Abstract
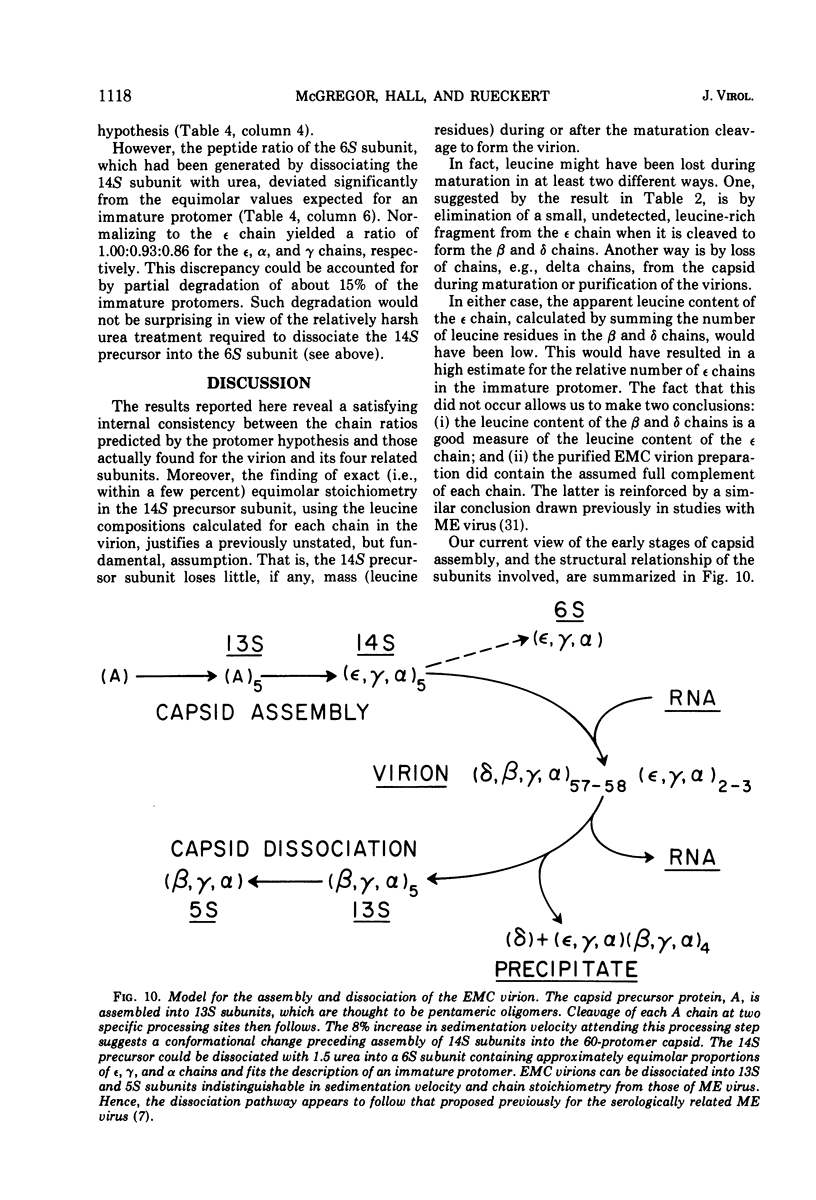
Two capsid precursor subunits, which sediment on glycerol gradients at 13S and 14S, respectively, have been identified in cytoplasmic extracts of encephalomyocarditis virus-infected HeLa cells. The 13S subunit, which was detected after a 10-min pulse label with -3H-labeled amino acids, contained only capsid precursor chain A (mol wt 100,000). When the 10-min pulse label in such cells was chased for 20 min, the A-containing 13S subunit in the cytoplasmic extracts was replaced by a 14S subunit containing equimolar proportions of three chains: epsilon, gamma, and alpha. This (epsilon, gamma, alpha)-containing 14S subunit could be dissociated into 6S subunits with the same polypeptide composition. The sedimentation properties and the polypeptide stoichiometry of these three precursor subunits, when compared with those of the 13S, (beta, gamma, alpha)(5), and 5S, (beta, gamma, alpha), subunits derived by acid dissociation of purified virions, suggest the following structural assignments: 13S, (A)(5); 14S, (epsilon, gamma, alpha)(5), 6S, (epsilon, gamma, alpha). The molecular weights of the individually isolated capsid chains were determined by gel filtration in 6 M guanidine hydrochloride to be: epsilon, 36,000; alpha, 32,000; beta, 29,500; gamma, 26,500; and delta, 7,800. With the exception of the delta-chain, these values are in reasonable agreement with the values previously determined by electrophoresis on sodium dodecyl sulfatepolyacrylamide gels. These data support the hypothesis that picornavirus capsids are assembled from identical protomers according to the following scheme: (A) leads to (A)(5) leads to (epsilon, gamma, alpha)(5) leads to (delta, beta, gamma, alpha)60-n(epsilon, gamma, alpha)n where n is the number of immature protomers per virion.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Britten R. J., Roberts R. B. High-Resolution Density Gradient Sedimentation Analysis. Science. 1960 Jan 1;131(3392):32–33. doi: 10.1126/science.131.3392.32. [DOI] [PubMed] [Google Scholar]
- Burness A. T., Fox S. M., Pardoe I. U. The polypeptide composition of the encephalomyocarditis virus particle. J Gen Virol. 1974 Jun;23(3):225–236. doi: 10.1099/0022-1317-23-3-225. [DOI] [PubMed] [Google Scholar]
- Butterworth B. E., Hall L., Stoltzfus C. M., Rueckert R. R. Virus-specific proteins synthesized in encephalomyocarditis virus-infected HeLa cells. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3083–3087. doi: 10.1073/pnas.68.12.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth B. E., Rueckert R. R. Kinetics of synthesis and cleavage of encephalomyocarditis virus-specific proteins. Virology. 1972 Nov;50(2):535–549. doi: 10.1016/0042-6822(72)90405-9. [DOI] [PubMed] [Google Scholar]
