardiol. 2011 doi: 10.1016/j.amjcard.2011.02.340. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Alexander B, Warner-Schmidt J, Eriksson TM, Tamminga C, Arango-Lievano M, Ghose S, Vernov M, Stavarache M, Musatov S, Flajolet M, Svenningsson P, Greengard P, Kaplitt MG. Reversal of depressed behaviors in mice by p11 gene therapy in the nucleus accumbens. Science Translational Medicine. 2010;54:1–9. doi: 10.1126/scitranslmed.3001079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amstadter AB, Nugent NR, Yang BZ, Miller A, Siburian R, Moorjani P, Haddad S, Basu A, Fagernesss J, Saxe G, Smoller JW, Koenen KC. Corticotropin-releasing hormone type 1 receptor gene (CRHR1) variants predict posttraumatic stress disorder onset and course in pediatric injury patients. Dis Markers. 2011;30:89–99. doi: 10.3233/DMA-2011-0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APA . Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition, Text Revision American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- Baker DG, West SA, Nicholson WE, Ekhator NN, Kasckow JW, Hill KK, Bruce AB, Orth DN, Geracioti TD. Serial CSF CRH levels and adrenocortical activity in combat veterans with posttraumatic stress disorder. Am J Psychiatry. 1999;156:585–588. doi: 10.1176/ajp.156.4.585. [DOI] [PubMed] [Google Scholar]
- Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Bauer MS, Altshuler L, Evans DR, Beresford T, Williford WO, Hauger R. Prevalence and distinct correlates of anxiety, substance, and combined comorbidity in a multi-site public sector sample with bipolar disorder. J Affect Dis. 2005;85:301–315. doi: 10.1016/j.jad.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Bayatti N, Hermann H, Lutz B, Behl C. Corticotropin-releasing hormone-mediated induction of intracellular signaling pathways and brain-derived neurotrophic factor expression is inhibited by the activation of the endocannabinoid system. Endocrinology. 2005;146:1205–1213. doi: 10.1210/en.2004-1154. [DOI] [PubMed] [Google Scholar]
- Binder EB. The role of FKBP5, a co-chaperone of the glucocorticoid receptor in pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology. 2009;34S:S186–S195. doi: 10.1016/j.psyneuen.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, Tang Y, Gillespie CF, Heim CM, Nemeroff CB, Schwartz AC, Cubells JF, Ressler KJ. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299:1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Licinio J, Darnell A, Krystal JH, Owens MJ, Southwick SM, Nemeroff CB, Charney DS. Elevated CSF CRF concentrations in PTSD. Am J Psychiatry. 1997;154:624–629. doi: 10.1176/ajp.154.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N, Chilcoat HD, Kessler RC, Davis GC. Previous exposure to trauma and PTSD effects of subsequent trauma: results from the Detroit area survery of trauma. Am J Psychiatry. 1999;156:902–907. doi: 10.1176/ajp.156.6.902. [DOI] [PubMed] [Google Scholar]
- Dahlhoff M, Siegmund A, Golub Y, Wolf E, Holsboer F, Wotjak CT. AKT/GSK-3β/β-catenin signaling within hippocampus and amygdala reflects genetically determined differences in posttraumatic stress disorder like symptoms. Neuroscience. 2010;169:1216–1226. doi: 10.1016/j.neuroscience.2010.05.066. [DOI] [PubMed] [Google Scholar]
- Dautzenberg FM, Braun S, Hauger RL. GRK3 mediates desensitization of CRF1 receptors: a potential mechanism regulating stress adaptation. Am J Physiol. 2001;280:R935–R946. doi: 10.1152/ajpregu.2001.280.4.R935. [DOI] [PubMed] [Google Scholar]
- Dauztenberg FM, Hauger RL. G-protein-coupled receptor kinase 3- and protein kinase C mediated desensitization of the PACAP receptor type 1 in human Y-79 retinoblastoma cells. Neuropharmacology. 2001;40:394–407. doi: 10.1016/s0028-3908(00)00167-2. [DOI] [PubMed] [Google Scholar]
