Abstract
Background
Low circulating levels of Coenzyme Q10 (CoQ10) have been associated with increased cancer incidence and poor prognosis for a number of cancer types, while a recent prospective study observed a positive association for CoQ10 with breast cancer risk.
Methods
We prospectively examined the association of plasma CoQ10 with breast cancer risk in a nested case-control study of Chinese women within the Shanghai Women's Health Study (SWHS). Pre-diagnostic plasma samples were obtained from 340 cases and 653 age-matched controls and analyzed for total CoQ10.
Results
A borderline significant inverse association for breast cancer incidence with plasma CoQ10 level was observed using a conditional logistic regression model adjusted for age and age at first live birth, which became significant after elimination of cases diagnosed within one year of blood draw (ptrend = 0.03). This association was independent of menopausal status. Plasma CoQ10 levels were also observed to be significantly associated with circulating γ-tocopherol (r = 0.50; p < 0.0001) and with α-tocopherol (r =0.38; p < 0.0001) levels.
Conclusions
Circulating levels of CoQ10 were generally low in this population and the observed association with breast cancer risk may be limited to those women with exceptionally low values.
Impact
This study reports an inverse relationship between circulating CoQ10 and breast cancer risk, while the only other prospective study of CoQ10 and breast cancer to date found a positive association. Lower levels of CoQ10 in the SWHS population suggests that the two studies may not be contradictory and indicates a possible non-linear (U-shaped) association of CoQ10 with risk.
Introduction
Coenzyme Q10 (CoQ10) was isolated and identified fifty years ago as an essential (rate-limiting) component of the mitochondrial electron transport system leading to ATP production and is the only major lipid-soluble antioxidant synthesized by humans (1, 2). All mammalian cells are capable of synthesizing CoQ10 (or closely related molecules) in a complex biosynthetic pathway involving the mevalonate pathway (also responsible for cholesterol and dolichol synthesis) and tyrosine, in a process dependent upon eight essential vitamins and nutrients (3,4). Mitochondrial energy production is essential for eukaryotic cell survival and CoQ10 is a key molecule in all energy requiring processes, including proliferation, apoptosis, angiogenesis, and immune function (5–8), suggesting the potential for multiple roles in the initiation and progression of cancer. Despite the critical role of CoQ10 in many cellular functions, its potential relationship with cancer development and progression has not received appropriate attention. Epidemiological or clinical studies of plasma or tissue CoQ10 are rare in the literature and have involved limited numbers of subjects. Folkers, et al. (9), reported reduced circulating total CoQ10 levels in breast cancer (n=17) and myeloma (n=15) patients. Palan et al., (10) in a cross-sectional study (n=230), reported an inverse association between cervical intraepithelial neoplasia and cervical cancer with total circulating CoQ10, as well as with α-tocopherol (αT) and γ-tocopherol (γT). Rusciani, et al. (11) reported a highly significant association between low plasma total CoQ10 levels and metastasis and progression in 117 melanoma patients. Recently, in the largest epidemiologic study to date of CoQ10 involving the Multiethnic Cohort, a positive association was observed for prediagnostic circulating total CoQ10 and breast cancer risk in postmenopausal women (12).
Administration of CoQ10 (as the oxidized quinone) to humans has been associated with a number of favorable clinical outcomes in the treatment of hypertension (13), heart failure (14), migraines (15), and myopathies associated with statin use (16). In the latter case there is growing concern for the long-term effects of statin use, resulting in decreased cellular CoQ10 synthesis and Boudroux, et al. reported a non significant increasing risk for breast cancer in women as a function of length of time on statins (17). Positive effects have been reported for CoQ10 in the treatment of breast cancer (18–20), however, these clinical studies were conducted on small numbers of patients and lacked adequate design.
Cellular and tissue levels of CoQ10 decrease with age, and cellular levels below a critical threshold are incompatible with life (21). In contrast, plasma levels of CoQ10 are reported by some to rise as a function of age (22), and are higher in postmenopausal women (23). Supplemental CoQ10 increases circulating α-T levels in animals (24) and humans (25), however, the determinants of circulating CoQ10 and its physiological regulation in vivo are unknown. The objective of the current study was to determine if an association exists between prediagnostic circulating CoQ10 and breast cancer risk among Chinese women from the Shanghai Women’s health Study (SWHS).
Materials and Methods
Study Population and Data Collection
The Shanghai Women’s Health Study (SWHS) is a cohort of approximately 75,000 adult Chinese women between the ages of 40 and 70 in Shanghai, China (26). Subject recruitment was initiated in June 1997 and completed in May 2000. The cohort is being actively followed through a combination of record linkage with the files collected in the Shanghai Cancer Registry and Vital Statistic Unit and a biannual home visit. Nearly all cohort members were successfully followed, with the response rates for first in-person follow-up being 99.8% (2000–2002), second 98.7% (2002–2004), and third 96.7% (2004–2007). All possible matches identified by record linkage were verified by home visits. Medical charts from the diagnostic hospitals were reviewed to verify the diagnosis, and pathological characteristics of the tumor were recorded. Breast cancer cases were defined as women for whom breast cancer was the first cancer diagnosis (ICD-9, code of 174).
