Abstract
Cross-talk between NK cells and dendritic cells (DCs) is critical for the potent therapeutic response to dsRNA, but the receptors involved remained controversial. We show in this paper that two dsRNAs, polyadenylic-polyuridylic acid and polyinosinic-polycytidylic acid [poly(I:C)], similarly engaged human TLR3, whereas only poly(I:C) triggered human RIG-I and MDA5. Both dsRNA enhanced NK cell activation within PBMCs but only poly(I:C) induced IFN-γ. Although myeloid DCs (mDCs) were required for NK cell activation, induction of cytolytic potential and IFN-γ production did not require contact with mDCs but was dependent on type I IFN and IL-12, respectively. Poly(I:C) but not polyadenylic-polyuridylic acid synergized with mDC-derived IL-12 for IFN-γ production by acting directly on NK cells. Finally, the requirement of both TLR3 and Rig-like receptor (RLR) on mDCs and RLRs but not TLR3 on NK cells for IFN-γ production was demonstrated using TLR3- and Cardif-deficient mice and human RIG-I–specific activator. Thus, we report the requirement of cotriggering TLR3 and RLR on mDCs and RLRs on NK cells for a pathogen product to induce potent innate cell activation.
Natural killer cells are potent cytotoxic cells capable of killing infected or tumor cells (1) and are also able of producing proinflammatory cytokines including IFN-γ and TNF. These cytokines are crucial in the enhancement of the innate resistance by activating macrophages and neutrophils but also regulate the adaptive immune response (2).
Although NK cells can directly sense infections and cellular transformation, in part by recognizing stress-associated surface markers, many studies have outlined the importance of accessory cells (ACs) in modulating their function (3). Activated NK cells induce dendritic cell (DC) maturation (4, 5) through both TNF/IFN-γ secretion and cell–cell contact involving NKp30 (5–7). In turn, cytokine production by activated DCs enhances NK cell IFN-γ production, proliferation, and cytotoxic potential (4, 5, 8–10). IL-12 is essential for IFN-γ production by innate lymphocytes (11) and the enhancement of antitumor and antiviral activity in vivo (12). This DC/NK cross-talk has been reported to be critical in Th1 and CTL responses (13–15).
DCs sense viral infection through motifs that are conserved between large classes of pathogens and bind to germline-encoded receptors (16). Among these receptors, the TLR3 (17) and more recently the cytosolic helicase MDA5 (18, 19) have been described to sense synthetic dsRNA or viral infection, leading to strong type I IFN production and contributing to inflammatory cytokine production (20). Furthermore, a mouse vaccination study showed that the production of type I IFN by DCs upon engagement of MDA5 and TLR3 by polyinosinic-polycytidylic acid [poly(I:C)] was required for Th1 driving CD4+ T cell immunity (21), illustrating the therapeutic potential of poly(I:C) in vaccination relative to other TLR agonists.
The role of ACs in dsRNA-mediated NK cells activation remains unclear. Both ACs-dependent (5, 22, 23) and -independent activation (24–27) of NK cells have been reported. The aim of the current study was to decipher the involvement of ACs in dsRNA-mediated NK cells response and to characterize the dsRNA receptors on DC and NK involved in this response. We used three synthetic ligands: poly (I:C) dsRNA that triggers both TLR3 and Rig-like receptor (RLR) pathways, polyadenylic-polyuridylic acid [poly(A:U)] dsRNA that displays specificity for TLR3, and 5′-triphosphate ssRNA (3pRNA) that engages only the RIG-I helicase. We demonstrated that mDCs are required for NK cells activation in response to dsRNA. Most importantly, the specificity of the three ligands and the use of TLR3−/− and Cardif−/− mice allowed us to demonstrate that the full activation of NK cells and especially the production of IFN-γ, in response to dsRNA required the triggering of both TLR3 and RLR on myeloid DCs (mDCs) and RLRs on NK cells. Our study deciphers the mechanisms of dsRNA-induced NK cell physiological response and supports the novel concept that a single pathogen product can lead to potent innate cell activation, because it engages different classes of receptors expressed by different cell types.
Materials and Methods
Reagents
dsRNA or recombinant cytokines were used at indicated concentrations: 1300 kDa poly(I:C) (InvivoGen, San Diego, CA), standardized high molecular mass poly(A:U) (Innate-Pharma, Marseille, France), IFN-β (PBL Biomedical Laboratories, Piscataway, NJ), IFN-α2b (IntronA; Schering-Plough, Kenilworth, NJ), IL-12, or IL-15 (R&D Systems, Minneapolis, MN). 3pRNA was synthesized and purified with the MEGAscript and MEGAclear kits, respectively (Ambion, Austin, TX). The following neutralizing mAbs were used: 20 μg/ml mouse anti-human IFN-αβR2 (PBL Biomedical Laboratories) or 10 μg/ml mouse anti-human IL-12p70 (clone 20C2) and mouse anti-human IL-15 (R&D Systems). Twenty micrograms per mililiter mouse IgG was used as control (R&D Systems). All these mAbs were previously validated using the corresponding recombinant cytokine on relevant NK activation parameters (data not shown).
Cell purification
From human blood of healthy donors
NK cells were selected by depletion using the NK cells isolation kit, whereas mDCs and monocytes were positively selected with, respectively, anti-BDCA1 plus -BDCA3 and -CD14 microbeads (Miltenyi Biotec, Auburn, CA). Purities of NK cells, monocytes, and mDCs were 98.5 ± 0.8, 99.1 ± 0.2, and 94.2 ± 2.6%, respectively. In some experiments, whole PBMCs or depleted in monocytes, mDCs, or plasmacytoid DCs (pDCs) with, respectively, anti-CD14, -BDCA1 plus -BDCA3, and -BDCA4 microbeads (Miltenyi Biotec) were treated with dsRNA.
For real-time PCR, NK cells and mDCs were stained with anti-CD56PE plus -CD3FITC (for NK) or anti-CD3, -CD14, -CD56, and CD35FITC (lineage) plus -CD4PECy5.5 plus CD11cAPC mAbs (BD Biosciences, San Jose, CA) (for mDCs) and sorted with a FACSVantage cell sorter (BD Biosciences) by gating on CD56+CD3− (NK cells) or lineage-CD4+ CD11c+ (mDCs) cells. Final purity of NK cells and mDCs were 99.5 ± 0.3 and 98.7 ± 0.5%.
From spleen of C57BL/6 mice
Spleens from wild-type, TLR3−/− (17), Cardif−/−, (28), and C57BL/6 mice were dilacerated and B, T, and erythroid cells depleted with anti-CD19, -CD3 -Ter119 (BD Biosciences), and sheep anti-rat Ig Dynabeads (Dynal, Oslo, Norway). DX5+ NK cells and CD11c+ DCs were then positively selected with anti-DX5 and anti-CD11c microbeads, respectively (Miltenyi Biotec). Purities of NK cells and DCs were 87.8 ± 2.4 and 86.4 ±6.1%, respectively.
dsRNA-activated DC supernatant generation
MACS-sorted mDCs or in vitro-generated monocyte-derived DCs (MdDCs) were incubated with 30 μg/ml poly(I:C) or poly(A:U) for 24 h (continuous treatment) or pulsed for 4 h, washed three times in PBS, and incubated for 20 supplementary h (pulse treatment). Supernatants (SNs) were harvested, filtered through a 0.22-μm membrane, and used to stimulate purified NK cells diluted as indicated, alone or in combination with 30 μg/ml poly(I:C) or poly(A:U).
Cell activation follow-up
CD107-based degranulation assay
Twenty-hour–activated effectors (PBMCs, NK cells alone, or with mDCs) were seeded in 96-well plates with HCC1806 breast cancer target cell line (American Type Culture Collection, Manassas, VA) at an E:T ratio of 10:1 for PBMCs and 1:1 for NK cells. Anti–CD107aPE-Cy5 mAb (1/20) and monensin (1/1500) (BD Biosciences) were added. After 4 h at 37°C, cells were stained with anti-CD56PE and -CD3FITC and analyzed by flow cytometry. Percentage of CD107a-expressing cells among CD56+CD3− NK cells was determined.
