The rate of nontreatment varies by cancer type and stage and is higher in patients receiving initial recommendations in nonaccredited cancer programs than in accredited cancer programs.
Abstract
Purpose:
Little has been published on nontreatment of cancer, yet the National Cancer Data Base (NCDB) indicates that 9.2% of patients receive no first course of treatment. Because the NCDB is limited to accredited cancer programs, there is potential for the actual rate to differ. We sought to understand the rate and characteristics of patients with cancer who receive no first course of treatment in a more population-representative data source.
Materials and Methods:
The Iowa Cancer Registry (ICR) strives to capture 100% of newly diagnosed cancer cases among Iowa residents, regardless of where they are diagnosed or treated.
Results:
In the ICR from 2004 to 2010, 12.3% of newly diagnosed patients with cancer did not receive a first course of treatment, which is 48% higher than the NCDB data for the state of Iowa (8.3%) during the same time period. Logistic regression indicated that nontreatment was more common in certain cancers (ie, small-cell and non–small-cell lung/bronchial cancers and low-grade non-Hodgkin lymphoma), advanced stages, older patients, those receiving treatment recommendations at nonaccredited cancer programs, and patients who never consulted an oncologist, radiation therapist, or surgeon. Distance to treatment facilities was not related to nontreatment.
Conclusion:
The rate of nontreatment varies by cancer type and stage and is higher in patients receiving initial treatment recommendations in nonaccredited cancer programs than in accredited cancer programs. This pattern seems to be correlated with patient characteristics but also may be related to provider and facility characteristics available to people locally that influence both patient and provider decision making.
Introduction
The American Society of Clinical Oncology (ASCO) contracted with the University of Iowa to conduct the ASCO Study of Geographic Access to Oncology Care, funded by Susan G. Komen for the Cure. The goals of the study are to look at patient and physician locations to determine if gaps exist in geographic distribution of physicians and access to treatment sites that may contribute to disparities in cancer care. Study findings will be released in early 2013. While analyzing results from the larger study, the authors noted that there is a cohort with a cancer diagnosis who received no treatment. Analysis of the data resulted in the interesting findings reported herein. Although the data are sufficient to correlate nontreatment decisions with characteristics relevant to patient access to care, they are insufficient to explain causation and are therefore not discussed in this article.
The National Cancer Data Base (NCDB), a joint program of the Commission on Cancer (COC) of the American College of Surgeons and the American Cancer Society, compiles data from COC-accredited cancer program registries covering 70% of all newly diagnosed patients with cancer in the United States.1 The NCDB collects information on the treatment modality or modalities used in the initial management of the disease, including surgery; irradiation; systemic, other, and combination treatments; and no treatment. Across 11 types of cancer, the NCDB reports that between 2000 and 2010, 9.2% of patients received no first course of treatment.1 In the NCDB, nontreatment rates were highest for lung/bronchial and non-Hodgkin lymphoma cancers (approximately 20%); approximately 10% for prostate, kidney/renal, rectal, and uteran cancers; and approximately 5% for breast, cervical, and colon cancers.1
Beyond the NCDB data, we could find no other report on the national rate of nontreatment in patients with prevalent forms of cancer. Because the NCDB lacks data from the 30% of patient cases of cancer that are not represented in COC-accredited cancer programs, there is potential for the actual rate to differ. We sought to understand the rate and characteristics of patients with cancer who have no first course of treatment in a more complete data source that includes accredited and nonaccredited sources of care.
Materials and Methods
The Iowa Cancer Registry2 (ICR) participates in the National Cancer Institute (NCI) SEER,3 a cancer registry representing 28% of the US population and widely used to track cancer incidence, treatment patterns, and mortality. The ICR strives to capture all newly diagnosed cancer cases among Iowa residents, regardless of where they are diagnosed or treated. We accessed data on all newly diagnosed patient cases of invasive cancer from 2004 to 2010. Between 2004 and 2010, the ICR provided 303,707 records from a total of 113,885 invasive cancer diagnoses in 106,603 distinct Iowa residents (over the course of the study, a patient may have > one cancer). These 113,885 patient cases form the basis for this study. The ICR tracks the first course of treatment until its completion. If no treatment occurs within a year, the ICR captures the reasons for nontreatment. We used the same codes as those used in the NCDB to identify patients with the 11 cancer sites reported there, plus a category for other cancer sites. The number of nontreated patient cases for uteran cancer was small in the ICR, so these were included in the other cancer group. For all patient cases, the ICR provides information on the derived American Joint Committee on Cancer stage group. Physicians involved in diagnosis and first-treatment recommendations were coded in the ICR, and their self-identified specialty was determined by links to the Iowa Physician Information System.4 Patient residence was classified as urban or rural based on the rural-urban commuting area (RUCA) code associated with the patient's zip code at time of diagnosis. Developed by the Economic Research Service of the US Department of Agriculture, RUCA codes5 are based on census tract population and commuting data. Using the approach described by the WWAMI (Washington, Wyoming, Alaska, Montana, and Idaho) Rural Health Research Center,6 zip codes were assigned to one of four geographic classifications: urban, large rural, small rural, and isolated rural. Analysis of the ICR data for this study was approved by the University of Iowa Institutional Review Board.
