Abstract
Background: In low-birth-weight girls, obesity increases the risk of premature adrenarche and metabolic complications. However, the consistency of this association in normal-birth-weight children and its potential mediators remain unknown.
Objectives: The objectives were to assess the associations between obesity indicators and dehydroepiandrosterone sulfate (DHEAS) at 7 y of age and to evaluate the role of hormonal markers on these associations.
Design: We assessed in 969 participants (6.9 y; 48% girls; all Tanner I) in the Growth and Obesity Chilean Cohort Study the associations between DHEAS and weight, BMI, waist circumference (WC), waist-to-height ratio, skinfold thickness, and percentage total fat (bioimpedance) and determined whether these associations were related to insulin, insulin-like growth factor I (IGF-I), and leptin. We also compared BMI and height growth from 0 to 7 y of age in nonobese and obese children with normal and high DHEAS (≥75th percentile) at 7 y.
Results: DHEAS concentrations were similar between girls (30.3 ±1.86 μg/dL) and boys (29.4 ±1.73 μg/dL) (P > 0.05); 17.3% of children were obese (BMI-for-age z score ≥2 SD). Adiposity indicators were positively and similarly associated with DHEAS [ie, BMI, β standardized regression coefficient: 0.23 (95% CI: 0.17, 0.29); WC, β standardized regression coefficient: 0.23 (95% CI: 0.16, 0.30)]; these associations were only partially related to IGF-I and leptin. Obese children had twice the risk of high DHEAS (OR: 2.16; 95% CI: 1.51, 3.09); at 7 y, obese children with high DHEAS were fatter and more centrally obese than their counterparts (P < 0.05), although their previous growth was similar (P > 0.05). None of the results differed by sex (P > 0.05).
Conclusion: In children of normal birth weight, obesity is positively associated with DHEAS at 7 y of age.
INTRODUCTION
Adrenarche is a maturational event resulting from the activation of the zona reticularis of the adrenal gland, which leads to an increase in the production of the adrenal androgens dehydroepiandrosterone and dehydroepiandrosterone sulfate (DHEAS)4 (1). It is identified by an increase in DHEAS production after the age of 5 y (1–4). Premature adrenarche (PA) is recognized when signs of androgen action (eg, pubic hair, adult-type body odor, and seborrhea) appear prematurely (before 8 y in girls, before 9 y in boys) and are accompanied by elevated serum DHEAS concentrations traditionally >40 μg/dL.
Emerging evidence links PA in girls to an increased risk of developing the metabolic syndrome. Areas of controversy include the higher risk of polycystic ovary syndrome in girls with PA and whether low birth weight increases the risk of developing PA (5–8). Prepubertal obesity has also been associated with insulin resistance, altered lipid and glucose metabolism (9), and increased adrenal androgen production (10), which raises the possibility that PA may be a contributing factor to the development of obesity-related metabolic complications (11, 12).
However, the relation between obesity and DHEAS has been described mainly in girls born with low birth weight (13, 14), and most obese children have a normal or an increased birth weight (15). In the general population, some studies have failed to show an association between adiposity and DHEAS (16), whereas others have shown an association (10, 17, 18). Importantly, the mechanisms that might explain this association have not been elucidated. Insulin and insulin-like growth factor I (IGF-I) have been suggested to play a role in girls but not in boys (19, 20). Leptin has also been implicated, but, overall, the potential mediators of this association remain largely unknown (1). A better understanding of the associations between obesity and adrenal maturation may contribute to an understanding of the pathways that link childhood obesity to metabolic complications. Thus, our aim was to assess the associations between serum DHEAS and obesity indicators, growth, and potential hormonal mediators at 7 y of age in a well-characterized cohort of Chilean children.
SUBJECTS AND METHODS
Our study sample was drawn from children enrolled in the Growth and Obesity Chilean Cohort Study, which assesses the association of early growth and development of adiposity and metabolic risk (21, 22). Children were eligible for the study if they were 3.0–4.9 y of age and attending Junta Nacional de Jardines Infantiles nursery schools from the south area of Santiago, Chile, in September 2006; were singletons; had a gestational age of 37–42 wk; had a birth weight ≥2500 g (data retrieved from medical registries); and had no physical or psychological conditions that could severely affect growth. Almost 85% of the total eligible population agreed to participate (n = 1196), and no significant differences in age, sex, birth, or anthropometric measures were observed at 4 y between participants and nonparticipants. Thereafter, annual evaluations have been carried out. In 2009, 1044 children of the original cohort were evaluated (∼87%). For the current analyses, we excluded 36 girls who had breast buds (breast Tanner II), 37 children for whom we were unable to obtain a blood sample, and 2 children with implausible DHEAS concentrations; thus, our final sample size was 969 children. Assuming 80% power and a 2-tailed significance level of 0.05, we were able to detect small effect sizes (23). The study protocol was approved by the Institutional Review Board of the Institute of Nutrition and Food Technology of the University of Chile. Informed consent was obtained from all parents or guardians of the children.
BMI and linear growth from 0 to 7 y of age
From 0 to 3 y, weight and height (recumbent length in children <2 y of age) data were abstracted from health records. The validity of these data were verified (21). Thereafter, a dietitian measured weight, height, and waist circumference (WC) annually by using standardized procedures. At 7 y of age, a single dietitian carried out all of the measurements. Weight was measured with a TANITA BC-418 device with a precision of 0.1 kg, height was measured with a wall-mounted Harpenden stadiometer (Holtain) to the nearest 0.1 cm, and WC (ie, minimum circumference between the iliac crest and the rib cage) was measured with a metal inextensible tape (model W606PM; Lufkin) to the closest 0.1 cm. The intraobserver technical error of measurement and the mean average bias of the observer were within the limits suggested by WHO in the growth reference study (24).
Body composition at 7 y
A single specially trained registered dietitian measured triceps, biceps, subscapular, suprailiac, and abdominal skinfold thicknesses using standardized procedures. Skinfold thicknesses were measured in triplicate on the right side of the body with a Lange caliper to the nearest 0.5 mm; the mean value was used in the analyses. Bioimpedance analysis (BIA) measurements were collected by a single dietitian using a TANITA Segmental Body Composition Analyzer (model BC-418).
Pubertal development at 7 y
A single pediatric endocrinologist (VM) assessed breast and genital development by palpation and classified breast and testes according to the Tanner stages (25).