Cytokine quantification
SNs of 20-h–activated human PBMCs, mDCs, or NK cells ± mDC cultures were collected and production of IFN-γ, IL-12p40, IL-12p70, IL-15 (BD Biosciences), and IFN-β (BioSource International, Camarillo, CA) was determined by ELISA. Mouse IFN-γ (BD Biosciences) was also quantified by ELISA in SNs of 20-h–activated mouse NK cells ± conventional DC (cDC) cultures.
In some experiments, IFN-γ was quantified by cytometry-based intracellular staining. A total of 2 × 106 PBMCs were activated or not with poly(I:C) for 2 h before addition of brefeldin A (BD Biosciences) for the last 6 h of culture. Cells were then stained with anti-CD56PE, -CD3PE-Cy5.5, -CD4PE-Cy7 (BD Biosciences), and -Vγ9FITC (Innate Pharma) mAbs. Cells were then fixed and permeabilized using Cytofix-Cytoperm kit, stained with anti–IFN-γ APC mAb (BD Biosciences), and analyzed by flow cytometry. Cells were gated on the IFN-γ–positive cells, and the percentage of γδ T cells, NK cells, NKT-activated T cells, and CD4+ and CD8+ T cells was determined.
Stable expression in HEK293T
HEK293T cell line (American Type Culture Collection) was transfected using FuGene 6 (Roche, Basel, Switzerland) with 5 μg pISRE-TA-luciferase plasmid (BD Clontech, Palo Alto, CA) and 500 ng of the pTK-Hyg plasmid (Invitrogen, Carlsbad, CA) containing hygromycin resistance gene. After an overnight incubation, transfected cells were selected with 150 μg/ml hygromycin B (Invitrogen), cloned by limiting dilution. and analyzed for luciferase activity in response to IFN-α2b. A responding clone was transfected as described above with 2 μg pUNO-hTLR3, pUNO-hMDA5, and pUNO-hRIG-I plasmids (InvivoGen) containing blasticydin resistance gene. Transfected cells were selected with 10 μg/ml blasticydin (Invitrogen), cloned, and analyzed for specific gene expression by real-time PCR.
Luciferase assay
A total of 4 × 104 TLR3-, RIG-I–, and MDA5-expressing cells or control cells were seeded into 96-well plates (MicroClear-96; Greiner Bio One, Courtaboeuf, France) and incubated for 20 h at 37°C. Cells were then activated for 6 h at 37°C with poly(I:C), poly(A:U), 3pRNA, or IFN-α2b (100 UI/ml). Luciferase activity was measured on a TopCount NXT apparatus (Packard Instrument, Meriden, CT) after addition of the Steady-Glo reporter assay reagent (Promega, Madison, WI). Results were expressed as a ratio between stimulated and nonstimulated cells. In some experiments, results were normalized to the IFN-α2b response to compare ligands efficacy.
Biacore assay
Biacore T100 apparatus (Biacore Life Sciences, GE Healthcare, Fairfield, CT) was used for these experiments. Recombinant human TLR3 (rhTLR3; R&D Systems) was immobilized onto the dextran layer of a Biacore CM5 Series S sensor chip by injecting rhTLR3 (10 μg/ml) until reaching the desired immobilization level (~3000 RU). A total of 10 μg/ml poly(A:U) or poly(I:C) were injected onto rhTLR3 surface at a flow rate of 10 μl/min. Injection was performed for 120 s, followed by a dissociation period of 180 s. Sensorgam curves were analyzed using BiaEvaluation software version 4.1.
Real-time PCR
Total mRNA was extracted from cells of interest and cDNA was synthesized using RNeasy Plus and QuantiTect RT Kits, respectively (Qiagen, Valencia, CA). GAPDH was used as a housekeeping gene. PCRs were performed with the QuantiTect SYBR Green PCR kit (Qiagen), primers, and 20 ng cDNA on a MX3000P thermocycler (Stratagene, La Jolla, CA). GAPDH, RIG-I, and MDA5 primers were purchased from Qiagen. TLR3 primer sequences were as follows: 5′-TGGTTGGGCCACCTAGAAGTA-3′ (forward) and 5′-TCTCCATTCCTGGCCTGTG-3′ (reverse). Cycling conditions were as follows: 1 cycle of 10 min at 95°C; 40 cycles of 30 s at 95°C, 1 min at 60°C and 30 s at 72°C; and 1 cycle of 1 min at 95°C, 30 s at 55°C, and 30 s at 95°C. Because of using primer pairs that amplified two or more exons from a unique gene, genomic contamination was excluded by melting curve analysis. Gene expression was calculated as the ratio between gene of interest and GAPDH using the MxPRO QPCR software (Stratagene).
Statistical analysis
Statistical analyses were performed using GraphPad Prism version 5.1 software. Student t test (two-tailed) was used in the study.
Results
Both poly(I:C) and poly(A:U) signal via TLR3 but only poly(I:C) triggers RIG-I or MDA5
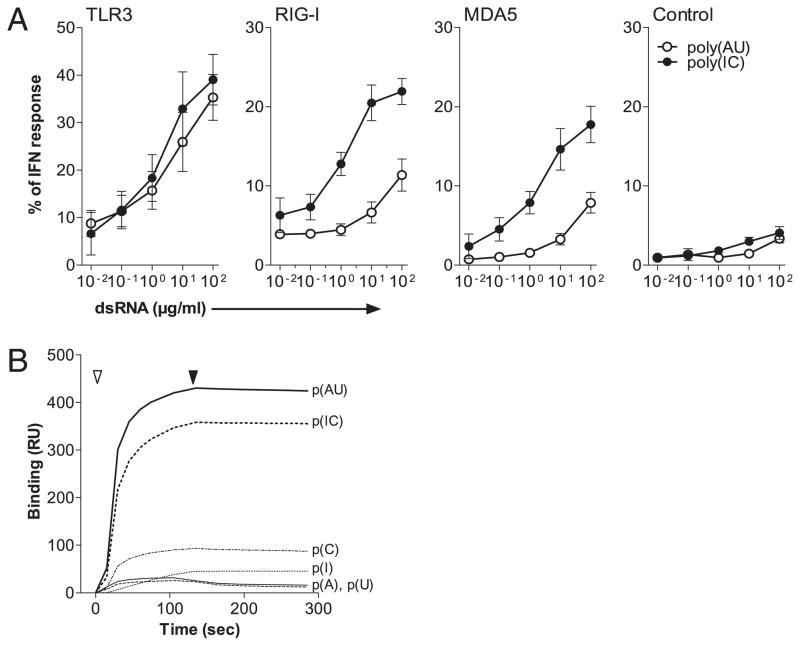
To determine the ability of dsRNA to trigger type I IFN production through TLR3 and RLR, HEK293T cells stably expressing the pISRE-TA-luciferase plasmid were transfected with plasmids encoding human TLR3, RIG-I, or MDA5. The transfected or control cell lines were stimulated for 6 h with different doses of poly(I:C) and poly(A:U) dsRNA or with 100 UI/ml IFN-α2b as internal calibrator and luciferase activity were measured. The activity of dsRNA was normalized to the response to IFN-α2b. Both dsRNAs triggered TLR3 with the same efficiency, but a specific response in RIG-I– and MDA5-transfected cells was observed only with poly(I:C) (Fig. 1A). A high concentration (100 μg/ml) of poly(A:U) only induced a weak response in RIG-I– and MDA5-expressing cell lines comparable to that also detected on cells expressing ISRE-TA-luciferase, only suggesting activation of endogenous TLR3 that appeared to be expressed at low levels in 293T cell line, as detected by real-time PCR (Supplemental Fig. 1).
FIGURE 1.
P(AU) and p(IC) show an identical potency for TLR3 engagement whereas only p(IC) triggers RLRs. A, A total of 4 × 105/ml human HEK293T cells stably expressing the pISRE-TA-luciferase plasmid were transfected with plasmids encoding human TLR3, RIG-I, or MDA5. Transfected or control HEK293T were stimulated with p(IC), p(AU), or with IFN-α (100 IU/ml), and luciferase activity was determined. Results were expressed as a ratio between stimulated and nonstimulated cells and normalized to IFN-α response. Data represent mean + SD of five independent experiments. B, p(AU) or p(IC) and control ssRNA poly(A), poly(U), poly(I), and poly(C) were injected onto rhTLR3-coated chip and analyzed on a Biacore T100 apparatus. Injection (open arrowhead) was performed for 120 s, followed by a dissociation period (filled arrowhead) of 180 s. Data are presented as one of three representative experiment. p(AU), poly(A:U); p(IC), poly(I:C).