Results
Rate of Nontreatment in the ICR
In the ICR, the vast majority of patient cases (83.3%) included data indicating that patients received treatment for their diagnosis at a reporting facility. A much smaller proportion of patient cases (4.4%) included data indicating treatment had been received at an unknown location and/or the treatment variables were indeterminate. The 13,977 remaining patient cases, which constituted 12.3% of the 113,885 invasive cancer cases, were identified as patients not receiving a first course of treatment. Overall, there were 48% more patient cases in the ICR (12.3%) with no first course of treatment than shown in the NCDB for the state of Iowa (8.3%) during the same time period.
Reasons for Nontreatment
As summarized in Table 1, for patients not receiving a first course of treatment, the data indicate the percentages where treatment was not recommended, treatment was recommended but not administered for various reasons, or treatment was refused. Surgery was the treatment modality that was most commonly contraindicated or not administered with no reason given. Surgery and chemotherapy were the recommended treatment modalities that were most commonly refused. Overall (data not shown), of those patients who did not receive treatment, 1.4% died before receiving a planned therapy, and 9.0% refused treatment (refusal by patient, parent, or guardian). For the remaining 89.6% of patient cases, no treatment was planned, it was contraindicated, or no reason was provided for not treating.
Table 1.
Reasons Specific Treatment Modalities Were Not Used: Iowa Cancer Registry Data, 2004 to 2010 (n = 13,977)*
| Reason | Irradiation (%)† | Surgery (%)‡ | Chemotherapy (%) | Hormone Therapy (%)§ | Immunotherapy (%)‖ | Hematopoietic Transplantation/Endocrine Therapy (%)¶ | Other (%)# |
|---|---|---|---|---|---|---|---|
| Treatment was not performed; no reason given | — | 15.2 | 0.2 | < 0.1 | < 0.1 | 0 | — |
| Death occurred before planned treatment | — | 0.5 | 0.9 | < 0.1 | 0 | 0 | — |
| Treatment was contraindicated | — | 6.8 | 2.8 | 0.1 | < 0.1 | 0 | — |
| Treatment was recommended but refused | 1.8 | 4.9 | 4.7 | 0.4 | < 0.1 | < 0.1 | < 0.1 |
| Specific treatment was not planned | 98.2 | 72.6 | 91.4 | 99.4 | 99.9 | 99.9 | 99.9 |
This table describes the 13,977 patients who did not receive treatment. Because the various treatment modalities apply to all patients, the vast majority of whom did not have the specific treatment modality planned (shown in final row), each column sums to 100%, and the total number for each column is 13,977.
Includes any of a number of types or combinations of radiation therapy, including beam radiation, radioactive implants, and radioisotopes.
Includes only surgery intended as part of treatment; does not include surgical procedures for diagnosis/staging.
Hormonal agents administered as treatment.
Immunotherapeutic agents (biologic response modifiers) include biologic or chemical agents designed to alter the immune system or change the host response to tumor cells.
Includes bone marrow transplantation, stem-cell harvests, and surgical and/or radiation endocrine therapy.
Any therapy that cannot be defined as surgery, irradiation, or systemic therapy according to the definitions used by North American Association of Central Cancer Registries.
Patient Characteristics in Nontreated Patient Cases
Because factors affecting nontreatment were of particular interest, the few patient cases where death occurred before planned treatment were excluded from subsequent analyses. Bivariate and multivariable logistic regression analyses were conducted to examine patient and facility characteristics associated with nontreatment. As summarized in Table 2, for patient characteristics, bivariate logistic regressions predicting no receipt of treatment showed significant relationships for cancer site, stage, age, sex, and residence location. In particular, patients with lung/bronchial cancer (both small- and non–small-cell cancers), low-grade non-Hodgkin lymphoma, and prostate cancer were significantly more likely to not be treated than those with other sites of cancer. In contrast, patients with breast, cervical, colon, melanoma, and rectal cancers were significantly less likely to not be treated. Not receiving treatment was significantly more common in male patients across these cancer types because of the prevalence of prostate cancer. But when the comparison was limited to cancers that predominantly or exclusively occur in both sexes (ie, excluding breast, cervical, prostate, and uteran cancers), nontreatment was significantly more common in female patients in bivariate analyses. Not receiving treatment was significantly more common in rural residents than in urban residents. Not receiving treatment increased significantly with age and cancer stage. In fact, patients with stage II or III disease were twice as likely to not receive treatment as those with early-stage disease (stage I), and dramatically, stage IV cancers were six times more likely to not be treated. With the exception of cervical and prostate cancers, all relationships were statistically significant when all variables were included in multivariable logistic regression. There were relatively few cases of cervical cancer, and nontreatment seemed to be explained better by variables other than disease type. Nontreatment in prostate cancer varied by stage but not in a linear pattern.
