Abstract
Background: The role of one-carbon metabolism nutrients in colorectal carcinogenesis is not fully understood. Associations might be modified by mandated folic acid (FA) fortification or alcohol intake.
Objective: We investigated associations between intakes of folate, riboflavin, vitamin B-6, and vitamin B-12 and colorectal cancer (CRC) in the Women's Health Initiative Observational Study, stratified by time exposed to FA fortification and alcohol intake.
Design: A total of 88,045 postmenopausal women were recruited during 1993–1998; 1003 incident CRC cases were ascertained as of 2009. Quartiles of dietary intakes were compared; HRs and 95% CIs were estimated by Cox proportional hazards models.
Results: Dietary and total intakes of vitamin B-6 in quartile 4 compared with quartile 1 (HR: 0.80; 95% CI: 0.66, 0.97 and HR: 0.80; 95% CI: 0.66, 0.99, respectively) and total intakes of riboflavin (HR: 0.81; 95% CI: 0.66, 0.99) were associated with reduced risk of CRC overall and of regionally spread disease. In current drinkers who consumed <1 drink (13 g alcohol)/wk, B vitamin intakes were inversely associated with CRC risk (P-interaction < 0.05). Dietary folate intake was positively associated with CRC risk among women who had experienced the initiation of FA fortification for 3 to <9 y (P-interaction < 0.01).
Conclusions: Vitamin B-6 and riboflavin intakes from diet and supplements were associated with a decreased risk of CRC in postmenopausal women. Associations of B vitamin intake were particularly strong for regional disease and among women drinkers who consumed alcohol infrequently. Our study provides new evidence that the increased folate intake during the early postfortification period may have been associated with a transient increase in CRC risk.
INTRODUCTION
Colorectal cancer (CRC)5 is the third most common type of cancer for both women and men in the United States, with an expected incidence of >160,000 cases in 2011 (1). Although better diagnostic means and treatment options have contributed to higher survival rates, the diagnosis still accounts for 9% of cancer-related deaths.
B vitamins, including folate, riboflavin, pyridoxine (vitamin B-6), and cobalamin (vitamin B-12), are essential for methylation reactions (including DNA methylation), nucleotide synthesis, and DNA stability and repair (2–4). Low folate intakes or biomarkers of low folate status have been implicated in higher CRC risk (5–8). Whether the associations are mainly from dietary folate or folic acid (FA) in supplements is under debate (9, 10). In 1998, FA fortification of enriched cereal-grain products was mandated by the US Food and Drug Administration (FDA), which led to a new dietary source of FA in addition to other available sources and resultant higher blood-folate concentrations (11). Because folate has tumor growth–promoting effects, concern has been raised about the potential risks associated with high folate intakes in the postfortification era, in particular because intakes often exceed dietary recommendations (7, 12–14). However, evidence from prospective, person-level data on this issue is very limited (8, 15, 16).
To date, there have been several investigations of other B vitamins as risk factors for neoplasia of the colon, and there is strong support for a role of vitamin B-6 in the prevention of colon tumorigenesis by reduction in cell proliferation, oxidative stress, and angiogenesis (17–19). Accordingly, in some studies, higher vitamin B-6 intakes or serum concentrations were associated with decreased risk of CRC (20–23). However, the effects of vitamin B-6 on rectal cancer may differ from those on colon cancer, because 2 studies have linked an increased risk in rectal cancer to high vitamin B-6 intake (24, 25).
In addition, high alcohol intake has been implicated as a risk factor for CRC (26). Alcohol functions as a folate antagonist by limiting the absorption and metabolism of this nutrient (27). Thus, alcohol intake also needs to be considered when evaluating the relation between one-carbon metabolism and CRC.
In this study, we investigated the relation between intakes of one-carbon nutrients (from diet and supplements) and risk of CRC by tumor site and stage in one of the largest US study populations of postmenopausal women. We also examined whether FA fortification and alcohol use are effect modifiers of this association.
SUBJECTS AND METHODS
Study population
The Women's Health Initiative Observational Study (WHI-OS) is a prospective cohort study that enrolled 93,676 women between 1993 and 1998 at 40 US clinical institutions. The study was approved by human subjects review committees at each of the participating institutions, and written informed consent was obtained from each study participant. The uniformity of data collection procedures was ensured through detailed protocols, centralized training of staff, and local and studywide quality-control mechanisms, including quality assurance visits by the Clinical Coordinating Center. Women were eligible for the WHI-OS if they were postmenopausal, not participating in any clinical trial, aged 50–79 y at the time of enrollment, unlikely to relocate within 3 y, and unlikely to die of a preexisting medical condition within 3 y. Characteristics of the WHI-OS cohort and the study design have been described in detail elsewhere (28). Women were excluded from the analyses presented here if they reported a history of CRC at enrollment (n = 959), if no follow-up was available (n = 490), if they were diagnosed with in situ CRC during follow-up (n = 46), or if a death certificate provided the first and only report of CRC (n = 52). Women with extreme reported intakes on the food-frequency questionnaire (FFQ) results (n = 3852; defined as dietary energy intake <600 or >5000 kcal/d) or with extreme values of BMI (in kg/m2) (n = 502; ≤15.0 or ≥50.0) were also excluded, which left n = 88,045 women in the study.
Data collection and case identification
Self-reported data on demographic and health-related characteristics (age, household income, race-ethnicity, medical history, family history, medication use, smoking status, alcohol intake, and recreational physical activity) were collected at baseline. Information on postmenopausal hormone therapy was assessed via interview and categorized as current, past, or never use. Height and weight were measured at a baseline clinic visit, and BMI was computed as weight (kg)/height (m)2.
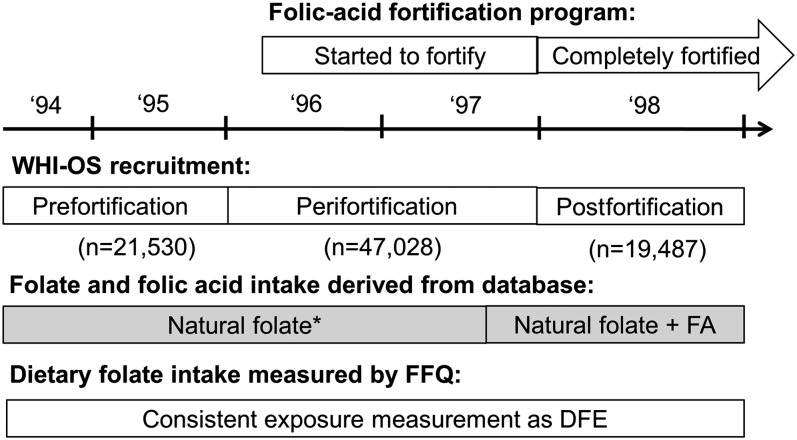
At baseline, women completed a self-administered FFQ, which was developed specifically for the Women's Health Initiative (WHI). This FFQ included 122 items for individual foods and food groups. The nutrient database used to convert food information into nutrients was derived from the Nutrition Data Systems for Research (NDS-R, version 2006), University of Minnesota Nutrition Coordinating Center food and nutrient database, and augmented with information from food manufacturers. The time sequence of the national program of FA fortification, WHI-OS recruitment, and FFQ databases used for measuring FA intake are shown in Figure 1. FDA-mandated FA fortification of enriched cereal was fully implemented on 1 January 1998. Baseline FFQs collected before fortification reflected both natural folate plus FA that was in a limited number of foods before full-scale fortification. For FFQs administered on or after 1 August 1997, intakes of both natural folate and synthetic FA added to enriched grains were computed. The WHI Clinical Coordinating Center determined the cutoff date because manufacturers might have completed fortification before the mandatory deadline. Dietary supplement use was determined by using an inventory-type questionnaire in which study staff recorded nutrients (including multivitamins, multivitamins with minerals, B vitamins) from participants’ vitamin bottles brought to a clinic visit. Total nutrient intake was summed from dietary and supplemental sources. To account for the higher bioavailability of synthetic FA, dietary folate equivalent, or DFE, was used as the unit for dietary folate intake from food and total folate intake. Thus, dietary folate intake from food was the sum of natural folate from food plus 1.7 times FA in fortification (29). Total folate intake was the sum of dietary folate plus 1.7 times FA in supplements (30).
FIGURE 1.
Time sequence of the national program of FA fortification, WHI-OS recruitment, and FFQ databases used for measuring FA intake. *The value reflected both natural folate plus FA that was in a limited number of foods. DFE, dietary folate equivalent; FA, folic acid; FFQ, food-frequency questionnaire; WHI-OS, Women's Health Initiative Observational Study.
Clinical outcomes were reported annually by self-administered medical history update questionnaires and a clinic follow-up visit in year 3. Medical records were centrally reviewed, and major diagnoses were confirmed by physician adjudicators (31). Median follow-up was 11 y, and 4% of women were lost to follow-up or left the study early. Cancer cases were identified as proximal colon [cecum, ascending colon, hepatic flexure, transverse colon, or splenic flexure, corresponding to International Classification of Diseases for Oncology, second edition (ICD-O-2) codes C180, C182, C183, C184, or C185], distal colon (descending and sigmoid colon; codes C186 and C187), or rectal adenocarcinomas (codes C199 and C209) and classified by using Surveillance, Epidemiology, and End Results program guidelines (32). The present study included 1003 incident cases of CRC, and 843 cases remained when restricted to cases with >2 y of follow-up.
Statistical analyses
HRs and 95% CIs were estimated by Cox's proportional hazards models. The proportionality assumption was examined by using log-log plots and Schoenfeld residuals, and the assumption was fulfilled. Days from study enrollment were treated as the basic time variable. Case women contributed follow-up time to the first postenrollment diagnosis of CRC. Otherwise, women were censored at the last documented follow-up contact, at death, or on 14 August 2009 (whichever occurred first). All models were adjusted for age as a continuous variable. Multivariate models included, in addition to age, a set of baseline variables that were chosen a priori for adjustment of potential confounding, including BMI (<25.0, 25.0 to <30.0, 30.0 to <35.0, or ≥35.0), race or ethnicity (white, black, or other), past medical history of colonoscopy (yes or no), smoking status (never, past, or current), physical activity (0 to <3, 3 to<11.75, or ≥11.75 metabolic equivalent task hours/wk), and postmenopausal hormone therapy use (never, past, or current). Alcohol intake was defined as nondrinker, past drinker, current drinker with <1 drink/wk, current drinker with 1 to <7 drinks/wk, and current drinker with ≥7 drinks/wk.
The following additional variables were evaluated as potential confounders: family income, education, US region, number of relatives with CRC, history of colon polyps, smoking pack-years, years of second-hand smoke exposure during child- and adulthood and at the workplace, duration of postmenopausal hormone use, nonsteroidal antiinflammatory drug use, and consumption of fruit, vegetables, animal protein, red meat, energy (total and percentage calories from fat), total fat, fiber, dietary calcium, and total calcium. None of these variables materially changed the risk estimates. Therefore, only age and the a priori set of covariates were included in the final models.
Quartile cutoffs were calculated on the basis of the distribution among all participants combined, and the lowest quartile served as the reference group. Tests of linear trend across increasing categories were conducted by modeling the median values of each category as a single continuous variable in the models and by assessing significance using the Wald test.