Bone age at 7 y
Bone age measurements were obtained from the left hand by using an ultrasound method (BonAge; Sunlight Co) (26, 27). This method is based on the differential transmission speed of ultrasound through cartilage and bone, and its use for research purposes has been validated (28). Measurements were obtained in duplicate and averaged to obtain a final bone age.
Blood sample at 7 y
A trained nurse collected a 25-mL fasting venous blood sample from the children at arrival to the nursery school. Mothers were contacted on the day before the sample was drawn to confirm the absence of fever (>37.5°C) or symptoms of acute infection in the children and to advise them not to provide foods or liquids to their children before arriving at the nursery the next day. These conditions were rechecked by the nurse at the time of the blood collection, and exams were rescheduled if the conditions were not met. The analyses were conducted at the Nutritional Laboratory of the Catholic University of Chile and Institute of Maternal and Child Research University of Chile. Serum dehydroepiandrosterone was measured by competitive specific binding radioimmunoassay supplied by Diagnostic System Laboratories; intra- and interassay CVs were 3.5% and 5.1%, respectively. Serum IGF-I was measured by using a standardized locally developed radioimmunoassay requiring sample extraction as a first step (sensitivity: 5 ng/mL; intra- and interassay CVs: 8.6% and 10.2%, respectively) (29). Serum glucose was measured by using enzymatic colorimetric techniques (HUMAN; Gesellschaft für Biochemica und Diagnostica), and serum insulin was measured with a commercial radioimmunoassay (Siemens Medical Solutions Diagnostics). The HOMA-IR was calculated as fasting glucose (mmol/L) × fasting insulin (μU/mL)/22.5. Serum leptin was measured by commercial radioimmunoassay (Millipore).
Computed indexes
Anthropometric and body-composition indicators
We divided weight (kg) by height squared (m2) to calculate BMI. We estimated weight-for-age, height-for-age, and BMI-for age (BAZ) z scores based on the WHO 2007 growth reference (30). We defined obesity as BAZ ≥2 SD and overweight as BAZ ≥1 SD. Central obesity was defined as WC ≥ NHANES III >75th percentile (girls: 63.0 cm; boys: 63.4 cm), because the use of WC 90th percentile gave a virtually identical categorization for general and central obesity (31). The waist-to-height ratio was based on the waist perimeter divided by height. Triceps and subscapular skinfold thicknesses were used to estimate body fat according to the Slaughter equation (32). Body fat (kg) estimated from skinfold thicknesses, by weight (kg) and height squared (m2), were used to calculate percentage fat (%fat = fat/weight) and fat mass index (FMI = fat/height2), respectively. Body fat estimated from BIA was also divided by height squared to calculate FMI (FMI BIA). We considered indicators of total adiposity: weight, BMI, %fat, FMI, and FMI BIA and WC and waist-to-height measurements as markers of central adiposity. IGF-I z scores were estimated based on a Chilean reference.
Maturation indicators
Measured bone age (BA) divided by chronologic age (CA) was used to assess standardized bone age (BA/CA). A high DHEAS concentration was defined based on sample distribution (75th percentile); the cutoffs were 42.0 μg/dL for girls and 45.1 μg/dL for boys (Table 1).
TABLE 1.
DHEAS distribution in 969 prepubertal (Tanner stage I) Chilean school-age (7 y) children, by sex1
| DHEAS (μg/dL) |
|||
| Percentile | Girls (n = 464) | Boys (n = 505) | All (n = 969) |
| 10th | 14.8 | 13.5 | 14.5 |
| 25th | 20.2 | 19.4 | 20.1 |
| 50th | 30.1 | 30.7 | 30.9 |
| 75th | 42.0 | 45.1 | 44.9 |
| 90th | 61.7 | 68.5 | 64.2 |
DHEAS, dehydroepiandrosterone sulfate.
Statistical analyses
We present the data as either means (or geometric means) and SDs (SDs = z score) or frequencies and 95% CIs; nonnormal distributions were log transformed. We tested differences by sex using Student's t test for continuous variables or chi-square and Fisher tests for dichotomous variables. Linear regression models using standardized coefficients were used to compare associations between the different adiposity indicators and DHEAS, and logistic regression models were used to assess the role of adiposity indicators as predictors of adrenarche. Generalized linear models served to assess 1) the association of IGF-I, insulin, and leptin and high DHEAS at 7 y and 2) differences in growth and adiposity and metabolic status at 7 y in nonobese and obese children with and without high DHEAS at 7 y. All analyses were adjusted by age. Interactions by sex were all nonsignificant; thus, the results are presented for both sexes combined. The associations were considered significant if P < 0.05. The analyses were conducted by using SAS (version 9.1; SAS Institute).
RESULTS
DHEAS distribution by sex is presented in Table 1. DHEAS concentrations were similar between girls and boys (P > 0.05). Adiposity, metabolic, and hormonal characteristics of the participants are presented in Table 2. A total of 969 children (∼50% girls) provided information. The mean age was ∼7 y; bone age was slightly advanced (BA/CA: 1.12). On average, children had close to +1 SDs BMI (BAZ) relative to the WHO reference, and 40% of the children had excess weight (BMI SDs >1) with no sex differences (P > 0.05). BMI measurements suggested that obesity (BAZ >2) was more prevalent in boys (21.2 compared with 13.1%; P < 0.05); however, estimations made with the use of more direct measurements of adiposity, such as skinfold thickness (%fat or FMI) or BIA (FMI BIA), showed the opposite (both P < 0.05). In both sexes, WC was close to 59.0 cm, waist-to-height ratio was on average <0.5 (0.49 ± 0.05), and ∼20% of children had central obesity (waist >75th percentile). Sixteen percent (n = 28) of the obese children (n = 168) did not have central obesity, whereas 24% (n = 42) of the children meeting the criteria for central obesity (n = 178) were not obese (data not shown). Mean IGF-I concentrations were higher in girls than in boys (175.2 ± 42.7 compared with 161 ± 42.9 ng/mL), but comparisons with a reference population (SDs) showed that concentrations were higher in boys than in girls. The boys had higher glucose concentrations, but insulin, HOMA-IR, and leptin did not differ by sex (P > 0.05).
TABLE 2.