Blood samples were collected from 56,900 subjects (76% of the cohort) during the baseline survey period. Over an approximately average 7.5 years follow-up, the number of incident breast cancer cases initially available for analysis was 386 with two controls for each index case (772) selected randomly from the group of cohort members who were free of cancer at the time of cancer diagnosis of the index case. The controls were matched to the index case by age (± 2 years), menopausal status at baseline (yes, no), date of sample collection (± 30 days), time of sample collection (morning or afternoon), time interval after the last meal (± 2 hours), and recent antibiotic use (yes, no). After exclusion of samples with inadequate plasma available, incomplete matching information, or analytical interference, 340 cases and 653 controls were used in the subsequent analysis. Cases without controls or controls without cases were deleted from the analysis.
Laboratory Assays
Plasma samples were stored at −75°C, thawed and then aliquoted in a dark room for analysis. Plasma samples were extracted using hexane after addition of δ-tocopheryl laurate as an internal standard. The extracts were then stored at −80 °C prior to subsequent analysis for total CoQ10 by HPLC (Model Spectra, ThermoFisher, San Jose, CA) with pre-column electrochemical oxidation (guard cell from ESA, Model 5020, Chelmsford, MA) and post-column UV detection at 275 nm (as described previously 12, 27). The separation was performed on a Gemini C18 analytical and guard column (150 mm × 2.0 mm, 3 µm and 4mm × 3.0mm, 10 µm, respectively; Phenomenex, Torrance, CA) with a mixture of sodium acetate trihydrate, glacial acetic acid, 2-propanol, hexane, and methanol. The range of inter-assay variability was 5 – 7 %. Plasma tocopherols were measured as described previously (28). Data for the distribution of CoQ10 levels among women was obtained from the current study and from another study (12) of CoQ10 and breast cancer utilizing the Multiethnic Cohort (MEC) performed by the same method in the same laboratory and provided by the authors of that study.
Statistical analysis
Conditional logistic regression, with matched sets as strata, was used to compute odds ratios (ORs) and 95% confidence intervals (CIs) whereby controls were matched to the index case by age, menopausal status at baseline, date of sample collection, time of sample collection, time interval after the last meal, and recent antibiotic use. CoQ10 levels were categorized into quintiles or quartiles based on the distribution of controls. The third quintile/quartile was chosen as the reference category to allow for a better comparison with the previous MEC study, in which the lowest tertile (median CoQ10 = 668 ng/ml) was used as a reference (12). In addition to matching variables, many potential confounding factors or effect modifiers have been obtained from survey or other studies (26,29). We conducted analyses to additionally adjust for age at first child birth, educational achievement, body mass index, regular physical activity (yes, no), number of full-term pregnancies, age at menarche, months of breast feeding, smoking status, and alcohol drinking. However, except for age at first live birth, adjusting for other covariates did not materially change the estimates. Stratified analyses were conducted by menopausal status and plasma concentration of γT (≤1948.9; >1948.9). Sensitivity analyses were conducted by excluding those whose blood samples were collected within one year of cancer diagnosis to reduce the effects possible pre-clinical cases. P values of <0.05 (2 sided probability) were interpreted as being statistically significant. Tests for trend were performed by entering the categorical variables as a continuous variable in the model. Statistical analyses were conducted using SAS statistical software (version 9.1; SAS Institute, Cary, NC).
Results
Baseline characteristics of patients and matched controls are shown in Table 1. Significant differences between cases and controls in the direction expected for this population were observed for education, age at menarche, age at first birth, months of breast feeding, and family history of breast cancer. Mean and median CoQ10 levels overall were slightly lower in cases compared to controls (Table 2), however the difference was not statistically significant. When stratified by menopausal status, postmenopausal women were observed to have approximately 20% higher average circulating CoQ10 levels compared to premenopausal women (p = 0.07 among controls).
Table 1.
Characteristics of breast cancer cases and controls analyzed for CoQ10 in a nested case-control study within the Shanghai Women’s Health Study (SWHS), 1997–2006.