We then tested both dsRNA on an rhTLR3-coated sensor chip analyzed using a Biacore T100 apparatus. Poly(A:U) and poly(I:C) displayed an identical avidity for binding to TLR3 as indicated by the association/dissociation curves of the dsRNA (Fig. 1B), confirming that poly(A:U) and poly(I:C) triggered TLR3 with the same efficiency.
Altogether, these results demonstrate that poly(A:U) and poly(I:C) signal via TLR3 with the same efficiency, but only poly(I:C) triggers the RLR pathway via RIG-I and/or MDA5.
Within PBMCs, poly(I:C) and poly(A:U) lead to different qualitative NK cell activation
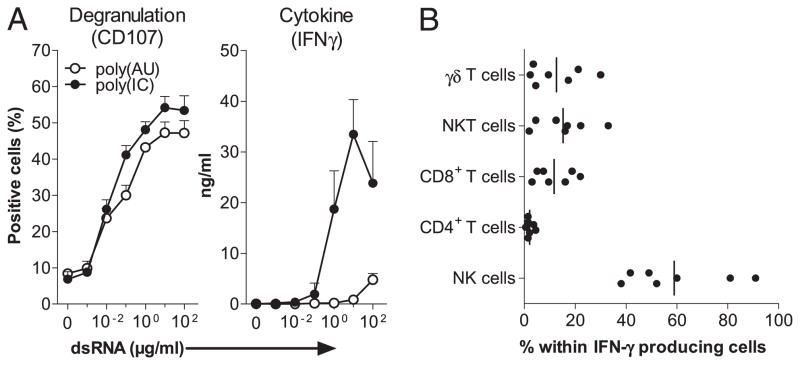
Human PBMCs were cultured with either dsRNA. After 20 h, IFN-γ was quantified in the SN, and following an additional 4-h coculture with HCC1806 breast cancer target cells, degranulating CD56+ CD3− NK cells were identified by the expression of CD107. The two dsRNAs enhanced NK cell degranulation with the same efficiency (Fig. 2A, left panel), but poly(I:C) was much more efficient than poly(A:U) (p < 0.0001 between 1 and 100 μg/ml dsRNA) to induce IFN-γ production (Fig. 2A, right panel). As detected by intracellular staining, NK cells represented the majority of IFN-γ–producing cells in poly(I:C)-activated PBMCs with a minor participation of CD8+, CD3+CD56+, and γδ T cells (Fig. 2B).
FIGURE 2.
Both poly(I:C) and poly(A:U) trigger NK cell activation within PBMCs, but only poly(I:C) induces high IFN-γ production by NK cells. A, A total of 2 × 106/ml human PBMCs were stimulated for 20 h with poly(I:C) and poly(A:U). Culture SNs were collected to determine the production of IFN-γ by ELISA (right panel). Cells were additionally incubated for 4 h with the HCC1806 target cell line to quantify the degranulating NK cells (CD56+CD3−) in a CD107 flow cytometry-based assay (left panel). Data are from one representative experiment (with SD on culture triplicates) of five performed with five different donors. B, A total of 2 × 106/ml human PBMCs were stimulated for 2 h with 30 μg/ml poly(I:C) before addition of brefeldin A for the last 6 h of culture. Cells were stained as described in Materials and Methods analyzed by flow cytometry by gating on the IFN-γ–positive fraction. Data are expressed as the percentage of different cell subsets within IFN-γ–producing cells. Data represent independent values of seven different donors, and horizontal bars represent the median.
This differential activity of dsRNA suggests that the induction of degranulation is mainly dependent on the TLR3 pathway activation shared by poly(I:C) and poly(A:U), whereas optimal IFN-γ induction required additional signaling through the RLR engaged by poly(I:C) exclusively.
mDCs are required for NK cell activation in response to dsRNA
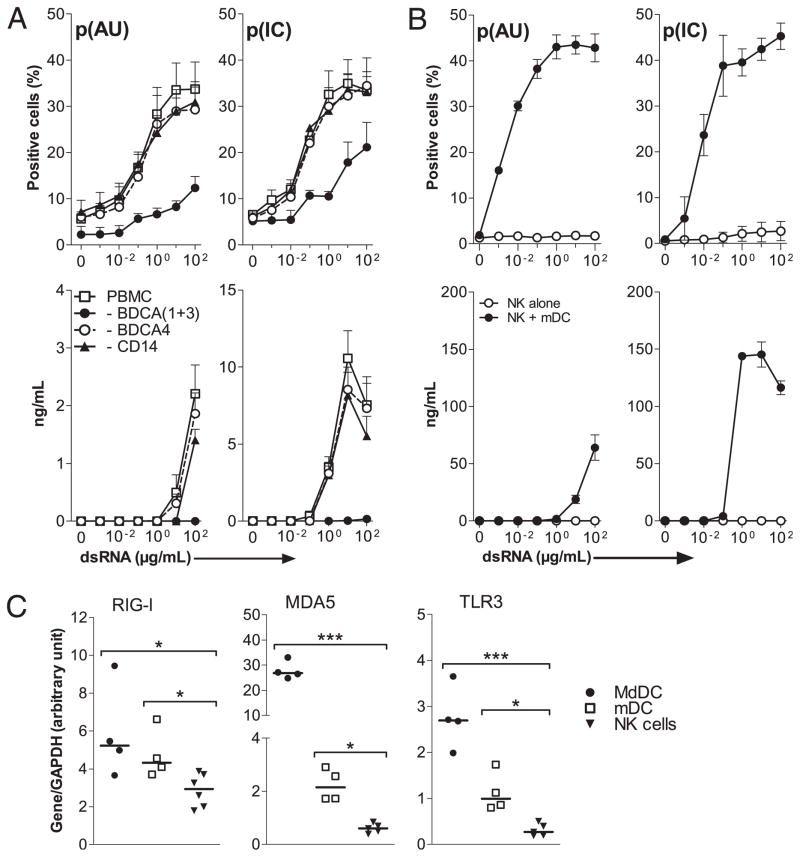
To analyze the requirement of ACs in the human NK cell response to dsRNA, PBMCs were immunomagnetically depleted of various cell populations before culture with poly(I:C) or poly(A:U), and CD107 expression and IFN-γ production were analyzed. Depletion of mDCs (BDCA1+3) before activation with either dsRNA dramatically decreased the cytolytic activity and completely abrogated the production of IFN-γ (Fig. 3A), whereas depletion of monocytes (CD14) and pDCs (BDCA4) had no effect. To further confirm the role of mDCs in dsRNA-mediated activation of NK cells, both cell types were purified by immunomagnetic sorting and activated either separately or in coculture. Using purified NK cells, the two dsRNA did not induce IFN-γ production or CD107 (Fig. 3B); however, in the presence of mDCs, both dsRNA similarly enhanced the cytotoxic activity of purified NK cells, whereas poly(I: C) was much more efficient than poly(A:U) in triggering IFN-γ production.
FIGURE 3.
mDCs are required for NK cells activation in response to dsRNA. NK cells activation was evaluated by CD107a staining (upper panels) and the IFN-γ production (lower panels), as described (Fig. 1). A, A total of 2 × 106/ml whole human PBMCs or depleted in myeloid cells (CD33), monocytes (CD14), pDCs (BDCA4), or mDCs (both BDCA1 and BDCA3 subtypes) were activated with the indicated concentrations of p(IC) and p(AU). B, 1 × 106/ml MACS-purified human NK cells were activated with the indicated concentrations of p(IC) and p(AU) with or without 2 × 105/ml MACS-purified blood mDC (both BDCA1 and BDCA3 subtypes). Data from A and B represent independent values of four and three different donor, respectively. C, RIG-I, MDA5, and TLR3 mRNA levels were determined in the three different cell types using real-time PCR. Results are expressed as arbitrary units normalized to value for the housekeeping gene GAPDH. Data represent independent values of four to six different donors and horizontal bars the median. *p < 0.05; ***p < 0.001. p(AU), poly(A:U); p(IC), poly(IC).