Anthropometric, metabolic, and hormonal indicators of 969 prepubertal Chilean school-age children, by sex1
| Boys (n = 505) | Girls (n = 464) | P2 | |
| Age (mo) | 82.6 ± 5.43 | 81.9 ± 5.2 | 0.16 |
| Bone age/chronologic age | 1.13 ± 0.13 | 1.12 ± 0.15 | 0.001 |
| Height (cm) | 121.2 ± 5.51 | 120.2 ± 5.20 | 0.02 |
| Height-for-age z score | 0.04 ± 0.93 | 0.06 ± 0.90 | 0.29 |
| Adiposity indicators | |||
| Weight (kg) | 25.5 ± 4.88 | 24.8 ± 4.50 | 0.33 |
| Weight-for-age z score | 0.69 ± 1.2 | 0.63 ± 1.01 | 0.96 |
| BMI (kg/m2) | 17.2 ± 2.39 | 17.1 ± 2.26 | 0.87 |
| BAZ | 0.93 ± 1.25 | 0.79 ± 1.02 | 0.21 |
| BAZ ≥1 [% (n)] | 44.3 (223) | 39.7 (185) | 0.12 |
| BAZ ≥2 [% (n)] | 21.2 (107) | 13.1 (61) | 0.001 |
| Percentage fat (%)4 | 15.0 ± 5.1 | 16.6 ± 4.4 | <0.0001 |
| Fat mass index (kg/m2)4 | 2.69 ± 1.33 | 2.92 ± 1.17 | <0.0001 |
| Fat mass index, BIA (kg/m2) | 3.98 ± 1.38 | 4.38 ± 1.33 | <0.0001 |
| WC (cm) | 59.0 ± 6.66 | 58.6 ± 6.30 | 0.98 |
| WC ≥75th percentile [% (n)]5 | 18.1 (91) | 18.7 (87) | 0.49 |
| Waist-height ratio | 0.49 ± 0.05 | 0.49 ± 0.04 | 0.28 |
| Hormonal and metabolic markers | |||
| DHEAS (μg/dL)6 | 30.3 ± 1.9 | 29.4 ± 1.73 | 0.92 |
| Glucose (mg/dL) | 90.3 ± 6.3 | 88.8 ± 6.05 | 0.001 |
| Insulin (μg/dL)6 | 5.31 ± 1.19 | 5.43 ± 1.22 | 0.07 |
| HOMA-IR6 | 1.19 ± 1.22 | 1.20 ± 1.24 | 0.57 |
| IGF-I (ng/mL) | 161.0 ± 42.9 | 175.2 ± 42.7 | <0.0001 |
| IGF-I (z score) | −0.13 ± 0.69 | −0.50 ± 0.72 | <0.0001 |
| Leptin (ng/mL)6 | 4.81 ± 1.75 | 4.91 ± 1.80 | 0.30 |
Anthropometric z scores are based on WHO 2007. BAZ, BMI-for-age z score; BIA, bioimpedance analysis (by TANITA BC-418); DHEAS, dehydroepiandrosterone sulfate; IGF-I, insulin-like growth factor I; WC, waist circumference.
Differences between the sexes were estimated by using a t test and chi-square tests.
Mean ± SD (all such values).
Based on skinfold thicknesses determined by using the Slaughter equation (32).
NHANES III percentiles: Fernandez (75th percentile = 63.0 cm for girls and 63.4 cm for boys) (31).
Variables not normally distributed were log transformed.
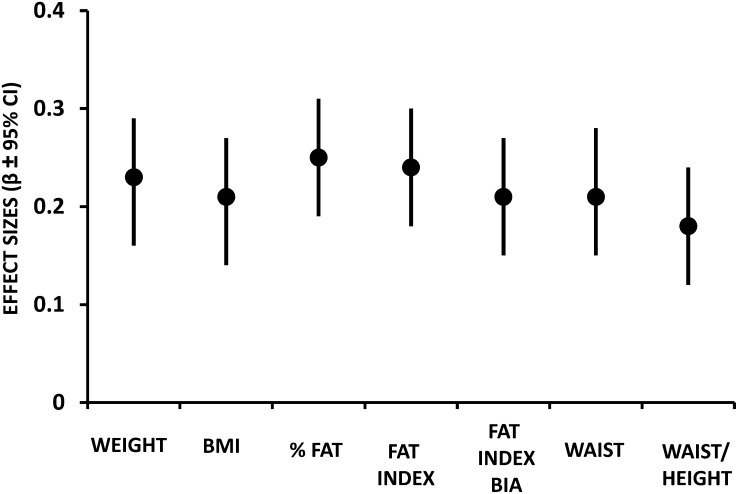
The standardized regression coefficients (and 95% CIs) for DHEAS concentrations at 7 y of age, per sample-specific 1-SD increments in adiposity indicators at 7 y, are shown in Figure 1. All total and central adiposity indicators related similarly to DHEAS concentrations [eg, WC, β standardized regression coefficient: 0.23 (95% CI: 0.16, 0.30); waist-to-height ratio, β standardized regression coefficient: 0.19 (95% CI: 0.13, 0.26)]; overall, the effect sizes were small (all <0.3).
FIGURE 1.
Standardized regression coefficients (and 95% CIs) for DHEAS concentrations at 7 y of age per sample-specific 1-SD increments in adiposity indicators at 7 y in 969 prepubertal Chilean children. Multiple linear models adjusted by age and sex. Fat index = fat mass based on skinfold thickness/height2; fat mass index BIA = fat mass based on bioimpedance/height2; waist/height = waist-to-height ratio. Each of the following values corresponds to the standard deviation: DHEAS: 1.83; weight: 4.71; BMI: 2.33; %fat: 4.82; fat mass index: 1.26; fat mass index BIA: 1.33; waist: 6.49; waist-to-height ratio: 0.05. BIA, bioimpedance analysis; DHEAS, dehydroepiandrosterone sulfate.
The associations of obesity, metabolic, and hormonal indicators with high DHEAS at 7 y are shown in Table 3. Obese children had twice the risk of high DHEAS (OR: 2.16; 95% CI: 1.51, 3.09). IGF-I SDs and leptin were also associated with high DHEAS [OR: 1.35 (95% CI: 1.16, 1.57) and 1.04 (95% CI: 1.01, 1.08), respectively], but only the effect of IGF-I (OR: 1.30; 95% CI: 1.11, 1.52) and not of leptin (OR: 1.01; 95% CI: 0.97, 1.05) was independent of obesity. Insulin was not associated with high DHEAS at 7 y (P > 0.05). Obesity remained associated with high DHEAS (OR: 2.05; 95% CI: 1.36, 3.09), even after all of the studied metabolic indicators were accounted for.