| Characteristics | Cases (n=340) |
Controls (n=653) |
P- value# |
|---|---|---|---|
| Age at blood draw (years), mean (SD)* | 52.4 ± 9.0 | 52.4 ± 9.0 | 0.15 |
| Current hormone therapy use, n (%) | 13 (3.8) | 9 (1.4) | 0.04 |
| Education, n (%) | <0.01 | ||
| Elementary and under | 52 (15.3) | 151 (23.1) | |
| Middle school | 121 (35.7) | 267 (40.9) | |
| High school | 116 (34.2) | 168 (25.7) | |
| College and above | 50 (14.7) | 67 (10.3) | |
| Body mass index (kg/m2), mean (SD) | |||
| All Women | 24.2 ± 3.6 | 24.4 ± 3.3 | 0.29 |
| Premenopausal Women | 23.4 ± 3.2 | 23.6 ± 3.1 | 0.48 |
| Postmenopausal Women | 25.1 ± 3.7 | 25.3 ± 3.3 | 0.54 |
| Physically active, n (%) | 122 (35.9) | 222 (34.0) | 0.67 |
| Nulliparous, n (%) | 15 (4.4) | 24 (3.7) | 0.29 |
| Number of full term pregnancies, mean (SD) | 1.7 ± 1.1 | 1.8 ± 1.1 | 0.05 |
| Age @ first child birth, mean (SD) | 26.3 ± 4.1 | 25.6 ± 4.2 | 0.01 |
| Age @ menarche, mean (SD) | 14.8 ± 1.8 | 15.0 ± 1.7 | 0.03 |
| Months of breast feeding | 13.7 ± 15.6 | 16.3 ± 18.4 | <0.01 |
| Smoking status, n (%) | 0.39 | ||
| Never | 335 (98.5) | 634 (97.1) | |
| Former | 0 (0) | 1 (0.1) | |
| Current | 5 (1.5) | 18 (2.8) | |
| Mother or sister with breast cancer, n (%) | 14 (4.12) | 10 (1.5) | 0.01 |
| Alcohol use, n (%) | 0.71 | ||
| Never | 333 (97.9) | 634 (97.1) | |
| Former | 1 (0.3) | 2 (0.3) | |
| Current | 6 (1.8) | 17 (2.6) | |
| Deaths, n (%) | 40 (11.7) | 20 (3.1) | <0.01 |
| Postmenopausal, n (%) | 165 (48.5) | 320 (49.0) | 0.06 |
SD Standard deviation
Conditional logistic regression model for categorical variables or ANOVA test for continuous variables
Table 2.
Comparison of plasma Q10 (ng/mL) levels between breast cancer cases and controls, a nested case-control study within the Shanghai Women’s Health Study (SWHS), 1997–2006.
| Plasma CoQ10 concentration (ng/mL) |
Cases | Controls | P- value |
|---|---|---|---|
| All women (340 pairs) | |||
| Mean ± SD | 605.4 ± 241.0 | 619.2 ± 185.4 | 0.25a |
| Median (25th, 75th) | 560.0 (435.0, 728.0) | 597.0 (500.0, 714.0) | 0.16b |
| All women with cases diagnosed > 1 year after blood draw (303 pairs) | |||
| Mean ± SD | 603.7 ± 242.8 | 622.9 ± 187.4 | 0.12a |
| Median (25th, 75th) | 553.0 (434.0, 739.0) | 597.5 (502.5, 714.5) | 0.09b |
| Premenopausal women (171 pairs) | |||
| Mean ± SD | 544.6 ± 223.5 | 554.9 ± 153.0 | 0.38a |
| Median (25th, 75th) | 508.0 (382.0, 649.0) | 554.0 (450.5, 644.0) | 0.13b |
| Postmenopausal women (169 pairs) | |||
| Mean ± SD | 667.0 ± 243.0 | 684.3 ± 192.9 | 0.45a |
| Median (25th, 75th) | 621.0 (494.0, 788.0) | 649.5 (550.0, 789.0) | 0.55b |
Paired test using log-transformed values for cases and the average of two matched controls.
Paired Wilcoxon signed rank test for cases and the average of two matched controls.
As shown in Table 3, there was a borderline significant increased risk for all women in the lowest quintile of plasma CoQ10 compared to the third quintile. After exclusion for cases diagnosed within one year of blood draw to reduce possible overt pre-clinical cases, a significant inverse association for plasma CoQ10 with breast cancer risk was observed (p for trend = 0.03), with significantly increased risk for women in the 1st quintile (OR =1.90; 95% CI, 1.14–3.16) relative to the third quintile of plasma CoQ10. We found plasma levels of CoQ10 significantly decreased with older age at first live birth (p<0.01). After including age at first live birth in the model, the OR (95% confidence interval) for the lowest plasma level of CoQ10 relative to the third quintile increased from 1.73 (1.07–2.80) to 1.90 (1.14–3.16) in the analyses excluding cases diagnosed within one year of blood draw. Stratification by menopausal status (Table 3) revealed similar trends by quartile with women in the lowest quartile of CoQ10 at elevated risk relative to the third quartile for both pre and postmenopausal women (p for interaction = 0.40). However, sample size became smaller and results did not reach significance in stratified analyses. Adjustment for tocopherols did not change the observed associations.
Table 3.