To link these biological activities with the receptor selectivity of poly(I:C) and poly(A:U), we then determined the levels of dsRNA receptor expression by real-time PCR on nonactivated cell populations. The three studied mRNAs were detected on MdDCs and mDCs but, except RIG-I, were much less expressed in NK cells (Fig. 3C).
These observations demonstrated that dsRNA-mediated activation of NK cells required the participation of mDCs that were likely activated by poly(I:C) and poly(A:U) through engagement of TLR3. However, the higher activity of poly(I:C) than poly(A:U) for IFN-γ induction suggested the contribution of another dsRNA receptor (likely an RLR) on mDCs and/or NK cells.
IL-12 and IFN-β produced by dsRNA-activated mDCs contribute to activate NK cells
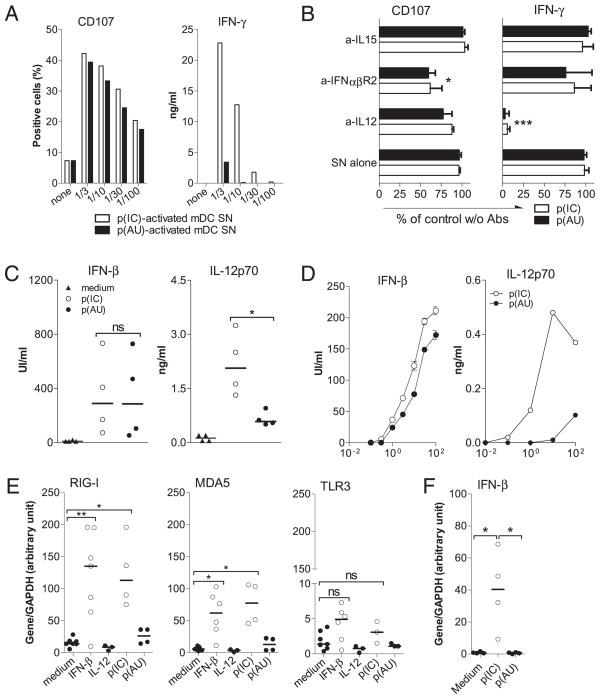
We next addressed whether soluble mediators produced by human dsRNA-activated mDCs were involved in the NK cell response to dsRNA. dsRNA-activated mDC SNs generated by treating cells for 20 h were used to stimulate purified NK cells. SNs of mDCs activated for 20 h with the same dose of either dsRNA were sufficient to activate NK cell cytolytic activity (Fig. 4A), whereas SNs generated with poly(A:U) were much less efficient than those generated with poly(I:C) to induce IFN-γ.
FIGURE 4.
IL-12 and type I IFN secreted by dsRNA-activated mDCs mediate NK cells activation. A, SNs from MACS-sorted mDCs (BDCA1 and BDCA3) activated with p(IC) (open histograms) or p(AU) (filled histograms) were used in a dose range to activate 1 × 106/ml MACS-purified human NK cells. Degranulation and IFN-γ production are evaluated as described (Fig. 2). Data are from of one of three representative experiments (mean of duplicate culture with <10% variation) performed with NK cell from three independent donors and two batches of mDC SNs. B, A total of 1 × 106/ml MACS-purified human NK cells were cultured with 10% p(IC)- or p(AU)-activated mDC SNs in the absence or presence of neutralizing mouse anti–IL-12p70, anti–IFN-αβR2, anti–IL-15, or mouse IgG as control (cont. mAb). Data obtained in the presence of the Abs are expressed as percentage of the activation observed in the absence of Abs and represent mean ± SD from three independent NK cells donors activated with two different SN batches. C and D, IFN-β and IL-12p70 concentrations were measured by specific ELISA in SNs of MACS-purified human mDCs (5 × 105/ml) stimulated 20 h with 50 μg/ml p(IC) or p(AU) (C) or of whole PBMCs (1 × 106/ml) stimulated 20 h with a dose range of both dsRNA (D). Symbols represent individual results from four different donors and the horizontal bars the median (C), and curves represent one representative experiment from one of four donors (D). *p <0.05; ***p <0.001. E and F, RIG-I, MDA5, TLR3, and IFN-β mRNA levels were determined by real-time PCR on FACS-sorted NK cells activated with IFN-β (1000 UI/ml), IL-12 (10 ng/ml), p(IC), p(AU) (both 50 μg/ml) (E) or only p(IC) or p(AU) (F). Results are expressed as arbitrary units normalized to value for the housekeeping gene GAPDH. Data represent independent values of three to eight different donors and horizontal bars represent the median. *p < 0.05; **p < 0.01. p(AU), poly(A:U); p(IC), poly(I:C).
NK cells were treated with dsRNA-activated DC SNs with or without neutralizing Abs. The blockade of type I IFNR significantly diminished NK cell degranulation [p = 0.048 for poly(I:C) and p = 0.010 for poly(A:U)] but not the production of IFN-γ (Fig. 4B). IL-12 neutralization had no effect on the CD107 staining, whereas the production of IFN-γ was completely abrogated [p = 0.0005 for poly(I:C) and p = 0.0009 for poly(A:U)]. No effect was observed when IL-15 was blocked.
Poly(I:C) and poly(A:U) were equally effective in inducing type I IFNs production by purified mDCs (Fig. 4C) or whole PBMCs (Fig. 4D), whereas poly(I:C) was a stronger inducer of IL-12p70 than poly(A:U). Of note, when PBMCs were depleted of their mDC fraction (BDCA1+3), IFN-β production was totally abrogated and dramatically decreased in response to poly(A:U) and poly(I:C), respectively (Supplemental Fig. 2), suggesting that mDCs were the main producers of type I IFN within PBMCs in response to dsRNA.
Finally, upon activation with IFN-β or poly(I:C), but not with poly(A:U), the levels of RIG-I and MDA5 transcripts in highly purified FACS-sorted NK cells were increased 50- to 100-fold, whereas the level of TLR3 transcripts remained very low (Fig. 4E). In addition, as shown in Fig. 4F, poly(I:C) significantly increased the level of IFN-β mRNA in purified NK cells as compared with medium (p = 0.022) or poly(A:U) (p = 0.021), indicating that the regulation of an RLR was presumably dependent on type I IFN.
These observations demonstrated that soluble mediators produced by dsRNA-activated mDCs participated in NK response to dsRNA and that type I IFN mainly contributed to the enhancement of cytotoxic activity and IL-12 to IFN-γ production. Furthermore, poly(I: C) and poly(A:U) engaged at least TLR3 on mDCs, leading to IFN-β secretion that upregulated the expression of RLRs on NK cells.
Requirement of direct effect of poly(I:C) on NK for IFN-γ production
Because, in the experiments described above we could not rule out the presence of remaining dsRNA within the mDC SNs, the contribution of mDC-produced soluble factors was mimicked using rIL-12 and type I IFN to decipher a possible contribution of direct activation of NK cells through one of the dsRNA receptor. We treated MACS-sorted NK cells with a different doses of rIL-12 or IFN-β alone or in the presence of poly(I:C) or poly(A:U). IFN-β alone triggered a strong cytolytic activity of NK cells but did not induce IFN-γ (Fig. 5A, upper panel). IL-12 alone was a weak NK cell activator in both readouts (Fig. 5A, lower panel). However, poly(I:C) but not poly(A:U) synergized with IL-12 to induce a strong activation of purified NK cells, suggesting that poly(I:C) directly engaged NK cells for the production of IFN-γ and that NK cells do not express receptors for poly(A:U) even after priming with IL-12 or IFN-β.
FIGURE 5.