- Dautzenberg FM, Hauger RL. The CRF peptide family and their receptors: yet more partners discovered. Trends Pharmacol Sci. 2002;23:71–77. doi: 10.1016/s0165-6147(02)01946-6. [DOI] [PubMed] [Google Scholar]
- Dautzenberg FM, Wille S, Braun S, Hauger RL. GRK3 regulation during CRF- and urocortin-inducedCRF1 receptor desensitization. Biochem Biophys Res Commun. 2002;298:303–308. doi: 10.1016/s0006-291x(02)02463-4. [DOI] [PubMed] [Google Scholar]
- De Wire SM, Violin JD. Biased ligands for better cardiovascular drugs: dissecting G-protein-coupled receptor pharmacology. Circ Res. 2011;109:205–216. doi: 10.1161/CIRCRESAHA.110.231308. [DOI] [PubMed] [Google Scholar]
- Duric V, Banasr M, Licznerski P, Schmidt HD, Stockmeier CA, Simen AA, Newton SS, Duman RS. A negative regulator of MAP kinase causes depressive behavior. Nature Medicine. 2010;16:1328–1332. doi: 10.1038/nm.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott E, Ezra-Nevo G, Regev L, Neufeld-Cohen A, Chen A. Resilience to social stress coincides with functional DNA methylation of the CRF gene in adult mice. Nat Neurosci. 2010;13:1351–1353. doi: 10.1038/nn.2642. [DOI] [PubMed] [Google Scholar]
- Feder A, Nestler EJ, Charney DS. Psychobiology and molecular genetics of resilience. Nat Rev Neurosci. 2009;10:446–457. doi: 10.1038/nrn2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman NJ, Ament AS, Oppermann M, Stoffel RH, Exum ST, Lefkowitz RJ. Phosphorylation and desensitization of human endothelin A and B receptors: evidence for G protein-coupled receptor kinase specificity. J Biol Chem. 1997;272:17734–17743. doi: 10.1074/jbc.272.28.17734. [DOI] [PubMed] [Google Scholar]
- Gillespie CF, Phifer J, Bradley B, Ressler KJ. Risk and resilience: genetic and environmental influences on development of the stress response. Depression and Anxiety. 2009;26:984–992. doi: 10.1002/da.20605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauger RL, Dautzenberg FM, Flaccus A, Liepold T, Spiess J. Regulation of corticotropin-releasing factor receptor function in human Y-79 retinoblastoma cells: rapid and reversible homologous desensitization but prolonged recovery. J Neurochem. 1997;68:2308–2316. doi: 10.1046/j.1471-4159.1997.68062308.x. [DOI] [PubMed] [Google Scholar]
- Hauger RL, Gresack JE, Wallace C, Bordson S, Risbrough VB. Neuroscience Meeting Planner. Society for Neuroscience; San Diego, CA: 2010. Corticotropin-releasing factor (CRF) receptor signal transduction and startle hyperreactivity are mediated by protein kinase C (PKC), but not extracellular-regulated kinase (ERK) second messenger systems. Program No. 90.17. 2010. Online. [Google Scholar]
- Hauger RL, Smith RD, Braun S, Dautzenberg FM, Catt KJ. Rapid agonist-induced phosphorylation of the human CRF receptor, type 1: a potential mechanism for homologous desensitization. Biochem Biophys Res Comm. 2000;268:572–576. doi: 10.1006/bbrc.2000.2183. [DOI] [PubMed] [Google Scholar]
- Hauger RL, Risbrough V, Brauns O, Dautzenberg FM. Corticotropin releasing factor (CRF) receptor signaling in the central nervous system: new molecular targets. CNS & Neurological Disorders – Drug Targets. 2006;5:453–479. doi: 10.2174/187152706777950684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauger RL, Risbrough V, Oakley RH, Olivares-Reyes JA, Dautzenberg FM. Role of CRF receptor signaling in stress vulnerability, anxiety, and depression. Ann NY Acad Sci. 2009;1179:120–143. doi: 10.1111/j.1749-6632.2009.05011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Roman CW, Lezak KR, Kocho-Shellenberg M, Grimmig B, Falls WA, Braas K, May V. Roles for pituitary adenylate cyclase-activating peptide (PACAP) expression and signaling in the bed nucleus of the stria terminalis (BNST) in mediating the behavioral consequences of chronic stress. J Mol Neurosci. 