To test effect modification by FA fortification, a variable indicating time exposed to FA fortification was calculated as time from 1 August 1997 to the date of CRC diagnosis, death, or last follow-up. We chose 1 August 1997 as the cutoff date because the WHI Clinical Coordinating Center incorporated fortified FA values into dietary folate intake amounts for the FFQs completed on that date and onward. The variable was categorized into <3, 3 to <6, 6 to <9, and ≥9 y. Effect modification was evaluated by Wald tests of a product term between the ordinal trend variables (folate and B vitamins intake) and each effect modifier (time exposed to FA fortification and alcohol intake). This is equivalent to testing the differences in slopes of the associations of folate and B vitamins intake with CRC risk.
We also examined associations within subgroups of cases by tumor site and stage of disease. We evaluated the associations overall as well as among women with ≥2 y of follow-up (n = 86,820, 843 cases). We reported results from the latter group, because this allows for a greater time period of exposure and reduces the likelihood that underlying disease would affect outcomes, and because their associations were generally stronger. Significance was defined as P < 0.05, and all statistical tests were 2-sided. Analyses were conducted by using SAS (version 9.2; SAS Institute Inc).
RESULTS
Demographic and lifestyle characteristics of the WHI-OS participants with ≥2 y of follow-up are shown in Table 1. The associations of one-carbon nutrient intake with CRC incidence are shown in Table 2. After age adjustment, reduced risks of CRC were observed for the highest compared with the lowest quartiles of intake of dietary folate (HR: 0.79; 95% CI: 0.65, 0.96; P-trend = 0.03) and vitamin B-6 (HR: 0.77; 95% CI: 0.63, 0.93; P-trend < 0.01). A higher intake of riboflavin from supplements was also associated with a decreased risk of CRC (HR: 0.77; 95% CI: 0.61, 0.97; P-trend = 0.03). The total (dietary and supplemental) intakes of vitamin B-6 and riboflavin were inversely associated with CRC risk when comparing highest and lowest groups (HR for vitamin B-6: 0.73; 95% CI: 0.60, 0.89; P-trend < 0.01; HR for riboflavin: 0.75; 95% CI: 0.62, 0.92; P-trend < 0.01). After multivariate adjustment, there was a trend toward risk reduction for higher dietary and total intakes of vitamin B-6 (HR: 0.80; 95% CI: 0.66, 0.97; P-trend = 0.03; and HR: 0.80; 95% CI: 0.66, 0.99; P-trend = 0.051, respectively). Total intakes of riboflavin (HR: 0.81; 95% CI: 0.66, 0.99; P-trend = 0.04) remained inversely associated with CRC risk.
TABLE 1.
Selected characteristics by total folate intake among participants with ≥2 y of follow-up in the Women's Health Initiative Observational Study1
| Total folate intake (DFE) |
||||||||
| <242 |
242 to <542 |
542 to <939 |
≥939 |
|||||
| Characteristic | n | Value | n | Value | n | Value | n | Value |
| Age (y) | 21,667 | 62.9 ± 7.52 | 21,696 | 63.4 ± 7.4 | 21,726 | 63.6 ± 7.4 | 21,731 | 64.2 ± 7.1 |
| BMI (%) | ||||||||
| <25 kg/m2 | 8202 | 38.3 | 8431 | 39.3 | 9319 | 43.4 | 9509 | 44.2 |
| 25 to <30 kg/m2 | 7377 | 34.5 | 7383 | 34.4 | 7317 | 34.1 | 7288 | 33.9 |
| 30 to <35 kg/m2 | 3707 | 17.3 | 3478 | 16.2 | 3184 | 14.8 | 3091 | 14.4 |
| ≥35 kg/m2 | 2120 | 9.9 | 2143 | 10.0 | 1653 | 7.7 | 1610 | 7.5 |
| Race-ethnicity (%) | ||||||||
| White | 17,260 | 79.7 | 18,087 | 83.4 | 18,836 | 86.7 | 19,315 | 88.9 |
| Black | 2273 | 10.5 | 1802 | 8.3 | 1218 | 5.6 | 1025 | 4.7 |
| Other | 2134 | 9.8 | 1807 | 8.3 | 1672 | 7.7 | 1391 | 6.4 |
| Family income (%) | ||||||||
| <$20,000 | 3847 | 19.2 | 3065 | 15.3 | 2829 | 13.9 | 2487 | 12.3 |
| $20,000–$34,999 | 4799 | 24.0 | 4624 | 23.1 | 4790 | 23.6 | 4521 | 22.3 |
| $35,000–$49,999 | 3893 | 19.4 | 4049 | 20.2 | 4093 | 20.2 | 4289 | 21.2 |
| $50,000–$74,999 | 3777 | 18.9 | 4020 | 20.1 | 4285 | 21.1 | 4418 | 21.8 |
| ≥$75,000 | 3701 | 18.5 | 4277 | 21.3 | 4297 | 21.2 | 4554 | 22.5 |
| No. of relatives with CRC (%) | ||||||||
| None | 16,460 | 84.4 | 16,514 | 83.8 | 16,670 | 84.4 | 16,551 | 83.9 |
| 1 | 2713 | 13.9 | 2840 | 14.4 | 2762 | 14.0 | 2832 | 14.4 |
| ≥2 | 338 | 1.7 | 352 | 1.8 | 314 | 1.6 | 346 | 1.8 |
| History of colonoscopy (%) | ||||||||
| No | 10,618 | 50.1 | 10,086 | 47.2 | 9606 | 44.8 | 8617 | 40.0 |
| Yes | 10,567 | 49.9 | 11,285 | 52.8 | 11,850 | 55.2 | 12,917 | 60.0 |
| Smoking status (%) | ||||||||
| Never | 10,782 | 50.5 | 11,025 | 51.5 | 10,871 | 50.7 | 10,903 | 50.8 |
| Past | 8774 | 41.1 | 9090 | 42.5 | 9330 | 43.5 | 9648 | 45.0 |
| Current | 1794 | 8.4 | 1273 | 6.0 | 1231 | 5.7 | 893 | 4.2 |
| Pack-years of smoking (%) | ||||||||
| Never-smoker | 10,782 | 51.6 | 11,025 | 52.8 | 10,871 | 51.9 | 10,903 | 52.1 |
| <5 | 2994 | 14.3 | 3100 | 14.8 | 3136 | 15.0 | 3199 | 15.3 |
| 5 to <20 | 2957 | 14.1 | 3062 | 14.7 | 2993 | 14.3 | 3016 | 14.4 |
| ≥20 | 4170 | 19.9 | 3713 | 17.8 | 3941 | 18.8 | 3803 | 18.2 |
| Dietary alcohol intake (g) | 21,667 | 4.9 ± 10.8 | 21,696 | 5.7± 11.9 | 21,726 | 5.4 ± 10.9 | 21,731 | 5.7 ± 11.6 |
| Alcohol use (%) | ||||||||
| Nondrinker | 2738 | 12.7 | 2408 | 11.2 | 2146 | 9.9 | 1993 | 9.2 |
| Past drinker | 4295 | 20.0 | 3802 | 17.6 | 3753 | 17.4 | 3879 | 17.9 |
| <1 drink/mo | 2590 | 12.0 | 2455 | 11.4 | 2550 | 11.8 | 2302 | 10.6 |
| <1 drink/wk | 4220 | 19.6 | 4211 | 19.5 | 4485 | 20.8 | 4452 | 20.6 |
| 1 to <7 drinks/wk | 5097 | 23.7 | 5752 | 26.7 | 5872 | 27.2 | 6000 | 27.8 |
| ≥7 drinks/wk | 2574 | 12.0 | 2922 | 13.6 | 2803 | 13.0 | 2991 | 13.8 |
| Physical activity (MET-h/wk) | 21,293 | 11.4 ± 13.2 | 21,433 | 14.2 ± 14.5 | 21,542 | 13.8 ± 14.1 | 21,589 | 16.1 ± 15.2 |
| Postmenopausal hormone use (%) | ||||||||
| None | 9484 | 43.8 | 9521 | 43.9 | 7940 | 36.6 | 7621 | 35.1 |
| Past | 3147 | 14.5 | 3121 | 14.4 | 3313 | 15.3 | 3349 | 15.4 |
| Current estrogen | 5059 | 23.4 | 5141 | 23.7 | 5730 | 26.4 | 5829 | 26.8 |
| Current estrogen + progesterone | 3951 | 18.3 | 3888 | 17.9 | 4725 | 21.8 | 4919 | 22.6 |
| NSAID use (%) | ||||||||
| No | 15,026 | 69.3 | 14,736 | 67.9 | 13,672 | 62.9 | 13,171 | 60.6 |
| Yes | 6641 | 30.7 | 6960 | 32.1 | 8054 | 37.1 | 8559 | 39.4 |
| Multivitamin supplement use (%) | ||||||||
| No | 21,620 | 99.8 | 20,494 | 94.5 | 5194 | 23.9 | 2984 | 13.7 |
| Yes | 47 | 0.2 | 1202 | 5.5 | 16,532 | 76.1 | 18,747 | 86.3 |
| Red meat intake (medium portions/d) | 21,667 | 0.6 ± 0.5 | 21,696 | 0.7 ± 0.6 | 21,726 | 0.6 ± 0.5 | 21,731 | 0.6 ± 0.5 |
| Total energy intake (kcal) | 21,667 | 1295 ± 441 | 21,696 | 1762 ± 614 | 21,726 | 1454 ± 551 | 21,731 | 1773 ± 621 |
| Dietary folate intake (DFE) | 21,667 | 175.3 ± 43.8 | 21,696 | 324.4 ± 82.1 | 21,726 | 261.2 ± 167.0 | 21,731 | 400.6 ± 189.2 |
| Supplemental folic acid intake (μg) | 21,667 | 0.2 ± 3.3 | 21,696 | 10.3 ± 36.5 | 21,726 | 331.9 ± 135.9 | 21,731 | 481.6 ± 285.6 |
| Folate, total intake (DFE) | 21,667 | 175.7 ± 42.7 | 21,696 | 341.9 ± 79.5 | 21,726 | 825.5 ± 102.6 | 21,731 | 1219.3 ± 477.6 |
| Dietary vitamin B-12 intake (μg) | 21,667 | 4.7 ± 2.6 | 21,696 | 6.5 ± 3.7 | 21,726 | 5.4 ± 3.3 | 21,731 | 6.6 ± 4.0 |
| Supplemental vitamin B-12 intake (μg) | 21,667 | 7.6 ± 59.4 | 21,696 | 9.7 ± 61.2 | 21,726 | 20.2 ± 76.3 | 21,731 | 32.9 ± 99.0 |
| Vitamin B-12 intake, total (μg) | 21,667 | 12.3 ± 59.4 | 21,696 | 16.2 ± 61.2 | 21,726 | 25.5 ± 76.3 | 21,731 | 39.5 ± 98.9 |
| Dietary vitamin B-6 intake (mg) | 21,667 | 1.3 ± 0.5 | 21,696 | 1.8 ± 0.7 | 21,726 | 1.5 ± 0.6 | 21,731 | 1.9 ± 0.7 |
| Supplemental vitamin B-6 intake (mg) | 21,667 | 2.8 ± 22.7 | 21,696 | 4.9 ± 35.3 | 21,726 | 7.8 ± 31.6 | 21,731 | 14.6 ± 41.8 |
| Vitamin B-6 intake, total (mg) | 21,667 | 4.1 ± 22.7 | 21,696 | 6.7 ± 35.3 | 21,726 | 9.3 ± 31.6 | 21,731 | 16.5 ± 41.8 |
| Dietary riboflavin intake (mg) | 21,667 | 1.6 ± 0.6 | 21,696 | 2.2 ± 0.9 | 21,726 | 1.8 ± 0.8 | 21,731 | 2.3 ± 0.9 |
| Supplemental riboflavin intake (mg) | 21,667 | 1.2 ± 15.5 | 21,696 | 2.2 ± 16.7 | 21,726 | 4.8 ± 16.5 | 21,731 | 10.5 ± 28.0 |
| Riboflavin intake, total (mg) | 21,667 | 2.7 ± 15.5 | 21,696 | 4.5 ± 16.7 | 21,726 | 6.6 ± 16.5 | 21,731 | 12.8 ± 27.9 |
Values do not add up to 100% because of missing values and rounding; numbers are frequencies and percentages unless otherwise noted. CRC, colorectal cancer; DFE, dietary folate equivalent; MET-h, metabolic equivalent task hours; NSAID, nonsteroidal antiinflammatory drug.