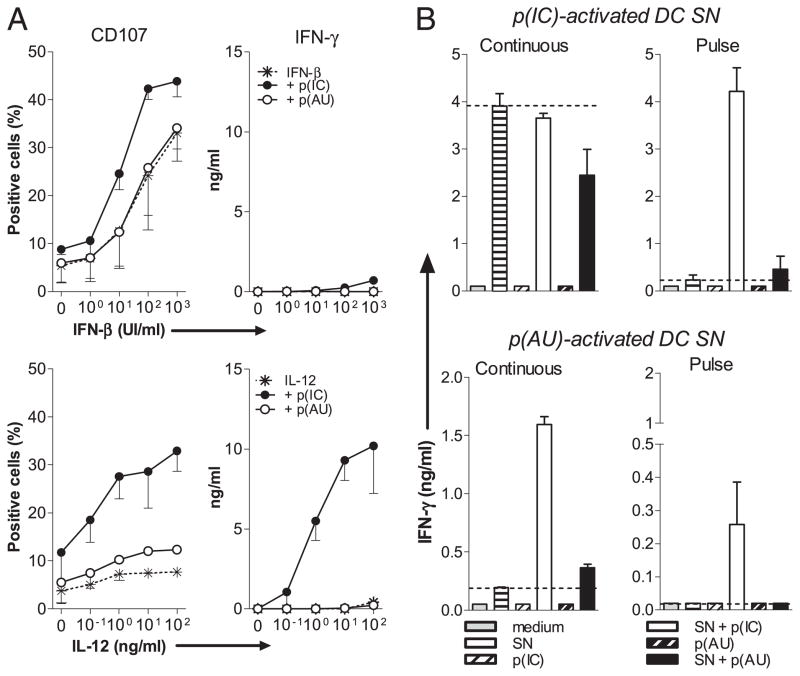
P(IC) but not p(AU) directly activates NK cells primed with IL-12, type I IFN, or soluble mediators from dsRNA-stimulated MdDCs. A, A total of 1 × 106/ml MACS-purified human NK cells were stimulated with the indicated concentrations of IFN-β (A, upper panels) or IL-12 (A, lower panels) with or without 30 μg/ml p(IC) or p(AU). Degranulation (left) and IFN-γ (right) production are evaluated as described (Fig. 1). Data are from one of three representative experiments with three different donors (mean ± SD of culture triplicates). B, A total of 2 × 106/ml MdDCs were pulsed for 4 h with p(IC) or p(AU), washed, and cultured for an additional 20 h (pulse-SN) or continuously treated for 24 h with either dsRNA (continuous-SN). A total of 1 × 106/ml MACS-sorted human NK cells were stimulated with or without 10% pulsed- or continued-SN from MdDCs alone or in presence of either dsRNA. IFN-γ production was studied as described (Fig. 1). Data are from one representative experiment from one of four donors (mean ± SD of culture triplicates). p(AU), poly(A:U); p(IC), poly(I:C).
We next produced SNs from mDCs pulsed with dsRNA for 4 h, extensively washed and cultured for an extra 20 h to avoid contaminating dsRNA in the SNs. Although SNs from pulsed mDCs induced CD69 expression, they were unable to induce IFN-γ production unlike SNs from mDCs continuously stimulated with dsRNA (Supplemental Fig. 3A).
We next performed experiments with MdDC SNs that displayed almost the same efficiency than mDC SNs to activate NK cells (Supplemental Fig. 3B). Poly(I:C) synergized with poly(I:C)-pulsed MdDC SNs to trigger the production of IFN-γ by MACS-sorted NK cells (Fig. 5B), with an 18-fold increase compared with SN alone. Using poly(A:U) continuously treated MdDC SNs, we observed a similar synergy with poly(I:C) for IFN-γ production by NK cells. In contrast, the addition of poly(A:U) on NK cells did not synergize with any of the pulsed DC SNs (Fig. 5B), in line with the absence of poly(A:U)-interacting receptors on NK cells.
Collectively, our results suggest that dsRNA treatment of mDCs induced the production of IL-12 that synergized with a TLR3-independent and probably RLR-dependent direct effect of poly(I: C) on purified NK cells for IFN-γ production.
Synergy between TLR3 and RLR engagement on mDCs and RLRs on NK cells for IFN-γ production
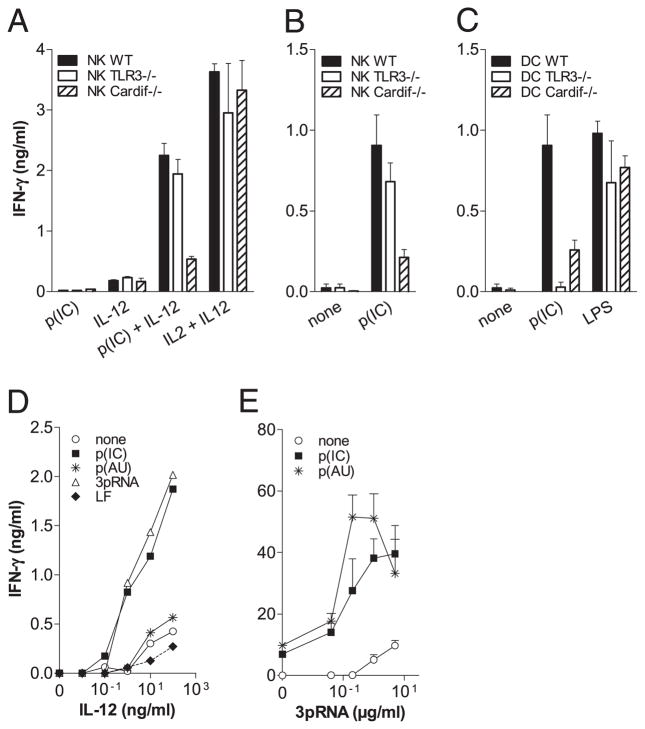
As in humans, poly(I:C) synergized with IL-12 to induce the production of IFN-γ by purified mouse NK cells (Fig. 6A). With the aim of confirming that the direct effect of poly(I:C) on NK cells was TLR3 independent, we performed experiments with mouse DX5+ NK cells and CD11c+ DCs purified from TLR3 or Cardif-deficient and -sufficient mice. The synergy between poly(I:C) and IL-12 was observed with WT and TLR3−/− but not Cardif−/− NK cells, demonstrating that the direct effect of poly(I:C) on NK cells was RLR dependent but TLR3 independent. As a control, IL-2 used in combination with IL-12 showed that WT, TLR3−/−, and Cardif−/− NK cells produced almost the same amount of IFN-γ (Fig. 6A). In coculture experiment using purified NK cells from either WT, TLR3−/−, or Cardif−/− and DCs from WT mouse spleen, IFN-γ production induced by poly(I:C) also required the RLR but not the TLR3 pathway on NK cells (Fig. 6B). Finally, IFN-γ production was completely abrogated and 70% reduced when WT NK cells were cocultured with DCs from TLR3−/− and Cardif−/− mice, respectively (Fig. 6C). When activated with LPS (TLR4), DCs from WT, TLR3−/−, or Cardif−/− mice induced IFN-γ production in NK cells. These observations demonstrated the crucial requirement of both TLR3 and RLR on DCs but of only the RLR on NK for production of IFN-γ in response to poly(I:C).
FIGURE 6.
Engagement of TLR3 and RLR on cDCs and RLRs on NK cells is essential for IFN-γ production. A, A total of 5 × 105/ml MACS-sorted mouse NK cells from TLR3−/−, Cardif−/−, and WT mice were stimulated with 100 ng/ml IL-12 or 100 μg/ml p(IC) alone or in combination; 10 ng/ml IL-2 + 100 ng/ml IL-12 were used as positive control. B, A total of 5 × 105/ml MACS-sorted mouse NK cells from TLR3−/−, Cardif/−, and WT mice were cultured in the presence of 105/ml MACS-sorted mouse CD11C+ cDCs from WT mice with or without 100 μg/ml p(IC). C, A total of 5 × 105/ml MACS-sorted mouse NK cells from WT mice were cultured with 105/ml MACS-sorted cDCs from either WT, TLR3−/−, or Cardif−/− in the presence of 100 μg/ml p(IC) or 100 ng/ml LPS. A–C, Data are from one of three representative experiments (mean ± SD of triplicate cultures). Each experiment was performed with the pool of spleens from at least five animals. D, A total of 1 × 106/ml MACS-sorted human NK cells were activated with the indicated concentrations of rIL-12 alone or in combination with p(IC), p(AU), or 3pRNA complexed with Lipofectamine 2000. E, A total of 1 × 106/ml MACS-purified human NK cells were stimulated for 6 h with Lipofectamine 2000-associated 3pRNA. Cells were then washed twice with ice-cold PBS and incubated for an additional 20 h with 2 × 105/ml autologous MACS-purified human mDCs with or without p(A:U) or p(I:C). D and E, Data are from one of three representative experiments performed with three independent donors (mean ± SD of culture triplicates). p(AU), poly(A:U); p(IC), poly(I:C).