TABLE 3.
Association of adiposity and hormonal and metabolic markers and high DHEAS at 7 y of age in 969 prepubertal Chilean children1
| OR | 95% CI | |
| BAZ ≥2 | ||
| Model 1 | 2.16 | 1.51, 3.09 |
| Model 3 | 2.05 | 1.36, 3.09 |
| Insulin | ||
| Model 1 | 1.02 | 0.93, 1.12 |
| Model 2 | 0.97 | 0.88, 1.07 |
| Model 3 | 0.95 | 0.86, 1.06 |
| IGF-I (z score) | ||
| Model 1 | 1.35 | 1.16, 1.57 |
| Model 2 | 1.30 | 1.11, 1.52 |
| Model 3 | 1.31 | 1.12, 1.53 |
| Leptin | ||
| Model 1 | 1.04 | 1.01, 1.08 |
| Model 2 | 1.01 | 0.97, 1.05 |
| Model 3 | 1.00 | 0.96, 1.05 |
A high DHEAS concentration is defined as ≥42.0 μg/dL in girls and ≥45.1 μg/dL in boys, based on sample distribution. Model 1: age + sex + one anthropometric or hormonal/metabolic marker; model 2: age + sex + BAZ ≥2 + one hormonal/metabolic marker; model 3: age + sex + BAZ ≥2 + all hormonal/metabolic markers. BAZ, BMI-for-age z score; DHEAS, dehydroepiandrosterone sulfate; IGF-I, insulin-like growth factor I.
The interactions between obesity and high DHEAS were not significant for any of the anthropometric, metabolic, or hormonal outcomes; nonetheless, the differences between the nonobese and obese children with normal or elevated DHEAS at 7 y are shown in Table 4 for illustrative purposes only. Obese children with higher DHEAS concentrations at 7 y had more total (%fat: 24.3 compared with 22.8; P < 0.05) and central (WC: 70.3 cm compared with 68.5 cm; waist-to-height ratio: 0.568 compared with 0.558; P < 0.05) adiposity and lower insulin (5.75 compared with 6.11 μg/dL; P < 0.05) and HOMA-IR values (1.30 compared with 1.41; P < 0.05) than did their nonobese counterparts, but no differences in sex distribution, standardized bone age, height, or other metabolic markers were observed (P > 0.05). Nonobese children with elevated DHEAS at 7 y were also generally and centrally fatter (%fat: 2.55 compared with 2.29; WC: 57.6 cm compared with 56.4 cm; P < 0.05) than their nonobese counterparts with normal DHEAS, and they also had more advanced bone age (BA/CA: 1.15 compared with 1.11; P < 0.05) and higher IGF-I concentrations (190.3 ng/mL compared with 174.7 ng/mL; P < 0.05). All variables, except for age, were significantly higher in obese than in nonobese children (P < 0.05).
TABLE 4.
Adiposity, metabolic, and hormonal differences among 801 nonobese and 168 obese Chilean children with normal and high DHEAS at 7 y of age1
| BAZ <2 SDs |
BAZ ≥2 SDs |
|||||
| Normal DHEAS (n = 619) | High DHEAS2 (n = 182) | P3 | Normal DHEAS (n = 103) | High DHEAS2 (n = 65) | P3 | |
| Age (mo) | 81.8 (81.4, 82.2) | 83.7 (83.0, 84.5) | <0.0001 | 81.9 (80.9, 83.0) | 83.1 (81.9, 84.5) | 0.14 |
| Female [% (n)] | 50.0 (309) | 53.3 (97) | 0.44 | 37.9 (39) | 32.3 (21) | 0.39 |
| Bone age | 1.11 (1.10, 1.13) | 1.15 (1.13, 1.17) | 0.004 | 1.21 (1.19, 1.24) | 1.24 (1.21, 1.28) | 0.14 |
| Height (cm) | 120.0 (119.7, 120.4) | 120.8 (120.1, 121.5) | 0.06 | 122.8 (121.9, 123.7) | 123.7 (122.6, 124.9) | 0.22 |
| Height-for-age z score | −0.08 (−0.15, −0.01) | 0.06 (−0.07, 0.19) | 0.06 | 0.44 (0.27, 0.62) | 0.61 (0.40, 0.83) | 0.22 |
| Adiposity indicators | ||||||
| BMI-for-age z score | 0.42 (0.36, 0.48) | 0.67 (0.56, 0.79) | 0.0002 | 2.63 (2.48, 2.78) | 2.84 (2.65, 3.04) | 0.09 |
| Percentage fat (%)4 | 13.9 (13.6, 14.1) | 15.1 (14.7, 15.6) | <0.0001 | 22.8 (22.2, 23.4) | 24.3 (23.5, 25.1) | 0.003 |
| Fat mass index (kg/m2)4 | 2.29 (2.22, 2.35) | 2.55 (2.44, 2.66) | <0.0001 | 4.81 (4.66, 4.96) | 5.25 (5.06, 5.44) | 0.0003 |
| Fat mass index, BIA (kg/m2) | 3.61 (3.54, 3.67) | 3.84 (3.72, 3.96) | <0.0001 | 6.20 (6.03, 6.36) | 6.61 (6.40, 6.81) | 0.002 |
| Waist circumference (cm) | 56.4 (56.0, 56.7) | 57.6 (57.0, 58.2) | 0.0005 | 68.5 (67.7, 69.4) | 70.3 (69.3, 71.4) | 0.008 |
| WC ≥75th percentile [% (n)]5 | 3.2 (20) | 9.8 (18) | 0.0002 | 82.4 (84) | 87.5 (56) | 0.31 |
| Waist-height ratio | 0.469 (0.467, 0.472) | 0.477 (0.473, 0.482) | 0.005 | 0.558 (0.552, 0.564) | 0.568 (0.560, 0.575) | 0.05 |
| Hormonal and metabolic markers | ||||||
| DHEAS (μg/dL)6 | 22.9 (22.2, 23.6) | 60.3 (57.4, 64.1) | <0.0001 | 25.5 (23.6, 27.7) | 63.4 (58.0, 70.1) | <0.0001 |
| Glucose (mg/dL) | 88.9 (88.4, 89.4) | 89.6 (88.7, 90.5) | 0.16 | 92.6 (91.4, 93.8) | 91.3 (89.8, 92.8) | 0.19 |
| Insulin (μg/dL)6 | 5.26 (5.16, 5.31) | 5.31 (5.16, 5.47) | 0.36 | 6.11 (5.93, 6.36) | 5.75 (5.47, 5.99) | 0.03 |
| HOMA-IR6 | 1.15 (1.13, 1.17) | 1.17 (1.14, 1.21) | 0.23 | 1.41 (1.35, 1.46) | 1.30 (1.22, 1.36) | 0.01 |
| IGF-I (ng/mL) | 174.7 (170.5, 179.6) | 190.3 (182.7, 197.9) | 0.001 | 198.8 (188.9, 208.8) | 203.5 (190.4, 216.5) | 0.39 |
| IGF-I (z score) | −0.19 (−0.26, −0.11) | 0.07 (−0.06, 0.20) | 0.002 | 0.18 (0.01, 0.36) | 0.28 (0.05, 0.51) | 0.29 |
| Leptin (ng/mL)6 | 4.4 (4.2, 4.5) | 4.4 (4.1, 4.8) | 0.77 | 8.0 (7.2, 8.9) | 8.5 (7.5, 9,7) | 0.46 |
Values are means; 95% CIs in parentheses. Anthropometric z scores are based on WHO 2007. BAZ, BMI-for-age z score; BIA, bioimpedance analysis (by TANITA BC-418); DHEAS, dehydroepiandrosterone sulfate; IGF-I, insulin-like growth factor I; WC, waist circumference.
