Referrals to all types of cancer specialists increased the likelihood of treatment with standard therapies for patients with non–small-cell lung cancer, particularly stage III. But racial and income disparities still prevent optimal referrals to cancer specialists.
Abstract
Purpose:
Little is known about how referrals to different cancer specialists influence cancer care for non–small-cell lung cancer (NSCLC). Among Medicare enrollees, we identified factors of patients and their primary care physician that were associated with referrals to cancer specialists, and how the types of cancer specialists seen correlated with delivery of guideline-based therapies (GBTs).
Methods:
Data from patients with stages III and IV NSCLC included in the SEER-Medicare database were linked to their physicians in the American Medical Association Masterfile database. Using logistic regression, we (1) identified patient and physician factors that were associated with referrals to cancer specialists (medical oncologists, radiation oncologists, and surgeons); (2) identified the types of referral to cancer specialists that predicted greater likelihood of receiving GBT (per National Comprehensive Cancer Network guidelines).
Results:
A total of 28,977 patients with NSCLC diagnosed from January 1, 2000 to December 31, 2005 met eligibility criteria. Younger age, white race, higher income, and primary physician specialty other than family practice predicted higher likelihood of referrals to medical oncologists (P < .01 for all predictors). Seeing the three types of cancer specialists predicted higher likelihood of GBT (stage IIIA: odds ratio [OR] = 20.6; P < .001; IIIB: OR = 77.2; P < .001; and IV: OR = 1.2; P = .011), compared with seeing a medical oncologist only. Use of GBTs increased over the study period (42% to 48% from 2000 to 2005; P < .001).
Conclusion:
Referrals to all types of cancer specialists increased the likelihood of treatment with standard therapies, particularly in stage III patients. However, racial and income disparities still prevent optimal referrals to cancer specialists.
Introduction
Lung cancer is a common and serious disease, with more than 221,000 new cases and 157,000 deaths nationwide in 2011.1 Non–small-cell lung cancer (NSCLC) comprises approximately 85% of lung cancer cases, and 75% of NSCLC cases are diagnosed at stages III and IV.1–3 Treatments for stage III NSCLC include combinations of surgery, radiotherapy, and chemotherapy, with cure rates of 10% to 25%.4–6 Treatment for stage IV NSCLC consists primarily of palliative chemotherapy, with palliative radiation therapy or surgery used to control symptomatic distant tumor sites, including bone and brain metastasis.7–11 Although lung cancer treatments are provided by cancer specialists, including medical oncologists, radiation oncologists, and surgeons, most patients diagnosed with NSCLC first present to other health care providers, who subsequently refer them to cancer specialists.12,13 The manner in which newly diagnosed NSCLC patients are referred to specialists and how such referrals ultimately influence care are important but understudied aspects of the quality of care provided to these patients. In addition, because treatments for stage III and IV NSCLC may involve multiple modalities, it is important to understand whether referral to particular cancer specialists influences the likelihood that a patient with NSCLC receives the array of services that are recommended by treatment guidelines, on the basis of stage at diagnosis.
To address these issues, we conducted a retrospective study using the SEER-Medicare database. The study had the following goals: (1) to identify factors influencing the likelihood of referrals to cancer specialists among patients with stage III and IV NSCLC; (2) to describe the initial treatments delivered to these patients; 3) To identify how referrals to particular cancer specialists correlate with delivery of guideline-based therapies for stages III and IV NSCLC.
Methods
Patient Population
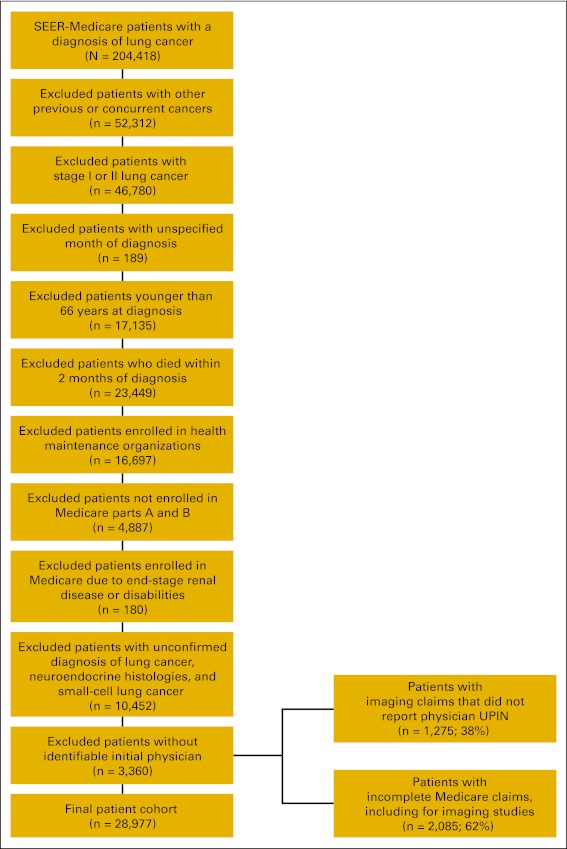
The study included patients in the SEER-Medicare database with histologically or cytologically proven stages IIIA, IIIB and IV NSCLC diagnosed from January 1, 2000, to December 31, 2005. The SEER-Medicare database captures 94% of all Medicare enrollees diagnosed with cancer within the 16 SEER registries existing in the study period, providing patient data on sociodemographic and tumor characteristics, as well as longitudinal data on health care resource utilization from Medicare claims.14 The SEER registries cover 26% of the US population, providing a nationally representative sample of lung cancer cases.15 We included patients who were 66 years or older at diagnosis in order to determine noncancer comorbidity that could influence referrals and care, based on 12 months of Medicare claims preceding the cancer diagnosis.16 Patients had to have complete Medicare part A and B claims available (ie, patients enrolled in HMOs were excluded). We excluded patients diagnosed with previous or concurrent cancers, patients enrolled in Medicare as a result of end-stage renal disease or disability, patients who died within 2 months of diagnosis, patients whose month of diagnosis was unknown, and patients whose initial physician could not be identified.
Patient Data Collection
SEER records provided information on patient histology (adenocarcinoma, squamous cell carcinoma, large-cell carcinoma, or NSCLC not otherwise specified) and stage according to the American Joint Committee on Cancer (AJCC) sixth edition. For a relatively small number of patients (N = 1,961), only SEER historical staging information was available. For these patients, we defined the SEER stage “regional” as AJCC stage IIIB, and “distant” as AJCC stage IV. We assigned patients to their census geographic area (northeast, midwest, west, and south) on the basis of SEER registry areas. From the SEER-Medicare database, we obtained patient sociodemographic variables, in addition to treatment information up to 6 months after diagnosis based on the Healthcare Common Procedure Coding System, International Classification of Diseases ninth revision, and revenue center codes included in Medicare claims. We defined surgical treatments as codes for lobectomy, sleeve lobectomy, bi-lobectomy, and pneumonectomy. Radiation therapy included all codes for external beam radiation therapy. Because these codes do not specify the site irradiated (eg, chest, brain, bones) or the indication for radiation (curative v palliative), we assumed that radiation therapy was given to the chest with curative intent in patients with stage III disease and that patients with stage IV received palliative radiation therapy to symptomatic metastatic sites.17 We based chemotherapy treatments on all chemotherapy administration codes and on specific codes for cisplatin, carboplatin, gemcitabine, etoposide, paclitaxel, docetaxel, vinblastine, vinorelbine, and bevacizumab.18 The study received approval from the institutional review board.