Mean ± SD (all such values).
TABLE 2.
Age- and multivariate-adjusted HRs and 95% CIs for the associations of baseline intakes of nutrients involved in one-carbon metabolism with colorectal cancer1
| Age adjusted |
Multivariate adjusted2 |
|||||||
| Cases | HR | (95% CI) | P-trend | Cases3 | HR | (95% CI) | P-trend | |
| Dietary folate | ||||||||
| <189 DFE | 229 | 1.00 | (Ref) | 0.03 | 221 | 1.00 | (Ref) | 0.14 |
| 189 to <252 DFE | 209 | 0.87 | (0.72, 1.04) | 194 | 0.86 | (0.71, 1.05) | ||
| 252 to <343 DFE | 224 | 0.92 | (0.76, 1.10) | 216 | 0.96 | (0.80, 1.17) | ||
| ≥343 DFE | 181 | 0.79 | (0.65, 0.96) | 177 | 0.83 | (0.68, 1.01) | ||
| Supplemental folic acid | ||||||||
| 0 μg | 444 | 1.00 | (Ref) | 0.10 | 425 | 1.00 | (Ref) | 0.59 |
| >0–400 μg | 356 | 0.88 | (0.76, 1.01) | 341 | 0.94 | (0.81, 1.09) | ||
| >400 μg | 43 | 0.90 | (0.66, 1.23) | 42 | 1.01 | (0.74, 1.39) | ||
| Folate, total | ||||||||
| <242 DFE | 230 | 1.00 | (Ref) | 0.06 | 218 | 1.00 | (Ref) | 0.51 |
| 242 to <542 DFE | 208 | 0.88 | (0.73, 1.06) | 199 | 0.92 | (0.76, 1.12) | ||
| 542 to <939 DFE | 211 | 0.88 | (0.73, 1.06) | 204 | 0.96 | (0.79, 1.16) | ||
| ≥939 DFE | 194 | 0.81 | (0.66, 0.97) | 187 | 0.90 | (0.74, 1.10) | ||
| Dietary vitamin B-12 | ||||||||
| <3.52 μg | 226 | 1.00 | (Ref) | 0.65 | 216 | 1.00 | (Ref) | 0.42 |
| 3.52–5.14 μg | 203 | 0.89 | (0.73, 1.07) | 192 | 0.88 | (0.72, 1.06) | ||
| 5.15–7.27 μg | 202 | 0.87 | (0.72, 1.05) | 194 | 0.86 | (0.71, 1.04) | ||
| >7.27 μg | 212 | 0.94 | (0.78, 1.13) | 206 | 0.91 | (0.75, 1.10) | ||
| Supplemental vitamin B-12 | ||||||||
| 0 μg | 433 | 1.00 | (Ref) | 0.07 | 415 | 1.00 | (Ref) | 0.27 |
| >0–6.0 μg | 312 | 0.90 | (0.78, 1.04) | 299 | 0.96 | (0.83, 1.12) | ||
| >6.0 μg | 98 | 0.81 | (0.65, 1.01) | 94 | 0.88 | (0.70, 1.10) | ||
| Vitamin B-12, total (μg) | ||||||||
| <5.13 (μg) | 215 | 1.00 | (Ref) | 0.051 | 203 | 1.00 | (Ref) | 0.17 |
| 5.13–8.98 (μg) | 225 | 1.02 | (0.85, 1.23) | 217 | 1.04 | (0.86, 1.26) | ||
| 8.99–12.87 (μg) | 217 | 0.96 | (0.79, 1.16) | 210 | 1.02 | (0.84, 1.23) | ||
| >12.87 (μg) | 186 | 0.84 | (0.69, 1.03) | 178 | 0.88 | (0.72, 1.08) | ||
| Dietary vitamin B-6 | ||||||||
| <1.16 μg | 236 | 1.00 | (Ref) | <0.01 | 225 | 1.00 | (Ref) | 0.03 |
| 1.16–1.53 μg | 213 | 0.87 | (0.72, 1.05) | 203 | 0.90 | (0.74, 1.09) | ||
| 1.54–1.99 μg | 203 | 0.81 | (0.67, 0.98) | 197 | 0.86 | (0.71, 1.04) | ||
| >1.99 μg | 191 | 0.77 | (0.63, 0.93) | 183 | 0.80 | (0.66, 0.97) | ||
| Supplemental vitamin B-6 | ||||||||
| 0 mg | 436 | 1.00 | (Ref) | 0.06 | 418 | 1.00 | (Ref) | 0.23 |
| >0–2.0 mg | 306 | 0.88 | (0.76, 1.02) | 293 | 0.94 | (0.81, 1.09) | ||
| >2.0 mg | 101 | 0.80 | (0.64, 0.99) | 97 | 0.87 | (0.69, 1.08) | ||
| Vitamin B-6, total | ||||||||
| <1.52 mg | 231 | 1.00 | (Ref) | <0.01 | 218 | 1.00 | (Ref) | 0.051 |
| 1.52–2.74 mg | 210 | 0.87 | (0.72, 1.05) | 205 | 0.93 | (0.76, 1.12) | ||
| 2.75–3.88 mg | 223 | 0.89 | (0.74, 1.07) | 215 | 0.98 | (0.81, 1.19) | ||
| >3.88 mg | 179 | 0.73 | (0.60, 0.89) | 170 | 0.80 | (0.66, 0.99) | ||
| Dietary riboflavin | ||||||||
| <1.37 mg | 210 | 1.00 | (Ref) | 0.42 | 200 | 1.00 | (Ref) | 0.41 |
| 1.37–1.84 mg | 217 | 0.99 | (0.82, 1.20) | 205 | 0.99 | (0.81, 1.20) | ||
| 1.85–2.43 mg | 211 | 0.96 | (0.79, 1.16) | 203 | 0.95 | (0.78, 1.16) | ||
| >2.43 mg | 205 | 0.93 | (0.77, 1.13) | 200 | 0.93 | (0.76, 1.13) | ||
| Supplemental riboflavin | ||||||||
| 0 mg | 443 | 1.00 | (Ref) | 0.03 | 426 | 1.00 | (Ref) | 0.13 |
| >0–1.7 mg | 315 | 0.88 | (0.76, 1.02) | 301 | 0.94 | (0.81, 1.09) | ||
| >1.7 mg | 85 | 0.77 | (0.61, 0.97) | 81 | 0.83 | (0.65, 1.05) | ||
| Riboflavin, total | ||||||||
| <1.80 mg | 221 | 1.00 | (Ref) | <0.01 | 209 | 1.00 | (Ref) | 0.04 |
| 1.80–2.87 mg | 216 | 0.94 | (0.78, 1.13) | 212 | 0.97 | (0.80, 1.17) | ||
| 2.88–3.97 mg | 229 | 0.97 | (0.80, 1.16) | 216 | 1.00 | (0.82, 1.21) | ||
| >3.97 mg | 177 | 0.75 | (0.62, 0.92) | 171 | 0.81 | (0.66, 0.99) | ||
DFE, dietary folate equivalent; Ref, reference.
Cox model adjusted for age, BMI (in kg/m2; <25, 25 to <30, 30 to <35, or ≥35), race-ethnicity (white, black, or other), past medical history of colonoscopy (yes or no), smoking status (never, past, or current), physical activity (0–3, 3 to <11.75, or ≥11.75 metabolic task equivalent hours/wk), and postmenopausal hormone use (never, past, or current).
Fewer cases were in the multivariable analyses because women with missing data on any one or more covariates were excluded from the analysis.
Associations by tumor stage are presented in Table 3. No significant differences were detected for localized and distant disease. However, there was an inverse association between the dietary intakes of folate (HR: 0.66; 95% CI: 0.48, 0.91) and CRC risk among those with regionally spread CRC, although the difference in risks between tumor stages was not significant (P = 0.37). Similarly, vitamin B-6 intake [total (HR: 0.65; 95% CI: 0.47, 0.90), dietary (HR: 0.73; 95% CI: 0.53, 0.99), and supplemental (HR: 0.66; 95% CI: 0.45, 0.96)] and riboflavin intake [total (HR: 0.71; 95% CI: 0.51, 0.99) and supplemental (HR: 0.66; 95% CI: 0.45, 0.99)] were inversely associated with regionally spread disease. No site-specific differences for proximal colon, distal colon, or rectum were seen (Table S1 under “Supplemental data” in the online issue).
TABLE 3.