Table 2.
Patient and Facility Characteristics Related to Not Receiving Treatment: Iowa Cancer Registry Data, 2004 to 2010
| Characteristic | Total Patient Cases | Bivariate Logistic Regression |
Multivariable Logistic Regression* |
||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | ||
| Disease site | |||||||
| Breast | 14,898 | 0.185 | 0.17 to 0.20 | < .001 | 0.423 | 0.37 to 0.48 | < .001 |
| Cervix | 684 | 0.349 | 0.25 to 0.49 | < .001 | 1.440 | 0.93 to 2.24 | .1047 |
| Colon | 9,060 | 0.584 | 0.54 to 0.63 | < .001 | 0.591 | 0.53 to 0.66 | < .001 |
| Kidney and renal pelvis | 3,762 | 0.914 | 0.83 to 1.01 | .0768 | 1.209 | 1.06 to 1.38 | .0057 |
| Lung/bronchus | |||||||
| Small cell | 2,367 | 1.433 | 1.29 to 1.60 | < .001 | 1.675 | 1.45 to 1.93 | < .001 |
| Non–small cell | 11,518 | 1.483 | 1.41 to 1.60 | < .001 | 2.465 | 2.28 to 2.66 | < .001 |
| Melanoma | 3,859 | 0.461 | 0.41 to 0.52 | < .001 | 0.304 | 0.25 to 0.37 | < .001 |
| Non-Hodgkin lymphoma | |||||||
| Low grade | 1,419 | 2.095 | 1.85 to 2.40 | < .001 | 3.049 | 2.59 to 3.60 | < .001 |
| High grade | 2,034 | 1.107 | 0.98 to 1.26 | 0.1091 | 1.252 | 1.07 to 1.47 | .0058 |
| Prostate | 14,432 | 1.303 | 1.24 to 1.37 | < .001 | 0.953 | 0.86 to 1.05 | .3304 |
| Rectum | 2,349 | 0.724 | 0.63 to 0.84 | < .001 | 1.199 | 1.01 to 1.43 | .0429 |
| Other | 42,059 | Ref† | |||||
| Stage of disease at diagnosis | |||||||
| II | 25,141 | 2.070 | 1.93 to 2.22 | < .001 | 1.691 | 1.53 to 1.87 | < .001 |
| III | 14,402 | 2.059 | 1.90 to 2.23 | < .001 | 2.725 | 2.47 to 3.01 | < .001 |
| IV | 18,613 | 6.448 | 6.05 to 6.89 | < .001 | 7.964 | 7.31 to 8.68 | < .001 |
| Unknown | 19,242 | 6.216 | 5.84 to 6.62 | < .001 | 4.962 | 4.57 to 5.39 | < .001 |
| I | 31,043 | Ref† | |||||
| Age at diagnosis, years | 1.057 | 1.06 to 1.08 | .0122 | 1.053 | 1.05 to 1.06 | < .001 | |
| Sex‡ | |||||||
| Female | 52,532 | 1.053 | 1.01 to 1.10 | .0164 | 0.916 | 0.88 to 0.97 | .0014 |
| Male | 55,906 | Ref† | |||||
| Patient residence | |||||||
| Large rural city/town | 16,569 | 1.253 | 1.19 to 1.32 | < .001 | 1.193 | 1.11 to 1.28 | < .001 |
| Small rural town | 20,844 | 1.106 | 1.05 to 1.16 | < .001 | 0.809 | 0.75 to 0.87 | < .001 |
| Isolated small rural | 19,440 | 1.049 | 0.10 to 1.10 | .0594 | 0.750 | 0.70 to 0.81 | < .001 |
| Urban | 51,582 | Ref† | |||||
| Institution type | |||||||
| Comprehensive or academic cancer center | 47,914 | 1.410 | 1.32 to 1.51 | < .001 | 2.975 | 2.61 to 3.39 | < .001 |
| Community or integrated cancer center | 9131 | 2.135 | 1.96 to 2.33 | < .001 | 3.575 | 3.08 to 4.14 | < .001 |
| PPS hospital§ | 21,064 | 2.467 | 2.30 to 2.65 | < .001 | 4.450 | 3.90 to 5.08 | < .001 |