2010;42:327–340. doi: 10.1007/s12031-010-9364-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mietzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Holmes KD, Babwah AV, Dale LB, Poulter MO, Ferguson SS. Differential regulation of corticotropin releasing factor 1alpha receptor endocytosis and trafficking by beta-arrestins and Rab GTPases. J Neurochem. 2006;96:934–949. doi: 10.1111/j.1471-4159.2005.03603.x. [DOI] [PubMed] [Google Scholar]
- Hunsberger JG, Austin DR, Chen G, Manji HK. Cellular mechanisms underlying affective resiliency: The role of glucocorticoid receptor- and mitochondrially-mediated plasticity. Brain Res. 2009;1293:76–84. doi: 10.1016/j.brainres.2009.06.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine . Treatment of Posttraumatic Stress Disorder: An Assessment of the Evidence. The National Academies Press; Washington DC: 2008. [Google Scholar]
- Juster R-P, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehav Rev. 2010;35:2–16. doi: 10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Keen-Rhinehart E, Michopoulos V, Toufexis DJ, Martin EI, Nair H, Ressler KJ, Davis M, Owens MJ, Nemeroff CB, Wilson ME. Continuous expression of corticotrophin-releasing factor in the central nucleus of the amygdala emulates the dysregulation of the stress and reproductive axes. Mol Psychiatry. 2008;14:37–50. doi: 10.1038/mp.2008.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the national comorbidity survey. Arch Gen Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Koo JW, Russo SJ, Ferguson D, Nestler EJ, Duman RS. Nuclear factor-κB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proc Natl Acad Sci (USA) 2010;107:2669–2674. doi: 10.1073/pnas.0910658107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasel C, Bunemann M, Lorenz K, Lohse MJ. β-Arrestin binding to the β2-adrenergic receptor requires both receptor phosphorylation and receptor activation. J Biol Chem. 2005;280:9528–9535. doi: 10.1074/jbc.M413078200. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Han M-H, Mazei-Robison M, Iniguez SD, Ables JL, Vialou V, Berton O, Ghose S, Covington HE, Wiley MD, Henderson RP, Neve RL, Eisch AJ, Tamminga CA, Russo SJ, Bolanos CA, Nestler EJ. AKT signaling within the ventral tegmental area regulates cellular and behavioral responses to stressful stimuli. Biol Psychiatry. 2008;64:691–700. doi: 10.1016/j.biopsych.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Han F, Liu D, Shi Y. Changes of Bax, Bcl-2 and apoptosis in hippocampus in the rat model of post-traumatic stress disorder. Neurol Res. 2010;232:579–586. doi: 10.1179/016164110X12556180206194. [DOI] [PubMed] [Google Scholar]
- Liapakis G, Venihaki M, Margioris A, Grigoriadis D, Gkountelias K. Members of CRF family and their receptors: from past to future. Curr Med Chem. 2011;18:2583–2600. doi: 10.2174/092986711795933704. [DOI] [PubMed] [Google Scholar]
- Licznerski P, Duric V, Banasr M, Alavian KN, Jonas EA, Duman RS. Neuroscience Meeting Planner. Society for Neuroscience; San Diego, CA: 2010. Serum- and glucocorticoid-induced kinase-1 (SGK1) in post-traumatic stress disorder and depression. Program No. 666.6. 2010. Online. [Google Scholar]