Multivariate HRs and 95% CIs for the associations of one-carbon metabolism nutrient intake with colorectal cancer by tumor stage1
| Localized |
Regional |
Distant |
||||||||||
| n | HR | (95% CI) | P-trend | n | HR | (95% CI) | P-trend | n | HR | (95% CI) | P-trend | |
| Dietary folate | ||||||||||||
| <189 DFE | 93 | 1.00 | (Ref) | 0.65 | 101 | 1.00 | (Ref) | 0.04 | 21 | 1.00 | (Ref) | 0.48 |
| 189 to <252 DFE | 80 | 0.85 | (0.63, 1.14) | 74 | 0.70 | (0.52, 0.95) | 33 | 1.65 | (0.95, 2.85) | |||
| 252 to <343 DFE | 87 | 0.93 | (0.69, 1.25) | 97 | 0.92 | (0.69, 1.21) | 27 | 1.37 | (0.77, 2.44) | |||
| ≥343 DFE | 79 | 0.89 | (0.66, 1.21) | 67 | 0.66 | (0.48, 0.91) | 27 | 1.43 | (0.80, 2.54) | |||
| Supplemental folic acid | ||||||||||||
| 0 μg | 178 | 1.00 | (Ref) | 1.00 | 183 | 1.00 | (Ref) | 0.23 | 51 | 1.00 | (Ref) | 0.39 |
| >0–400 μg | 139 | 0.91 | (0.72, 1.14) | 142 | 0.90 | (0.72, 1.12) | 52 | 1.25 | (0.85, 1.86) | |||
| >400 μg | 22 | 1.25 | (0.80, 1.95) | 14 | 0.78 | (0.45, 1.34) | 5 | 1.05 | (0.42, 2.65) | |||
| Folate, total | ||||||||||||
| <242 DFE | 87 | 1.00 | (Ref) | 0.99 | 97 | 1.00 | (Ref) | 0.12 | 27 | 1.00 | (Ref) | 0.26 |
| 242 to <542 DFE | 84 | 0.98 | (0.73, 1.33) | 87 | 0.88 | (0.66, 1.18) | 23 | 0.90 | (0.51, 1.57) | |||
| 542 to <939 DFE | 88 | 1.03 | (0.77, 1.39) | 81 | 0.84 | (0.62, 1.13) | 29 | 1.16 | (0.68, 1.97) | |||
| ≥939 DFE | 80 | 0.97 | (0.71, 1.32) | 74 | 0.77 | (0.57, 1.05) | 29 | 1.23 | (0.72, 2.09) | |||
| Dietary vitamin B-12 | ||||||||||||
| <3.52 μg | 95 | 1.00 | (Ref) | 0.59 | 85 | 1.00 | (Ref) | 0.73 | 31 | 1.00 | (Ref) | 0.40 |
| 3.52–5.14 μg | 77 | 0.79 | (0.58, 1.07) | 84 | 0.97 | (0.72, 1.31) | 27 | 0.88 | (0.53, 1.48) | |||
| 5.15–7.27 μg | 78 | 0.78 | (0.57, 1.05) | 84 | 0.94 | (0.69, 1.27) | 25 | 0.80 | (0.47, 1.37) | |||
| >7.27 μg | 89 | 0.89 | (0.66, 1.19) | 86 | 0.95 | (0.70, 1.29) | 25 | 0.80 | (0.47, 1.36) | |||
| Supplemental vitamin B-12 | ||||||||||||
| 0 (μg) | 174 | 1.00 | (Ref) | 0.98 | 178 | 1.00 | (Ref) | 0.10 | 51 | 1.00 | (Ref) | 0.99 |
| >0–6.0 (μg) | 120 | 0.91 | (0.72, 1.15) | 127 | 0.94 | (0.74, 1.18) | 44 | 1.21 | (0.80, 1.82) | |||
| >6.0 (μg) | 45 | 0.99 | (0.71, 1.38) | 34 | 0.73 | (0.51, 1.06) | 13 | 1.02 | (0.55, 1.89) | |||
| Vitamin B-12, total | ||||||||||||
| <5.13 μg | 88 | 1.00 | (Ref) | 0.58 | 79 | 1.00 | (Ref) | 0.19 | 31 | 1.00 | (Ref) | 0.85 |
| 5.13–8.98 μg | 88 | 0.97 | (0.72, 1.31) | 96 | 1.17 | (0.87, 1.58) | 24 | 0.78 | (0.46, 1.33) | |||
| 8.99–12.87 μg | 82 | 0.91 | (0.67, 1.23) | 97 | 1.19 | (0.88, 1.60) | 27 | 0.91 | (0.54, 1.53) | |||
| >12.87 μg | 81 | 0.92 | (0.68, 1.25) | 67 | 0.84 | (0.61, 1.17) | 26 | 0.89 | (0.53, 1.51) | |||
| Dietary vitamin B-6 | ||||||||||||
| <1.16 mg | 95 | 1.00 | (Ref) | 0.25 | 92 | 1.00 | (Ref) | 0.02 | 33 | 1.00 | (Ref) | 0.96 |
| 1.16–1.53 mg | 84 | 0.88 | (0.65, 1.18) | 95 | 1.01 | (0.75, 1.34) | 19 | 0.61 | (0.35, 1.07) | |||
| 1.54–1.99 mg | 79 | 0.81 | (0.60, 1.10) | 81 | 0.83 | (0.62, 1.13) | 29 | 0.94 | (0.56, 1.55) | |||
| >1.99 mg | 81 | 0.84 | (0.62, 1.13) | 71 | 0.73 | (0.53, 0.99) | 27 | 0.88 | (0.52, 1.48) | |||
| Supplemental vitamin B-6 | ||||||||||||
| 0 mg | 177 | 1.00 | (Ref) | 0.80 | 180 | 1.00 | (Ref) | 0.03 | 49 | 1.00 | (Ref) | 0.23 |
| >0–2.0 mg | 117 | 0.88 | (0.69, 1.11) | 127 | 0.93 | (0.74, 1.17) | 41 | 1.18 | (0.77, 1.80) | |||
| >2.0 mg | 45 | 0.94 | (0.67, 1.30) | 32 | 0.66 | (0.45, 0.96) | 18 | 1.42 | (0.82, 2.44) | |||
| Vitamin B-6, total | ||||||||||||
| <1.52 mg | 92 | 1.00 | (Ref) | 0.25 | 92 | 1.00 | (Ref) | 0.01 | 29 | 1.00 | (Ref) | 0.21 |
| 1.52–2.74 mg | 88 | 0.94 | (0.70, 1.27) | 89 | 0.93 | (0.69, 1.24) | 20 | 0.72 | (0.41, 1.28) | |||
| 2.75–3.88 mg | 84 | 0.90 | (0.67, 1.21) | 98 | 1.03 | (0.77, 1.37) | 28 | 1.04 | (0.61, 1.76) | |||
| >3.89 mg | 75 | 0.84 | (0.61, 1.14) | 60 | 0.65 | (0.47, 0.90) | 31 | 1.20 | (0.72, 2.02) | |||
| Dietary riboflavin | ||||||||||||
| <1.37 mg | 83 | 1.00 | (Ref) | 0.96 | 81 | 1.00 | (Ref) | 0.40 | 30 | 1.00 | (Ref) | 0.49 |
| 1.37–1.84 mg | 85 | 0.97 | (0.72, 1.32) | 86 | 1.02 | (0.75, 1.38) | 31 | 1.05 | (0.64, 1.75) | |||
| 1.85–2.43 mg | 79 | 0.88 | (0.65, 1.20) | 94 | 1.07 | (0.80, 1.45) | 20 | 0.67 | (0.38, 1.19) | |||
| >2.43 mg | 92 | 1.01 | (0.75, 1.37) | 78 | 0.87 | (0.64, 1.20) | 27 | 0.90 | (0.53, 1.54) | |||
| Supplemental riboflavin | ||||||||||||
| 0 mg | 181 | 1.00 | (Ref) | 0.52 | 182 | 1.00 | (Ref) | 0.048 | 51 | 1.00 | (Ref) | 0.50 |
| >0–1.7 mg | 121 | 0.88 | (0.70, 1.11) | 129 | 0.93 | (0.74, 1.17) | 43 | 1.18 | (0.78, 1.78) | |||
| >1.7 mg | 37 | 0.88 | (0.62, 1.26) | 28 | 0.66 | (0.45, 0.99) | 14 | 1.24 | (0.68, 2.25) | |||
| Riboflavin, total | ||||||||||||
| <1.80 mg | 85 | 1.00 | (Ref) | 0.30 | 84 | 1.00 | (Ref) | 0.04 | 35 | 1.00 | (Ref) | 0.90 |
| 1.80–2.87 mg | 92 | 1.03 | (0.76, 1.38) | 92 | 1.03 | (0.77, 1.39) | 18 | 0.51 | (0.29, 0.91) | |||
| 2.88–3.97 mg | 86 | 0.97 | (0.71, 1.31) | 101 | 1.14 | (0.85, 1.53) | 26 | 0.78 | (0.46, 1.30) | |||
| >3.97 mg | 76 | 0.87 | (0.64, 1.19) | 62 | 0.71 | (0.51, 0.99) | 29 | 0.88 | (0.53, 1.46) | |||
A Cox model adjusted for age, BMI (in kg/m2; <25, 25 to <30, 30 to <35, or ≥35), race-ethnicity (white, black, or other), past medical history of colonoscopy (yes or no), smoking status (never, past, or current), physical activity (0 to 3, 3 to <11.75, or ≥11.75 metabolic equivalent task hours/wk), and postmenopausal hormone use (never, past, or current). DFE, dietary folate equivalent; Ref, reference.
To investigate whether FDA-mandated FA fortification influenced CRC risk, we conducted analyses stratified by time exposed to FA fortification (Table 4). For dietary folate intake of ≥343 compared with <189 μg DFE/d, increased risks were observed specifically in participants exposed to fortification for 3 to <6 y (HR: 1.54; 95% CI: 1.09, 2.16; P-trend < 0.01) and 6–9 y (HR: 3.24; 95% CI: 2.23, 4.72; P-trend < 0.01) but not those exposed <3 y or ≥9 y. Trends by time exposed to FA fortification were significantly different (P-interaction < 0.01). The trend was evident among both supplemental FA takers and non–supplement takers (data not shown). These differences by fortification period were observed only for dietary folate and not for other nutrients.
TABLE 4.