Odds ratios (ORs) and 95% confidence intervals (CI) for risk of breast cancer associated with plasma level of Q10 and stratified by menopausal status, a nested case-control study within the Shanghai Women’s Health Study (SWHS), 1997–2006.
| OR (95% CI) by quintile of plasma concentration of CoQ10 |
|||||||
|---|---|---|---|---|---|---|---|
| Case- control pairs |
Q11 | Q21 | Q31 | Q41 | Q51 | P for trend |
|
| All women | |||||||
| 340 | 1.55 (0.97–2.48) | 1.14 (0.72–1.80) | 1.00 (Ref) | 1.11 (0.71–1.74) | 0.97 (0.60–1.60) | 0.09 | |
| Women with cases diagnosed > 1 year after blood draw | |||||||
| 303 | 1.90 (1.14–3.16) | 1.41 (0.87–2.30) | 1.00 (Ref) | 1.15 (0.71–1.87) | 1.13 (0.66–1.91) | 0.03 | |
| OR (95% CI) by menopausal status and quartile of CoQ10 | |||||||
| Q12 | Q22 | Q32 | Q42 | P for Trend | |||
| Premenopausal women3 | |||||||
| All | 171 | 1.62 (0.91–2.89) | 1.38 (0.78–2.44) | 1.00 (Ref) | 1.15 (0.65–2.02) | 0.16 | |
| >1 year | 152 | 1.89 (1.01–3.54) | 1.70 (0.91–3.16) | 1.00 (Ref) | 1.25 (0.68–2.32) | 0.09 | |
| Postmenopausal women3 | |||||||
| All | 169 | 1.35 (0.79–2.28) | 1.04 (0.58–1.88) | 1.00 (Ref) | 0.96 (0.52–1.79) | 0.24 | |
| >1 year | 151 | 1.71 (0.95–3.09) | 1.14 (0.60–2.15) | 1.00 (Ref) | 1.15 (0.59–2.23) | 0.14 | |
20th, 40th, 60th and 80th percentiles were 429.0, 536.0, 629.0, and 796.0 ng/ml, respectively for all subjects.
25th, 50th, and 75th percentiles were 417, 537, and 665 ng/ml respectively for premenopausal women; and 517.5, 633, and 825 ng/ml respectively for postmenopausal women.
P for interactions was 0.40.
A conditional logistic regression model was used whereby controls were matched to the index case by age, menopausal status at baseline, date of sample collection, time of sample collection, time interval after the last meal, and recent antibiotic use and additionally adjusted for age at 1st live birth (continuous).
As shown in Figure 1, plasma CoQ10 levels were highly positively correlated with both plasma γT (r = 0.50; p < 0.0001) and αT (r = 0.38; p < 0.0001) levels. Circulating γT and αT levels were not correlated with one another. The distribution of values for plasma CoQ10 for the women analyzed in the SWHS is shown in Figure 2. Comparison data from a similar study of postmenopausal women in the MEC (12) are plotted for comparison. Significantly greater CoQ10 levels (approximately 60% higher) were observed in the MEC samples compared to the SWHS (means ± SD were 1,007 ± 387 and 631 ± 254 ng/ml, respectively, p < 0.00001). Comparing only post menopausal women, the median CoQ10 level in the MEC samples was 934 ng/ml compared to 633 ng/ml in the SWHS. In contrast, γT levels in women from the SWHS (median = 1.95 µg/ml) were nearly twice those observed for women in the MEC, where a median value of 1.07 µg/ml was reported (12).
Figure 1.
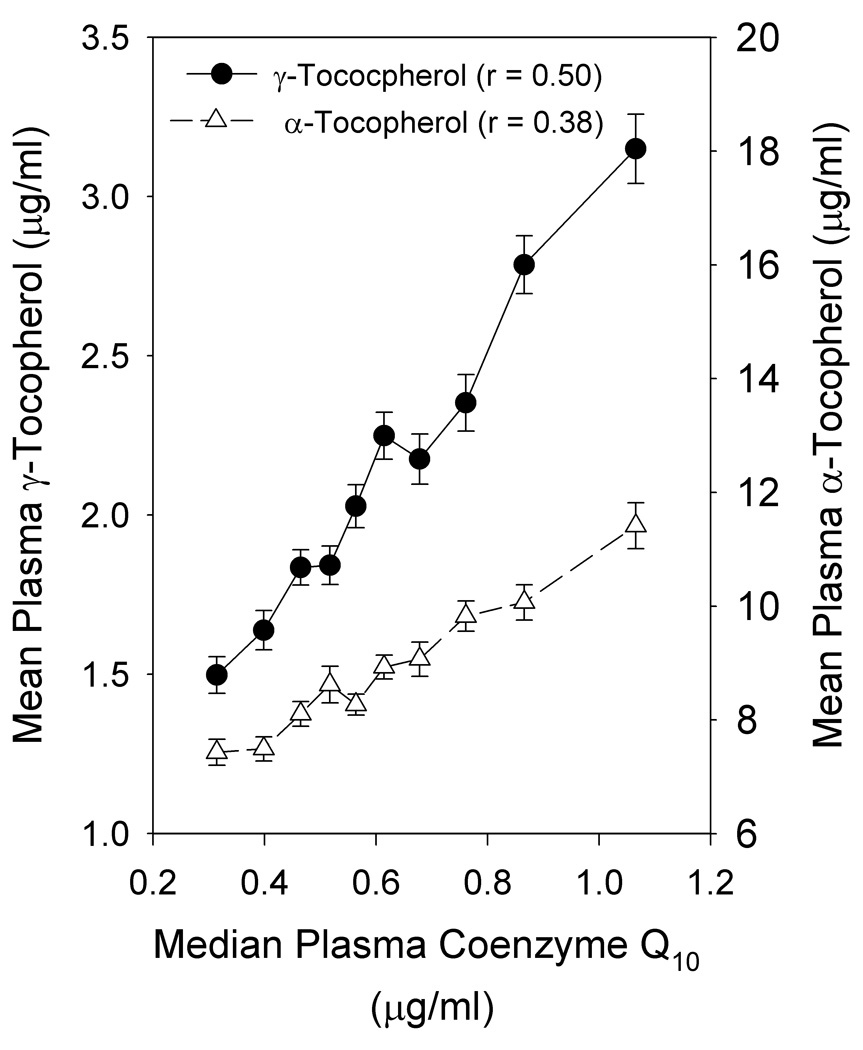
Association of CoQ10 with tocopherols in plasma. All subjects (n=1,113) were stratified by plasma CoQ10 into deciles and α- and γ-tocopherol (mean ± SEM) plotted as a function of the median CoQ10 level for each decile. Correlation coefficients were calculated for the association of each tocopherol with CoQ10.
