Abstract
Background: Few studies have evaluated the association of diet and weight status with head and neck cancer outcomes.
Objective: The purpose of this study was to determine whether pretreatment dietary patterns and weight status are associated with head and neck cancer prognosis.
Design: This was a longitudinal study of 542 patients with newly diagnosed head and neck cancer who completed food-frequency questionnaires and health surveys before treatment. Clinical data were abstracted from medical records and the Social Security Death Index. Dietary patterns were identified by using principal component analysis. Cox proportional hazard models were used to examine the association of derived dietary patterns (fit by quintiles of exposure) and weight status with time to recurrence and survival, with control for covariates.
Results: During the study period, there were 229 deaths and 184 recurrences. Two dietary patterns were identified: a whole-foods pattern (characterized by high intakes of vegetables, fruit, fish, poultry, and whole grains) and a Western pattern (characterized by high intakes of red and processed meats, refined grains, potatoes, and French fries). In multivariable analyses, significantly fewer deaths were observed in subjects most adherent to the whole-foods pattern (HR: 0.56; 95% CI: 0.34, 0.92; P-trend = 0.01). Subjects classified as overweight or obese had significantly fewer deaths (HR: 0.65; 95% CI: 0.49, 0.85; P = 0.001) and recurrences (HR: 0.70; 95% CI: 0.52, 0.95; P = 0.02) than did normal-weight or underweight subjects.
Conclusion: Consumption of a diet rich in vegetables, fruit, fish, poultry, and whole grains and being overweight before diagnosis with head and neck cancer are associated with a better prognosis.
INTRODUCTION
Often combined into a single category, head and neck squamous cell carcinoma (HNSCC)4 is a heterogeneous disease that includes squamous cell cancers of the oral cavity, oropharynx, nasopharynx, hypopharynx, and larynx. Five-year HNSCC survival rates have remained steady at ∼60%, likely because of late detection and high rates of persistent or recurrent disease. An estimated 50% of patients develop recurrence within the first 2 y after diagnosis (1, 2). Evidence suggests that poor nutritional status and low consumption of foods and nutrients with chemopreventive properties increase the risk of developing HNSCC (3), but little research has investigated the role of diet and weight status in HNSCC prognosis. Modifiable lifestyle factors, such as diet and nutritional status, may present a feasible approach for improving head and neck cancer survival and recurrence rates.
A small number of studies have examined the association of HNSCC prognosis with individual food groups (eg, fruit and vegetables) (4, 5) or nutrients (6–9). Most of these studies had small sample sizes (5–8) and yielded inconclusive or null results (4, 5, 7, 9), and none have examined overall dietary patterns. Our group previously reported that low pretreatment fruit intake is negatively associated with survival in bivariate analyses (4). It may be more informative to examine the combined, rather than the individual, effects of dietary exposures on HNSCC prognosis because it is likely that the many nutrients and nutritive compounds in food interact and work synergistically to produce a stronger effect than any individually (10). Examining overall dietary patterns is advantageous because it allows for the ability to relate usual intake of a range of nutrients to disease prognosis and can be used as the basis for translational research aimed at developing population-specific nutritional interventions and dietary recommendations.
Weight status, as measured by BMI [weight (kg)/height (m)2], may be inversely associated with mortality, because patients classified as overweight or obese (BMI >25) at the time of HNSCC diagnosis have a longer survival time than do similar patients with a lower BMI (11–14). Previous studies that investigated this association had either a small sample size (13), measured as weight status several years before diagnosis (11), or did not consider recurrence as an outcome (11, 12).
The objective of this study was to characterize pretreatment dietary patterns and to determine whether dietary patterns and weight status predict recurrence and survival among HNSCC patients; other known prognostic factors were controlled for, such as demographic characteristics, smoking, problem drinking, cancer site and stage, comorbidities, and treatment. The hypotheses were that diets high in fruit, vegetables, lean proteins, and unprocessed foods as well as overweight or obese status would be associated with better prognoses, whereas diets high in fat, processed foods, and red meat and normal or underweight status would predict worse prognoses.
SUBJECTS AND METHODS
Design
This was a prospective study of patients enrolled in the University of Michigan Head and Neck Specialized Program of Research Excellence (UM HN-SPORE). The independent variables of interest were pretreatment dietary patterns and weight status. Control variables were age, sex, cancer site and stage, treatment, comorbidities, smoking, and total energy intake. The dependent (outcome) variables were disease recurrence and all-cause survival.