| Critical access hospital | 9788 | 3.851 | 3.56 to 4.16 | < .001 | 6.531 | 5.67 to 7.52 | < .001 |
| Clinic or physician office | 3233 | 26.576 | 24.19 to 29.20 | < .001 | 81.325 | 68.97 to 95.90 | < .001 |
| NCI cancer center | 17,311 | Ref† | |||||
| Oncologist seen‖ | |||||||
| Yes | 39,146 | 0.545 | 0.52 to 0.57 | < .001 | 0.415 | 0.39 to 0.44 | < .001 |
| No | 47,771 | Ref† | |||||
| Radiation therapist seen | |||||||
| Yes | 24,126 | 0.173 | 0.16 to 0.19 | < .001 | 0.165 | 0.15 to 0.18 | < .001 |
| No | 62,791 | Ref† | |||||
| Surgeon seen | |||||||
| Yes | 31,959 | 0.333 | 0.32 to 0.35 | < .001 | 0.455 | 0.43 to 0.47 | < .001 |
| No | 54,958 | Ref† | |||||
Abbreviations: NCI, National Cancer Institute; OR, odds ratio; PPS, prospective payment system; Ref, reference.
Multivariable logistic regression included all variables in the model.
Ref indicates the reference group within each bivariate and multivariable logistic regression analysis.
Bivariate analysis of sex was conducted excluding predominately sex-specific cancers (ie, breast, cervical, prostate, and uterus cancers).
Indicates PPS hospitals that are not Commission on Cancer accredited.
Oncologist includes medical, gynecologic, pediatric, and hematologic oncologists but does not include radiation oncologists.
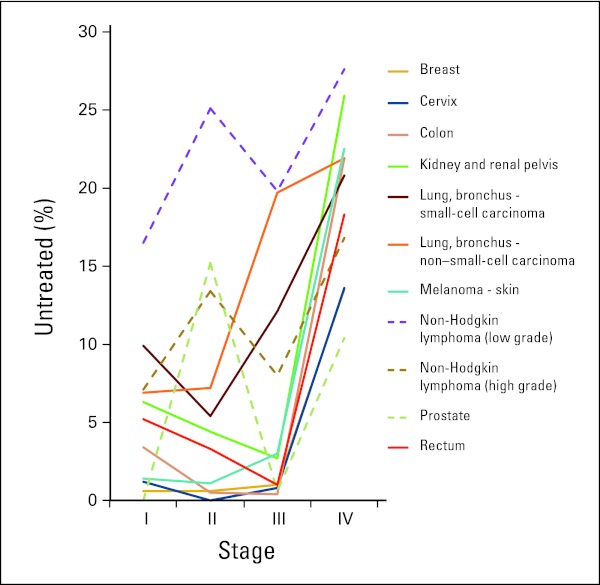
In the ICR, nearly 19% of patients with lung/bronchial cancer and non-Hodgkin lymphoma and more than 16% of patients with prostate cancer received no treatment for their disease. Figure 1 shows the rate of nontreatment by cancer site and stage. Within non-Hodgkin lymphoma, patients with low-grade disease were much less likely to receive treatment than those with high-grade disease. Regardless of cancer site, patients diagnosed at stage IV were less likely to be treated. More than 25% of those with stage IV low-grade non-Hodgkin lymphoma and kidney/renal pelvic cancer and more than 20% of those with stage IV colon, lung/bronchial, and melanoma cancers were not treated. A much smaller proportion of those with stage I disease were not treated regardless of cancer site. Also apparent in Figure 1 are cancer sites that showed a nonlinear pattern across stages. In particular, patient cases of prostate cancer and non-Hodgkin lymphoma (shown in dashed lines) had noticeably higher rates of nontreatment in stage II than in stage III. Because the pattern across stages differed dramatically for these two types of cancer, multivariable analyses were repeated with patient cases of prostate cancer and non-Hodgkin lymphoma excluded, and the results were similar.
Figure 1.
Percentage of nontreated patient cases by cancer site at each stage (Iowa Cancer Registry data, 2004 to 2010).