Figure 2.
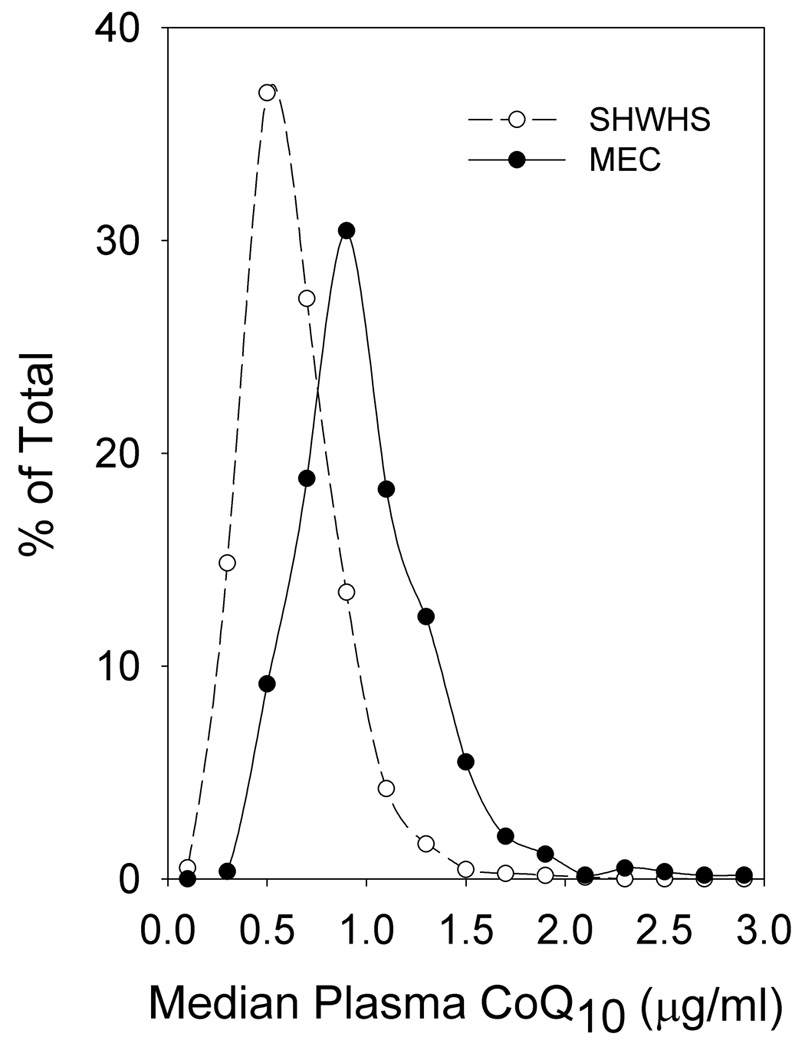
Distribution of CoQ10 levels found in cases and controls of the Shanghai Cohort: comparison with postmenopausal women from the Multiethnic Cohort study of CoQ10 and breast cancer (12). The number of women with CoQ10 values were determined for each 0.2 µg/ml increase in plasma CoQ10 level and plotted as a percentage of the total number of women analyzed in the SWHS. For comparison, the distribution of plasma CoQ10 levels in women analyzed for a study of CoQ10 in the Multiethnic Cohort (12) are also shown.