- Caliguiri L. A., Tamm I. The role of cytoplasmic membranes in poliovirus biosynthesis. Virology. 1970 Sep;42(1):100–111. doi: 10.1016/0042-6822(70)90242-4. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunker A. K., Rueckert R. R. Fragments generated by pH dissociation of ME-virus and their relation to the structure of the virion. J Mol Biol. 1971 May 28;58(1):217–235. doi: 10.1016/0022-2836(71)90242-7. [DOI] [PubMed] [Google Scholar]
- Dunker A. K., Rueckert R. R. Observations on molecular weight determinations on polyacrylamide gel. J Biol Chem. 1969 Sep 25;244(18):5074–5080. [PubMed] [Google Scholar]
- FAULKNER P., MARTIN E. M., SVED S., VALENTINE R. C., WORK T. S. Studies on protein and nucleic acid metabolism in virus-infected mammalian cells. 2. The isolation, crystallization and chemical characterization of mouse encephalomyocarditis virus. Biochem J. 1961 Sep;80:597–605. doi: 10.1042/bj0800597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish W. W., Mann K. G., Tanford C. The estimation of polypeptide chain molecular weights by gel filtration in 6 M guanidine hydrochloride. J Biol Chem. 1969 Sep 25;244(18):4989–4994. [PubMed] [Google Scholar]
- Gilson W., Gilson R., Rueckert R. R. An automatic high-precision acrylamide gel fractionator. Anal Biochem. 1972 Jun;47(2):321–328. doi: 10.1016/0003-2697(72)90125-x. [DOI] [PubMed] [Google Scholar]
- Hall L., Rueckert R. R. Infection of mouse fibroblasts by cardioviruses: premature uncoating and its prevention by elevated pH and magnesium chloride. Virology. 1971 Jan;43(1):152–165. doi: 10.1016/0042-6822(71)90233-9. [DOI] [PubMed] [Google Scholar]
- Jacobson M. F., Asso J., Baltimore D. Further evidence on the formation of poliovirus proteins. J Mol Biol. 1970 May 14;49(3):657–669. doi: 10.1016/0022-2836(70)90289-5. [DOI] [PubMed] [Google Scholar]
- Jacobson M. F., Baltimore D. Morphogenesis of poliovirus. I. Association of the viral RNA with coat protein. J Mol Biol. 1968 Apr 28;33(2):369–378. doi: 10.1016/0022-2836(68)90195-2. [DOI] [PubMed] [Google Scholar]
- KERR I. M., MARTIN E. M., HAMILTON M. G., WORK T. S. STUDIES ON PROTEIN AND NUCLEIC ACID METABOLISM IN VIRUS-INFECTED MAMMALIAN CELLS. THE FORMATION OF A VIRUS-SPECIFIC ANTIGEN IN KREBS II ASCITES-TUMOUR CELLS INFECTED WITH ENCEPHALOMYOCARDITIS VIRUS. Biochem J. 1965 Feb;94:337–344. doi: 10.1042/bj0940337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas-Lenard J. Cleavage of mengovirus polyproteins in vivo. J Virol. 1974 Aug;14(2):261–269. doi: 10.1128/jvi.14.2.261-269.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Mak T. W., Colter J. S., Scraba D. G. Structure of the Mengo virion. II. Physicochemical and electron microscopic analysis of degraded virus. Virology. 1974 Feb;57(2):543–553. doi: 10.1016/0042-6822(74)90193-7. [DOI] [PubMed] [Google Scholar]