To further confirm that the triggering of the RLR pathway on human NK cells was involved in the production of IFN-γ, we stimulated MACS-sorted NK cells with 3pRNA, a newly described RIG-I–specific ligand (29, 30) (Supplemental Fig. 4). We then observed that 3pRNA synergized with IL-12 to enhance IFN-γ production by NK cells similarly to poly(I:C) but unlike poly(A: U) (Fig. 6D). In addition, the combination of 3pRNA and poly(A: U) induced IFN-γ production in NK-DC cocultures at levels comparable to those induced by poly(I:C) (Fig. 6E). Altogether, these results demonstrate that TLR3 and RLR engagement on DCs and RIG-I on NK are required for optimal IFN-γ production by NK. The mechanisms deciphered along the study concluding that the optimal production of IFN-γ by NK cells required the concomitant engagement of TLR3 and RLRs on mDCs and RLRs on NK cells by dsRNA are depicted in Fig. 7.
FIGURE 7.
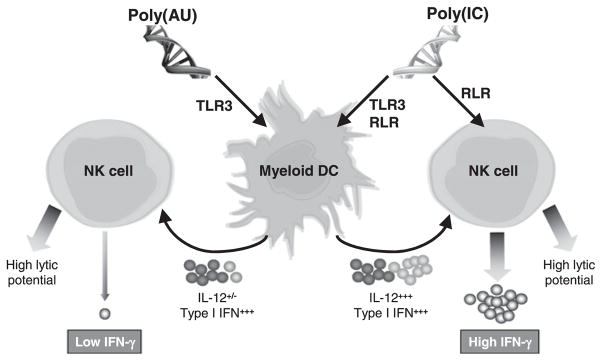
Mechanisms that induced optimal production of IFN-γ by NK cells: requirement of concomitant engagement of TLR3 and RLRs on mDCs and RLRs on NK cells by dsRNA. Poly(I:C) that triggered both TLR3 and RLR pathway led to high level of IL-12 and type I IFN production by mDCs, whereas poly(A:U) that activated only TLR3 induced a high amount of type I IFN but low IL-12. In parallel, poly(I:C)—but not poly(A:U)—was able to directly activate NK cells via RLRs and allowed them to produce high level of IFN-γ in combination with IL-12. Cytotoxic potential of NK cells mostly dependent of the type I IFN produced by dsRNA-activated mDCs and not of a direct triggering by dsRNA was comparable with both poly(I:C) and poly(A:U).
Discussion
In the current study, we report the first evidence that optimal NK cell response to synthetic dsRNA requires the triggering of both TLR3 and RLR on mDCs and RIG-I on NK cells (Fig. 7). In particular, we show that 1) NK cell activation in response to dsRNA required the presence of mDCs; 2) poly(I:C) dsRNA (an agonist for both TLR3 and RLR) and 3pRNA (an agonist for RIG-I) but not poly(A:U) dsRNA (an agonist for TLR3 only) directly activated NK cells leading to high IFN-γ production in synergy with mDC-released soluble mediators induced by either dsRNA; and 3) TLR3 and RLR expression were required on mDCs, whereas RLR was needed on NK cells for IFN-γ production in response to dsRNA.
In Biacore experiments and in the luciferase assay with TLR3-transfected cell line (Fig. 1A, 1B), poly(A:U) and poly(I:C) showed similar efficiency (identical avidity and potency) to bind to and trigger TLR3. In contrast, using RLR-overexpressing cell lines, only poly(I:C) activated the two RLRs, RIG-I and MDA5. Our results are in agreement with the study of Kato et al. (31), showing that dsRNA is recognized by either RIG-I or MDA5, although in a length-dependent way.
DC depletion and NK-DC coculture experiments established that mDCs were critical for dsRNA-mediated NK cell activation. These observations are in agreement with several studies both in humans (5) and in mouse models (22) showing the mandatory role of mDCs or conventional CD11c+ DCs, respectively, for NK cells activation upon treatment with synthetic poly(I:C). Numerous reports deciphered the interplay between NK cells and DCs leading to the complete activation of both cell types (4, 6, 9, 10, 32) and to the establishment of the innate and adaptive immune responses involved in the control of viral infection (33, 34) or tumor growth (8). In particular, using an inducible and selective ablation system of CD11c+ DCs, Lucas et al. (35) have shown that naive NK cells do not acquire effector functions without prior priming by contact with cDCs in draining lymph nodes.
We show in our experimental model, that for DC/NK cooperation, cell-to-cell contact was not required. Type I IFN and IL-12 produced by either poly(I:C)- or poly(A:U)-activated mDCs were sufficient to enhance the cytolytic potential and IFN-γ production by NK cells, respectively. This is in agreement with previous reports that highlighted the respective role for IL-12 and type I IFN in the production of IFN-γ and the cytolytic potential of NK cells (5, 12, 36). In several other experimental settings, a direct contact between NK cells and DCs (membrane IL-15 and NKp30) was shown to participate in efficient NK cells triggering (8, 32). To examine the role of dsRNA receptors on NK, our study focused on soluble mediators, but a role for membrane interaction between NK cells and DC was not excluded.
The fact that both poly(A:U) and poly(I:C) induced the secretion of soluble mediators from mDCs leading to NK cell activation clearly suggested that the dsRNA activity on mDCs was at least dependent on TLR3. This observation is in agreement with several studies demonstrating the involvement of TLR3 on mDC response to poly(I:C) (4, 5, 22). However, although both dsRNAs induce comparable levels of type I IFN, poly(I:C) induced significantly higher levels of IL-12p70 suggesting the engagement of a receptor was distinct from TLR3 for the optimal IL-12p70 production by mDCs. Experiments performed with mouse CD11c+ DCs deficient for TLR3 or Cardif expression confirmed that TLR3 and also RLRs on DCs were required for the dsRNA-mediated DC-dependent NK cell activation, notably IFN-γ production. McCartney et al. (37) showed that production of IL-12p40 in response to an in vivo injection of poly(I:C) was totally abrogated in TLR3−/− mice but not in MDA−/− mice. This observation might appear in contradiction with our results showing an abrogation or a dramatic diminution of IFN-γ production by NK cells when cocultured with DC from TLR3−/− or Cardif−/− mice, respectively. However, the involvement of RIG-I was not explored in the study of McCartney et al. (37).
The synergistic activity between DC-derived soluble mediators and poly(I:C) acting on purified NK cells demonstrated a direct effect of dsRNA on NK cells. Furthermore, the biological responses observed with purified human NK cells and mouse TLR3- or Cardif-deficient NK cells unambiguously demonstrated that the direct effect of poly(I:C) on NK cells is RLR dependent and TLR3 independent. The fact that the RIG-I agonist 3pRNA mimicked the ability of poly(I:C) to activate NK cells further confirm the role of RLRs on human NK cells for induction of IFN-γ in synergy with IL-12. In addition, upon treatment of NK with type I IFN or poly(I:C), the expression of both RIG-I and MDA5 was significantly enhanced, whereas TLR3 was still only marginally expressed (Figs. 3C, 4E). Similarly to our results, Tu et al. (23) showed that poly(I:C)-treated Kupffer cells allowed NK cells to produce IFN-γ after a direct sensing of poly(I:C), but these authors did not identify the dsRNA receptor involved on NK cells.