Discussion
In the SWHS we observed a significant inverse association for low circulating CoQ10 with subsequent incidence of breast cancer for women whose breast cancer was diagnosed > one year after obtaining blood specimens with the highest risk associated with women in the lowest quintile of circulating CoQ10. The results are consistent with previous reports of associations of low CoQ10 with increased risk for various cancers and their progression (9–11). However, a recent prospective study of postmenopausal women utilizing the MEC found a significant positive association between plasma CoQ10 and risk of breast cancer risk (12). That study (MEC) utilizing the same analytical laboratory as the current study found overall significantly higher levels of circulating CoQ10 in a multiethnic American population compared to the current SWHS study (Figure 2). The median CoQ10 for the reference tertile in the MEC study (668 ng/ml) was similar to the values for the SWHS cohort (536–629 ng/ml) where minimal risk was also observed. Significantly increased risk for breast cancer was observed for the MEC study at CoQ10 levels >1,000 ng/ml, a level found in very few women in the SWHS. A possible explanation reconciling these opposing results is that women at either extreme of CoQ10 may be at increased risk for breast cancer. The Shanghai cohort encompasses the low end of what may be a U-shaped curve for CoQ10 and the MEC study (12) captures the high end (Figure 2). Both prospective studies appear consistent in that women with circulating CoQ10 levels in the range of 500–800 ng/ml have the lowest risk for developing breast cancer. It is unlikely that differences in sample collection or handling would account for any differences in CoQ10 levels between these two populations as all CoQ10 was oxidized to the stable quinone prior to analysis and measured as total CoQ10 by the same method and laboratory.
Because cells are capable of synthesizing CoQ10 endogenously, the question arises as to the source and physiological meaning of circulating CoQ10. While the source and physiologic determinants of CoQ10 in the blood are unknown, the close relationship between CoQ10 and circulating tocopherols may provide some insight. The tocopherols were found to be highly associated with circulating CoQ10 levels, suggesting either a causal relationship or a common regulatory mechanism. The mechanism of regulation of circulating tocopherol levels is also unknown, however, tocopherols, particularly γT, are known to rise in response to inflammation (30, 31). The strong association between circulating CoQ10 and tocopherols suggests that CoQ10 level in the blood may also be mediated by systemic and/or localized inflammation (32). Increased release and/or retention of CoQ10 into the circulatory system may, like γT, be a response to processes such as inflammation, apoptosis, and cellular necrosis. Low circulating CoQ10 levels may represent inadequate cellular levels, low inflammation, enhanced excretion, and/or inadequate immune function. The immune system can participate in cancer etiology in two opposing manners (33, 34). Chronic inflammation with an overactive immune system can result in cellular DNA damage and the development of tumors over time, while an inadequate immune response can lead to decreased immune surveillance and allow tumors to progress and metastasize.
The SWHS population appears to be quite unique (Table 1) with few participants who were ever smokers (1.5% for cases, 2.9% for controls), ever drinkers (2.1% for cases, 2.9% for controls), and current hormone therapy use (3.8% for cases vs 1.4% for controls), indicating that the population is quite unique relative to Western societies, thus limiting comparisons with the results of Chai, et al. where considerably higher smoking, alcohol and HRT use were reported (12). Differences in diet and supplement use may account for the stronger association observed between γT and CoQ10 in the SWHS. Unlike studies in U.S. populations, where αT supplementation is more prevalent, no inverse association was observed between circulating γT and αT in women of the SWHS, which may account for the stronger association observed for both tocopherols with CoQ10. In the study by Chai, et al. (12) the positive association between CoQ10 and breast cancer risk was strongest in women with low γT levels. In contrast, women in the SWHS were found to have generally higher γT levels and lower CoQ10 values (median γ-tocopherol of 1.95 µg/ml in the SWHS vs 1.07 µg/ml for the MEC women, 12). As was the case for CoQ10, all tocopherols were measured in the same laboratory and the lower levels of γT observed in the MEC are likely related to αT supplementation which significantly lowers γT, but does not affect CoQ10.
In conclusion, the current SWHS study, with relatively larger sample size and longer follow-up time suggests an inverse association for plasma CoQ10 levels with breast cancer risk in Chinese women. The opposing relationships observed in the two prospective studies (SWHS vs the MEC), requires further research to verify the hypothesis that extreme levels of CoQ10 in the plasma are indicators of risk. Additional study into the physiologic significance and regulation of plasma CoQ10 and its relationship to tocopherols is needed. The present study does not address the role, if any, of supplemental CoQ10 in the prevention and treatment of cancer. Future intervention studies that can assess the physiological effects of supplementation will be necessary to identify the likely cause and effect relationships and determine the possible therapeutic benefits or potential harm of supplementation of CoQ10.
Acknowledgments
Grant support: This work was supported by NIH Grants CA132149 (RVC), CA106591 (QD), CA90956 (WC), CA71789 (AAF), and CA70867 (WZ). Sample preparations were performed at the Survey and Biospecimen Core, which is supported in part by the Vanderbilt-Ingram Cancer Center (P30 CA68485).