Multivariate HRs and 95% CIs for the associations of one-carbon metabolism nutrient intake with colorectal cancer by time since exposure to folic acid fortification1
| Fortification (total patient-years) |
|||||||||||||
| <3 y (5123)2 |
3 to <6 y (21,434) |
6 to <9 y (163,885) |
≥9 y (693,016) |
||||||||||
| Cases | HR | (95% CI) | Cases | HR | (95% CI) | Cases | HR | (95% CI) | Cases | HR | (95% CI) | P-interaction3 | |
| Dietary folate | <0.01 | ||||||||||||
| <189 DFE | 55 | 1.00 | (Ref) | 83 | 1.00 | (Ref) | 48 | 1.00 | (Ref) | 35 | 1.00 | (Ref) | |
| 189 to <252 DFE | 52 | 0.16 | (0.79, 1.70) | 67 | 0.96 | (0.69, 1.33) | 42 | 0.98 | (0.64, 1.48) | 33 | 0.91 | (0.56, 1.47) | |
| 252 to <343 DFE | 33 | 0.77 | (0.49, 1.20) | 71 | 1.17 | (0.85, 1.61) | 70 | 1.74 | (1.20, 2.52) | 42 | 1.18 | (0.75, 1.86) | |
| ≥343 DFE | 15 | 0.85 | (0.48, 1.52) | 58 | 1.54 | (1.09, 2.16) | 71 | 3.24 | (2.23, 4.72) | 33 | 1.36 | (0.84, 2.22) | |
| P-trend | 0.31 | <0.01 | <0.01 | 0.12 | |||||||||
| Supplemental folic acid | 0.57 | ||||||||||||
| 0 μg | 83 | 1.00 | (Ref) | 156 | 1.00 | (Ref) | 115 | 1.00 | (Ref) | 71 | 1.00 | (Ref) | |
| >0–400 μg | 67 | 1.04 | (0.75, 1.45) | 113 | 1.05 | (0.82, 1.34) | 101 | 1.15 | (0.88, 1.51) | 60 | 0.97 | (0.69, 1.37) | |
| >400 μg | 5 | 0.69 | (0.28, 1.73) | 10 | 0.85 | (0.45, 1.63) | 15 | 1.55 | (0.90, 2.66) | 12 | 1.72 | (0.93, 3.19) | |
| P-trend | 0.80 | 0.98 | 0.11 | 0.36 | |||||||||
| Folate, total | 0.09 | ||||||||||||
| <242 DFE | 57 | 1.00 | (Ref) | 82 | 1.00 | (Ref) | 47 | 1.00 | (Ref) | 32 | 1.00 | (Ref) | |
| 242 to <542 DFE | 27 | 0.68 | (0.43, 1.10) | 73 | 1.25 | (0.91, 1.72) | 62 | 1.69 | (1.16, 2.48) | 37 | 1.26 | (0.78, 2.02) | |
| 542 to <939 DFE | 49 | 1.09 | (0.73, 1.61) | 64 | 1.06 | (0.76, 1.48) | 53 | 1.44 | (0.97, 2.14) | 38 | 1.25 | (0.78, 2.01) | |
| ≥939 DFE | 22 | 0.70 | (0.42, 1.16) | 60 | 1.42 | (1.01, 1.99) | 69 | 2.48 | (1.69, 3.62) | 36 | 1.36 | (0.84, 2.21) | |
| P-trend | 0.87 | 0.23 | <0.01 | 0.30 | |||||||||
| Dietary vitamin B-12 | 0.18 | ||||||||||||
| <3.52 μg | 47 | 1.00 | (Ref) | 74 | 1.00 | (Ref) | 57 | 1.00 | (Ref) | 38 | 1.00 | (Ref) | |
| 3.52–5.14 μg | 37 | 0.86 | (0.56, 1.34) | 75 | 1.22 | (0.88, 1.69) | 53 | 1.02 | (0.70, 1.48) | 27 | 0.70 | (0.43, 1.14) | |
| 5.15–7.27 μg | 39 | 0.81 | (0.52, 1.24) | 64 | 1.01 | (0.72, 1.42) | 53 | 0.98 | (0.67, 1.43) | 38 | 0.95 | (0.61, 1.50) | |
| >7.27 μg | 32 | 0.73 | (0.47, 1.16) | 66 | 0.86 | (0.62, 1.21) | 68 | 1.23 | (0.86, 1.76) | 40 | 1.03 | (0.66, 1.62) | |
| P-trend | 0.18 | 0.20 | 0.23 | 0.54 | |||||||||
| Supplemental vitamin B-12 | 0.14 | ||||||||||||
| 0 mg | 79 | 1.00 | (Ref) | 151 | 1.00 | (Ref) | 115 | 1.00 | (Ref) | 70 | 1.00 | (Ref) | |
| >0–6.0 mg | 65 | 1.21 | (0.86, 1.69) | 96 | 1.03 | (0.80, 1.34) | 82 | 1.07 | (0.80, 1.43) | 56 | 1.05 | (0.73, 1.49) | |
| >6.0 mg | 11 | 0.55 | (0.29, 1.04) | 32 | 1.00 | (0.68, 1.48) | 34 | 1.26 | (0.86, 1.86) | 17 | 0.91 | (0.54, 1.56) | |
| P-trend | 0.06 | 0.99 | 0.28 | 0.75 | |||||||||
| Vitamin B-12 intake, total | 0.11 | ||||||||||||
| <5.13 μg | 44 | 1.00 | (Ref) | 79 | 1.00 | (Ref) | 49 | 1.00 | (Ref) | 31 | 1.00 | (Ref) | |
| 5.13–8.98 μg | 42 | 0.97 | (0.63, 1.49) | 70 | 0.95 | (0.68, 1.31) | 65 | 1.35 | (0.93, 1.96) | 40 | 1.25 | (0.78, 1.99) | |
| 8.99–12.87 μg | 44 | 1.02 | (0.67, 1.56) | 74 | 1.18 | (0.86, 1.63) | 55 | 1.27 | (0.86, 1.87) | 37 | 1.17 | (0.73, 1.90) | |
| >12.87 μg | 25 | 0.65 | (0.39, 1.07) | 56 | 0.82 | (0.58, 1.16) | 62 | 1.43 | (0.98, 2.09) | 35 | 1.14 | (0.70, 1.86) | |
| P-trend | 0.10 | 0.36 | 0.11 | 0.76 | |||||||||
| Dietary vitamin B-6 | 0.07 | ||||||||||||
| <1.16 mg | 49 | 1.00 | (Ref) | 92 | 1.00 | (Ref) | 50 | 1.00 | (Ref) | 34 | 1.00 | (Ref) | |
| 1.16–1.53 mg | 44 | 1.05 | (0.70, 1.59) | 61 | 0.74 | (0.53, 1.02) | 63 | 1.32 | (0.91, 1.92) | 35 | 1.00 | (0.62, 1.61) | |
| 1.54–1.99 mg | 37 | 0.86 | (0.56, 1.34) | 70 | 0.89 | (0.65, 1.22) | 52 | 1.07 | (0.72, 1.58) | 38 | 1.05 | (0.66, 1.68) | |
| >1.99 mg | 25 | 0.65 | (0.40, 1.06) | 56 | 0.69 | (0.49, 0.97) | 66 | 1.36 | (0.94, 1.97) | 36 | 1.01 | (0.63, 1.62) | |
| P-trend | 0.06 | 0.07 | 0.21 | 0.95 | |||||||||
| Supplemental vitamin B-6 | 0.09 | ||||||||||||
| 0 mg | 81 | 1.00 | (Ref) | 152 | 1.00 | (Ref) | 115 | 1.00 | (Ref) | 70 | 1.00 | (Ref) | |
| >0–2.0 mg | 65 | 1.20 | (0.86, 1.68) | 92 | 0.99 | (0.76, 1.28) | 83 | 1.07 | (0.81, 1.43) | 53 | 1.00 | (0.69, 1.43) | |
| >2.0 mg | 9 | 0.47 | (0.24, 0.95) | 35 | 1.07 | (0.73, 1.55) | 33 | 1.24 | (0.84, 1.83) | 20 | 1.05 | (0.64, 1.73) | |
| P-trend | 0.03 | 0.73 | 0.33 | 0.84 | |||||||||
| Vitamin B-6 intake, total | 0.27 | ||||||||||||
| <1.52 mg | 48 | 1.00 | (Ref) | 78 | 1.00 | (Ref) | 58 | 1.00 | (Ref) | 34 | 1.00 | (Ref) | |
| 1.52–2.74 mg | 36 | 0.93 | (0.60, 1.45) | 76 | 1.07 | (0.78, 1.47) | 61 | 1.04 | (0.72, 1.49) | 32 | 0.91 | (0.56, 1.48) | |
| 2.75–3.88 mg | 51 | 1.24 | (0.83, 1.87) | 68 | 1.10 | (0.79, 1.53) | 54 | 1.03 | (0.71, 1.50) | 42 | 1.21 | (0.76, 1.91) | |
| >3.88 mg | 20 | 0.56 | (0.33, 0.95) | 57 | 0.97 | (0.69, 1.38) | 58 | 1.15 | (0.79, 1.66) | 35 | 1.04 | (0.64, 1.68) | |
| P-trend | 0.07 | 0.83 | 0.47 | 0.68 | |||||||||
| Dietary riboflavin | 0.11 | ||||||||||||
| <1.37 mg | 51 | 1.00 | (Ref) | 74 | 1.00 | (Ref) | 48 | 1.00 | (Ref) | 27 | 1.00 | (Ref) | |
| 1.37–1.84 mg | 35 | 0.77 | (0.50, 1.20) | 67 | 1.05 | (0.75, 1.46) | 60 | 1.30 | (0.89, 1.90) | 43 | 1.46 | (0.90, 2.37) | |
| 1.85–2.43 mg | 40 | 0.76 | (0.50, 1.16) | 71 | 1.18 | (0.85, 1.65) | 63 | 1.33 | (0.91, 1.94) | 29 | 0.95 | (0.56, 1.61) | |
| >2.43 mg | 29 | 0.59 | (0.37, 0.94) | 67 | 1.03 | (0.74, 1.45) | 60 | 1.20 | (0.81, 1.76) | 44 | 1.43 | (0.88, 2.32) | |
| P-trend | 0.03 | 0.78 | 0.49 | 0.35 | |||||||||
| Supplemental riboflavin | 0.20 | ||||||||||||
| 0 mg | 82 | 1.00 | (Ref) | 156 | 1.00 | (Ref) | 117 | 1.00 | (Ref) | 71 | 1.00 | (Ref) | |
| >0–1.7 mg | 64 | 1.14 | (0.81, 1.59) | 96 | 1.00 | (0.77, 1.29) | 85 | 1.07 | (0.80, 1.42) | 56 | 1.03 | (0.72, 1.47) | |
| >1.7 mg | 9 | 0.49 | (0.25, 0.99) | 27 | 0.96 | (0.63, 1.45) | 29 | 1.18 | (0.78, 1.77) | 16 | 0.94 | (0.54, 1.62) | |
| P-trend | 0.046 | 0.84 | 0.43 | 0.80 | |||||||||
| Riboflavin intake, total | 0.07 | ||||||||||||
| <1.80 mg | 49 | 1.00 | (Ref) | 78 | 1.00 | (Ref) | 52 | 1.00 | (Ref) | 30 | 1.00 | (Ref) | |
| 1.80–2.87 mg | 37 | 0.83 | (0.54, 1.27) | 77 | 1.14 | (0.83, 1.57) | 58 | 1.09 | (0.75, 1.58) | 40 | 1.23 | (0.77, 1.98) | |
| 2.88–3.97 mg | 48 | 1.09 | (0.72, 1.64) | 65 | 1.06 | (0.76, 1.48) | 62 | 1.28 | (0.88, 1.85) | 41 | 1.28 | (0.79, 2.05) | |
| >3.97 mg | 21 | 0.48 | (0.28, 0.81) | 59 | 1.00 | (0.71, 1.42) | 59 | 1.21 | (0.83, 1.77) | 32 | 0.99 | (0.60, 1.64) | |
| P-trend | 0.01 | 0.87 | 0.29 | 0.78 | |||||||||
Colorectal cancer risks for dietary, supplemental, and total intake of each nutrient were estimated in separate models. A Cox model adjusted for age, BMI (in kg/m2; <25, 25 to <30, 30 to <35, or ≥35), race-ethnicity (white, black, or other), past medical history of colonoscopy (yes or no), smoking status (never, past, or current), physical activity (0 to 3, 3 to <11.75, or ≥11.75 metabolic equivalent task hours/wk), and postmenopausal hormone use (never, past, or current). DFE, dietary folate equivalent; Ref, reference.
For the majority of women who were exposed to <3 y of folic acid fortification, dietary folate intake likely consisted of natural folate only.
P value for test of interaction between the linear trend variable of interest and fortification year categories.
The associations of B vitamin intakes with CRC incidence by alcohol intake are shown in Table 5. In nondrinkers and current drinkers who consumed ≥1 alcoholic drinks/wk, no associations between the amounts of nutrient intakes could be seen. However, we observed inverse associations between CRC risk and total intakes for all analyzed B vitamins (HR for folate: 0.71; 95% CI: 0.51, 0.98; HR for vitamin B-12: 0.62; 95% CI: 0.43, 0.88; HR for vitamin B-6: 0.50; 95% CI: 0.35, 0.72; HR for riboflavin: 0.57; 95% CI: 0.40, 0.81; all P < 0.01) in current drinkers who consumed <1 drink/wk. The trends of these associations among current drinkers who consumed <1 drink/wk were significantly different from those among nondrinkers or among current drinkers who consumed ≥1 drink/wk (P for differences in slopes <0.05).
TABLE 5.