Facility and Provider Characteristics in Nontreated Patient Cases
The ICR records location of diagnosis and first treatment for all Iowa residents with cancer, regardless of where the facility is located. First-treatment decisions occurred at COC-accredited centers in 68.6% of patient cases, most commonly at accredited comprehensive or academic cancer programs (44.2%) or at NCI-designated comprehensive cancer centers (16.0%). However, 31.4% of Iowa residents with cancer received first-treatment recommendations at a facility that was not accredited by the COC. Accreditation by the COC is optional, and COC categories are assigned based on facility or organization type, services provided, and patient cases accessioned.7 Comparing treatment rates across facility types, bivariate logistic regression indicated a statistically significant monotonic increase in the likelihood of not receiving treatment, going from higher classifications of institutions to lower classifications of institutions. In particular, compared with NCI-designated comprehensive cancer centers, the odds ratios for nontreatment were 1.41 for comprehensive community cancer centers or academic comprehensive cancer centers, 2.14 for community cancer programs or integrated cancer programs, 2.47 for nonaccredited prospective payment–system hospitals, 3.85 for critical access hospitals, and 26.58 for clinics and physician offices. These relationships held when patient cases of non-Hodgkin lymphoma and prostate cancer were excluded. Characteristics of patients with cancer diagnosed at these facilities varied in expected ways. In particular, age of patient with cancer increased monotonically from higher classifications of institutions (eg, 19.3% of patientes were age > 75 years at NCI-designated comprehensive cancer centers) to lower classifications of institutions (eg, 52.1% of patients were age > 75 years at critical access hospitals). Late-stage cancers were somewhat more common at higher classifications of institutions (eg, 34.8% at NCI-designated comprehensive cancer centers) than at lower classifications of institutions (eg, 27.0% at critical access hospitals). Even though patient characteristics varied across facilities, the institution effect remained statistically significant when both patient and facility variables were included in the multivariable regression analysis.
The identity of physicians involved in diagnosis and first treatment was recorded for approximately 80% of patient cases in the ICR. Their self-identified specialty was determined by links to the Iowa Physician Information System.4 Analyses indicated that for diagnosis or initial treatment recommendations, an oncologist was seen in 45% of patient cases, a radiation therapist in 28%, and a surgeon in 37%. These percentages sum to more than 100% because patients often saw more than one type of provider. Bivariate logistic regression analysis indicated that in patient cases in which an oncologist (medical, gynecologic, or pediatric) was seen, patients were 0.55 times as likely to not receive treatment as those who never saw an oncologist. Likewise, in patient cases in which a surgeon was seen, patients were 0.33 times as likely to not receive treatment as those who never saw a surgeon. And in patient cases in which a radiation therapist (radiation oncologist, nuclear medicine physician) was seen, patients were 0.17 times as likely to not receive treatment as those who never saw a radiation therapist. These relationships remained statistically significant in the multivariable regression analysis. Because no physician was identified in approximately 20% of patient cases, the multivariable regression analysis was repeated with these patient cases excluded, and the overall result pattern remained.
Access to Treatment
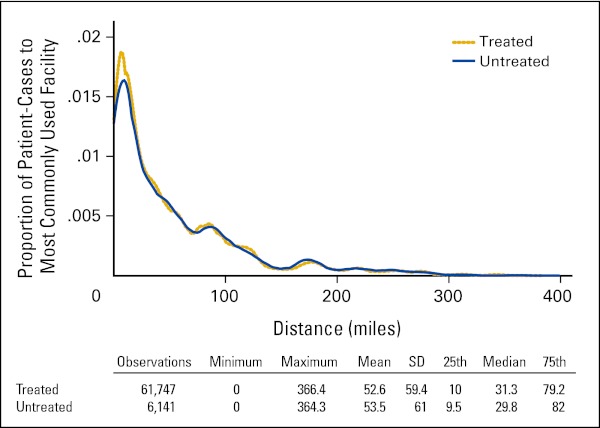
Using RUCA codes to define rural and urban populations,5 53.4% of the population of Iowa lives in urbanized areas and 46.6% in rural areas.6 In the ICR data, the proportion of patient cases in which patients resided in urban areas was 47.6% at the time of first-treatment decision. Analyses indicated that rural residents were 13% more likely not to be treated than urban residents. Thus, access to treatment by rural residents was examined as a possible explanation. To do so, in each zip code where a patient diagnosed with cancer resided, the facilities where patients with the same disease type most frequently received treatment were identified, and the travel distance was calculated. These travel distances were then attributed to patients with the same cancer site residing in the same zip code who did not receive treatment. As shown in Appendix Figure A1 (online only), median travel differences across these cancer sites were 31.3 miles for treated patients and 29.8 miles for those who did not receive treatment. These differences suggest that there were no significant differences in travel distance between all treated and nontreated patients. Fourteen percent of cancer types (ie, breast, colon, non–small-cell lung/bronchial, and prostate cancers) involved residents of zip codes from which no other patients with the same type of cancer were treated, and therefore, no travel distance to a likely treatment site could be imputed for these nontreated patients. With this potential limitation, no evidence was found that travel distance as an index of access was a factor in nontreatment.