Initial Physician Data
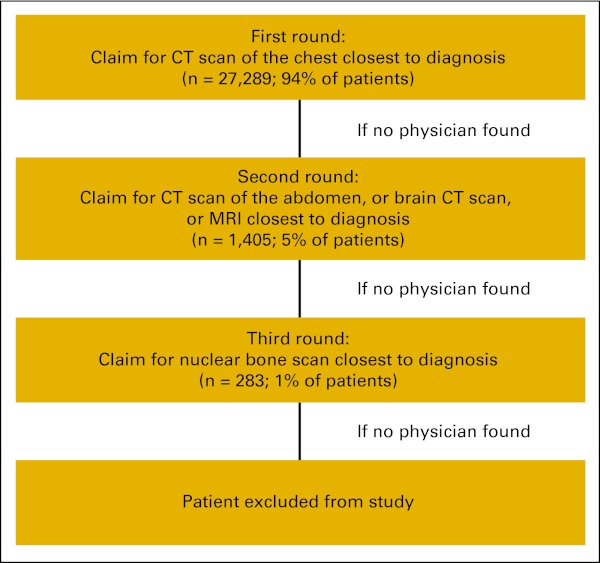
We developed an algorithm to identify the physician who was initially involved in the management of the patient's NSCLC (Appendix Figure A1, online only). We identified these physicians using Unique Physician Identification Numbers (UPIN) that were included in claims for imaging studies in the time period near to the diagnosis of NSCLC (from 12 weeks before diagnosis to 6 weeks after diagnosis). Because SEER reports only the month and year of diagnosis, we set the date of diagnosis as the first day of the month. In order to capture studies that were performed before the diagnosis, we included imaging study claims up to 6 weeks after the set date of diagnosis. The first round of the algorithm captured the computed tomography (CT) scan of the chest taken closest to the time of diagnosis (94% of the final cohort of patients). If no claims were found, the second round of the algorithm captured CT scans of the abdomen or CT scans or magnetic resonance imaging (MRI) of the brain, whichever was closest to diagnosis, assuming these tests were the initial work-up for lung cancers diagnosed through distant metastatic sites (5% of patients). If no claims were found, the third round of the algorithm captured claims for nuclear bone scans (1% of patients). The use of positron emission tomography (PET) scans was not frequent in the study period, and we did not include this imaging modality in the algorithm.
To determine physician specialty and other relevant physician characteristics, we linked the UPINs captured by the algorithm to the UPINs included in the American Medical Association (AMA) Masterfile database. The AMA Masterfile database includes member and nonmember physician (MDs and doctors of oncology [Dos]) information collected since 1906, and represents a validated data source of individual physician characteristics for linking with Medicare claims.19,20 Physician data included age, sex, office geographic location, MD versus DO degree, receipt of the AMA Physician Recognition Award, country of medical school graduation, primary specialty, years since graduation from medical school, primary type of practice (eg, direct patient care v administrative), and employment setting (eg, solo practice v group practice).
Referral to Cancer Specialists
We defined cancer specialists as medical oncologists or hematologists-oncologists (hereafter medical oncologists), radiation oncologists, thoracic surgeons, and general surgeons. We based referral episodes on claims for outpatient and inpatient visits that occurred after the initial imaging study or cancer diagnosis (whichever was first) and on all treatment claims up to 6 months after diagnosis. We linked the UPINs contained in these claims to the AMA Masterfile to identify the primary specialty of each cancer specialist. We also identified referrals to pulmonologists before referrals to cancer specialists on the basis of UPINs for outpatient or inpatient visit claims billed by pulmonologists.
Definition of Guideline-Based Therapy
Guideline-based therapies were defined using the current version of the National Comprehensive Cancer Network (NCCN) practice guidelines.7 For broad treatment categories used in this study, (surgery, radiation, and chemotherapy), the current NCCN recommendations are the same as during the study period. Appendix Table A1 (online only) provides the stage-specific treatment recommendations endorsed by NCCN. For stage IIIA, these recommendations included different combinations and sequences of chemotherapy, radiation, and surgery; for stage IIIB, the NCCN recommends concurrent chemoradiotherapy or chemotherapy followed by radiation; and for stage IV, chemotherapy alone or with palliative radiation for symptomatic metastatic sites.7
Statistical Analyses
We used uni- and multivariable multilevel mixed logistic regression models to test associations of patient and initial physician characteristics with referrals to (1) medical oncologists (because chemotherapy is indicated for stages III and IV NSCLC) and (2) all cancer specialists (medical oncologists, radiation oncologists, and thoracic or general surgeons, because seeing all cancer specialist could potentially enhance adherence to standard therapies). We used the initial physician UPIN as a random-effect variable to account for patient clustering around the same initial physician (1.6 patients per physician on average). We entered all covariates that were statistically significant at a P value less than .05 in univariablee analysis into the final multivariable model. We omitted the initial physician age and office geographic area from the final models as a result of collinearity with years of graduation from medical school and patient geographical area, respectively.
We used a multivariable logistic model to identify types of referrals to cancer specialists that were associated with higher likelihood of receipt of guideline-based therapies, stratified by stage, and adjusted for patient-specific factors. We used χ2 tests to test the association of patient stage with receipt of guideline-based therapies, and tested all models for potential interactions. P values less than .05 were considered statistically significant.
To evaluate the effect of calendar time on referral and treatment trends, we added year of diagnosis as a discrete variable into all multivariable models. For the referral models, we assumed a fixed effect for each study period year relative to the reference year (2000). For the guideline-based therapy model, we tested the individual effect of each year of diagnosis relative to the year of 2000.
Results
The SEER-Medicare database included 157,638 patients with stages III and IV NSCLC diagnosed from January 1, 2000, to December 31, 2005. We excluded 3,360 patients as a result of a lack of identifiable initial physician data in Medicare claims or in the AMA Masterfile. After applying all eligibility criteria, the final study cohort included 28,977 patients (Appendix Figure A2, online only).
Appendix Table A2 (online only) describes the characteristics of patients and their initial physicians (N = 18,605). Mean patient age was 76 years; 53% were male, 83% were white, and 51% had stage IV NSCLC. Initial physician mean age was 49 years; 86% were male, 55% had internal medicine or family practice as their primary specialty, 75% have been in practice for 15 years or more, and 90% worked primarily with patient care. Of the 1,204 (4%) patients who had no referrals to any cancer specialists, mean age was 79 years, 78% were white, 49% were male, 38% had no comorbidities, and 49% had stage IV NSCLC.