Our present conclusion that in NK cells poly(I:C)-mediated activation of RLRs and in particular RIG-I but not TLR3 play an important role in enhancing the production of IFN-γ in response to DC-produced cytokines such as IL-12 is based on several lines of evidence both in human and mouse cells. However, this conclusion may appear in contradiction with previous studies suggesting that NK cells directly respond to dsRNA in a TLR3-dependent manner (25, 26, 38, 39). Sivori et al. (26), in particular, demonstrated a convincing correlation between poly(I:C)-mediated activation and TLR3 mRNA expression in in vitro-expanded clones of IL-2–activated NK cells. However, these results were obtained using in vitro-expanded NK cell clones that may have upregulated TLR3 upon activation and, thus, do not contradict our own results that have been obtained with freshly purified resting NK cells and that may more closely reproduce the physiological NK response to infection in naive organisms. Most other studies did not exclude the contribution of other dsRNA receptors expressed by NK cells. The discovery that viral dsRNA and poly(I:C) are capable of triggering receptors other than TLR3 and in particular the RLRs family is recent (18, 31, 40, 41), and most other studies on NK cells have not directly tested the contribution of RLRs. However, our results demonstrating a critical role for RLRs on NK cells are in contradiction with Miyake et al. (42) and McCartney et al. (37) recent reports that failed to identify a role of either RLR or MDA5 on NK cells for IFN-γ production in DC-NK cultures in response to poly(I:C). The reason for this discrepancy is unclear but might be due to different experimental conditions that may mask the requirement for RLRs on NK cells.
Taken together with the recent work of Lucas et al. (35), our observations would suggest that in vivo mDCs activated through TLR3 migrate into the draining lymph node and produce cytokines, leading to NK cell priming. Then, upon recirculation and access to the inflamed tissues, primed NK cells would directly respond to viral invasion through lytic synapse and pathogens components transfer and sensing through cytosolic helicases. This secondary activation of the primed NK cells would further enhance their ability to kill infected or transformed cells and would induce the production of inflammatory cytokines contributing to the shaping of the adaptive immune response (39). These observations are consistent with the concept that, to trigger a productive innate immune response, viruses need to engage different classes of receptors either constitutively expressed or induced on different cell types. Other known examples that support this concept include imidazoquinolines (43) compounds that mimic microbial components by triggering TLR7/8 and cryopyrin/NALP3 (44) as well as peptidoglycan, which triggers several receptors including TLR2 (40) and NOD1 (41, 42). Thus, suggesting that this concept may also apply to some bacteria; however, in our experimental conditions, we could not detect such cooperation for LPS-induced IFN-γ by NK cells. A similar scenario involving synergy between different TLRs expressed within different cellular compartments on a unique DC type (45) or cooperation between different DC types (39) have been suggested as a requirement to mount an appropriate immune response against a single pathogen. Our model strengthens the notion that powerful but also potentially harmful immune effector mechanisms are elicited only in response to those invading pathogens that the immune system senses cooperatively through multiple cells, compartments, and receptors. Furthermore, Longhi et al. (21) showed that the production of type I IFN by DCs in response to poly(I:C) as adjuvant that triggered both TLR3 and RLR pathways leads to a strong adaptive immunity. This study clearly highlighted the clinical potential of a drug with specificity for TLR3 and RIG-I/MDA5 or of two drugs specific for each pathway. The combination of different products that act individually on each receptor will allow to tune very finely the immune response and to ensure the safety of the treatment.
Supplementary Material
Acknowledgments
We thank N. Bendriss-Vermare, N. Goutagny, and J. Valladeau (Institut National de la Santé et de la Recherche Médicale Unité 590) for all scientific discussions and critical review of the manuscript, N. Anfossi, P. André, and F. Romagné (Innate Pharma) for helpful advice on NK cell biology, and M.-C. Michallet (Institut National de la Santé et de la Recherche Médicale Unité 851) for help in obtaining and establishing the Cardif-deficient colony.
Abbreviations used in this paper
- AC
accessory cell
- cDC
conventional dendritic cell
- DC
dendritic cell
- mDC
myeloid dendritic cell
- MdDC
monocyte-derived dendritic cell
- p(AU), poly(A:U); poly(A:U)
polyadenylic-polyuridylic acid
- pDC
plasmacytoid dendritic cell
- p(IC), poly(I:C); poly(I:C)
polyinosinic-polycytidylic acid
- 3pRNA
5′-triphosphate ssRNA
- rhTLR3
recombinant human TLR3
- RLR
Rig-like receptor
- SN
supernatant
Footnotes
The online version of this article contains supplemental material.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moretta A, Marcenaro E, Parolini S, Ferlazzo G, Moretta L. NK cells at the interface between innate and adaptive immunity. Cell Death Differ. 2008;15:226–233. doi: 10.1038/sj.cdd.4402170. [DOI] [PubMed] [Google Scholar]
- 3.Walzer T, Dalod M, Robbins SH, Zitvogel L, Vivier E. Natural-killer cells and dendritic cells: “l’union fait la force”. Blood. 2005;106:2252–2258. doi: 10.1182/blood-2005-03-1154. [DOI] [PubMed] [Google Scholar]
- 4.Gerosa F, Baldani-Guerra B, Nisii C, Marchesini V, Carra G, Trinchieri G. Reciprocal activating interaction between natural killer cells and dendritic cells. J Exp Med. 2002;195:327–333. doi: 10.1084/jem.20010938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerosa F, Gobbi A, Zorzi P, Burg S, Briere F, Carra G, Trinchieri G. The reciprocal interaction of NK cells with plasmacytoid or myeloid dendritic cells profoundly affects innate resistance functions. J Immunol. 2005;174:727–734. doi: 10.4049/jimmunol.174.2.727. [DOI] [PubMed] [Google Scholar]
- 6.Ferlazzo G, Tsang ML, Moretta L, Melioli G, Steinman RM, Münz C. Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. J Exp Med. 2002;195:343–351. doi: 10.1084/jem.20011149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vitale M, Della Chiesa M, Carlomagno S, Pende D, Aricò M, Moretta L, Moretta A. NK-dependent DC maturation is mediated by TNFα and IFNγ released upon engagement of the NKp30 triggering receptor. Blood. 2005;106:566–571. doi: 10.1182/blood-2004-10-4035. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez NC, Lozier A, Flament C, Ricciardi-Castagnoli P, Bellet D, Suter M, Perricaudet M, Tursz T, Maraskovsky E, Zitvogel L. Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune responses in vivo. Nat Med. 1999;5:405–411. doi: 10.1038/7403. [DOI] [PubMed] [Google Scholar]
- 9.Nishioka Y, Nishimura N, Suzuki Y, Sone S. Human monocyte-derived and CD83+ blood dendritic cells enhance NK cell-mediated cytotoxicity. Eur J Immunol. 2001;31:2633–2641. doi: 10.1002/1521-4141(200109)31:9<2633::aid-immu2633>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 10.Yu Y, Hagihara M, Ando K, Gansuvd B, Matsuzawa H, Tsuchiya T, Ueda Y, Inoue H, Hotta T, Kato S. Enhancement of human cord blood CD34+ cell-derived NK cell cytotoxicity by dendritic cells. J Immunol. 2001;166:1590–1600. doi: 10.4049/jimmunol.166.3.1590. [DOI] [PubMed] [Google Scholar]
- 11.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 12.Orange JS, Biron CA. An absolute and restricted requirement for IL-12 in natural killer cell IFN-γ production and antiviral defense: studies of natural killer and T cell responses in contrasting viral infections. J Immunol. 1996;156:1138–1142. [PubMed] [Google Scholar]
- 13.Adam C, King S, Allgeier T, Braumüller H, Lüking C, Mysliwietz J, Kriegeskorte A, Busch DH, Röcken M, Mocikat R. DC-NK cell cross talk as a novel CD4+ T-cell–independent pathway for antitumor CTL induction. Blood. 2005;106:338–344. doi: 10.1182/blood-2004-09-3775. [DOI] [PubMed] [Google Scholar]