Discussion
In the vast majority of patient cases of invasive cancer (83.3%) among Iowa residents diagnosed between 2004 and 2010, patients received treatment consisting of surgery, irradiation, chemotherapy, hormone therapy, immunotherapy, stem-cell transplantation, or endocrine therapy or a combination of these treatments. A much smaller proportion of patient cases (12.3%) were documented as patients not receiving a first course of treatment. This nontreatment rate (12.3%) from the ICR is 48% higher than the rate reported in the NCDB1 from COC-accredited cancer treatment centers in Iowa (8.3%). This difference in rates suggests that nontreatment is less common in patients seen in COC-accredited cancer programs and that nontreatment for cancer is more common than the NCDB data indicate.
In the ICR, bivariate and multivariable logistic regression indicated that receiving no treatment was significantly more likely in patients with advanced age and cancer stage, along with certain cancer sites (ie, small- and non–small-cell lung/bronchial cancer and low-grade non-Hodgkin lymphoma). In the ICR, approximately 20% of patient cases of lung/bronchial cancer and low-grade non-Hodgkin lymphoma and more than 15% of patient cases of prostate cancer and high-grade non-Hodgkin lymphoma involved no treatment for the disease. In the NCDB, the percentage of nontreatment is similar for most of these cancers but somewhat lower for rectal and kidney/renal pelvic cancers and remarkably lower for melanoma (0% in the NCDB v 5.6% in the ICR) and prostate cancer (6.8% in the NCDB v 16.4% in the ICR).
Limitations in the current study must be acknowledged in making comparisons between the ICR and NCDB. The ICR participates in SEER, with known and accepted methodologies for tracking cancer incidence, treatment patterns, and mortality. The ICR also accesses data from the NCDB sites in Iowa as part of its data collection activities. We purposely used NCDB codes for identifying specific cancer sites to facilitate comparisons. Yet, identifying nontreatment in the ICR was limited to records obtained 1 year after diagnosis; thus, a portion of the 12.3% of patients who did not seek treatment may have done so at a later date and been missed. Likewise, ICR coders are well trained, but there is always room for error from incomplete or confusing medical record documentation. Furthermore, data on interesting factors such as insurance coverage and socioeconomic status are not available in the ICR. And because 93% of the ICR patients are identified as white, race and ethnic group could not be included in the analysis.
On the plus side, ICR data permit analysis of multiple factors related to nontreatment. For example, codes specify the reasons that patients did not receive treatment. For those not receiving a first course of treatment, the data indicate that treatment was recommended, but the patient died before treatment in 1.4% of cases, that treatment was recommended but refused in 9% of cases, and that for the remaining 89.6% of these cases, no treatment was planned, it was contraindicated, or no reason was provided for not treating. Surgery was the treatment modality that was most commonly contraindicated or not completed for unknown reasons. Surgery and chemotherapy were the recommended treatment modalities that were most commonly refused.
We could find no published national rates of nontreatment. However, there are some published articles exploring the factors involved in treatment refusal. In adults, treatment refusal has been linked to patients' belief that conventional treatment is ineffective and harmful and that complementary and alternative medicine is a viable option8 and complaints about communication with physicians and health system discontinuities.9
With the overall rate of nontreatment at 12%, and up to 20% for some cancer sites, understanding the factors related to nontreatment is important. Not surprisingly, advanced stage and advanced age are related to nontreatment rates. Patient age has been reported to be a factor in cancer treatment because of both provider and patient perception of a low ratio of benefits to risks.10 Patient refusals of adjuvant treatment have been related to increasing age, comorbid illness, and lack of perceived clinical benefit.11 Newer US Food and Drug Administration–approved treatments that are better tolerated and more easily administered (eg, oral medications for kidney cancers) might be expected to reduce treatment refusal, but the ICR data over the 2004 to 2010 period showed no decrease in nontreatment rates over the time during which these newer treatments were introduced. These data raise the concern that adoption of new treatments may be slower than expected. Other patient characteristics related to nontreatment also emerged. In particular, nontreatment was notably higher in stage II prostate cancer and non-Hodgkin lymphoma than for these cancers at stage III. This finding seems to reflect the fairly common recommendation12 of watchful waiting or active surveillance in patients with certain non-Hodgkin lymphoma subtypes12 and prostate cancers,13,14 because they are usually slow to progress. With these cancers, in particular, watchful waiting or active surveillance could be considered the standard treatment approach. Unfortunately, the registry does not provide an option to indicate this approach as a treatment or reason for nontreatment.