Within 6 months from diagnosis, 24,462 patients (84%) saw at least a medical oncologist, and 9,053 patients (31%) saw all cancer specialists (medical oncologists, radiation oncologists, and thoracic or general surgeons). Of 14,870 patients with stage IV NSCLC, 88% saw at least a medical oncologist and 54% received guideline-based therapies (chemotherapy alone in 24% and chemotherapy plus palliative radiotherapy in 30% patients). Of 4,371 patients with stage IIIA, 41% saw all cancer specialists, 19% saw a medical oncologist plus a radiation therapist, 16% saw a medical oncologist plus a surgeon, and 45% received a combination of therapies that are consistent with guideline recommendations (chemoradiotherapy in 35%, chemotherapy plus surgery in 4%, and trimodality therapy in 6%). Of 9,376 patients with stage IIIB, 18% saw a medical oncologist and a radiation therapist, 32% saw all cancer specialists, and 30% received guideline-based therapies (chemoradiotherapy; Table 1). Although patients with stages IIIA and IIIB were more likely to see all types of cancer specialists than stage IV patients (41% and 32% v 28%, P < .001), patients with stages IIIA and IIIB were less likely to receive care consistent with guidelines than stage IV patients (45% and 30% v 54%, P < .001).
Table 1.
Patterns of Referral to Cancer Specialists and Treatments, by Stage
| Stage, Referral, and Treatment | No. | % |
|---|---|---|
| Stage IIIA | ||
| Referral type | ||
| Oncology and radiation oncology | 831 | 19.0 |
| Oncology and surgery (thoracic or general) | 710 | 16.2 |
| Oncology, radiation oncology, and surgery | 1,784 | 40.9 |
| Other specialty combinations* | 917 | 20.9 |
| No referrals | 129 | 3.0 |
| Total | 4,371 | 100.0 |
| Treatments | ||
| Chemotherapy and radiotherapy | 1,513 | 34.6 |
| Chemotherapy and surgery | 181 | 4.1 |
| Chemotherapy, radiotherapy, and surgery | 269 | 6.2 |
| Other treatment combinations† | 1,849 | 42.3 |
| No treatment | 559 | 12.8 |
| Total | 4,371 | 100.0 |
| Stage IIIB | ||
| Referral type | ||
| Oncology and radiation oncology | 1,709 | 17.6 |
| Oncology, radiation oncology, and surgery | 3,089 | 31.7 |
| Other specialty combinations‡ | 4,451 | 45.7 |
| No referrals | 487 | 5.0 |
| Total | 9,736 | 100.0 |
| Treatments | ||
| Chemotherapy and radiotherapy | 2,937 | 30.2 |
| Chemotherapy, radiotherapy, and surgery | 187 | 1.9 |
| Other treatment combinations§ | 4,525 | 46.5 |
| No treatment | 2,087 | 21.4 |
| Total | 9,736 | 100.0 |
| Stage IV | ||
| Referral type | ||
| Oncology with or without other specialties | 13,013 | 87.5 |
| Other specialties without oncology‖ | 1,268 | 8.5 |
| No referrals | 589 | 4.0 |
| Total | 14,870 | 100.0 |
| Treatments | ||
| Chemotherapy alone | 3,645 | 24.5 |
| Chemotherapy and radiotherapy | 4,437 | 29.8 |
| Other treatment combinations¶ | 3,954 | 26.6 |
| No treatment | 2,834 | 19.1 |
| Total | 14,870 | 100.0 |
Includes referrals to a single specialty (oncology, radiation oncology, or surgery), radiation oncology plus general surgery or thoracic surgery.
Includes single modality treatments (chemotherapy alone, radiation alone, or surgery alone), or radiation plus surgery.
Includes referrals to a single specialty (oncology, radiation oncology, or surgery), oncology plus general or thoracic surgery, or radiation oncology plus general or thoracic surgery.
Includes single modality treatments (chemotherapy alone, radiation alone, or surgery alone), chemotherapy plus surgery, or radiation plus surgery.
Includes referrals to a single specialty other than oncology (radiation oncology, general surgery, or thoracic surgery), or radiation oncology plus general or thoracic surgery.
Includes radiation alone, surgery alone, chemotherapy plus surgery, radiation plus surgery, or chemotherapy plus radiation plus surgery.
In the multivariable model, patients were less likely to see a medical oncologist if they were older, black, had higher comorbidities, or had initially seen a family practice physician versus a general internist. Patients were more likely to see a medical oncologist if they lived in areas of higher income, had stage IV versus IIIA or IIIB, were diagnosed in later study years, or were referred to a pulmonologist first (Table 2).
Table 2.
Multivariate Logistical Regression Analysis of Patient and Initial Physician Characteristics With Referrals to Medical Oncologists
| Characteristic | Referred* |
Unadjusted P | Adjusted OR | 95% CI | Adjusted P | |
|---|---|---|---|---|---|---|
| No. | % | |||||
| Patients (N = 28,977) | ||||||
| Age, years† | < .001 | 0.94‡ | 0.93 to 0.94 | < .001 | ||
| Mean | 75 | |||||
| SD | 6 | |||||
| Race/ethnicity | .010 | |||||
| White | 20,490 | 85 | Reference | |||
| Black | 1,891 | 81 | 0.79 | 0.69 to 0.90 | < .001 | |
| Hispanic | 857 | 84 | 0.96 | 0.79 to 1.17 | .691 | |
| Asian | 1,147 | 84 | 0.96 | 0.81 to 1.14 | .633 | |
| American Indian/Alaska Native | 55 | 82 | 0.91 | 0.45 to 1.84 | .790 | |
| Unknown | 22 | 69 | 0.34 | 0.14 to 0.81 | .015 | |
| Sex | .535 | |||||
| Male | 12,974) | 85 | ||||
| Female | 11,488 | 84 | ||||
| Stage | < .001 | |||||
| IV | 13,013 | 88 | Reference | |||
| IIIA | 3,585 | 82 | 0.65 | 0.59 to 0.72 | < .001 | |
| IIIB | 7,864 | 81 | 0.64 | 0.59 to 0.70 | < .001 | |
| Region | .307 | |||||
| West | 9,492 | 83 | ||||
| Northeast | 5,726 | 87 | ||||
| Midwest | 4,020 | 87 | ||||
| South | 5,224 | 82 | ||||
| Household income§ | < .001 | |||||
| Lower tertile | 7,847 | 82 | Reference | |||
| Medium tertile | 8,110 | 84 | 1.18 | 1.08 to 1.29 | < .001 | |
| Higher tertile | 8,403 | 87 | 1.48 | 1.35 to 1.62 | < .001 | |
| Unknown | 102 | 85 | 1.61 | 0.91 to 2.83 | .101 | |
| Charlson index | < .001 | |||||
| 0 | 11,163 | 86 | Reference | |||
| 1-2 | 10,326 | 83 | 0.83 | 0.77 to 0.90 | < .001 | |
| > 2 | 2,008 | 81 | 0.74 | 0.65 to 0.84 | < .001 | |
| Unknown | 965 | 82 | 0.62 | 0.52 to 0.74 | < .001 | |
| Year of diagnosis‖ | < .001 | |||||
| 2000 | 3,598 | 81 | Reference | |||
| 2001 | 3,807 | 83 | 1.11 | < .001 | ||
| 2002 | 3,995 | 84 | ||||
| 2003 | 4,369 | 84 | 1.08 to 1.13 | |||
| 2004 | 4,379 | 87 | ||||
| 2005 | 4,314 | 87 | ||||
| Initial Physicians (N = 18,605) | ||||||
| Degree | .081 | |||||
| MD | 22,671 | 84 | ||||
| DO | 1,791 | 86 | ||||