Multivariate HRs and 95% CIs for the associations of one-carbon metabolism nutrient intake with colorectal cancer by alcohol use1
| Nondrinkers(never or past drinkers) | Current drinkers(<1 drink/wk) | Current drinkers(≥1 drink/wk) | |||||||||||
| Cases | HR | (95% CI) | Cases | HR | (95% CI) | Cases | HR | (95% CI) | P-interaction2 | ||||
| Dietary folate | 0.89 | ||||||||||||
| <189 DFE | 65 | 1.00 | (Ref) | 84 | 1.00 | (Ref) | 71 | 1.00 | (Ref) | ||||
| 189 to <252 DFE | 62 | 1.12 | (0.79, 1.59) | 59 | 0.71 | (0.50, 0.99) | 73 | 0.85 | (0.61, 1.18) | ||||
| 252 to <343 DFE | 55 | 1.08 | (0.75, 1.55) | 78 | 0.95 | (0.70, 1.30) | 82 | 0.91 | (0.66, 1.26) | ||||
| ≥343 DFE | 48 | 0.86 | (0.59, 1.26) | 62 | 0.80 | (0.57, 1.12) | 67 | 0.85 | (0.61, 1.19) | ||||
| P-trend | 0.35 | 0.45 | 0.49 | ||||||||||
| Supplemental folic acid | 0.01 | ||||||||||||
| 0 μg | 126 | 1.00 | (Ref) | 164 | 1.00 | (Ref) | 134 | 1.00 | (Ref) | ||||
| >0–400 μg | 89 | 0.99 | (0.75, 1.30) | 110 | 0.75 | (0.59, 0.96) | 141 | 1.11 | (0.88, 1.41) | ||||
| >400 μg | 15 | 1.35 | (0.79, 2.32) | 9 | 0.54 | (0.28, 1.06) | 18 | 1.30 | (0.80, 2.14) | ||||
| P-trend | 0.56 | <0.01 | 0.23 | ||||||||||
| Folate, total | 0.02 | ||||||||||||
| <242 DFE | 71 | 1.00 | (Ref) | 89 | 1.00 | (Ref) | 57 | 1.00 | (Ref) | ||||
| 242 to <542 DFE | 53 | 0.88 | (0.62, 1.26) | 75 | 0.85 | (0.63, 1.16) | 71 | 1.11 | (0.78, 1.58) | ||||
| 542 to <939 DFE | 56 | 0.99 | (0.70, 1.41) | 58 | 0.63 | (0.45, 0.89) | 90 | 1.42 | (1.02, 1.98) | ||||
| ≥939 DFE | 50 | 0.94 | (0.65, 1.36) | 61 | 0.71 | (0.51, 0.98) | 75 | 1.17 | (0.82, 1.65) | ||||
| P-trend | 0.99 | <0.01 | 0.15 | ||||||||||
| Dietary vitamin B-12 | 0.28 | ||||||||||||
| <3.52 μg | 70 | 1.00 | (Ref) | 77 | 1.00 | (Ref) | 68 | 1.00 | (Ref) | ||||
| 3.52–5.14 μg | 57 | 0.96 | (0.67, 1.36) | 68 | 0.86 | (0.62, 1.19) | 67 | 0.86 | (0.61, 1.20) | ||||
| 5.15–7.27 μg | 44 | 0.74 | (0.51, 1.09) | 75 | 0.90 | (0.65, 1.24) | 74 | 0.91 | (0.65, 1.27) | ||||
| >7.27 μg | 59 | 0.90 | (0.63, 1.28) | 63 | 0.74 | (0.53, 1.04) | 84 | 1.12 | (0.81, 1.54) | ||||
| P-trend | 0.44 | 0.11 | 0.30 | ||||||||||
| Supplemental vitamin B-12 | 0.25 | ||||||||||||
| 0 μg | 121 | 1.00 | (Ref) | 159 | 1.00 | (Ref) | 134 | 1.00 | (Ref) | ||||
| >0–6.0 μg | 80 | 1.07 | (0.80, 1.42) | 97 | 0.78 | (0.60, 1.00) | 122 | 1.08 | (0.85, 1.39) | ||||
| >6.0 μg | 29 | 1.01 | (0.67, 1.52) | 27 | 0.64 | (0.42, 0.96) | 37 | 1.02 | (0.71, 1.47) | ||||
| P-trend | 0.97 | 0.04 | 0.96 | ||||||||||
| Vitamin B-12, total | 0.03 | ||||||||||||
| <5.13 μg | 65 | 1.00 | (Ref) | 77 | 1.00 | (Ref) | 60 | 1.00 | (Ref) | ||||
| 5.13–8.98 μg | 53 | 0.89 | (0.62, 1.28) | 90 | 1.10 | (0.81, 1.49) | 74 | 1.14 | (0.81, 1.60) | ||||
| 8.99–12.87 μg | 58 | 1.08 | (0.76, 1.54) | 66 | 0.81 | (0.58, 1.13) | 86 | 1.24 | (0.89, 1.73) | ||||
| >12.87 μg | 54 | 0.97 | (0.67, 1.40) | 50 | 0.62 | (0.43, 0.88) | 73 | 1.15 | (0.81, 1.62) | ||||
| P-trend | 0.93 | <0.01 | 0.49 | ||||||||||
| Dietary vitamin B-6 | 0.45 | ||||||||||||
| <1.16 mg | 71 | 1.00 | (Ref) | 84 | 1.00 | (Ref) | 69 | 1.00 | (Ref) | ||||
| 1.16–1.53 mg | 51 | 0.86 | (0.60, 1.23) | 75 | 0.91 | (0.66, 1.24) | 76 | 0.93 | (0.67, 1.29) | ||||
| 1.54–1.99 mg | 58 | 1.02 | (0.71, 1.44) | 68 | 0.79 | (0.57, 1.10) | 71 | 0.84 | (0.60, 1.18) | ||||
| >1.99 mg | 50 | 0.84 | (0.58, 1.21) | 56 | 0.67 | (0.48, 0.95) | 77 | 0.92 | (0.66, 1.28) | ||||
| P-trend | 0.50 | 0.02 | 0.60 | ||||||||||
| Supplemental vitamin B-6 | 0.07 | ||||||||||||
| 0 mg | 125 | 1.00 | (Ref) | 160 | 1.00 | (Ref) | 132 | 1.00 | (Ref) | ||||
| >0–2.0 mg | 79 | 1.02 | (0.77, 1.36) | 97 | 0.77 | (0.60, 1.00) | 117 | 1.06 | (0.83, 1.37) | ||||
| >2.0 mg | 26 | 0.86 | (0.56, 1.32) | 26 | 0.60 | (0.39, 0.91) | 44 | 1.16 | (0.82, 1.63) | ||||
| P-trend | 0.45 | 0.03 | 0.44 | ||||||||||
| Vitamin B-6, total | <0.01 | ||||||||||||
| <1.52 mg | 64 | 1.00 | (Ref) | 91 | 1.00 | (Ref) | 62 | 1.00 | (Ref) | ||||
| 1.52–2.74 mg | 63 | 1.16 | (0.82, 1.65) | 75 | 0.79 | (0.58, 1.08) | 67 | 0.94 | (0.67, 1.34) | ||||
| 2.75–3.88 mg | 58 | 1.16 | (0.81, 1.66) | 71 | 0.73 | (0.53, 1.00) | 86 | 1.17 | (0.84, 1.63) | ||||
| >3.88 mg | 45 | 0.93 | (0.63, 1.37) | 46 | 0.50 | (0.35, 0.72) | 78 | 1.10 | (0.79, 1.55) | ||||
| P-trend | 0.56 | <0.01 | 0.38 | ||||||||||
| Dietary riboflavin | 0.46 | ||||||||||||
| <1.37 mg | 66 | 1.00 | (Ref) | 72 | 1.00 | (Ref) | 61 | 1.00 | (Ref) | ||||
| 1.37–1.84 mg | 55 | 1.03 | (0.72, 1.48) | 68 | 0.89 | (0.63, 1.24) | 82 | 1.11 | (0.79, 1.54) | ||||
| 1.85–2.43 mg | 54 | 1.00 | (0.69, 1.44) | 80 | 0.98 | (0.71, 1.35) | 68 | 0.90 | (0.64, 1.28) | ||||
| >2.43 mg | 55 | 0.97 | (0.67, 1.40) | 63 | 0.74 | (0.52, 1.04) | 82 | 1.13 | (0.81, 1.58) | ||||
| P-trend | 0.85 | 0.11 | 0.65 | ||||||||||
| Supplemental riboflavin | 0.08 | ||||||||||||
| 0 mg | 128 | 1.00 | (Ref) | 162 | 1.00 | (Ref) | 135 | 1.00 | (Ref) | ||||
| >0–1.7 mg | 82 | 1.03 | (0.77, 1.36) | 98 | 0.77 | (0.59, 0.99) | 121 | 1.07 | (0.83, 1.37) | ||||
| >1.7 mg | 20 | 0.76 | (0.47, 1.21) | 23 | 0.60 | (0.39, 0.93) | 37 | 1.11 | (0.77, 1.60) | ||||
| P-trend | 0.24 | 0.03 | 0.57 | ||||||||||
| Riboflavin, total | 0.02 | ||||||||||||
| <1.80 mg | 66 | 1.00 | (Ref) | 82 | 1.00 | (Ref) | 60 | 1.00 | (Ref) | ||||
| 1.80–2.87 mg | 62 | 1.05 | (0.74, 1.49) | 82 | 0.90 | (0.66, 1.23) | 68 | 1.01 | (0.71, 1.42) | ||||
| 2.88–3.97 mg | 57 | 1.08 | (0.76, 1.56) | 66 | 0.72 | (0.52, 1.01) | 93 | 1.30 | (0.94, 1.80) | ||||
| >3.97 mg | 45 | 0.86 | (0.58, 1.26) | 53 | 0.57 | (0.40, 0.81) | 72 | 1.07 | (0.76, 1.51) | ||||
| P-trend | 0.41 | <0.01 | 0.60 | ||||||||||
A Cox model adjusted for age, BMI (in kg/m2; <25, 25 to <30, 30 to <35, or ≥35), race-ethnicity (white, black, or other), past medical history of colonoscopy (yes or no), smoking status (never, past, or current), physical activity (0 to 3, 3 to <11.75, or ≥11.75 metabolic equivalent task hours/wk), and postmenopausal hormone use (never, past, or current). DFE, dietary folate equivalent; Ref, reference.
P value for test of interaction between the linear trend variable of interest and alcohol use categories.
Several additional analyses were conducted: 1) investigating the associations of dietary intake with CRC risk among women with no multivitamin/one-carbon supplement use at baseline, 2) including all 4 one-carbon nutrients in one multivariate-adjusted model because the nutrient intakes were moderately to highly correlated (r = 0.64–0.81; see Table S2 under “Supplemental data” in the online issue), 3) using period-specific quartiles of intake instead of a unified quartile as the exposure for each of the FA fortification periods, and 4) including multivitamin use (yes or no) in the models of main effects and effect modification to assess a potential healthy behavior effect. None of these factors altered the patterns of associations (data not shown).
DISCUSSION
In a recent meta-analysis, a null finding for higher dietary folate intakes and CRC risk has been shown (HR: 0.92; 95% CI: 0.81, 1.05; 11 cohort studies, 8 providing CRC risks and 3 providing colon cancer risks) (33). Our observation that dietary, supplemental, and total folate intake were not associated with overall CRC risk is consistent with most of the literature. However, in the NIH-AARP Diet and Health Study, which was not included in the meta-analysis, with 525,488 individuals recruited in a similar time frame and age range as the WHI-OS cohort, inverse associations of total, dietary, and supplemental folate intake with CRC were observed for men and women when postfortification dietary folate values were applied. The study confirmed that both sources of folate have protective effects but potentially may exist in men only (34). The mechanism of this sex discrepancy may involve factors such as sexual hormone and folate demand and warrants further research.