Although patient characteristics seem to drive treatment choice in many situations, significant differences emerged across physician and facility types. Specifically, nontreatment rates were lower for patients who saw an oncologist, radiation therapist, or surgeon and for patients seen at COC-accredited facilities that offer more comprehensive services. There are several possible explanations for this pattern. It is possible that providers at facilities such as NCI-designated comprehensive cancer centers and comprehensive community cancer programs in academic and research facilities have access to more treatment options through clinical trials or their range of specialties and associated equipment.15,16 Adherence to guideline-recommended treatment has been shown to vary across facilities,17 although a recent national study found that guideline-recommended surgical care for cancer was more likely in younger, white, more affluent, and healthier patients with less-advanced disease.17 These findings suggest an interaction between patient and facility factors, an important consideration when attributing quality to facility characteristics.18
The rate of nontreatment in nonaccredited cancer programs was higher than in COC-accredited cancer programs, most likely related to patient characteristics influencing treatment and facility/provider choice. Nonaccredited cancer programs in Iowa are more commonly located in rural areas. Studies on rural/urban differences in cancer have differed, but several have shown higher incidence of some cancers and higher mortality, possibly related to later stage at diagnosis and nonoptimal treatment choices, in rural residents.19 However, rural and urban populations differ in numerous ways, and inconsistencies across studies likely result from failure to adjust for underlying population differences.20 For example, our data indicate that the age of patients with cancer increases substantially as one moves from accredited cancer programs offering more services to those with less services and to nonaccredited community facilities. Other patient characteristics not measured in this study must also be considered. One of these possible mediating factors is selective referral, which has been shown to explain better outcomes in patients with cancer undergoing surgery at high-volume hospitals.21 Patients with cancer who definitely want treatment choices are likely to seek out accredited, high-volume facilities that offer more comprehensive services. And in contrast, those with cancer who are elderly and have more comorbidities may be more likely to seek consultation in a smaller facility closer to home rather than travel to larger facilities. Thus, disentangling the “chicken and egg” of whether treatment or nontreatment is primarily driven by facility resources, provider options, or patient preferences is a substantial challenge when interpreting studies on this topic.
Interestingly, distance to treatment facilities did not seem to be a factor in nontreatment, on average. However, the role of distance as a barrier in individual choices cannot be determined in this study. The type of physician and facility from whom and at which a patient receives his or her initial treatment recommendation is related to nontreatment rates, but other factors that influence nontreatment choice cannot be examined in more depth with the data available. We speculate that this pattern is related to some patients (eg, older and/or with certain cancers) choosing providers and treatment facilities close to home and declining aggressive treatment, whereas other patients (eg, younger and/or with certain cancers) seek the best available treatment and choose specialists and accredited, high-volume cancer programs that offer more services. Overall, surprisingly little is known about the patterns of nontreatment, and more research is needed to elucidate additional factors and reasons for nontreatment to provide both patients and providers with the necessary information to make the best treatment choices.
Acknowledgment
Supported in part by the American Society of Clinical Oncology Study of Geographic Access to Oncology Care, funded by Susan G. Komen for the Cure and by National Cancer Institute Contract No. HHSN261201000032C. We thank the Iowa Cancer Registry and the Iowa Physician Information System for access to their data, Dan Olson for preparing the Iowa Cancer Registry data files, and Geoffrey Fairchild for computing road travel time distances between all zip codes in Iowa and cancer facilities in neighboring states used by Iowa residents.
Appendix
Figure A1.
Distance to facility for treated patients and imputed distance for nontreated patients (Iowa Cancer Registry Data, 2004 to 2010). SD, standard deviation.
Authors' Disclosures of Potential Conflicts of Interest
The author(s) indicated no potential conflicts of interest.
Author Contributions
Conception and design: Marcia M. Ward, Fred Ullrich, Gerard Rushton, Michael A. Goldstein, Dean F. Bajorin, Charles F. Lynch
Financial support: Michael A. Goldstein, Dean F. Bajorin, Amy Hanley
Provision of study material or patients: Charles F. Lynch
Collection and assembly of data: Marcia M. Ward, Fred Ullrich, Kevin Matthews, Gerard Rushton, Charles F. Lynch
Data analysis and interpretation: Marcia M. Ward, Fred Ullrich, Kevin Matthews, Gerard Rushton, Michael A. Goldstein, Dean F. Bajorin, Charles F. Lynch
Manuscript writing: All authors
Final approval of manuscript: All authors
References
- 1.American College of Surgeons. National Cancer Data Base: Public reports. http://cromwell.facs.org/BMarks/BMPub/Ver10/bm_reports.cfm.