| AMA Physician Recognition Award | .096 | |||||
| No | 22,783 | 84 | ||||
| Yes | 1,679 | 86 | ||||
| US medical school | .194 | |||||
| Yes | 18,084 | 84 | ||||
| No | 6,378 | 85 | ||||
| Primary specialty | < .001 | |||||
| Internal medicine | 9,114 | 84 | Reference | |||
| Family practice | 4,298 | 83 | 0.87 | 0.78 to 0.96 | .008 | |
| Pulmonology | 2,975 | 83 | 0.88 | 0.78 to 1.00 | .053 | |
| Emergency medicine | 1,206 | 83 | 0.85 | 0.72 to 1.01 | .062 | |
| Cardiology | 1,116 | 84 | 1.04 | 0.87 to 1.24 | .674 | |
| Oncology | 1,046 | 100 | N/A | N/A | ||
| General surgery | 551 | 84 | 0.93 | 0.73 to 1.19 | .581 | |
| Thoracic surgery | 329 | 84 | 0.94 | 0.68 to 1.29 | .690 | |
| Other | 3,827 | 85 | 0.99 | 0.89 to 1.11 | .865 | |
| Sex | .791 | |||||
| Male | 21,459 | 84 | ||||
| Female | 3,003 | 84 | ||||
| Years since graduation | .332 | |||||
| 0-9 | 2,282 | 85 | ||||
| 10-14 | 3,188 | 86 | ||||
| ≥ 15 | 18,992 | 84 | ||||
| Type of practice | .662 | |||||
| Direct patient care | 22,309 | 84 | ||||
| Administration | 188 | 82 | ||||
| Teaching | 173 | 80 | ||||
| Research | 181 | 82 | ||||
| Not active during study period | 1,556 | 85 | ||||
| Unknown | 55 | 79 | ||||
| Employment setting | .833 | |||||
| Self-employed/solo | 6,449 | 84 | ||||
| Group practice¶ | 13,992 | 85 | ||||
| Medical school | 237 | 85 | ||||
| Government hospital (VA/non-VA) | 1,142 | 81 | ||||
| Nongovernment hospital | 755 | 84 | ||||
| Other/unknown | 1,887 | 85 | ||||
| Pulmonology after initial physician | < .001 | |||||
| No | 9,748 | 83 | Reference | |||
| Yes | 14,714 | 85 | 1.20 | 1.12 to 1.29 | < .001 | |
| Initial physician random-effect coefficient | 0.83 | 0.73 to 0.94 | < .0001 | |||
| Total referred to oncologist | 24,462 | 84 | ||||
Abbreviations: AMA, American Medical Association; DO, doctor of oncology; OR, odds ratio; SD, standard deviation; VA, Veterans Affairs.
Percentages in parentheses indicate row proportions of patients referred to medical oncologists (as opposed to those not referred) for each category level.
Among patients who saw medical oncologists.
Odds ratio shows the effect of 1-year increase in age on the odds of referral to medical oncologist.
Median household income at the census tract or Zip code level.
We assumed a fixed effect for each subsequent year on referral to medical oncologists.
Group practice refers to two or more physicians working in the same clinic other than health maintenance organizations.
Patients were less likely to see all cancer specialists if they were older, black, female, had higher comorbidities, or if their initial physician graduated from a US medical school, was female, was a pulmonologist, worked in a teaching hospital, or worked in a government hospital (Veterans Affairs and other federally funded hospitals). Patients were more likely to see all cancer specialists if they had stage IIIA or IIIB versus IV; lived in areas other than the West; if their initial physician was an oncologist, general or thoracic surgeon; or if their initial physician graduated from medical school within 10 to 14 years before the date of diagnosis (Appendix Table A3, online only).
Patients with stage IIIA and IIIB NSCLC were more likely to receive guideline-based therapies if they saw a medical oncologist and radiation oncologist, compared with seeing a medical oncologist only. Stage III patients who saw all types of cancer specialists had the highest likelihood of receiving guideline-based therapies (Table 3).
Table 3.
Multivariate Logistic Regression Analysis of Patterns of Referral to Cancer Specialists and Treatment With Guideline-Based Therapies by Stage (adjusted for patient age, race, sex, household income, comorbidity, country region, and diagnosis year)
| Referral Type | Guideline-Based Therapy |
Odds Ratio | 95% CI | P | |
|---|---|---|---|---|---|
| No.* | % | ||||
| Stage IIIA | |||||
| Oncology | 27 | 10.4 | Reference | ||
| Oncology and radiotherapy | 475 | 57.1 | 13.4 | 8.7 to 20.7 | < .001 |
| Oncology and thoracic surgery | 150 | 27.9 | 2.7 | 1.7 to 4.3 | < .001 |
| Oncology and general surgery | 23 | 13.4 | 1.2 | 0.6 to 2.1 | .629 |
| Oncology, radiotherapy, thoracic surgery | 804 | 66.0 | 14.8 | 9.7 to 22.7 | < .001 |
| Oncology, radiotherapy, general surgery | 390 | 68.9 | 20.6 | 13.1 to 32.2 | < .001 |
| Nononcology referrals† or no referrals (n = 0) | 94 | 12.0 | 1.2 | 0.7 to 1.9 | .461 |
| Stage IIIB | |||||
| Oncology | 24 | 2.2 | Reference | ||
| Oncology and radiotherapy | 892 | 52.2 | 50.4 | 33.2 to 76.6 | < .001 |
| Oncology and thoracic surgery | 47 | 3.7 | 1.5 | 0.9 to 2.5 | .099 |
| Oncology and general surgery | 33 | 4.6 | 2.1 | 1.2 to 3.5 | .009 |
| Oncology, radiotherapy, thoracic surgery | 1,045 | 54.3 | 47.5 | 31.3 to 72.0 | < .001 |
| Oncology, radiotherapy, general surgery | 745 | 64.0 | 77.2 | 50.5 to 118.2 | < .001 |
| Nononcology referrals† or no referrals (n = 1) | 151 | 8.1 | 4.3 | 2.8 to 6.7 | < .001 |
| Stage IV | |||||
| Oncology | 1,367 | 57.9 | Reference | ||
| Oncology and radiotherapy | 1,867 | 50.5 | 0.6 | 0.6 to 0.7 | < .001 |
| Oncology and Thoracic surgery | 824 | 62.3 | 1.1 | 0.9 to 1.3 | .205 |
| Oncology and general surgery | 997 | 68.6 | 1.6 | 1.4 to 1.8 | < .001 |
| Oncology, radiotherapy, thoracic surgery | 1,162 | 61.3 | 0.9 | 0.8 to 1.1 | .257 |
| Oncology, radiotherapy, general surgery | 1,500 | 65.7 | 1.2 | 1.0 to 1.3 | .011 |
| Nononcology referrals† or no referrals (n = 88) | 365 | 19.7 | 0.2 | 0.1 to 0.2 | < .001 |
| Time-trend analysis: Stage III patients‡ | |||||
| Year of diagnosis | |||||
| 2000 | 782 | 33.3 | Reference | ||
| 2001 | 745 | 31.3 | 0.9 | 0.8 to 1.1 | .245 |
| 2002 | 813 | 33.0 | 1.0 | 0.9 to 1.2 | .624 |
| 2003 | 896 | 34.4 | 1.1 | 0.98 to 1.2 | .111 |
| 2004 | 859 | 39.1 | 1.4 | 1.3 to 1.6 | < .001 |
| 2005 | 805 | 38.1 | 1.3 | 1.2 to 1.5 | < .001 |
| Time-trend analysis: Stage IV patients‡ | |||||
| Year of diagnosis | |||||
| 2000 | 1,084 | 51.8 | Reference | ||
| 2001 | 1,152 | 51.6 | 1.0 | 0.9 to 1.1 | .789 |
| 2002 | 1,214 | 53.5 | 1.1 | 1.0 to 1.3 | .101 |
| 2003 | 1,469 | 57.1 | 1.3 | 1.1 to 1.4 | < .001 |
| 2004 | 1,551 | 54.8 | 1.2 | 1.0 to 1.3 | .009 |
| 2005 | 1,612 | 56.2 | 1.3 | 1.2 to 1.5 | < .001 |
Percentages refer to rows.
