Abstract
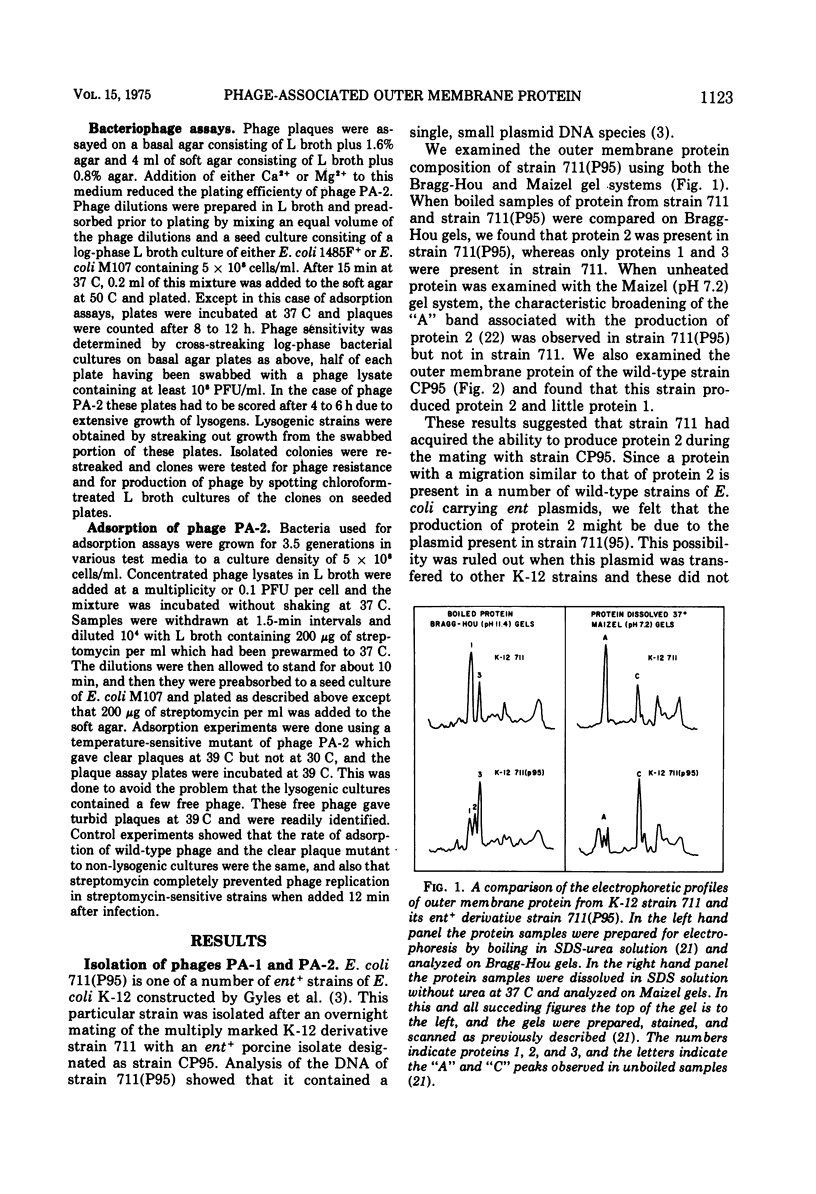
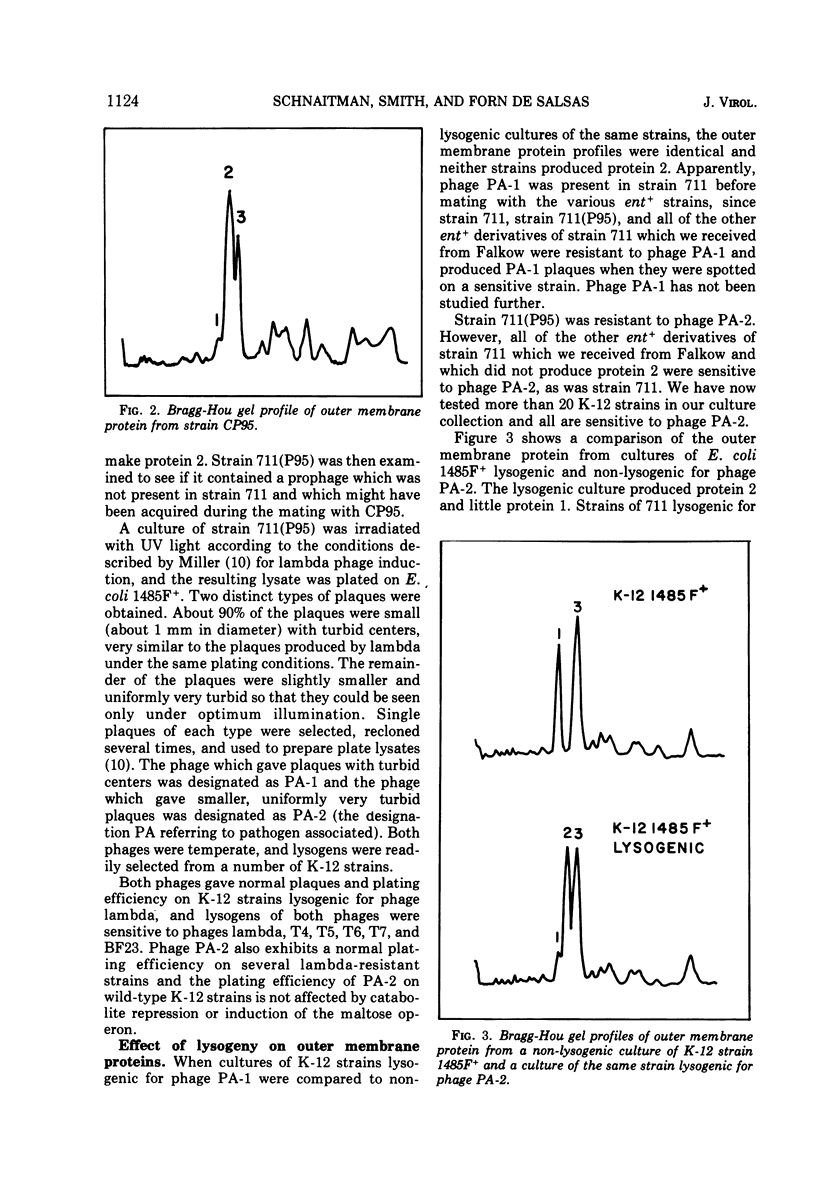
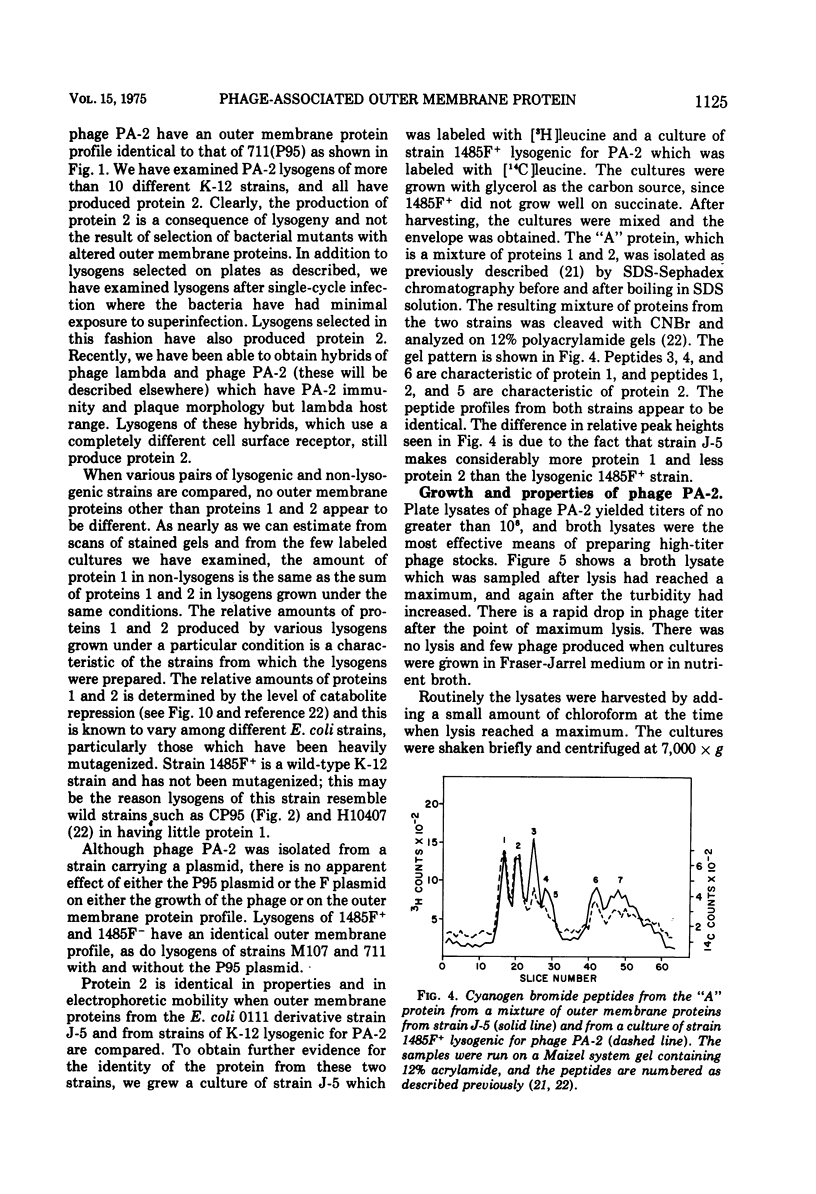
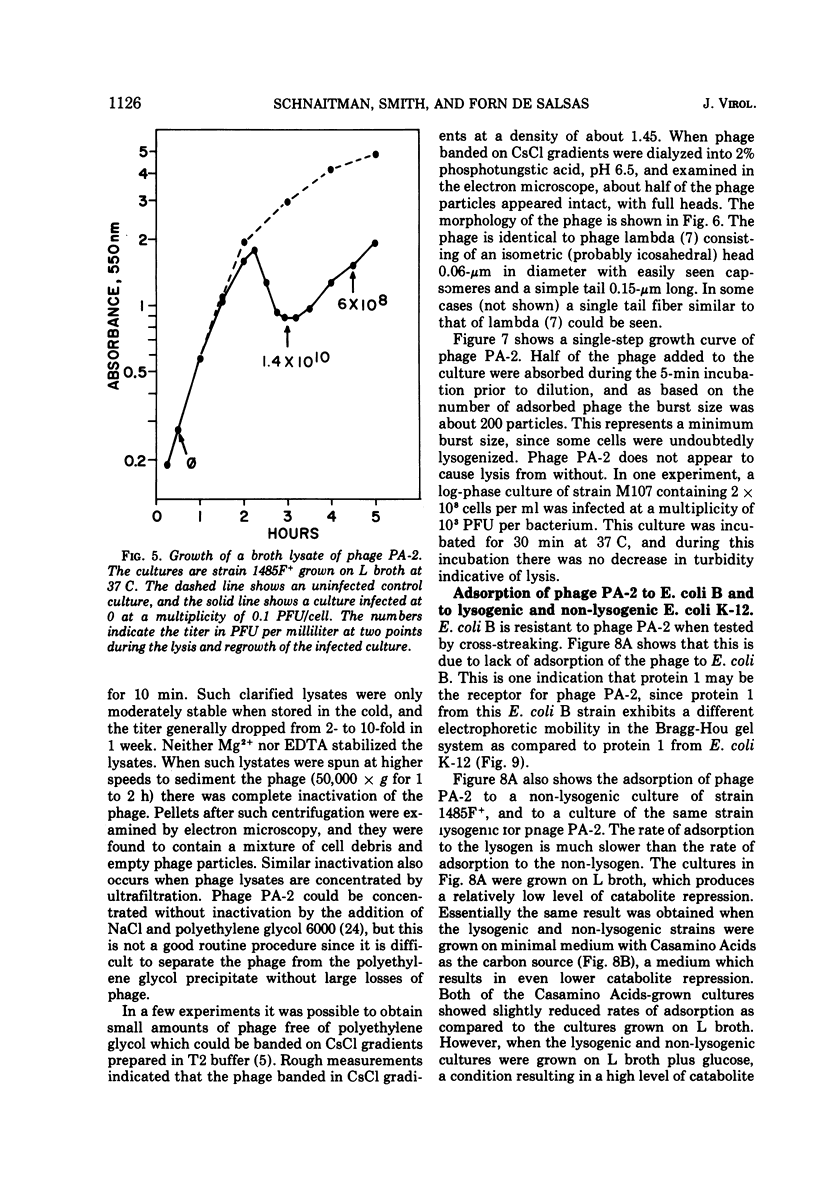
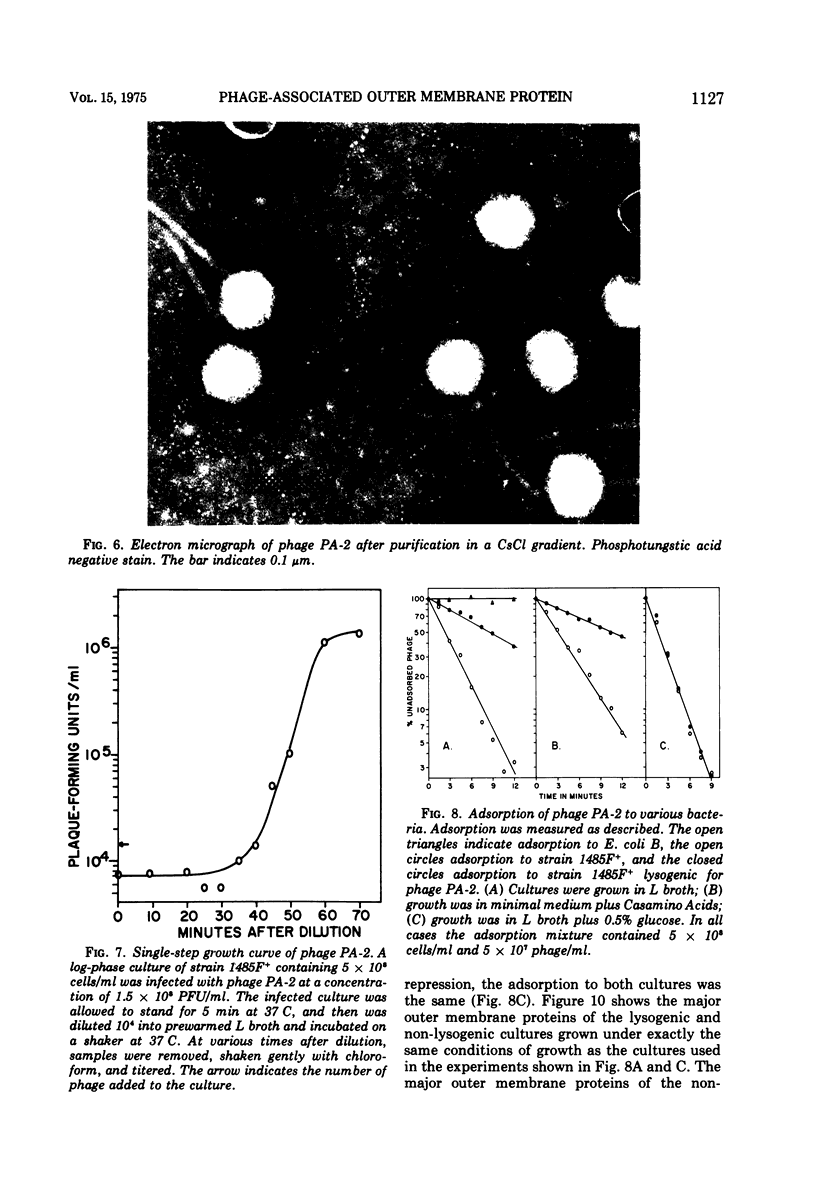
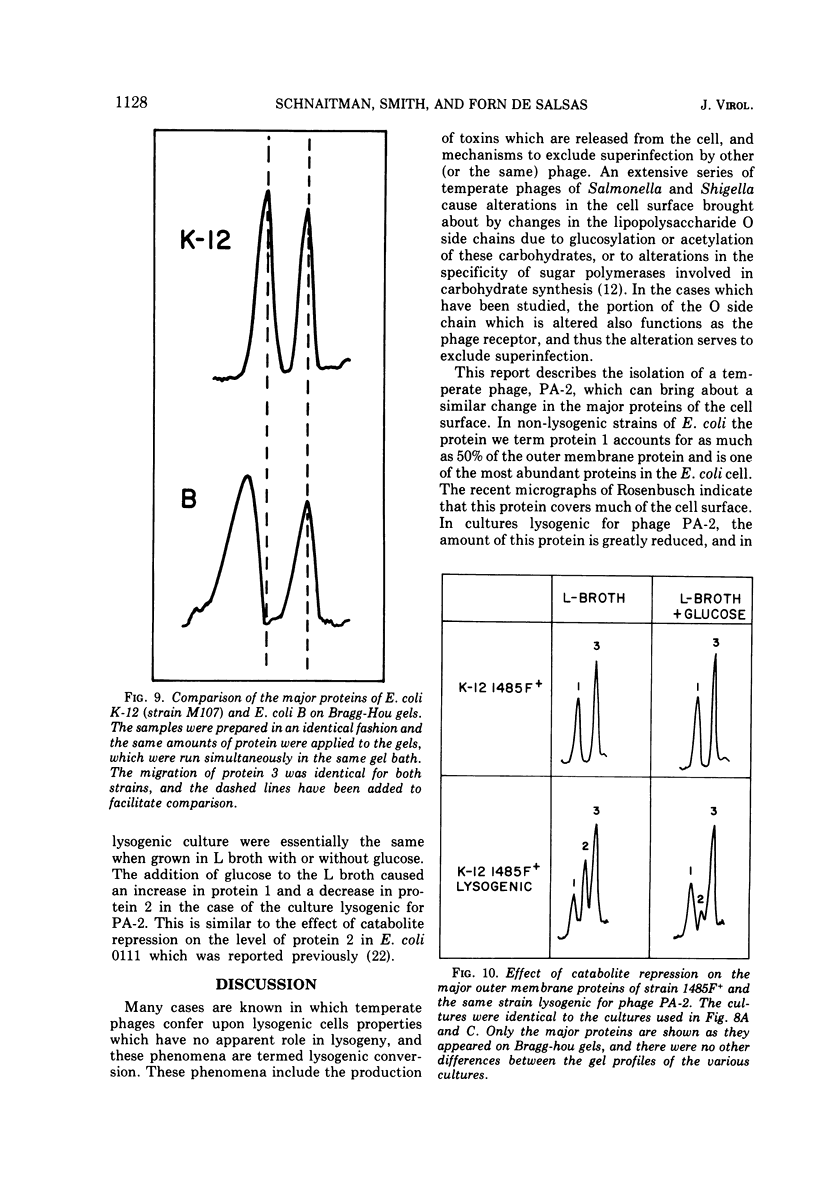
Under most conditions of growth, the most abundant protein in the outer membrane of most strains of Escherichia coli is a protein designated as “protein 1” or “matrix protein”. In E. coli B, this protein has been shown to be a single polypeptide with a molecular mass of 36,500 and it may account for more than 50% of the total outer membrane protein. E. coli K-12 contains a very similar, although probably not identical, species of protein 1. Some pathogenic E. coli strains contain very little protein 1 and, in its place, make a protein designated as protein 2 which migrates faster on alkaline polyacrylamide gels containing sodium dodecyl sulfate and which gives a different spectrum of CNBr peptides. An E. coli