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Literature Cited
- 1.Crane FL, Hatefi Y, Lester R, Widmer C. Isolation of a quinone from beef heart mitochondria. Biochim Biophys Acta. 1957;25:220–221. doi: 10.1016/0006-3002(57)90457-2. [DOI] [PubMed] [Google Scholar]
- 2.Turunen M, Olsson J, Dallner G. Metabolism and function of coenzyme Q. Biochim Biophys Acta. 2004;1660:171–199. doi: 10.1016/j.bbamem.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 3.Dallner G, Sindelar PJ. Regulation of ubiquinone metabolism. Free Radic Biol Med. 2000;29:285–294. doi: 10.1016/s0891-5849(00)00307-5. [DOI] [PubMed] [Google Scholar]
- 4.Tran UPC, Clarke CF. Endogenous synthesis of coenzyme Q in eukaryotes. Mitochondrion. 2007;7S:S62–S71. doi: 10.1016/j.mito.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glatthaar-Saalmuller B, Fallier-Becker P, Weiser M. Influence of homeopathically processed coenzyme Q10 on proliferation and redifferentiation of endothelial cells. Forsch Komplementarmed Klass Naturheilkd. 2004;11:267–273. doi: 10.1159/000082031. [DOI] [PubMed] [Google Scholar]
- 6.Lopez-Lluch G, Barasso MP, Martin SF, Fernandez-Ayala DLM, Gomez-Diaz C, Villalba JM, Navas P. Role of plasma membrane coenzyme Q10 on regulation of apoptosis. Biofactors. 1999;9:171–177. doi: 10.1002/biof.5520090212. [DOI] [PubMed] [Google Scholar]
- 7.Sachdanandam P. Antiangiogenic and hypolipidemic activity of coenzyme Q10 supplementation to breast cancer patients undergoing Tamoxifen therapy. Biofactors. 2008;32:151–159. doi: 10.1002/biof.5520320118. [DOI] [PubMed] [Google Scholar]
- 8.Bliznakov EG. Effect of stimulation of the host defense system by coenzyme Q10 on dibenzpyrene-induced tumours and infection with Friend leukemia virus in mice. Proc Natl Acad Sci. 1973;70:390–394. doi: 10.1073/pnas.70.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Folkers K, Osterborg A, Nylander M, Morita M, Mellstedt H. Activities of vitamin Q10 in animal models and a serious deficiency in patients with cancer. Biochem Biophys Res Commun. 1997;234:296–299. doi: 10.1006/bbrc.1997.6522. [DOI] [PubMed] [Google Scholar]
- 10.Palan PR, Mikhail MS, Shaban DW, Romney SL. Plasma concentrations of coenzyme Q10 and tocopherols in cervical intraepithelial neoplasia and cervical cancer. Eur J Cancer Prev. 2003;12:321–326. doi: 10.1097/00008469-200308000-00013. [DOI] [PubMed] [Google Scholar]
- 11.Rusciani L, Proietti I, Rusciani A, Paradisi A, Sbordoni G, Alfano C, Panunzi S, De Gaetano A, Lippa S. Low plasma coenzyme Q10 levels as an independent prognostic factor for melanoma progression. J Am Acad Dermatol. 2006;54:234–241. doi: 10.1016/j.jaad.2005.08.031. [DOI] [PubMed] [Google Scholar]
- 12.Chai W, Cooney RV, Franke AA, Shvetsov YB, Caberto CP, Wilkens LR, et al. Plasma Coenzyme Q10 levels and Postmenopausal Breast Cancer Risk: The Multiethnic Cohort Study. Cancer Epidemol Biomarkers Prev. 2010;19:2351–2356. doi: 10.1158/1055-9965.EPI-10-0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burke BE, Neuenschwander R, Olson RD. Randomized, double-blind, placebo-controlled trial of coenzyme Q10 in isolated systolic hypertension. South Med J. 2001;94:1112–1117. doi: 10.1097/00007611-200111000-00015. [DOI] [PubMed] [Google Scholar]
- 14.Sander S, Coleman CI, Patel AA, Kluger J, White CM. The impact of coenzyme Q10 on systolic function in patients with chronic heart failure. J. Cardiac Failure. 2006;12:464–472. doi: 10.1016/j.cardfail.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Sandor PS, Di Clemente L, Coppola G, Saenger U, Fumal A, Magis D, et al. Efficacy of coenzyme Q10 in migraine prophylaxis: a randomized controlled trial. Neurol. 2005;64:713–715. doi: 10.1212/01.WNL.0000151975.03598.ED. [DOI] [PubMed] [Google Scholar]
- 16.Silver MA, Langsjoen PH, Szabo S, Patil H, Zelinger A. Effect of atorvastatin on left ventricular diastolic function and ability of coenzyme Q10 to reverse that dysfunction. Am J Cardiol. 2004;94:306–310. doi: 10.1016/j.amjcard.2004.07.121. [DOI] [PubMed] [Google Scholar]
- 17.Boudreau DM, Yu O, Miglioretti DL, Buist DS, Heckbert SR, Daling JR. Statin use and breast cancer risk in a large population-based setting. Cancer Epidemiol Biomarkers Prev. 2007;16:416–421. doi: 10.1158/1055-9965.EPI-06-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lockwood K, Moesgaard S, Yamamoto T, Folkers K. Progress on therapy of breast cancer with vitamin Q10 and the regression of metastases. Biochem Biophys Res Comm. 1995;212:172–177. doi: 10.1006/bbrc.1995.1952. [DOI] [PubMed] [Google Scholar]