Nononcology referrals include thoracic surgery, general surgery, or radiation therapy, alone or in combination.
Time-trend analyses were performed as part of the same multivariate model, but with a simplified stage variable (III v IV, instead of IIIA/IIIB/IV).
Stage IV patients were less likely to receive guideline-based therapies if they saw a medical oncologist and radiation oncologist, compared with those who saw a medical oncologist alone (Table 3). Those who saw both a radiation therapist and a medical oncologist were more likely to be treated with radiation alone than those who saw only an oncologist (45% versus 3%, P < .001). Compared with seeing a medical oncologist alone, stage IV patients who saw all 3 specialties were slightly more likely to receive guideline-based therapies if the surgeon was a general surgeon, but not a thoracic surgeon. This difference is partly explained by a higher frequency of surgery among stage IV patients who saw a thoracic surgeon compared with those who saw a general surgeon (7% versus 1%, P < .001).
Patient referrals to medical oncologists steadily increased over the study period, from 81% in 2000% to 87% in 2005 (odds ratio = 1.1; P < .001 for each subsequent year; Table 2). Referrals to all three types of cancer specialists remained relatively constant over time, varying from 32% in 2000% to 30% in 2005 (odds ratio = 1.00; P = .536; Appendix Table A3).
Stage III patients diagnosed in 2004 and 2005 were more likely to receive guideline-based therapies than patients diagnosed in 2000. Stage IV patients diagnosed between 2003 and 2005 were more likely to receive guideline-based therapies than those diagnosed in 2000 (Table 3).
Discussion
Using a nationally representative claims database for Medicare patients, we identified factors associated with referral to cancer specialists and types of treatment received (in relation to recommendations) for patients with stages III and IV NSCLC. Our study suggests that most patients (84%) will see at least a medical oncologist, whereas 31% will see all cancer specialists. Patients who saw a medical oncologist, a radiation oncologist, and a surgeon had the highest likelihood of receiving treatments endorsed by the NCCN guidelines, a finding that was particularly relevant in patients with stage III NSCLC.
Patients diagnosed in more recent years were more likely to see medical oncologists and to receive recommended therapies. This increasing trend in adoption of evidence-based practices suggests improvements in supportive care, lower surgical morbidity, and increased dissemination of guideline recommendations through scientific events and multimedia tools, including Web-enabled electronic health records.
Several observational studies have shown that 45% to 90% of patients with NSCLC are referred to cancer specialists, and 20% to 65% receive recommended therapies for their disease stage.12,21–29 A common finding in these studies is that patients who are older or have a lower socioeconomic status are less likely to see cancer specialists and/or receive recommended cancer therapies, including surgery for early-stage or chemotherapy for advanced-stage NSCLC.
Consistent with these observations, our study showed a lower likelihood of referrals to medical oncologists in patients who were older, black, or lived in lower income areas. In addition, patients seen initially by family practice physicians were statistically less likely to see medical oncologists. These findings indicate that sociodemographic characteristics still represent access barriers to specialty care for NSCLC and that some general practitioners are not fully aware of the role of chemotherapy for stages III and IV NSCLC.30 Health care systems need to promote efforts that increase access to specialty care so that only medical factors and patient preferences determine the receipt of cancer therapy modalities.
Patients with stages IIIA and IIIB NSCLC were more likely to see all types of cancer specialists compared with stage IV patients (40% and 32% v 28%, respectively), and yet patients with stage III disease were less likely to receive guideline-based therapies than stage IV patients (45% and 30% v 54%, respectively). These differences could be partly explained by the higher complexity and toxicity of standard multimodality therapy for stage III compared with palliative chemotherapy for stage IV disease, which could result in a lower adherence to guideline-recommended treatments in stage III compared with stage IV. In addition, other factors not accounted for in the study could have influenced the differences observed in treatment adherence, including performance status, patient preferences, and other clinical characteristics not available in the SEER-Medicare database (eg, pulmonary function test results).
Several limitations apply to our study. Factors that could influence referrals and care that are not available in the SEER-Medicare database, including performance status, could have influenced the associations we found for referrals and treatments in this study. Treatment and referral data relied on claim codes and are therefore subject to unverifiable errors. Our algorithm to identify initial physicians has not been validated. Current claims for radiation therapy do not allow the distinction between treatments delivered with curative and those delivered with palliative intent, and this may have biased our estimates of receipt of guideline-based therapies. We did not explore associations of referrals or treatment patterns with overall survival, because substantial selection bias would probably prevent an accurate interpretation of survival outcomes.
In conclusion, our study suggests that sociodemographic disparities still prevent access to cancer specialists for patients with advanced NSCLC, and patients who see medical oncologists, radiation oncologists, and surgeons have the highest likelihood of receiving therapies endorsed by guidelines, particularly those with stage III NSCLC. As providers strive to improve quality of care, efforts should focus on decreasing disparities in access and elucidating other reasons for suboptimal therapy in patients appropriately referred to lung cancer specialists, including patient preferences and clinical characteristics.
Acknowledgment
Supported by Genentech Grant No. W677297. Presented in part at the 2011 International Association for the Study of Lung Cancer meeting, July 7, 2011 (abstract P4.057), and at the 2012 American Society of Clinical Oncology Meeting on June 2, 2012 (abstract 6007).
Appendix
Table A1.