- Mehta D, Binder EB. Gene x environment vulnerability factors for PTSD: the HPA-axis. Neuropharmacology. 2011 doi: 10.1016/j.neuropharm.2011.03.009. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Mehta D, Gonik M, Klengel T, Rex-Haffner M, Menke A, Rubel J, Mercer KB, Putz B, Bradley B, Holsboer F, Ressler KJ, Muller-Myhsok B, Binder EB. Using polymorphisms in FKBP5 to define biologically distinct subtypes of posttraumatic stress disorder. Arch Gen Psychiatry. 2011;68:901–910. doi: 10.1001/archgenpsychiatry.2011.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R, Adamec R, Sapolsky R. Resilience against predator stress and dendritic morphology of amygdala neurons. Behav Brain Res. 2009;205:535–543. doi: 10.1016/j.bbr.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mynard V, Latchoumanin O, Guignat L, Devin-Leclerc J, Bertagna X, Barre B, Fagart J, Coqueret O, Catelli MG. Synergistic signaling by corticotropin-releasing hormone and leukemia inhibitory factor bridged by phosphorylated 3′,5′-cyclic adenosine monophosphate response element binding protein at the Nur response element (NurRE)-signal transducers and activators of transcription (STAT) element of the proopiomelanocortin promoter. Mol Endocrinol. 2004;18:2997–3010. doi: 10.1210/me.2003-0417. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Bremner JD, Foa EB, Mayberg HS, North CS, Stein MG. Posttraumatic stress disorder: a state-of-the-science review. J Psychiatr Res. 2006;40:1–21. doi: 10.1016/j.jpsychires.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Oakley RH, Olivares-Reyes JA, Hudson CC, Flores-Vega F, Dautzenberg FM, Hauger RL. Carboxyl terminal and intracellular loop sites for CRF1 receptor phosphorylation and βarrestin2 recruitment: a mechanism regulating stress and anxiety responses. Am J Physiol. 2007;293:R209–R222. doi: 10.1152/ajpregu.00099.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan A, Epel E, Lin J, Wolkowitz O, Cohen B, Maquen S, Metzler T, Lenocci M, Blackburn E, Neylan TC. Childhood trauma associated with short leukocyte telomere length in posttraumatic stress disorder. Biol Psychiatry. 2011a;70:465–471. doi: 10.1016/j.biopsych.2011.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan A, Sun B, Cole S, Rempel H, Lenoci M, Pulliam L, Neylan T. Transcriptional control of monocyte gene expression in post-traumatic stress disorder. Dis Markers. 2011b;30:123–132. doi: 10.3233/DMA-2011-0768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace TW, Heim CM. A short review of the psychoneuroimmunology of posttraumatic stress disorder: from risk factors to medical comorbidities. Brain Behav Immun. 2011;25:6–13. doi: 10.1016/j.bbi.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Peeters PJ, Fierens FL, van den Wyngaert I, Goehlmann HW, Swagemakers SM, Kass SU, Langlois X, Pullan S, Stenzel-Poore MP, Steckler T. Gene expression profiles highlight adaptive brain mechanisms in corticotropin releasing factor overexpressing mice. Brain Res Mol Brain Res. 2004;129:135–150. doi: 10.1016/j.molbrainres.2004.06.038. [DOI] [PubMed] [Google Scholar]
- Perrin MH, Vale W. Chapter 25: Corticotropin-releasing factor receptors. In: Pangalos MN, Davies CH, editors. Understanding G protein-coupled receptors and their role in the CNS. Oxford University Press; New York: 2002. pp. 505–526. [Google Scholar]
- Perry SJ, Junger S, Kohout TA, Hoare SRJ, Struthers RS, Grigoriadis DE, Maki RA. Distinct conformations of the corticotropin releasing factor type 1 receptor adopted following agonist and antagonist binding are differentially regulated. J Biol Chem. 2005;280:11560–11568. doi: 10.1074/jbc.M412914200. [DOI] [PubMed] [Google Scholar]
- Polanczyk G, Caspi A, Williams B, Price TS, Danese A, Sugden K, Uher F, Poulton R, Moffitt TE. Protective effect of CRHR1 gene variants on the development of adult depression following childhood maltreatment. Arch Gen Psychiatry. 2009;66:978–985. doi: 10.1001/archgenpsychiatry.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmusson AM, Schnurr PP, Zukowska Z, Scioli E, Forman DE. Adaptation to extreme stress: posttraumatic stress disorder, neuropeptide Y and metabolic syndrome. Exp Biol Med (Maywood) 2010;235:1150–1162. doi: 10.1258/ebm.2010.009334. [DOI] [PubMed] [Google Scholar]