- 19.Premkumar VG, Yuvarag S, Vijayasarathy K, Gangadaran SGD, Sachdandam P. Effect of coenzyme Q10, riboflavin and niacin on serum CEA and CA15-3 levels in breast cancer patients undergoing tamoxifen therapy. Biol Pharm Bull. 2007;30:367–370. doi: 10.1248/bpb.30.367. [DOI] [PubMed] [Google Scholar]
- 20.Folkers K, Brown R, Judy WV, Morita M. Survival of cancer patients on therapy with coenzyme Q10. Biochem Biophys Res Commun. 1993;192:241–245. doi: 10.1006/bbrc.1993.1405. [DOI] [PubMed] [Google Scholar]
- 21.Navas P, Villalba JM, de Cabo R. The importance of plasma membrane coenzyme Q10 in aging and stress responses. Mitochondrion. 2007;7S:S34–S40. doi: 10.1016/j.mito.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 22.Miles MV, Horn PS, Tang PH, Morrison JA, Miles L, DeGrauw T, Pesce AJ. Age-related changes in plasma coenzyme Q10 concentrations and redox state in apparently healthy children and adults. Clin Chim Acta. 2004;347:139–144. doi: 10.1016/j.cccn.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 23.Palan PR, Connell K, Ramirez E, Inegbenijie C, Gavara RY, Ouseph JA, Mikhail MS. Effects of menopause and hormone replacement therapy on serum levels of coenzyme Q10 and other lipid-soluble antioxidants. Biofactors. 2005;25:61–66. doi: 10.1002/biof.5520250107. [DOI] [PubMed] [Google Scholar]
- 24.Lass A, Forster MJ, Sohal RS. Effects of coenzyme Q10 and α-tocopherol administration on their tissue levels in the mouse: Elevation of mitochondrial α-tocopherol by coenzyme Q10. Free Radical Biol Med. 1999;26:1375–1382. doi: 10.1016/s0891-5849(98)00330-x. [DOI] [PubMed] [Google Scholar]
- 25.Singh RB, Neki NS, Kartikey K, Pella D, Kumar A, Niaz MA, Thakur AS. Effect of coenzyme Q10 on risk of atherosclerosis in patients with recent myocardial infarction. Mol Cell Biochem. 2003;246:75–82. [PubMed] [Google Scholar]
- 26.Zheng W, Chow WH, Yang G, Jin F, Rothman N, Blair A, et al. The Shanghai Women’s Health Study; rationale, study design, and baseline characteristics. Am J Epidemiol. 2005;162:1123–1131. doi: 10.1093/aje/kwi322. [DOI] [PubMed] [Google Scholar]
- 27.Franke AA, Morrison CM, Bakke JL, Custer LJ, Li X, Cooney RV. Coenzyme Q10 in human blood: native levels and determinants of oxidation during processing and storage. Free Radic Biol Med. 2010;48:1610–1617. doi: 10.1016/j.freeradbiomed.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franke AA, Custer LJ, Cooney RV. Synthetic carotenoids as internal standards for plasma micronutrient analysis by high performance liquid chromatography. J Chromatog, B. 1993;614:43–57. doi: 10.1016/0378-4347(93)80222-p. [DOI] [PubMed] [Google Scholar]
- 29.Dorjgochoo T, Gao YT, Chow WH, Shu XO, Li H, Yang G, et al. Plasma carotenoids, tocopherols, retinol and breast cancer risk: results from the Shanghai Women Health Study (SWHS) Breast Cancer Res Treat. 2009;117(2):381–389. doi: 10.1007/s10549-008-0270-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanaka Y, Lesoon Wood LA, Cooney RV. Enhancement of intracellular γ-tocopherol levels in cytokine-stimulated C3H 10T1/2 fibroblasts: relation to NO synthesis, isoprostane formation and tocopherol oxidation. BMC Chemical Biology. 2007;7:2–13. doi: 10.1186/1472-6769-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang Q, Lykkesfeldt J, Shigenaga MK, Shigeno ET, Christen S, Ames BN. Gamma-tocopherol supplementation inhibits protein nitration and ascorbate oxidation in rats with inflammation. Free Radic Biol Med. 2002;33:1534–1542. doi: 10.1016/s0891-5849(02)01091-2. [DOI] [PubMed] [Google Scholar]
- 32.Menke T, Niklowitz P, Wiesel T, Andler W. Antioxidant level and redox status of coenzyme Q10 in the plasma and blood cells of children with diabetes mellitus type 1. Pediatric Diabetes. 2008;9:540–545. doi: 10.1111/j.1399-5448.2008.00389.x. [DOI] [PubMed] [Google Scholar]
- 33.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nature Reviews Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 34.Hussain SP, Harris CC. Inflammation and cancer: An ancient link with novel potentials. Int J Cancer. 2007;121:2373–2380. doi: 10.1002/ijc.23173. [DOI] [PubMed] [Google Scholar]