Not all of our observations on intake of vitamin B-6, vitamin B-12, and riboflavin are in agreement with previous literature. A meta-analysis of 9 cohort studies found a reduced risk of CRC for high blood vitamin B-6 concentrations (RR: 0.52; 95% CI: 0.38, 0.71) but not for high intakes (RR: 0.90; 95% CI: 0.75, 1.07) in men and women (35). However, total vitamin B-6 intake was associated with a 21% decrease in CRC risk when the differences in median total vitamin B-6 intake between the exposure and comparison groups were sufficiently large (>1.5 mg). In our study, the difference in median of total vitamin B-6 intake between the highest and lowest groups was 4.8 mg, and the strength of association was consistent with the meta-analysis. Our study further showed that vitamin B-6 intake from food only might be sufficient for risk reduction. For riboflavin, 3 cohort studies showed no associations between CRC incidence and riboflavin intakes for women (24, 36, 37). However, we observed an inverse association for total riboflavin. Our observation that both vitamin B-6 and riboflavin intakes were associated with reduced CRC risk is supported by a large study involving vitamin B-6 and riboflavin status (38). Vitamin B-6 and riboflavin are interrelated because flavin mononucleotide serves as a cofactor in the synthesis of pyridoxal-5′-phosphate. Suboptimal status of vitamin B-6 or riboflavin leads to the accumulation of homocysteine, a metabolite strongly linked with CRC (39, 40).
Evidence on whether one-carbon nutrients are associated with a specific tumor site is sparse. Within the WHI-OS cohort, we did not note any differences of one-carbon nutrient intakes and CRC risk by tumor site. The Iowa Women's Health Study reported that participants with high intakes of both vitamin B-12 and folate were at lower risk of cancer in the proximal colon (RR: 0.59; 95% CI: 0.39, 0.89) (25). Also, 2 cohort studies reported that high total vitamin B-6 intake might be associated with rectal cancer in women, one overall and the other in a low-folate-intake group (<350 μg/d) (24, 25). However, we observed a 30% nonsignificant risk reduction in rectal cancer in the WHI-OS cohort (HR: 0.68; 95% CI: 0.41, 1.13; total vitamin B-6 intake; see Table S1 under “Supplemental data” in the online issue). Interestingly, when stratifying by stage, we noted specific findings for regionally spread disease only—results that might be explained by different effects of one-carbon nutrients during carcinogenesis dependent on exposure time. Nevertheless, we were unable to investigate the timing for folate and tumor growth with the current data and study design.
WHI-OS provided a unique opportunity for investigating potential effects of FA fortification on CRC risk. The recruitment (1993–1998) and follow-up (1993–2009) straddled the initiation of the fortification program (1996 to 1 January 1998). We observed that dietary folate intake after fortification had started was associated with an increased CRC risk among women exposed to this source for 3 to <9 y. One explanation for the observed transient increased risk is that in the early postfortification period, total folate content of several fortified foods were reported to initially exceed the amount specified by federal regulations (41). Although there was no systematic analysis of enriched cereal-grain products, mean folate content was reported to have been reduced during 2000–2003 (42–44). In addition, before the implementation of fortification, there was an increase in consumer selection of high-folate foods and an increased availability of the numbers and types of nonstandardized folate-fortified foods, eg, breakfast cereals (44, 45). The national trend of blood folate also followed this pattern (44, 46). This transient increase in folate intake might have led to the promotion of latent CRC in our study population. Specifically, smaller cancers or advanced adenomas, which tend to upregulate folate receptors to sustain DNA synthesis, would have grown more rapidly in response to the increased folate and consequently become clinically detectable. A similar transient increase in CRC incidence was apparent in the US population (15). In addition, the continuing decrease in CRC incidence in women from 1992 to 1995 (−1.9%) was offset by an increase between 1995 and 1998 (2.0%), followed subsequently by another decrease between 1998 and 2007 (−2.3%) (47). Consistent with our hypothesis is that the effect modification by the transient increase in folate intake during the early postfortification period was observed in both supplement and nonsupplement users and was not associated with the intake of other one-carbon nutrients. This is the first study showing such a pattern of risk variation within an epidemiologic study.
Our finding that higher B vitamin intakes were associated with reduced CRC incidence among current alcohol drinkers with <1 drink/wk is noteworthy. Similar interactions were observed in other cohorts (34, 48, 49). The NIH-AARP Diet and Health Study showed total folate intake–associated CRC risk reductions among male and female drinkers (both >15 g/d and ≤15 g/d) but not nondrinkers. The effect modification of alcohol use is plausible because alcohol disturbs folate absorption and the one-carbon cycle. Therefore, drinkers have a higher folate requirement, and reduced CRC risks are more likely observed among those with high folate intake (50). However, our data suggest that moderate to heavy amounts of alcohol intake could completely nullify the beneficial association of folate intake with CRC risk. We have limited explanations for this finding, except for the differences in study population. The WHI recruited, on average, older and a higher percentage of postmenopausal women than did the NIH-AARP Diet and Health Study. The differences in age and postmenopausal status may have influenced the modification effect of alcohol intake.
Several strengths and limitations of this study should be noted. A major strength of our study was the large sample size that allowed subgroup analyses. The prospective design of the study eliminated the risk of recall bias. Simultaneous adjustments for factors known to alter cancer risk provided a thorough analysis of one-carbon–related nutrients and their role in colorectal carcinogenesis. We focused on cancer cases diagnosed after 2 y postrecruitment to account to some extent for the long period of CRC development and to eliminate confounding of changed health behaviors due to early symptoms of the disease. All hypotheses and analyses were a priori. However, there are some limitations of our study. First, there is a potential of residual confounding that we attempted to minimize through multivariate adjustments for several factors. Second, the measurement of nutrient intake by questionnaire can result in some exposure misclassification. However, folate and B vitamins assessed by the WHI FFQ had reasonable agreement with four 24-h recall and a 4-d food record (Pearson correlation coefficient: 0.28–0.69) (51). Third, the baseline nutrient intakes might not reflect representative intake patterns of the subjects over the entire course of follow-up. We acknowledge that dietary folate intake among those recruited during pre- and perifortification periods might have been underestimated because they experienced higher intakes from fortification during the majority of the follow-up period. Fourth, there might have been confounding if participants used FA supplementation when early lesions had formed. Recent research has emphasized the importance of timing of folate supplementation during carcinogenesis because folate can enhance tumor growth once preneoplastic lesions are present (52). Fifth, B vitamin intake amounts were moderately to highly correlated; however, this appeared not to affect the risk estimates on the basis of models of mutual adjustment for all B vitamin intakes. Last, a relatively large number of comparisons were made, although all of them were prehypothesized.
In conclusion, the results of this study support an inverse association for vitamin B-6 and riboflavin intakes and CRC risk among postmenopausal women. The associations of B vitamin intake are particularly strong for regionally spread disease and among those who consume <1 drink of alcohol/wk. Our study provides new evidence that the increased folate intake during the early postfortification period may have been associated with a transient increase in CRC risk.
Acknowledgments
We thank the participants of the WHI for their contributions. We also thank the Program Office (National Heart, Lung, and Blood Institute, Bethesda, MD: Jacques Rossouw, Shari Ludlam, Joan McGowan, Leslie Ford, and Nancy Geller); the Clinical Coordinating Center at Fred Hutchinson Cancer Research Center, Seattle, WA (Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg); the Investigators and Academic Centers (Brigham and Women's Hospital, Harvard Medical School, Boston, MA); JoAnn E Manson; MedStar Health Research Institute/Howard University, Washington, DC; Barbara V Howard; Stanford Prevention Research Center, Stanford, CA; Marcia L Stefanick; The Ohio State University, Columbus, OH; Rebecca Jackson; University of Arizona, Tucson/Phoenix, AZ; Cynthia A Thomson; University at Buffalo, Buffalo, NY; Jean Wactawski-Wende; University of Florida, Gainesville/Jacksonville, FL; Marian Limacher; University of Iowa, Iowa City/Davenport, IA; Robert Wallace; University of Pittsburgh, Pittsburgh, PA; Lewis Kuller; Wake Forest University School of Medicine, Winston-Salem, NC; and Sally Shumaker. The WHI program is funded by the National Heart, Lung, and Blood Institute, NIH, US Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C.
The authors’ responsibilities were as follows—CMU: designed and conducted the research and had primary responsibility for the final content; CMU, MLN, XS, SAAB, JWM, DRM, DL, and JMS: provided essential reagents or provided essential materials; YZ and RMR: analyzed data or performed statistical analyses; and SZ, T-YDC, and CMU: wrote the manuscript. All authors read and approved the final manuscript. The authors did not declare any conflicts of interest related to this study.
Footnotes
Abbreviations used: CRC, colorectal cancer; FA, folic acid; FDA, Food and Drug Administration; FFQ, food-frequency questionnaire; WHI, Women's Health Initiative; WHI-OS, Women's Health Initiative Observational Study.