K-12 strain which had been mated with a pathogenic strain was found to produce protein 2, and a temperate bacteriophage was isolated from this K-12 strain after induction with UV light. This phage, designated as PA-2, is similar in morphology and several other properties to phage lambda. When strains of E. coli K-12 are lysogenized by phage PA-2, they produce protein 2 and very little protein 1. Adsorption to lysogenic strains grown under conditions where they produce little protein 1 and primarily protein 2 is greatly reduced as compared to non-lysogenic strains which produce only protein 1. However, when cultures are grown under conditions of catabolite repression, protein 2 is reduced and protein 1 is increased, and lysogenic and non-lysogenic cultures grown under these conditions exhibit the same rate of adsorption. Phage PA-2 does not adsorb to E. coli B, which appears to have a slightly different protein 1 from K-12. These results suggest that protein 1 is the receptor for PA-2, and that protein 2 is made to reduce the superinfection of lysogens.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyles C., So M., Falkow S. The enterotoxin plasmids of Escherichia coli. J Infect Dis. 1974 Jul;130(1):40–49. doi: 10.1093/infdis/130.1.40. [DOI] [PubMed] [Google Scholar]
- HERSHEY A. D., CHASE M. Independent functions of viral protein and nucleic acid in growth of bacteriophage. J Gen Physiol. 1952 May;36(1):39–56. doi: 10.1085/jgp.36.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning U., Höhn B., Sonntag I. Cell envelope and shape of Escherichia coli K12. The ghost membrane. Eur J Biochem. 1973 Nov 1;39(1):27–36. doi: 10.1111/j.1432-1033.1973.tb03099.x. [DOI] [PubMed] [Google Scholar]
- Inouye M., Yee M. L. Homogeneity of envelope proteins of Escherichia coli separated by gel electrophoresis in sodium dodecyl sulfate. J Bacteriol. 1973 Jan;113(1):304–312. doi: 10.1128/jb.113.1.304-312.