- 2.University of Iowa. Iowa Cancer Registry: State Health Registry of Iowa. http://www.public-health.uiowa.edu/shri/index.html.
- 3.National Cancer Institute. Surveillance, Epidemiology, and End Results: Overview of the SEER program. http://seer.cancer.gov/about/overview.html.
- 4.University of Iowa Carver College of Medicine. Office of Statewide Clinical Education Programs. http://www.medicine.uiowa.edu/oscep/
- 5.US Department of Agriculture. Economic Research Service: Rural-urban community area codes. http://www.ers.usda.gov/briefing/Rurality/RuralUrbanCommutingAreas/
- 6.WWAMI Rural Health Research Center. RUCA data: Code definitions, version 2.0. http://depts.washington.edu/uwruca/ruca-codes.php.
- 7.American College of Surgeons. Cancer programs: Categories of accreditation. http://www.facs.org/cancer/coc/categories.html.
- 8.Shumay DM, Mascarinak G, Kakai H, et al. Why some cancer patients choose complementary and alternative medicine instead of conventional treatment. J Fam Pract. 2001;50:1067. [PubMed] [Google Scholar]
- 9.Sharf BF, Stelljes LA, Gordon HS. “A little bitty spot and I'm a big man”: Patients' perspectives on refusing diagnosis or treatment for lung cancer. Psychooncology. 2005;14:636–646. doi: 10.1002/pon.885. [DOI] [PubMed] [Google Scholar]
- 10.Samet J, Hunt WC, Key C, et al. Choice of cancer therapy varies with age of patient. JAMA. 1986;255:3385–3390. [PubMed] [Google Scholar]
- 11.Edwards BK, Brown ML, Wingo PA, et al. Annual report to the nation on the status of cancer, 1975-2002, featuring population-based trends in cancer treatment. J Natl Cancer Inst. 2005;97:1407–1427. doi: 10.1093/jnci/dji289. [DOI] [PubMed] [Google Scholar]
- 12.CancerTreatment.net. Watchful waiting cancer treatment. http://watchful-waiting.cancertreatment.net/
- 13.Hegarty J, Beirne PV, Walsh E, et al. Radical prostatectomy versus watchful waiting for prostate cancer. Cochrane Database Syst Rev. 2010;11:CD006590. doi: 10.1002/14651858.CD006590.pub2. [DOI] [PubMed] [Google Scholar]
- 14.Hayes JH, Ollendorf DA, Pearson SD, et al. Active surveillance compared with initial treatment for men with low-risk prostate cancer: A decision analysis. JAMA. 2010;304:2373–2380. doi: 10.1001/jama.2010.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bilimoria KY, Bentrem DJ, Stewart AK, et al. Comparison of commission on cancer-approved and -nonapproved hospitals in the United States: Implications for studies that use the National Cancer Data Base. J Clin Oncol. 2009;27:4177–4181. doi: 10.1200/JCO.2008.21.7018. [DOI] [PubMed] [Google Scholar]
- 16.Grilli R, Minozzi S, Tinazzi A, et al. Do specialists do it better? The impact of specialization on the processes and outcomes of care for cancer patients. Ann Oncol. 1998;9:365–374. doi: 10.1023/a:1008201331167. [DOI] [PubMed] [Google Scholar]
- 17.Greenberg CC, Lipsitz SR, Neville B, et al. Receipt of appropriate surgical care for Medicare beneficiaries with cancer. Arch Surg. 2011;146:1128–1134. doi: 10.1001/archsurg.2011.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.James PA, Li P, Ward MM. Comparing mortality rates in rural and urban hospitals for patients with myocardial infarction: Rethinking measures of quality of care. Ann Fam Med. 2007;5:105–111. doi: 10.1370/afm.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boulos DN, Ghali RR, Ibrahim EM, et al. An eight-year snapshot of geospatial cancer research (2002-2009): Clinic-epidemiological and methodological findings and trends. Med Oncol. 2011;28:1145–1162. doi: 10.1007/s12032-010-9607-z. [DOI] [PubMed] [Google Scholar]
- 20.Chan L, Hart LG, Goodman DC. Geographic access to health care for rural Medicare beneficiaries. J Rural Health. 2006;22:140–146. doi: 10.1111/j.1748-0361.2006.00022.x. [DOI] [PubMed] [Google Scholar]
- 21.Allareddy V, Ward MM, Wehby G, et al. The connection between selective referrals for radical cystectomy and radical prostatectomy and volume-outcome effects: An instrumental variables analysis. Am J Med Qual. 2012;27:434–440. doi: 10.1177/1062860611423728. [DOI] [PubMed] [Google Scholar]