- 14.Mailliard RB, Son YI, Redlinger R, Coates PT, Giermasz A, Morel PA, Storkus WJ, Kalinski P. Dendritic cells mediate NK cell help for Th1 and CTL responses: two-signal requirement for the induction of NK cell helper function. J Immunol. 2003;171:2366–2373. doi: 10.4049/jimmunol.171.5.2366. [DOI] [PubMed] [Google Scholar]
- 15.Martín-Fontecha A, Thomsen LL, Brett S, Gerard C, Lipp M, Lanzavecchia A, Sallusto F. Induced recruitment of NK cells to lymph nodes provides IFN-γ for T(H)1 priming. Nat Immunol. 2004;5:1260–1265. doi: 10.1038/ni1138. [DOI] [PubMed] [Google Scholar]
- 16.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 17.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 18.Trumpfheller C, Caskey M, Nchinda G, Longhi MP, Mizenina O, Huang Y, Schlesinger SJ, Colonna M, Steinman RM. The microbial mimic poly IC induces durable and protective CD4+ T cell immunity together with a dendritic cell targeted vaccine. Proc Natl Acad Sci USA. 2008;105:2574–2579. doi: 10.1073/pnas.0711976105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yount JS, Gitlin L, Moran TM, López CB. MDA5 participates in the detection of paramyxovirus infection and is essential for the early activation of dendritic cells in response to Sendai virus defective interfering particles. J Immunol. 2008;180:4910–4918. doi: 10.4049/jimmunol.180.7.4910. [DOI] [PubMed] [Google Scholar]
- 20.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 21.Longhi MP, Trumpfheller C, Idoyaga J, Caskey M, Matos I, Kluger C, Salazar AM, Colonna M, Steinman RM. Dendritic cells require a systemic type I interferon response to mature and induce CD4+ Th1 immunity with poly IC as adjuvant. J Exp Med. 2009;206:1589–1602. doi: 10.1084/jem.20090247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akazawa T, Ebihara T, Okuno M, Okuda Y, Shingai M, Tsujimura K, Takahashi T, Ikawa M, Okabe M, Inoue N, et al. Antitumor NK activation induced by the Toll-like receptor 3-TICAM-1 (TRIF) pathway in myeloid dendritic cells. Proc Natl Acad Sci USA. 2007;104:252–257. doi: 10.1073/pnas.0605978104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tu Z, Bozorgzadeh A, Pierce RH, Kurtis J, Crispe IN, Orloff MS. TLR-dependent cross talk between human Kupffer cells and NK cells. J Exp Med. 2008;205:233–244. doi: 10.1084/jem.20072195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lauzon NM, Mian F, MacKenzie R, Ashkar AA. The direct effects of Toll-like receptor ligands on human NK cell cytokine production and cytotoxicity. Cell Immunol. 2006;241:102–112. doi: 10.1016/j.cellimm.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt KN, Leung B, Kwong M, Zarember KA, Satyal S, Navas TA, Wang F, Godowski PJ. APC-independent activation of NK cells by the Toll-like receptor 3 agonist double-stranded RNA. J Immunol. 2004;172:138–143. doi: 10.4049/jimmunol.172.1.138. [DOI] [PubMed] [Google Scholar]
- 26.Sivori S, Falco M, Carlomagno S, Romeo E, Moretta L, Moretta A. Heterogeneity of TLR3 mRNA transcripts and responsiveness to poly (I:C) in human NK cells derived from different donors. Int Immunol. 2007;19:1341–1348. doi: 10.1093/intimm/dxm105. [DOI] [PubMed] [Google Scholar]
- 27.Sivori S, Falco M, Della Chiesa M, Carlomagno S, Vitale M, Moretta L, Moretta A. CpG and double-stranded RNA trigger human NK cells by Toll-like receptors: induction of cytokine release and cytotoxicity against tumors and dendritic cells. Proc Natl Acad Sci USA. 2004;101:10116–10121. doi: 10.1073/pnas.0403744101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michallet MC, Meylan E, Ermolaeva MA, Vazquez J, Rebsamen M, Curran J, Poeck H, Bscheider M, Hartmann G, König M, et al. TRADD protein is an essential component of the RIG-like helicase antiviral pathway. Immunity. 2008;28:651–661. doi: 10.1016/j.immuni.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 29.Hornung V, Ellegast J, Kim S, Brzózka K, Jung A, Kato H, Poeck H, Akira S, Conzelmann KK, Schlee M, et al. 5′-Triphosphate RNA is the ligand for RIG-I. Science. 2006;314:994–997. doi: 10.1126/science.1132505. [DOI] [PubMed] [Google Scholar]
- 30.Pichlmair A, Schulz O, Tan CP, Näslund TI, Liljeström P, Weber F, Reis e Sousa C. RIG-I–mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science. 2006;314:997–1001. doi: 10.1126/science.1132998. [DOI] [PubMed] [Google Scholar]
- 31.Kato H, Takeuchi O, Mikamo-Satoh E, Hirai R, Kawai T, Matsushita K, Hiiragi A, Dermody TS, Fujita T, Akira S. Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid-inducible gene-I and melanoma differentiation-associated gene 5. J Exp Med. 2008;205:1601–1610. doi: 10.1084/jem.20080091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Granucci F, Zanoni I, Pavelka N, Van Dommelen SL, Andoniou CE, Belardelli F, Degli Esposti MA, Ricciardi-Castagnoli P. A contribution of mouse dendritic cell-derived IL-2 for NK cell activation. J Exp Med. 2004;200:287–295. doi: 10.1084/jem.20040370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andoniou CE, van Dommelen SL, Voigt V, Andrews DM, Brizard G, Asselin-Paturel C, Delale T, Stacey KJ, Trinchieri G, Degli-Esposti MA. Interaction between conventional dendritic cells and natural killer cells is integral to the activation of effective antiviral immunity. Nat Immunol. 2005;6:1011–1019. doi: 10.1038/ni1244. [DOI] [PubMed] [Google Scholar]
- 34.Andrews DM, Scalzo AA, Yokoyama WM, Smyth MJ, Degli-Esposti MA. Functional interactions between dendritic cells and NK cells during viral infection. Nat Immunol. 2003;4:175–181. doi: 10.1038/ni880. [DOI] [PubMed] [Google Scholar]
- 35.Lucas M, Schachterle W, Oberle K, Aichele P, Diefenbach A. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity. 2007;26:503–517. doi: 10.1016/j.immuni.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orange JS, Biron CA. Characterization of early IL-12, IFN-αβ, and TNF effects on antiviral state and NK cell responses during murine cytomegalovirus infection. J Immunol. 1996;156:4746–4756. [PubMed] [Google Scholar]
- 37.McCartney S, Vermi W, Gilfillan S, Cella M, Murphy TL, Schreiber RD, Murphy KM, Colonna M. Distinct and complementary functions of MDA5 and TLR3 in poly(I:C)-mediated activation of mouse NK cells. J Exp Med. 2009;206:2967–2976. doi: 10.1084/jem.20091181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu C, Lou Y, Lizée G, Qin H, Liu S, Rabinovich B, Kim GJ, Wang YH, Ye Y, Sikora AG, et al. Plasmacytoid dendritic cells induce NK cell-dependent, tumor antigen-specific T cell cross-priming and tumor regression in mice. J Clin Invest. 2008;118:1165–1175. doi: 10.1172/JCI33583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning CJ. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by Toll-like receptor 2. J Biol Chem. 1999;274:17406–17409. doi: 10.1074/jbc.274.25.17406. [DOI] [PubMed] [Google Scholar]
- 40.Chamaillard M, Hashimoto M, Horie Y, Masumoto J, Qiu S, Saab L, Ogura Y, Kawasaki A, Fukase K, Kusumoto S, et al. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat Immunol. 2003;4:702–707. doi: 10.1038/ni945. [DOI] [PubMed] [Google Scholar]
- 41.Girardin SE, I, Boneca G, Carneiro LA, Antignac A, Jéhanno M, Viala J, Tedin K, Taha MK, Labigne A, Zähringer U, et al. Nod1 detects a unique muropeptide from Gram-negative bacterial peptidoglycan. Science. 2003;300:1584–1587. doi: 10.1126/science.1084677. [DOI] [PubMed] [Google Scholar]
- 42.Miyake T, Kumagai Y, Kato H, Guo Z, Matsushita K, Satoh T, Kawagoe T, Kumar H, Jang MH, Kawai T, et al. Poly I:C-induced activation of NK cells by CD8α+ dendritic cells via the IPS-1 and TRIF-dependent pathways. J Immunol. 2009;183:2522–2528. doi: 10.4049/jimmunol.0901500. [DOI] [PubMed] [Google Scholar]
- 43.Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, Horiuchi T, Tomizawa H, Takeda K, Akira S. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3:196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- 44.Kanneganti TD, Ozören N, Body-Malapel M, Amer A, Park JH, Franchi L, Whitfield J, Barchet W, Colonna M, Vandenabeele P, et al. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature. 2006;440:233–236. doi: 10.1038/nature04517. [DOI] [PubMed] [Google Scholar]
- 45.Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat Immunol. 2005;6:769–776. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.