Defined as ≥42.0 μg/dL in girls and ≥45.1 μg/dL in boys based on sample distribution.
P values for differences estimated from post hoc comparisons of generalized linear models (means) adjusted for age and sex and chi-square tests (%); interactions between obesity and high DHEAS concentrations were all nonsignificant (P > 0.05).
Based on skinfold thicknesses determined by using the Slaughter equation (32).
NHANES III percentiles: Fernandez (75th percentile = 63.0 cm for girls and 63.4 cm for boys) (31).
Variables not normally distributed were log transformed.
Growth of nonobese and obese children with and without high DHEAS at 7 y
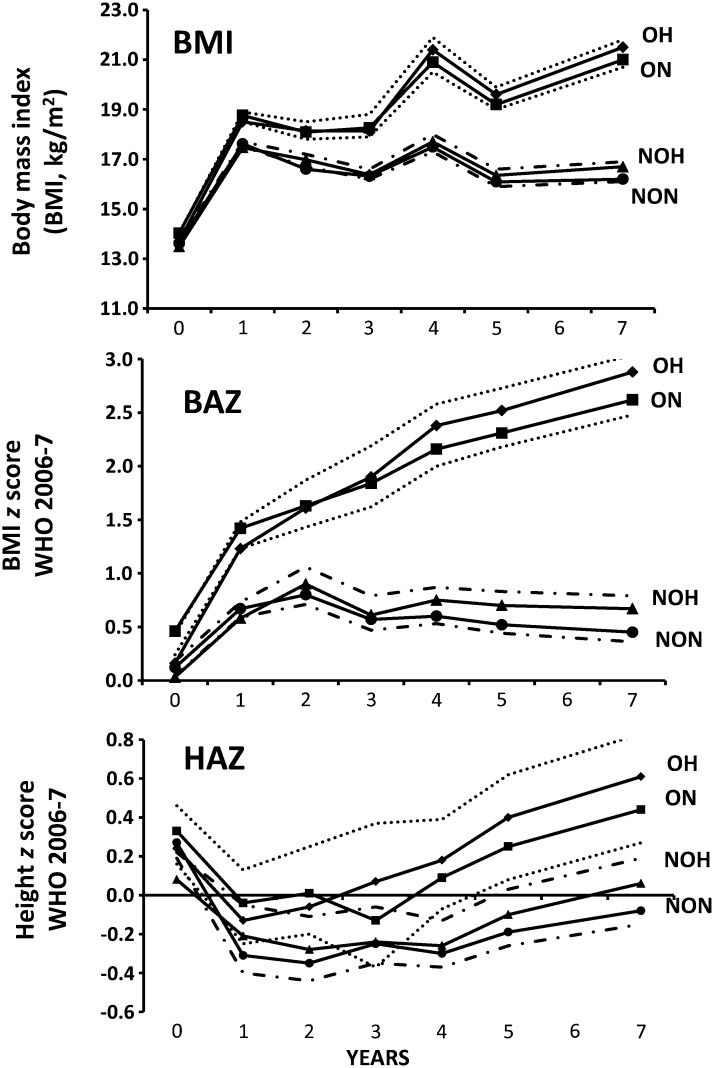
The ponderal and linear growth of nonobese and obese children who had high DHEAS at 7 y compared with those who did not is shown in Figure 2. We observed that children with high DHEAS (either nonobese or obese) have growth patterns similar to those of their counterparts (P > 0.05), whereas obese children were fatter and taller from 12 and 48 mo than were nonobese children, respectively (P < 0.05).
FIGURE 2.
BMI and linear growth of 996 prepubertal Chilean children by nutritional status and DHEAS concentrations at 7 y of age. Dotted lines indicate 95% superior CIs for OH and 95% inferior CIs for ON. Dotted and dashed lines indicate 95% superior CIs for NOH and 95% inferior CIs for NON. Generalized linear models were adjusted for age and sex. Obesity = BAZ ≥2, WHO 2007; anthropometric z scores are based on WHO 2006–2007. Differences between nonobese and obese children were significant (P < 0.05) from 12 mo on (BMI and BAZ) and from 48 mo on (HAZ); all remaining differences and interactions between obesity and high DHEAS concentrations were not significant (P > 0.05). BAZ, BMI-for-age z score; DHEAS, dehydroepiandrosterone sulfate; HAZ, height-for-age z score; NOH, nonobese high DHEAS; NON, nonobese normal DHEAS; OH, obese high DHEAS; ON, obese normal DHEAS.