- Mak T. W., O'Callaghan D. J., Kay C. M., Colter J. S. Studies of the protein subunit of pH-inactivated Mengo virus variants. II. Physicochemical properties. Virology. 1971 Mar;43(3):579–587. doi: 10.1016/0042-6822(71)90283-2. [DOI] [PubMed] [Google Scholar]
- Medappa K. C., McLean C., Rueckert R. R. On the structure of rhinovirus 1A. Virology. 1971 May;44(2):259–270. doi: 10.1016/0042-6822(71)90258-3. [DOI] [PubMed] [Google Scholar]
- PENMAN S., BECKER Y., DARNELL J. E. A CYTOPLASMIC STRUCTURE INVOLVED IN THE SYNTHESIS AND ASSEMBLY OF POLIOVIRUS COMPONENTS. J Mol Biol. 1964 Apr;8:541–555. doi: 10.1016/s0022-2836(64)80010-3. [DOI] [PubMed] [Google Scholar]
- Paucha E., Seehafer J., Colter J. S. Synthesis of viral-specific polypeptides in Mengo virus-infected L cells: evidence for asymmetric translation of the viral genome. Virology. 1974 Oct;61(2):315–326. doi: 10.1016/0042-6822(74)90269-4. [DOI] [PubMed] [Google Scholar]
- Perlin M., Phillips B. A. In vitro assembly of polioviruses. 3. Assembly of 14 S particles into empty capsids by poliovirus-infected HeLa cell membranes. Virology. 1973 May;53(1):107–114. doi: 10.1016/0042-6822(73)90469-8. [DOI] [PubMed] [Google Scholar]
- Phillips B. A., Fennell R. Polypeptide composition of poliovirions, naturally occurring empty capsids, and 14S precursor particles. J Virol. 1973 Aug;12(2):291–299. doi: 10.1128/jvi.12.2.291-299.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips B. A. In vitro assembly of polioviruses. I. Kinetics of the assembly of empty capsids and the role of extracts from infected cells. Virology. 1969 Dec;39(4):811–821. doi: 10.1016/0042-6822(69)90018-x. [DOI] [PubMed] [Google Scholar]
- Phillips B. A., Summers D. F., Maizel J. V., Jr In vitro assembly of poliovirus-related particles. Virology. 1968 Jun;35(2):216–226. doi: 10.1016/0042-6822(68)90262-6. [DOI] [PubMed] [Google Scholar]
- Reynolds J. A., Tanford C. The gross conformation of protein-sodium dodecyl sulfate complexes. J Biol Chem. 1970 Oct 10;245(19):5161–5165. [PubMed] [Google Scholar]
- Rueckert R. R., Dunker A. K., Stoltzfus C. M. The structure of mouse-Elberfeld virus: a model. Proc Natl Acad Sci U S A. 1969 Mar;62(3):912–919. doi: 10.1073/pnas.62.3.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltzfus C. M., Rueckert R. Capsid polypeptides of mouse Elberfeld virus. I. Amino acid compositions and molar ratios in the virion. J Virol. 1972 Sep;10(3):347–355. doi: 10.1128/jvi.10.3.347-355.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers D. F., Maizel J. V., Jr, Darnell J. E., Jr Evidence for virus-specific noncapsid proteins in poliovirus-infected HeLa cells. Proc Natl Acad Sci U S A. 1965 Aug;54(2):505–513. doi: 10.1073/pnas.54.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]