- Ren XR, Reiter E, Ahn S, Kim J, Chen W, Lefkowitz RJ. Different G protein-coupled receptor kinases govern G protein and beta-arrestin-mediated signaling of V2 vasopressin receptor. Proc Natl Acad Sci USA. 2005;102:1448–1453. doi: 10.1073/pnas.0409534102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ, Mercer KB, Bradley B, Jovanovic T, Mahan A, Kerley K, Norrholm SD, Kilaru V, Smith AK, Myers AJ, Ramirez M, Engel A, Hammack SE, Toufexis D, Braas KM, Binder EB, May V. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature. 2011;470:492–497. doi: 10.1038/nature09856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risbrough VB, Geyer MA, Hauger RL, Coste S, Stenzel-Poore M, Wurst W, Holsboer F. CRF1 and CRF2 receptors are required for potentiated startle to contextual but not discrete cues. Neuropsychopharmacology. 2009;34:1494–1503. doi: 10.1038/npp.2008.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risbrough VB, Hauger RL, Roberts AL, Vale WW, Geyer MA. Corticotropin releasing factor receptors CRF1 and CRF2 exert both additive and opposing influences on defensive startle behavior. J Neurosci. 2004;24:6545–6552. doi: 10.1523/JNEUROSCI.5760-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risbrough VB, Stein MB. Role of corticotropin releasing factor in anxiety disorders: a translational research perspective. Horm Behav. 2006;50:550–561. doi: 10.1016/j.yhbeh.2006.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sautter FJ, Bissette G, Wiley J, Manguno-Mire G, Schoenbachler B, Myers L, Johnson JE, Cerbone A, Malaspina D. CRF in PTSD with secondary psychotic symptoms, nonpsychotic PTSD, and healthy control subjects. Biol Psychiatry. 2003;54:1382–1388. doi: 10.1016/s0006-3223(03)00571-7. [DOI] [PubMed] [Google Scholar]
- Sheng H, Sun T, Cong B, He P, Zhang Y, Yan J, Lu C, Ni X. Corticotropin-releasing hormone stimulates SGK-1 kinase expression in cultured hippocampal neurons via CRH-R1. Am J Physiol. 2008;295:E938–E946. doi: 10.1152/ajpendo.90462.2008. [DOI] [PubMed] [Google Scholar]
- Shenoy SK, Lefkowitz RJ. β-Arrestin-mediated receptor trafficking and signal transduction. Trends Pharmacol Sci. 2011;32:521–533. doi: 10.1016/j.tips.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelton K, Ressler KJ, Norrholm SD, Jovanovic T, Bradley-Davino B. PTSD and gene variants: new pathways and new thinking. Neuropharmacology. 2011 doi: 10.1016/j.neuropharm.2011.02.013. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EM, Gregg M, Hashemi F, Schott L, Hughes TK. Corticotropin releasing factor (CRF) activation of NF-kappaB-directed transcription in leukocytes. Cell Mol Neurobiol. 2006;26:1021–1036. doi: 10.1007/s10571-006-9040-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steckler T. Developing small molecule nonpeptidergic drugs for the treatment of anxiety disorders: is the challenge still ahead? Curr Top Behav Neurosci. 2010;2:415–428. doi: 10.1007/7854_2009_14. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Chergui K, Rachleff I, Flajolet M, Zhang X, El Yacoubi M, Vaugeois J-M, Nomikos GG, Greengard P. Alterations in 5-HT1B receptor function by p11 in depression-like states. Science. 2006;311:77–80. doi: 10.1126/science.1117571. [DOI] [PubMed] [Google Scholar]
- Swinny JD, Valentino RJ. Corticotropin-releasing factor promotes growth of brain norepinephrine neuronal processes through Rho GTPase regulators of the actin cytoskeleton in rat. Eur J Neurosci. 2006;24:2481–2490. doi: 10.1111/j.1460-9568.2006.05129.x. [DOI] [PubMed] [Google Scholar]