Central obesity analyses (data not shown)
We repeated logistic analyses considering central obesity instead of obesity. The results were slightly stronger in magnitude and in the same direction than were the results of analyses using obesity [unadjusted model OR: 2.02 (95% CI: 1.46, 2.80); full model OR: 1.85 (95% CI: 0.99, 3.52)]. Differences in growth patterns between centrally obese children with high DHEAS concentrations and those without high concentrations were similar to those observed in generally obese children. We also repeated the analyses considering a cutoff for WC based on Cook et al (33), and the results were unchanged.
Breast Tanner II (exploratory analyses)
Girls with breast Tanner II (n = 36) had higher DHEAS concentrations and were fatter and had a higher standardized bone age than did prepubertal girls (see Tables S1 and S2 under “Supplemental data” in the online issue). Nonetheless, associations between adiposity and hormonal and metabolic markers and high DHEAS concentrations at 7 y (61.2 μg/dL) were similar in magnitude and direction than in prepubertal girls (see Table S3 under “Supplemental data” in the online issue).
DISCUSSION
In this large cohort of normal-birth-weight children, we found that indicators of total and central adiposity were positively and similarly related to DHEAS concentrations at 7 y in both girls and boys; these association were only partially related to IGF-I and leptin concentrations. Obese children had almost twice the risk of having high DHEAS concentrations at 7 y than did normal-weight children. Obese children with higher DHEAS concentrations at 7 y were fatter than their counterparts, but they did not differ in their current metabolic/hormonal status and their earlier ponderal and linear growth.
In our sample, obesity and central obesity were relatively common (∼20%), as would be expected for a posttransitional urban population such as that of Santiago, Chile. Obesity has been shown to be positively associated with adrenal maturation (10, 17, 18); however, not all of the studies have reported positive findings (16). For example, one small longitudinal study found an association between changes in BMI and increases in DHEAS but failed to show a cross-sectional association between the 2 indicators (10), even when using a more specific indicator of adiposity such as skinfold thickness. In our study, we found a small positive association among 3 different indicators of adiposity (BMI, skinfold thickness, and WC) and DHEAS concentrations at 7 y. Central adiposity tended to be more strongly associated to DHEAS than did general adiposity. This finding is consistent with a potential role of cortisol as an initiator of adrenal maturation, because central obesity is associated with increased cortisol secretions (34, 35). Nonetheless, these conclusions have to be tempered given that only ∼7% of the children were discordant (ie, were obese but not centrally obese). We found that associations between adiposity and DHEAS were of similar magnitude and direction in both girls and boys, even after Tanner II girls were excluded from the analyses. Other authors have reported similar results in prepuberty (1, 36). It was also shown that the effect of early growth on adrenal maturation is of similar magnitude in both sexes (37). We add to the existing literature by showing that the effects of adiposity on adrenal maturation do not show evidence of sexual dimorphism at this age.
Several reports recognize the association of obesity as a “switch” for adrenarche. However, the direct mechanisms through which this process starts are still unknown. In vitro studies suggest a role for insulin and IGF-I; however, in vivo studies have shown conflicting results (38). Correlations between insulin and IGF-I and DHEAS have been shown in prepubertal girls but not in boys (19, 20). Children with PA have higher insulin and IGF-I and lower IGF binding protein-1 concentrations (39, 40), although this finding has not been consistent (41). In this study, we showed that IGF-I had a small effect on DHEAS that remained significant after adjustment for BMI and insulin concentrations. This observation agrees with the studies of Baquedano et al (42), who demonstrated the presence of IGF-I/IGF-I receptor in the outer zone of the adrenal cortex during childhood and adolescence, but no evidence of a direct action of IGF-I on the zona reticularis. Leptin has been also explored as a trigger of adrenarche; however, the evidence remains inconsistent (43). In our model, we found a significant relation between leptin and DHEAS that disappeared after adjustment for indicators of adiposity. Conversely, the strength of the associations between adiposity indicators and DHEAS decreased when leptin was added to the model, which suggests a mediator role. Overall, the hormonal markers that we explored (insulin, IGF-I, and leptin) only partially explained the obesity and DHEAS associations, which suggests that other factors may be implicated.
We found, that obesity was positively associated with DHEAS, although not all obese children had high DHEAS concentrations. In our cohort, children with higher DHEAS concentrations were fatter (in general and centrally) than their counterparts and tended to be taller and have a higher bone age, although these differences were not always significantly different. It has been shown that a progressive increase in serum concentrations of DHEAS roughly parallel an increase in bone age in both healthy and obese populations (17, 44). Metabolic markers also did not differ among obese persons with and without adrenarche, although a higher concentration of IGF-I was suggested. Further follow-up of these children will allow assessment of how DHEAS concentrations at this age will affect the metabolic health of these children.
In our population-based cohort, in contrast with what has been shown in clinical settings (41), we observed that children with high DHEAS concentrations at 7 y grew similarly to their normal counterparts from 0 to 7 y of age. It was suggested that obese children with high DHEAS concentrations had an earlier adiposity rebound than did the rest of the obese children; however, we were unable to further explore this observation because our data were limited to yearly measurements, which do not allow a precise estimation of the age at which adiposity rebound occurs.
Our study had the same limitations inherent with the interpretation of cross-sectional analyses, which does not allow firm conclusions on the direction of the associations and thus cannot be used to infer causality. Nonetheless, we had a large population that was restricted in terms of age, which allowed us to explore the association of adiposity and adrenal maturation considering different indicators of adiposity and potential hormonal mediators assessing the effect of potential sex differences and controlling a potential confounder role of age. We expect that further follow-up of these children will allow us to better disentangle the timing of the different events.
In conclusion, childhood obesity has reached epidemic proportions on a global scale (45). This is a matter of concern given the number of complications and conditions associated with childhood obesity, such as metabolic, cardiovascular, and cancer disease. PA has also been linked to the emergence of metabolic and other health complications (5, 14, 39). We showed here, in a population of normal-birth-weight school-age children, that obesity and DHEAS are positively associated (11). It remains to be determined how high DHEAS concentrations will affect the emergence of the metabolic complications of obesity, but it might be possible that avoiding the development of PA may be a manner of decreasing childhood obesity burden and the potential lifelong metabolic derangements derived from this condition.