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koplow J., Goldfine H. Alterations in the outer membrane of the cell envelope of heptose-deficient mutants of Escherichia coli. J Bacteriol. 1974 Feb;117(2):527–543. doi: 10.1128/jb.117.2.527-543.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- Randall-Hazelbauer L., Schwartz M. Isolation of the bacteriophage lambda receptor from Escherichia coli. J Bacteriol. 1973 Dec;116(3):1436–1446. doi: 10.1128/jb.116.3.1436-1446.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reithmeier R. A., Bragg P. D. Purification and characterization of heat-modifiable protein from the outer membrane of Escherichia coli. FEBS Lett. 1974 May 1;41(2):195–198. doi: 10.1016/0014-5793(74)81210-x. [DOI] [PubMed] [Google Scholar]
- Sabet S. F., Schnaitman C. A. Purification and properties of the colicin E3 receptor of Escherichia coli. J Biol Chem. 1973 Mar 10;248(5):1797–1806. [PubMed] [Google Scholar]
- Schnaitman C. A. Comparison of the envelope protein compositions of several gram-negative bacteria. J Bacteriol. 1970 Dec;104(3):1404–1405. doi: 10.1128/jb.104.3.1404-1405.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Effect of ethylenediaminetetraacetic acid, Triton X-100, and lysozyme on the morphology and chemical composition of isolate cell walls of Escherichia coli. J Bacteriol. 1971 Oct;108(1):553–563. doi: 10.1128/jb.108.1.553-563.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Outer membrane proteins of Escherichia coli. 3. Evidence that the major protein of Escherichia coli O111 outer membrane consists of four distinct polypeptide species. J Bacteriol. 1974 May;118(2):442–453. doi: 10.1128/jb.118.2.442-453.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Outer membrane proteins of Escherichia coli. I. Effect of preparative conditions on the migration of protein in polyacrylamide gels. Arch Biochem Biophys. 1973 Aug;157(2):541–552. doi: 10.1016/0003-9861(73)90673-5. [DOI] [PubMed] [Google Scholar]
- Schnaitman C. A. Outer membrane proteins of Escherichia coli. IV. Differences in outer membrane proteins due to strain and cultural differences. J Bacteriol. 1974 May;118(2):454–464. doi: 10.1128/jb.118.2.454-464.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Protein composition of the cell wall and cytoplasmic membrane of Escherichia coli. J Bacteriol. 1970 Nov;104(2):890–901. doi: 10.1128/jb.104.2.890-901.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Solubilization of the cytoplasmic membrane of Escherichia coli by Triton X-100. J Bacteriol. 1971 Oct;108(1):545–552. doi: 10.1128/jb.108.1.545-552.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. A., Lennarz W. J., Schnaitman C. A. Distribution of lipids in the wall and cytoplasmic membrane subfractions of the cell envelope of Escherichia coli. J Bacteriol. 1972 Feb;109(2):686–690. doi: 10.1128/jb.109.2.686-690.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]