Treatment Recommendations for Stage III and IV Non–Small-Cell Lung Cancer Endorsed by the National Comprehensive Cancer Network Guidelines
| Stage IIIA | T3 N1 M0 | Surgery followed by chemotherapy with or without postoperative radiation (the latter only if positive margins) |
| T1-T3 N2 M0 | Neoadjuvant chemotherapy followed by surgery Neoadjuvant chemotherapy followed by surgery and postoperative radiation (if positive margins) Neoadjuvant chemoradiation followed by surgery Definitive concurrent chemoradiation Sequential chemotherapy followed by radiation (if borderline performance status) |
|
| Stage IIIB | T4 any N M0 | Definitive concurrent chemoradiation Sequential chemotherapy followed by radiation (if borderline performance status) |
| Any T N3 M0 | Definitive concurrent chemoradiation Sequential chemotherapy followed by radiation (if borderline performance status) |
|
| Stage IV | Any T any N M1 | Chemotherapy alone Chemotherapy and palliative radiation |
Recommendations are based on the American Joint Committee on Cancer 6th Edition Staging System.
Table A2.
Patient and Initial Physician Characteristics
| Characteristic | No. | % |
|---|---|---|
| Patients (N = 28,977) | ||
| Age, years | 75.6 | |
| Mean | 6.1 | |
| SD | ||
| Sex | ||
| Male | 15,346 | 53.0 |
| Female | 13,631 | 47.0 |
| Race/ethnicity | ||
| White | 24,161 | 83.4 |
| Black | 2,336 | 8.1 |
| Hispanic | 1,021 | 3.5 |
| Asian | 1,360 | 4.7 |
| American Indian/Alaskan Native | 67 | 0.2 |
| Unknown | 32 | 0.1 |
| SEER registry area | ||
| San Francisco-Oakland | 974 | 3.4 |
| Connecticut | 2,151 | 7.4 |
| Detroit | 2,539 | 8.8 |
| Hawaii | 409 | 1.4 |
| Iowa | 2,064 | 7.1 |
| New Mexico | 497 | 1.7 |
| Seattle/Puget Sound | 1,666 | 5.8 |
| Utah | 401 | 1.4 |
| Atlanta | 777 | 2.7 |
| San Jose | 614 | 2.1 |
| Los Angeles | 1,867 | 6.4 |
| Rural Georgia | 91 | 0.3 |
| Greater California | 5,025 | 17.3 |
| Kentucky | 3,041 | 10.5 |
| Louisiana | 2,449 | 8.5 |
| New Jersey | 4,412 | 15.2 |
| 2004 | 5,030 | 17.4 |
| 2005 | 4,982 | 17.2 |
| Initial Physicians (N = 18,605) | ||
| Median annual household income, $* | ||
| Lower tertile | ≤ 36,600 | |
| Medium tertile | 36,601-52,700 | |
| Higher tertile | 52,701-200,000 | |
| Charlson comorbidity score | ||
| 0 | 12,949 | 44.7 |
| 1-2 | 12,380 | 42.7 |
| > 2 | 2,469 | 8.5 |
| Unknown | 1,179 | 4.1 |
| Stage | ||
| IIIA | 4,371 | 15.1 |
| IIIB | 9,736 | 33.6 |
| IV | 14,870 | 51.3 |
| Year of diagnosis | ||
| 2000 | 4,442 | 15.3 |
| 2001 | 4,610 | 15.9 |
| 2002 | 4,737 | 16.3 |
| 2003 | 5,176 | 17.9 |
| Age, years | ||
| Mean | 49.4 | |
| SD | 9.9 | |
| Sex | ||
| Male | 15,899 | 85.5 |
| Female | 2,706 | 14.5 |
| Degree | ||
| MD | 17,258 | 92.8 |
| DO | 1,347 | 7.2 |
| AMA Physician Recognition Award | ||
| Yes | 1,308 | 7.0 |
| No | 17,297 | 93.0 |
| US medical school graduate | ||
| Yes | 13,856 | 74.5 |
| No | 4,749 | 25.5 |
| Primary medical specialty | ||
| Internal medicine | 6,713 | 36.1 |
| Family practice | 3,543 | 19.0 |
| Pulmonology | 1,304 | 7.0 |
| Emergency medicine | 1,170 | 6.3 |
| Cardiology | 973 | 5.2 |
| Hematology-oncology or oncology | 652 | 3.5 |
| General surgery | 527 | 2.8 |
| Thoracic surgery | 244 | 1.4 |
| Other/unknown | 3,479 | 18.7 |
| Office geographic region | ||
| West | 7,183 | 38.6 |
| Northeast | 3,853 | 20.7 |
| Midwest | 2708 | 14.6 |
| South | 3,830 | 20.6 |
| Unknown | 1,031 | 5.5 |
| Years since graduation from medical school† | ||
| 0-9 | 2,074 | 11.1 |
| 10-14 | 2,599 | 14.0 |
| ≥ 15 | 13,932 | 74.9 |
| Type of primary practice | ||
| Direct patient care | 16,822 | 90.4 |
| Administrative | 154 | 0.8 |
| Medical teaching | 164 | 0.9 |
| Medical research | 138 | 0.7 |
| Not currently active | 1,276 | 6.9 |
| Unknown | 51 | 0.3 |
| Practice setting | ||
| Self-employed/solo | 4,755 | 25.6 |
| Group‡ | 10,440 | 56.1 |
| HMO | 23 | 0.1 |
| Teaching hospital | 223 | 1.2 |
| VA/non-VA government hospital | 991 | 5.3 |
| Nongovernment hospital | 578 | 3.1 |
| Other or unknown | 1,595 | 8.6 |
Abbreviations: AMA, American Medical Association; DO, doctor of oncology; HMO, health maintenance organization; SD, standard deviation; VA, Veterans Affairs.
Household income at the census tract or Zip code level.
At the time of initiation of study period (January 1, 2000).
Group practice includes two or more physicians working in the same clinic, excluding HMOs.
Table A3.