Acknowledgments
We thank Daniela González for her work as study coordinator.
The authors’ responsibilities were as follows—CC, UR, and MV: designed the research and wrote the manuscript; and CC: analyzed the data and had primary responsibility for the final content. All authors read and approved the final manuscript. None of the authors declared a conflict of interest.
Footnotes
Abbreviations used: BA, bone age; BAZ, BMI-for-age z score; BIA, bioimpedance analysis; CA, chronologic age; DHEAS, dehydroepiandrosterone sulfate; FMI, fat mass index; IGF-I, insulin-like growth factor I; PA, premature adrenarche; WC, waist circumference; %fat, percentage fat.
REFERENCES
- 1.Havelock JC, Auchus RJ, Rainey WE. The rise in adrenal androgen biosynthesis: adrenarche. Semin Reprod Med 2004;22:337–47 [DOI] [PubMed] [Google Scholar]
- 2.Kelnar CJ, Brook CG. A mixed longitudinal study of adrenal steroid excretion in childhood and the mechanism of adrenarche. Clin Endocrinol (Oxf) 1983;19:117–29 [DOI] [PubMed] [Google Scholar]
- 3.Palmert MR, Hayden DL, Mansfield MJ, Crigler JF, Jr, Crowley WF, Jr, Chandler DW, Boepple PA. The longitudinal study of adrenal maturation during gonadal suppression: evidence that adrenarche is a gradual process. J Clin Endocrinol Metab 2001;86:4536–42 [DOI] [PubMed] [Google Scholar]
- 4.Remer T, Boye KR, Hartmann MF, Wudy SA. Urinary markers of adrenarche: reference values in healthy subjects, aged 3-18 years. J Clin Endocrinol Metab 2005;90:2015–21 [DOI] [PubMed] [Google Scholar]
- 5.Ibáñez L, Dimartino-Nardi J, Potau N, Saenger P. Premature adrenarche—normal variant or forerunner of adult disease? Endocr Rev 2000;21:671–96 [DOI] [PubMed] [Google Scholar]
- 6.Rosenfield RL. Clinical review: identifying children at risk for polycystic ovary syndrome. J Clin Endocrinol Metab 2007;92:787–96 [DOI] [PubMed] [Google Scholar]
- 7.Idkowiak J, Lavery GG, Dhir V, Barrett TG, Stewart PM, Krone N, Arlt W. Premature adrenarche: novel lessons from early onset androgen excess. Eur J Endocrinol 2001;165(2):189–207 [DOI] [PubMed] [Google Scholar]
- 8.Williams RM, Ward CE, Hughes IA. Premature adrenarche. Arch Dis Child 2012;97:250–4 [DOI] [PubMed] [Google Scholar]
- 9.D'Adamo E, Santoro N, Caprio S. Metabolic syndrome in pediatrics: old concepts revised, new concepts discussed. Pediatr Clin North Am 2011;58(5):1241–55 [DOI] [PubMed] [Google Scholar]
- 10.Remer T, Manz F. Role of nutritional status in the regulation of adrenarche. J Clin Endocrinol Metab 1999;84:3936–44 [DOI] [PubMed] [Google Scholar]
- 11.Jean AM, Hassoun A, Hughes J, Pomeranz C, Fennoy I, McMahon DJ, Oberfield SE. Utility of early insulin response and proinsulin to assess insulin resistance. J Pediatr 2009;155(6):893–9. [DOI] [PMC free article] [PubMed]
- 12.Rittmaster RS, Deshwal N, Lehman L. The role of adrenal hyperandrogenism, insulin resistance, and obesity in the pathogenesis of polycystic ovarian syndrome. J Clin Endocrinol Metab 1993;76:1295–300 [DOI] [PubMed] [Google Scholar]
- 13.Charkaluk ML, Trivin C, Brauner R. Premature pubarche as an indicator of how body weight influences the onset of adrenarche. Eur J Pediatr 2004;163:89–93. [DOI] [PubMed] [Google Scholar]
- 14.Ibáñez L, Potau N, Marcos MV, de Zegher F. Exaggerated adrenarche and hyperinsulinism in adolescent girls born small for gestational age. J Clin Endocrinol Metab 1999;84:4739–41 [DOI] [PubMed] [Google Scholar]
- 15.Yu ZB, Han SP, Zhu GZ, Zhu C, Wang XJ, Cao XG, Guo XR. Birth weight and subsequent risk of obesity: a systematic review and meta-analysis. Obes Rev 2011;12:525–42 [DOI] [PubMed] [Google Scholar]
- 16.Gonzales GF, Villena A, Gonez C, Zevallos M. Relationship between body mass index, age, and serum adrenal androgen levels in Peruvian children living at high altitude and at sea level. Hum Biol 1994;66:145–53 [PubMed] [Google Scholar]
- 17.Sopher AB, Jean AM, Zwany SK, Winston DM, Pomeranz CB, Bell JJ, McMahon DJ, Hassoun A, Fennoy I, Oberfield SE. Bone age advancement in prepubertal children with obesity and premature adrenarche: possible potentiating factors. Obesity (Silver Spring) 2011;19(6):1259–64. [DOI] [PMC free article] [PubMed]
- 18.Genazzani AR, Pintor C, Corda R. Plasma levels of gonadotropins, prolactin, thyroxine, and adrenal and gonadal steroids in obese prepubertal girls. J Clin Endocrinol Metab 1978;47:974–9 [DOI] [PubMed] [Google Scholar]
- 19.Guercio G, Rivarola MA, Chaler E, Maceiras M, Belgorosky A. Relationship between the GH/IGF-I axis, insulin sensitivity, and adrenal androgens in normal prepubertal and pubertal boys. J Clin Endocrinol Metab 2002;87:1162–9 [DOI] [PubMed] [Google Scholar]
- 20.Guercio G, Rivarola MA, Chaler E, Maceiras M, Belgorosky A. Relationship between the growth hormone/insulin-like growth factor-I axis, insulin sensitivity, and adrenal androgens in normal prepubertal and pubertal girls. J Clin Endocrinol Metab 2003;88:1389–93 [DOI] [PubMed] [Google Scholar]
- 21.Corvalán C, Uauy R, Stein AD, Kain J, Martorell R. Effect of growth on cardiometabolic status at 4 y of age. Am J Clin Nutr 2009;90:547–55 [DOI] [PubMed] [Google Scholar]
- 22.Kain J, Corvalan C, Lera L, Galvan M, Uauy R. Accelerated growth in early life and obesity in preschool Chilean children. Obesity (Silver Spring) 2009;17:1603–8 [DOI] [PubMed] [Google Scholar]
- 23.Cohen J. Statistical power analysis for the behavioral sciences. Revised ed. New York, NY: Academic Press, 1977.