REFERENCES
- 1.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin 2011;61:212–36 [DOI] [PubMed] [Google Scholar]
- 2.Rampersaud GC, Kauwell GP, Hutson AD, Cerda JJ, Bailey LB. Genomic DNA methylation decreases in response to moderate folate depletion in elderly women. Am J Clin Nutr 2000;72:998–1003 [DOI] [PubMed] [Google Scholar]
- 3.Kim YI. Folate and DNA methylation: a mechanistic link between folate deficiency and colorectal cancer? Cancer Epidemiol Biomarkers Prev 2004;13:511–9 [PubMed] [Google Scholar]
- 4.Ulrich CM. Folate and cancer prevention–where to next? Counterpoint. Cancer Epidemiol Biomarkers Prev 2008;17:2226–30 [DOI] [PubMed] [Google Scholar]
- 5.Giovannucci E. Epidemiologic studies of folate and colorectal neoplasia: a review. J Nutr 2002;132:2350S–5S [DOI] [PubMed] [Google Scholar]
- 6.Giovannucci E, Stampfer MJ, Colditz GA, Rimm EB, Trichopoulos D, Rosner BA, Speizer FE, Willett WC. Folate, methionine, and alcohol intake and risk of colorectal adenoma. J Natl Cancer Inst 1993;85:875–84 [DOI] [PubMed] [Google Scholar]
- 7.Ulrich CM, Potter JD. Folate supplementation: too much of a good thing? Cancer Epidemiol Biomarkers Prev 2006;15:189–93 [DOI] [PubMed] [Google Scholar]
- 8.Stevens VL, McCullough ML, Sun J, Jacobs EJ, Campbell PT, Gapstur SM. High levels of folate from supplements and fortification are not associated with increased risk of colorectal cancer. Gastroenterology 2011;141:98–105 [DOI] [PubMed] [Google Scholar]
- 9.Sanjoaquin MA, Allen N, Couto E, Roddam AW, Key TJ. Folate intake and colorectal cancer risk: a meta-analytical approach. Int J Cancer 2005;113:825–8 [DOI] [PubMed] [Google Scholar]
- 10.Kim DH, Smith-Warner SA, Spiegelman D, Yaun SS, Colditz GA, Freudenheim JL, Giovannucci E, Goldbohm RA, Graham S, Harnack L, et al. Pooled analyses of 13 prospective cohort studies on folate intake and colon cancer. Cancer Causes Control 2010;21:1919–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pfeiffer CM, Caudill SP, Gunter EW, Osterloh J, Sampson EJ. Biochemical indicators of B vitamin status in the US population after folic acid fortification: results from the National Health and Nutrition Examination Survey 1999–2000. Am J Clin Nutr 2005;82:442–50 [DOI] [PubMed] [Google Scholar]
- 12.Institute of Medicine Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Washington, DC: National Academy Press, 1998 [PubMed] [Google Scholar]
- 13.Choumenkovitch SF, Selhub J, Wilson PW, Rader JI, Rosenberg IH, Jacques PF. Folic acid intake from fortification in United States exceeds predictions. J Nutr 2002;132:2792–8 [DOI] [PubMed] [Google Scholar]
- 14.Kim YI. Will mandatory folic acid fortification prevent or promote cancer? Am J Clin Nutr 2004;80:1123–8 [DOI] [PubMed] [Google Scholar]
- 15.Mason JB, Dickstein A, Jacques PF, Haggarty P, Selhub J, Dallal G, Rosenberg IH. A temporal association between folic acid fortification and an increase in colorectal cancer rates may be illuminating important biological principles: a hypothesis. Cancer Epidemiol Biomarkers Prev 2007;16:1325–9 [DOI] [PubMed] [Google Scholar]
- 16.Hirsch S, Sanchez H, Albala C, de la Maza MP, Barrera G, Leiva L, Bunout D. Colon cancer in Chile before and after the start of the flour fortification program with folic acid. Eur J Gastroenterol Hepatol 2009;21:436–9 [DOI] [PubMed] [Google Scholar]
- 17.Komatsu S, Watanabe H, Oka T, Tsuge H, Kat N. Dietary vitamin B6 suppresses colon tumorigenesis, 8-hydroxyguanosine, 4-hydroxynonenal, and inducible nitric oxide synthase protein in azoxymethane-treated mice. J Nutr Sci Vitaminol (Tokyo) 2002;48:65–8 [DOI] [PubMed] [Google Scholar]
- 18.Komatsu SI, Watanabe H, Oka T, Tsuge H, Nii H, Kato N. Vitamin B-6-supplemented diets compared with a low vitamin B-6 diet suppress azoxymethane-induced colon tumorigenesis in mice by reducing cell proliferation. J Nutr 2001;131:2204–7 [DOI] [PubMed] [Google Scholar]
- 19.Shen J, Lai CQ, Mattei J, Ordovas JM, Tucker KL. Association of vitamin B-6 status with inflammation, oxidative stress, and chronic inflammatory conditions: the Boston Puerto Rican Health Study. Am J Clin Nutr 2010;91:337–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishihara J, Otani T, Inoue M, Iwasaki M, Sasazuki S, Tsugane S. Low intake of vitamin B-6 is associated with increased risk of colorectal cancer in Japanese men. J Nutr 2007;137:1808–14 [DOI] [PubMed] [Google Scholar]
- 21.Larsson SC, Giovannucci E, Wolk A. Vitamin B6 intake, alcohol consumption, and colorectal cancer: a longitudinal population-based cohort of women. Gastroenterology 2005;128:1830–7 [DOI] [PubMed] [Google Scholar]
- 22.Le Marchand L, White KK, Nomura AM, Wilkens LR, Selhub JS, Tiirikainen M, Goodman MT, Murphy SP, Henderson BE, Kolonel LN. Plasma levels of B vitamins and colorectal cancer risk: the multiethnic cohort study. Cancer Epidemiol Biomarkers Prev 2009;18:2195–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang SM, Moore SC, Lin J, Cook NR, Manson JE, Lee IM, Buring JE. Folate, vitamin B6, multivitamin supplements, and colorectal cancer risk in women. Am J Epidemiol 2006;163:108–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Vogel S, Dindore V, van Engeland M, Goldbohm RA, van den Brandt PA, Weijenberg MP. Dietary folate, methionine, riboflavin, and vitamin B-6 and risk of sporadic colorectal cancer. J Nutr 2008;138:2372–8 [DOI] [PubMed] [Google Scholar]
- 25.Harnack L, Jacobs DR, Jr, Nicodemus K, Lazovich D, Anderson K, Folsom AR. Relationship of folate, vitamin B-6, vitamin B-12, and methionine intake to incidence of colorectal cancers. Nutr Cancer 2002;43:152–8 [DOI] [PubMed] [Google Scholar]
- 26.World Cancer Research Fund Diet, nutrition, and the prevention of cancer: a global perspective. Washington, DC: World Cancer Research Fund /American Institute for Cancer Research, 1997 [Google Scholar]
- 27.Halsted C. Alcohol and folate interactions: clinical implications. New York, NY: Marcel Dekker, 1995 [Google Scholar]
- 28.Langer RD, White E, Lewis CE, Kotchen JM, Hendrix SL, Trevisan M. The Women's Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol 2003;13:S107–21 [DOI] [PubMed] [Google Scholar]
- 29.Lewis CJ, Crane NT, Wilson DB, Yetley EA. Estimated folate intakes: data updated to reflect food fortification, increased bioavailability, and dietary supplement use. Am J Clin Nutr 1999;70:198–207 [DOI] [PubMed] [Google Scholar]
- 30.Institute of Medicine Dietary Reference Intakes: thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Washington, DC: National Academy Press, 1998 [PubMed] [Google Scholar]
- 31.Curb JD, McTiernan A, Heckbert SR, Kooperberg C, Stanford J, Nevitt M, Johnson KC, Proulx-Burns L, Pastore L, Criqui M, et al. Outcomes ascertainment and adjudication methods in the Women's Health Initiative. Ann Epidemiol 2003;13:S122–8 [DOI] [PubMed] [Google Scholar]
- 32.Ries LAGMD, Karpcho M, Mariotto A, Miller BA, Feuer EJ, Clegg L, Horner MJ, Howlader N, Eisner MP, Reichman M, et al. SEER cancer statistics review, 1975-2004. Bethesda, MD: National Cancer Institute, 2007 [Google Scholar]
- 33.Kennedy DA, Stern SJ, Moretti M, Matok I, Sarkar M, Nickel C, Koren G. Folate intake and the risk of colorectal cancer: a systematic review and meta-analysis. Cancer Epidemiol 2011;35:2–10 [DOI] [PubMed] [Google Scholar]
- 34.Gibson TM, Weinstein SJ, Pfeiffer RM, Hollenbeck AR, Subar AF, Schatzkin A, Mayne ST, Stolzenberg-Solomon R. Pre- and postfortification intake of folate and risk of colorectal cancer in a large prospective cohort study in the United States. Am J Clin Nutr 2011;94:1053–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Larsson SC, Orsini N, Wolk A. Vitamin B6 and risk of colorectal cancer: a meta-analysis of prospective studies. JAMA 2010;303:1077–83 [DOI] [PubMed] [Google Scholar]
- 36.Kabat GC, Miller AB, Jain M, Rohan TE. Dietary intake of selected B vitamins in relation to risk of major cancers in women. Br J Cancer 2008;99:816–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shrubsole MJ, Yang G, Gao YT, Chow WH, Shu XO, Cai Q, Rothman N, Gao J, Wagner C, Zheng W. Dietary B vitamin and methionine intakes and plasma folate are not associated with colorectal cancer risk in Chinese women. Cancer Epidemiol Biomarkers Prev 2009;18:1003–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eussen SJ, Vollset SE, Hustad S, Midttun O, Meyer K, Fredriksen A, Ueland PM, Jenab M, Slimani N, Boffetta P, et al. Plasma vitamins B2, B6, and B12, and related genetic variants as predictors of colorectal cancer risk. Cancer Epidemiol Biomarkers Prev 2010;19:2549–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ulvik A, Vollset SE, Hansen S, Gislefoss R, Jellum E, Ueland PM. Colorectal cancer and the methylenetetrahydrofolate reductase 677C -> T and methionine synthase 2756A -> G polymorphisms: a study of 2,168 case-control pairs from the JANUS cohort. Cancer Epidemiol Biomarkers Prev 2004;13:2175–80 [PubMed] [Google Scholar]
- 40.Kato I, Dnistrian AM, Schwartz M, Toniolo P, Koenig K, Shore RE, Akhmedkhanov A, Zeleniuch-Jacquotte A, Riboli E. Serum folate, homocysteine and colorectal cancer risk in women: a nested case-control study. Br J Cancer 1999;79:1917–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rader JI, Weaver CM, Angyal G. Total folate in enriched cereal-grain products in the United States following fortification. Food Chem 2000;70:275–89 [Google Scholar]
- 42.Johnston KE, Tamura T. Folate content in commercial white and whole wheat sandwich breads. J Agric Food Chem 2004;52:6338–40 [DOI] [PubMed] [Google Scholar]
- 43.Póo-Prieto R, Haytowitz DB, Holden JM, Rogers G, Choumenkovitch SF, Jacques PF, Selhub J. Use of the affinity/HPLC method for quantitative estimation of folic acid in enriched cereal-grain products. J Nutr 2006;136:3079–83 [DOI] [PubMed] [Google Scholar]
- 44.Pfeiffer CM, Johnson CL, Jain RB, Yetley EA, Picciano MF, Rader JI, Fisher KD, Mulinare J, Osterloh JD. Trends in blood folate and vitamin B-12 concentrations in the United States, 1988–2004. Am J Clin Nutr 2007;86:718–27 [DOI] [PubMed] [Google Scholar]
- 45.Food and Drug Administration Food labeling: health claims and label statements: folate and neural tube defects. Final rule. Fed Regist 1996;61:8752 [Google Scholar]
- 46.Bailey LB. The rise and fall of blood folate in the United States emphasizes the need to identify all sources of folic acid. Am J Clin Nutr 2007;86:528–30 [DOI] [PubMed] [Google Scholar]
- 47.Kohler BA, Ward E, McCarthy BJ, Schymura MJ, Ries LA, Eheman C, Jemal A, Anderson RN, Ajani UA, Edwards BK. Annual report to the nation on the status of cancer, 1975-2007, featuring tumors of the brain and other nervous system. J Natl Cancer Inst 2011;103:714–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Giovannucci E, Rimm EB, Ascherio A, Stampfer MJ, Colditz GA, Willett WC. Alcohol, low-methionine–low-folate diets, and risk of colon cancer in men. J Natl Cancer Inst 1995;87:265–73 [DOI] [PubMed] [Google Scholar]
- 49.Zhang SM, Willett WC, Selhub J, Hunter DJ, Giovannucci EL, Holmes MD, Colditz GA, Hankinson SE. Plasma folate, vitamin B6, vitamin B12, homocysteine, and risk of breast cancer. J Natl Cancer Inst 2003;95:373–80 [DOI] [PubMed] [Google Scholar]
- 50.Halsted CHM, Medici V, Esfandiari F. Influence of alcohol and folate status and methionine metabolism in relation to alcoholic liver disease. 2nd ed. Boca Raton, FL: CRC Press, 2010.
- 51.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women's Health Initiative food frequency questionnaire. Ann Epidemiol 1999;9:178–87 [DOI] [PubMed] [Google Scholar]
- 52.Choi SW, Mason JB. Folate and carcinogenesis: an integrated scheme. J Nutr 2000;130:129–32 [DOI] [PubMed] [Google Scholar]