Univariable and Multivariable Logistic Regression Analysis of Patient and Initial Physician Characteristics With Referrals to All Cancer Specialists (medical oncologists, radiation oncologists, and thoracic or general surgeons)
| Characteristic | Referred* |
Unadjusted P | Adjusted OR | 95% CI | Adjusted P | |
|---|---|---|---|---|---|---|
| No. | % | |||||
| Patients (N = 28,977) | ||||||
| Age, years† | < .001 | 0.94‡ | 0.94 to 0.95 | < .001 | ||
| Mean | 74 | |||||
| SD | 5 | |||||
| Race/ethnicity | < .001 | |||||
| White | 7,718 | 32 | Reference | |||
| Black | 690 | 30 | 0.79 | 0.72 to 0.87 | < .001 | |
| Hispanic | 274 | 27 | 0.90 | 0.77 to 1.05 | .169 | |
| Asian | 344 | 25 | 0.97 | 0.85 to 1.12 | .691 | |
| American Indian/Alaska Native | 22 | 33 | 1.34 | 0.78 to 2.30 | .292 | |
| Unknown | 5 | 16 | 0.42 | 0.15 to 1.13 | .087 | |
| Sex | < .001 | |||||
| Male | 4,945 | 32 | Reference | |||
| Female | 4,108 | 0.95 | 0.90 to 0.99 | .040 | ||
| Stage | 30 | < .001 | ||||
| IV | 4,180 | 28 | Reference | |||
| IIIA | 1,784 | 41 | 1.86 | 1.73 to 2.01 | < .001 | |
| IIIB | 3,089 | 32 | 1.27 | 1.20 to 1.35 | < .001 | |
| Region | < .001 | |||||
| West | 2,923 | 26 | Reference | |||
| Northeast | 2,201 | 34 | 1.53 | 1.42 to 1.64 | < .001 | |
| Midwest | 1,756 | 38 | 1.84 | 1.70 to 2.00 | < .001 | |
| South | 2,173 | 34 | 1.44 | 1.34 to 1.56 | < .001 | |
| Household income§ | .608 | |||||
| Lower tertile | 2,973 | 31 | ||||
| Medium tertile | 3,009 | 31 | ||||
| Higher tertile | 3,035 | 31 | ||||
| Unknown | 36 | 30 | ||||
| Charlson index | < .001 | |||||
| 0 | 4,193 | 32 | Reference | |||
| 1-2 | 3,841 | 31 | 0.92 | 0.87 to 0.98 | .006 | |
| > 2 | 688 | 28 | 0.78 | 0.70 to 0.86 | < .001 | |
| Unknown | 331 | 28 | 0.72 | 0.63 to 0.83 | < .001 | |
| Year of diagnosis‖ | .188 | |||||
| 2000 | 1,417 | 32 | Reference | |||
| 2001 | 1,433 | 31 | 1.00 | .536 | ||
| 2002 | 1,488 | 31 | ||||
| 2003 | 1,634 | 32 | 0.99 to 1.02 | |||
| 2004 | 1,572 | 31 | ||||
| 2005 | 1,509 | 30 | ||||
| Initial Physicians (N = 18,605) | ||||||
| Degree | .180 | |||||
| MD | 8,372 | 31 | ||||
| DO | 681 | 33 | ||||
| AMA Physician Recognition Award | .473 | |||||
| No | 8,427 | 31 | ||||
| Yes | 626 | 32 | ||||
| US medical school | .028 | |||||
| Yes | 2,422 | 32 | Reference | |||
| No | 6,631 | 31 | 0.93 | 0.87 to 0.99 | .026 | |
| Primary specialty | < .001 | |||||
| Internal medicine | 3,316 | 31 | Reference | |||
| Family practice | 1,614 | 31 | 0.98 | 0.90 to 1.06 | .549 | |
| Pulmonology | 1,002 | 28 | 0.84 | 0.77 to 0.92 | < .001 | |
| Emergency medicine | 408 | 28 | 1.00 | 0.88 to 1.15 | .962 | |
| Cardiology | 405 | 31 | 1.01 | 0.88 to 1.15 | .881 | |
| Oncology | 358 | 34 | 1.17 | 1.01 to 1.36 | .031 | |
| General surgery | 317 | 48 | 2.15 | 1.81 to 2.55 | < .001 | |
| Thoracic surgery | 192 | 49 | 2.01 | 1.61 to 2.52 | < .001 | |
| Other | 1,441 | 32 | 1.08 | 1.00 to 1.18 | .048 | |
| Sex | .002 | |||||
| Male | 8,021 | 32 | Reference | |||
| Female | 1,032 | 29 | 0.92 | 0.84 to 0.99 | .048 | |
| Years since graduation | .020 | |||||
| 0-9 | 776 | 29 | Reference | |||
| 10-14 | 1,179 | 32 | 1.13 | 1.01 to 1.27 | .040 | |
| ≥ 15 | 7,098 | 31 | 1.10 | 1.00 to 1.21 | .055 | |
| Type of practice | .697 | |||||
| Direct patient care | 8,248 | 31 | ||||
| Administration | 73 | 31 | ||||
| Teaching | 58 | 27 | ||||
| Research | 54 | 25 | ||||
| Not active during study period | 602 | 33 | ||||
| Unknown | 18 | 26 | ||||
| Employment setting | .010 | |||||
| Self-employed/solo | 2,459 | 32 | Reference | |||
| Group practice¶ | 5,181 | 31 | 0.95 | 0.89 to 1.01 | .094 | |
| Medical school | 74 | 26 | 0.74 | 0.55 to 0.99 | .039 | |
| Government hospital (VA/non-VA) | 397 | 28 | 0.81 | 0.71 to 0.93 | .003 | |
| Nongovernment hospital | 282 | 31 | 0.91 | 0.77 to 1.07 | .268 | |
| Other/unknown | 660 | 30 | 0.89 | 0.80 to 1.00 | .048 | |
| Pulmonology after initial physician | .281 | |||||
| No | 3709 | 32 | ||||
| Yes | 5,344 | 31 | ||||
| Initial physician random-effect coefficient | 0.40 | 0.31 to 0.53 | < .001 | |||
| Total referred to all specialists | 9,053 | 31 | ||||
Percentages in parenthesis indicate row proportions of patients referred to all cancer specialists (as opposed to those not referred) for each category level.
Among patients who saw all types of cancer specialists.
Odds ratio shows the effect of 1-year increase in age on the odds of referral to all cancer specialists.
Median household income at the census tract or Zip code level.
We assumed a fixed effect for each subsequent year on referral to all cancer specialists.
Group practice refers to two or more physicians working in the same clinic other than health maintenance organizations.
Figure A1.
Algorithm to identify physicians initially involved in the management of non–small-cell lung cancer cases (initial physician). CT, computed tomography; MRI, magnetic resonance imaging.
Figure A2.
Flow chart of patient selection criteria. UPIN, universal physician identification number.