- 24.de Onis M, Garza C, Victora CG, Onyango AW, Frongillo EA, Martines J. The WHO Multicentre Growth Reference Study: planning, study design, and methodology. Food Nutr Bull 2004;25(suppl):S15–26 [DOI] [PubMed] [Google Scholar]
- 25.Tanner JM. Growth at adolescense. Oxford, United Kingdom: Blackwell Scientific Publications, 1962 [Google Scholar]
- 26. Sunlight. Available from: http://www.sunlightnet.com/international/html/productBA.html (cited 20 January 2012)
- 27.Mentzel HJ, Vilser C, Eulenstein M, Schwartz T, Vogt S, Bottcher J, Yaniv I, Tsoref L, Kauf E, Kaiser WA. Assessment of skeletal age at the wrist in children with a new ultrasound device. Pediatr Radiol 2005;35:429–33 [DOI] [PubMed] [Google Scholar]
- 28.Khan KM, Miller BS, Hoggard E, Somani A, Sarafoglou K. Application of ultrasound for bone age estimation in clinical practice. J Pediatr 2009;154:243–7 [DOI] [PubMed] [Google Scholar]
- 29.Iñiguez G, Ong K, Bazaes R, Avila A, Salazar T, Dunger D, Mericq V. Longitudinal changes in insulin-like growth factor-I, insulin sensitivity, and secretion from birth to age three years in small-for-gestational-age children. J Clin Endocrinol Metab 2006;91:4645–9 [DOI] [PubMed] [Google Scholar]
- 30.World Health Organization. Available from: http://www.who.int/growthref/en/ (cited 15 June 2011)
- 31.Fernandez JR, Redden DT, Pietrobelli A, Allison DB. Waist circumference percentiles in nationally representative samples of African-American, European-American, and Mexican-American children and adolescents. J Pediatr 2004;145(4):439–44 [DOI] [PubMed] [Google Scholar]
- 32.Slaughter MH, Lohman TG, Boileau RA, Horswill CA, Stillman RJ, Van Loan MD, Bemben DA. Skinfold equations for estimation of body fatness in children and youth. Hum Biol 1988;60:709–23 [PubMed] [Google Scholar]
- 33.Cook S, Auinger P, Huang TT. Growth curves for cardio-metabolic risk factors in children and adolescents. J Pediatr 2009;155(3):S6 e15–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mericq V, Medina P, Bouwman C, Johnson MC, Godoy J, Lopez T, Iniguez G. Expression and activity of 11beta-hydroxysteroid dehydrogenase type 1 enzyme in subcutaneous and visceral adipose tissue of prepubertal children. Horm Res 2009;71(2):89–93 [DOI] [PubMed] [Google Scholar]
- 35.Topor LS, Asai M, Dunn J, Majzoub JA. Cortisol stimulates secretion of dehydroepiandrosterone in human adrenocortical cells through inhibition of 3betaHSD2. J Clin Endocrinol Metab 2011;96(1):E31–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rainey WE, Carr BR, Sasano H, Suzuki T, Mason JI. Dissecting human adrenal androgen production. Trends Endocrinol Metab 2002;13(6):234–9 [DOI] [PubMed] [Google Scholar]
- 37.Ong KK, Potau N, Petry CJ, Jones R, Ness AR, Honour JW, de Zegher F, Ibanez L, Dunger DB. Opposing influences of prenatal and postnatal weight gain on adrenarche in normal boys and girls. J Clin Endocrinol Metab 2004;89:2647–51 [DOI] [PubMed] [Google Scholar]
- 38.Smith CP, Dunger DB, Williams AJ, Taylor AM, Perry LA, Gale EA, Preece MA, Savage MO. Relationship between insulin, insulin-like growth factor I, and dehydroepiandrosterone sulfate concentrations during childhood, puberty, and adult life. J Clin Endocrinol Metab 1989;68:932–7 [DOI] [PubMed] [Google Scholar]
- 39.Ibáñez L, Potau N, Zampolli M, Rique S, Saenger P, Carrascosa A. Hyperinsulinemia and decreased insulin-like growth factor-binding protein-1 are common features in prepubertal and pubertal girls with a history of premature pubarche. J Clin Endocrinol Metab 1997;82:2283–8 [DOI] [PubMed] [Google Scholar]
- 40.Silfen ME, Manibo AM, Ferin M, McMahon DJ, Levine LS, Oberfield SE. Elevated free IGF-I levels in prepubertal Hispanic girls with premature adrenarche: relationship with hyperandrogenism and insulin sensitivity. J Clin Endocrinol Metab 2002;87:398–403 [DOI] [PubMed] [Google Scholar]
- 41.Utriainen P, Voutilainen R, Jaaskelainen J. Girls with premature adrenarche have accelerated early childhood growth. J Pediatr 2009;154(6):882–7 [DOI] [PubMed] [Google Scholar]
- 42.Baquedano MS, Berensztein E, Saraco N, Dorn GV, De Davila MT, Rivarola MA, Belgorosky A. Expression of the IGF system in human adrenal tissues from early infancy to late puberty: implications for the development of adrenarche. Ped Res 2005;58(3):451–8 [DOI] [PubMed] [Google Scholar]
- 43.l'Allemand D, Schmidt S, Rousson V, Brabant G, Gasser T, Gruters A. Associations between body mass, leptin, IGF-I and circulating adrenal androgens in children with obesity and premature adrenarche. Eur J Endocrinol 2002;146(4):537–43 [DOI] [PubMed] [Google Scholar]
- 44.Parker LN. Adrenarche. Endocrinol Metab Clin North Am 1991;20:71–83 [PubMed] [Google Scholar]
- 45.Popkin BM, Adair LS, Ng SW. Global nutrition transition and the pandemic of obesity in developing countries. Nutr Rev 2012;70:3–21 [DOI] [PMC free article] [PubMed] [Google Scholar]