- Taneja M, Salim S, Saha K, Happe HK, Qutna N, Petty F, Bylund DB, Eikenburg DC. Differential effects of inescapable stress on locus coeruleus GRK3, alpha2-adrenoceptor and CRF1 receptor levels in learned helpless and non-helpless rats: a potential link to stress resilience. Behav Brain Res. 2011;221:25–33. doi: 10.1016/j.bbr.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teli T, Markovic D, Levine MA, Hillhouse EW, Grammatopoulos DK. Regulation of corticotropin-releasing hormone (CRH) receptor type 1α signaling: structural determinants for G protein-coupled receptor kinase-mediated phosphorylation and agonist-mediated desensitization. Mol Endocrinol. 2005;19:474–490. doi: 10.1210/me.2004-0275. [DOI] [PubMed] [Google Scholar]
- Traver S, Marien M, Martin E, Hirsch EC, Michel PP. The phenotypic differentiation of locus coeruleus noradrenergic neurons mediated by brain-derived neurotrophic factor is enhanced by corticotropin releasing factor through the activation of cAMP-dependent signaling pathway. Mol Pharmacol. 2006;70:30–40. doi: 10.1124/mol.106.022715. [DOI] [PubMed] [Google Scholar]
- Tsatsanis C, Androulidaki A, Dermitzaki E, Charalampopoulos I, Spiess J, Gravanis A, Margioris AN. Urocortin 1 and urocortin 2 induce macrophage apoptosis via CRFR2. FEBS Lett. 2005;579:4259–5264. doi: 10.1016/j.febslet.2005.06.057. [DOI] [PubMed] [Google Scholar]
- Tyrka AR, Price LH, Gelernter J, Schepker C, Anderson GM, Carpenter LL. Interaction of childhood maltreatment with the corticotropin-releasing hormone receptor gene: effects on hypothalamic-pituitary-adrenal axis reactivity. Biol Psychiatry. 2009;66:681–685. doi: 10.1016/j.biopsych.2009.05.012. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Zuiden M, Geuze E, Willermen HL, Vermetten E, Maas M, Heijnen CJ, Kavelaars A. Pre-existing high glucocorticoid receptor number predicting development of posttraumatic stress symptoms after military deployment. Am J Psychiatry. 2011;168:89–96. doi: 10.1176/appi.ajp.2010.10050706. [DOI] [PubMed] [Google Scholar]
- Whalen EJ, Rajagopal S, Lefkowitz RJ. Therapeutic potential of β-arrestin- and G protein-biased agonists. Trends Mol Med. 2011;17:126–139. doi: 10.1016/j.molmed.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . International Classification of Diseases and related health problems. 10th rev World Health Organization; Geneva: 1992. [Google Scholar]
- Yehuda R. Status of glucocorticoid alterations in post-traumatic stress disorder. Ann NY Acad Sci. 2009;1179:56–59. doi: 10.1111/j.1749-6632.2009.04979.x. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Cai G, Golier JA, Sarapas C, Galea S, Ising M, Rein T, Schmeidler J, Muller-Myhsok B, Holsboer F, Buxbaum JD. Gene expression patterns associated with posttraumatic stress disorder following exposure to the world trade center attacks. Biol Psychiatry. 2009;66:708–711. doi: 10.1016/j.biopsych.2009.02.034. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Flory JD, Pratchett LC, Buxbaum J, Ising M, Holsboer F. Putative biological mechanisms for the association between early life adversity and the subsequent development of PTSD. Psychopharmacology (Berl) 2010;212:405–417. doi: 10.1007/s00213-010-1969-6. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Koenen KC, Galea S, Flory JD. The role of genes in defining a molecular biology of PTSD. Dis Markers. 2011;30:67–76. doi: 10.3233/DMA-2011-0794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Li H, Su TP, Barker JL, Maric D, Fullerton CS, Webster MJ, Hough CJ, Li XX, Ursano R. p11 is up-regulated in the forebrain of stress rats by glucocorticoid acting via two specific glucocorticoid response elements in the p11 promoter. Neuroscience. 2008;153:1126–1134. doi: 10.1016/j.neuroscience.2008.03.022. [DOI] [PubMed] [Google Scholar]