Authors' Disclosures of Potential Conflicts of Interest
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Carolina M. Reyes, Genentech, Inc. (C) Consultant or Advisory Role: Sacha Satram-Hoang, Genentech, Inc. (C) Stock Ownership: Carolina M. Reyes, Roche Honoraria: None Research Funding: Bernardo H.L. Goulart, Genentech; Catherine R. Fedorenko, Genentech, Inc.; David G. Mummy, Genentech, Inc.; Lisel M. Koepl, Genentech, Inc.; Scott D. Ramsey, Genentech, Inc. Expert Testimony: None Other Remuneration: None
Author Contributions
Conception and design: Bernardo H.L. Goulart, Carolina M. Reyes, Catherine R. Fedorenko, Sacha Satram-Hoang, David K. Blough, Scott D. Ramsey
Financial support: Carolina M. Reyes
Administrative support: Catherine R. Fedorenko, Lisel M. Koepl
Collection and assembly of data: Bernardo H.L. Goulart, Catherine R. Fedorenko, David G. Mummy, Lisel M. Koepl
Data analysis and interpretation: Bernardo H.L. Goulart, Carolina M. Reyes, Catherine R. Fedorenko, David G. Mummy, Sacha Satram-Hoang, David K. Blough
Manuscript writing: Bernardo H.L. Goulart, Carolina M. Reyes, Catherine R. Fedorenko, David G. Mummy, Sacha Satram-Hoang, Lisel M. Koepl, Scott D. Ramsey
Final approval of manuscript: All authors
References
- 1.Siegel R, Ward E, Brawley O, et al. Cancer statistics, 2011: The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Govindan R, Page N, Morgensztern D, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: Analysis of the Surveillance, Epidemiologic, and End Results database. J Clin Oncol. 2006;24:4539–4544. doi: 10.1200/JCO.2005.04.4859. [DOI] [PubMed] [Google Scholar]
- 3.Nair A, Klusmann MJ, Jogeesvaran KH, et al. Revisions to the TNM staging of non-small cell lung cancer: Rationale, clinicoradiologic implications, and persistent limitations. Radiographics. 2011;31:215–238. doi: 10.1148/rg.311105039. [DOI] [PubMed] [Google Scholar]
- 4.Albain KS, Swann RS, Rusch VW, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: A phase III randomised controlled trial. Lancet. 2009;374:379–386. doi: 10.1016/S0140-6736(09)60737-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Meerbeeck JP, Kramer GW, Van Schil PE, et al. Randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage IIIA-N2 non-small-cell lung cancer. J Natl Cancer Inst. 2007;99:442–450. doi: 10.1093/jnci/djk093. [DOI] [PubMed] [Google Scholar]
- 6.Curran WJ, Jr, Paulus R, Langer CJ, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: Randomized phase III trial RTOG 9410. J Natl Cancer Inst. 2011;103:1452–1460. doi: 10.1093/jnci/djr325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network. Non-Small Cell Lung Cancer version 2.2012, NCCN guidelines, NCCN, 2011. http://www.nccn.org.
- 8.NSCLC Meta-Analyses Collaborative Group. Chemotherapy in addition to supportive care improves survival in advanced non-small-cell lung cancer: A systematic review and meta-analysis of individual patient data from 16 randomized controlled trials. J Clin Oncol. 2008;26:4617–4625. doi: 10.1200/JCO.2008.17.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 10.Reck M, von Pawel J, Zatloukal P, et al. Overall survival with cisplatin-gemcitabine and bevacizumab or placebo as first-line therapy for nonsquamous non-small-cell lung cancer: Results from a randomised phase III trial (AVAiL) Ann Oncol. 2010;21:1804–1809. doi: 10.1093/annonc/mdq020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 12.Earle CC, Neumann PJ, Gelber RD, et al. Impact of referral patterns on the use of chemotherapy for lung cancer. J Clin Oncol. 2002;20:1786–1792. doi: 10.1200/JCO.2002.07.142. [DOI] [PubMed] [Google Scholar]
- 13.Beatty S, Stevens W, Stevens G, et al. Lung cancer patients in New Zealand initially present to secondary care through the emergency department rather than by referral to a respiratory specialist. N Z Med J. 2009;122:33–41. [PubMed] [Google Scholar]
- 14.Potosky AL, Riley GF, Lubitz JD, et al. Potential for cancer related health services research using a linked Medicare-tumor registry database. Med Care. 1993;31:732–748. [PubMed] [Google Scholar]
- 15.National Cancer Institute. SEER program overview, National Cancer Institute, 2011. http://seer.cancer.gov/
- 16.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 17.Hayman JA, Abrahamse PH, Lakhani I, et al. Use of palliative radiotherapy among patients with metastatic non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2007;69:1001–1007. doi: 10.1016/j.ijrobp.2007.04.059. [DOI] [PubMed] [Google Scholar]
- 18.Warren JL, Harlan LC, Fahey A, et al. Utility of the SEER-Medicare data to identify chemotherapy use. Med Care. 2002;40:55–61. doi: 10.1097/01.MLR.0000020944.17670.D7. [DOI] [PubMed] [Google Scholar]
- 19.Baldwin LM, Adamache W, Klabunde CN, et al. Linking physician characteristics and Medicare claims data: Issues in data availability, quality, and measurement. Med Care. 2002;40:82–95. doi: 10.1097/00005650-200208001-00012. [DOI] [PubMed] [Google Scholar]
- 20.Jang TL, Bekelman JE, Liu Y, et al. Physician visits prior to treatment for clinically localized prostate cancer. Arch Intern Med. 2010;170:440–450. doi: 10.1001/archinternmed.2010.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramsey SD, Howlader N, Etzioni RD, et al. Chemotherapy use, outcomes, and costs for older persons with advanced non-small-cell lung cancer: Evidence from Surveillance, Epidemiology and End Results-Medicare. J Clin Oncol. 2004;22:4971–4978. doi: 10.1200/JCO.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 22.Li X, Butts C, Fenton D, et al. Utilization of oncology services and receipt of treatment: A comparison between patients with breast, colon, rectal, or lung cancer. Ann Oncol. 2011;22:1902–1909. doi: 10.1093/annonc/mdq692. [DOI] [PubMed] [Google Scholar]
- 23.Podnos YD, Borneman TR, Koczywas M, et al. Symptom concerns and resource utilization in patients with lung cancer. J Palliat Med. 2007;10:899–903. doi: 10.1089/jpm.2006.0232. [DOI] [PubMed] [Google Scholar]
- 24.Vinod SK, O'Connell DL, Simonella L, et al. Gaps in optimal care for lung cancer. J Thorac Oncol. 2008;3:871–879. doi: 10.1097/JTO.0b013e31818020c3. [DOI] [PubMed] [Google Scholar]
- 25.Vinod SK, Simonella L, Goldsbury D, et al. Underutilization of radiotherapy for lung cancer in New South Wales, Australia. Cancer. 2010;116:686–694. doi: 10.1002/cncr.24762. [DOI] [PubMed] [Google Scholar]
- 26.Wang J, Kuo YF, Freeman J, et al. Increasing access to medical oncology consultation in older patients with stage II-IIIA non-small-cell lung cancer. Med Oncol. 2008;25:125–132. doi: 10.1007/s12032-007-9003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Winget M, Stanger J, Gao Z, et al. Predictors of surgery and consult with an oncologist for adjuvant chemotherapy in early stage NSCLC patients in Alberta, Canada. J Thorac Oncol. 2009;4:629–634. doi: 10.1097/JTO.0b013e31819ccf26. [DOI] [PubMed] [Google Scholar]
- 28.Earle CC, Venditti LN, Neumann PJ, et al. Who gets chemotherapy for metastatic lung cancer? Chest. 2000;117:1239–1246. doi: 10.1378/chest.117.5.1239. [DOI] [PubMed] [Google Scholar]
- 29.Dalton SO, Frederiksen BL, Jacobsen E, et al. Socioeconomic position, stage of lung cancer and time between referral and diagnosis in Denmark, 2001-2008. Br J Cancer. 2011;105:1042–1048. doi: 10.1038/bjc.2011.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wassenaar TR, Eickhoff JC, Jarzemsky DR, et al. Differences in primary care clinicians' approach to non-small cell lung cancer patients compared with breast cancer. J Thorac Oncol. 2007;2:722–728. doi: 10.1097/JTO.0b013e3180cc2599. [DOI] [PubMed] [Google Scholar]