Study population
Patients with newly diagnosed HNSCC were recruited to participate in this study as part of the UM HN-SPORE. Institutional Review Board approval was granted from the University of Michigan Health System (Ann Arbor, MI), the Veterans Affairs Health Care System (Ann Arbor, MI), and the Henry Ford Health System (Detroit, MI). Patients were recruited between January 2003 and December 2008. Of 1185 patients approached, 934 consented, yielding a response rate of 79%. Exclusion criteria included 1) <18 y of age, 2) pregnancy, 3) non–English speaking, 4) diagnosis of mental instability, or 5) diagnosis of another non–upper aerodigestive tract cancer. Patients who did not complete a baseline food-frequency questionnaire (FFQ; n = 300), had previously diagnosed HNSCC (n = 40), and did not have a signed informed consent on file (n = 3) were excluded. The Rosner method was used to detect statistically influential outliers based on reported daily energy intake (15); 5 participants were identified and excluded. Comparative analyses of participant characteristics (chi-square tests for categorical and t tests for continuous variables) indicated that women reporting consumption of >3500 kcal/d and men reporting >4000 kcal/d were more likely to report being current smokers and having an alcohol problem than were the rest of the study population. Because problem drinkers may be unreliable reporters, they were excluded from the analyses (n = 44) to avoid potential systematic confounding. Although conservative, these upper bounds are considered reasonable for making exclusions based on energy intake (16). We did not exclude a priori based on a lower energy bound because most of the participants presented to the clinic with advanced cancers that may have hindered dietary consumption before diagnosis. Nevertheless, the lowest reported daily energy intake was 519 kcal/d, which is greater than the usual lower bound of 500 kcal. The final sample size included 542 patients with newly diagnosed HNSCC.
Procedures
Participants completed a self-administered health questionnaire at baseline that collected data on demographic characteristics, tobacco use, alcohol use, physical activity, sleep, comorbidities, depression, and quality of life. A medical record review was completed at baseline and annually for each study participant, from which data on tumor site and stage, recurrence status, treatment modalities, and survival were abstracted.
Measures
Predictors: dietary intake and weight status
Dietary intake data were collected by using the self-administered, semiquantitative Harvard FFQ, which was designed to assess respondents’ usual dietary intake from food and supplements over the past year. The reproducibility and validity of the 131-item questionnaire was reported previously (16–18). The FFQ includes standard portion sizes for each item [eg, 1 apple or 3 oz (85 g) chicken], which allowed participants to choose their average frequency of consumption over the past year from a list of 9 choices ranging from “almost never” to “≥6 times per day.” Total energy and nutrient intake was estimated by summing intakes from each food based on the selected standard portion size, reported frequency of consumption, and nutrient content of each food item. Daily food group servings were estimated by summing the frequency weights of each food item based on reported daily frequencies of consumption (16). Weight status was classified as overweight/obese (BMI >25) or normal/underweight (BMI ≤25) based on self-reported height and weight.
Covariates
Demographic variables were age, sex, race, level of education, and marital status. Smoking data were categorized as current/former versus never smoking, and alcohol abuse was measured by using the previously validated Alcohol Use Disorders Identification Test (AUDIT); an AUDIT score ≥8 indicated problem drinking (19). Tumor site was recorded from operative notes and surgical pathology forms and categorized into 3 groups: 1) oral cavity, 2) pharynx, and 3) larynx. To increase the statistical power of stage-wise comparisons, cancer stage was categorized a priori into 3 groups: TNM stages 1 and 2 were collapsed, whereas stage 3 and stage 4 were considered separately. Comorbidities were measured by using the Adult Comorbidity Evaluation-27 and categorized into none or mild comorbidities compared with moderate to severe comorbidities (20). Depression was measured by using the 5-item Geriatric Depression Scale-Short Forms (21). Treatment was categorized into 6 groups to allow individual treatment approaches, including multimodal therapy, to be estimated separately: 1) surgery only, 2) radiation only, 3) surgery and radiation, 4) radiation and chemotherapy, 5) all treatment (surgery, radiation, and chemotherapy), or 6) unknown treatment or none.
Outcome: survival and recurrence
Outcome variables were recurrence and overall survival. The subjects were followed up every 3 mo, which allowed for tracking patient status (alive or deceased). Each participant was censored from their last annual chart review as alive or deceased and recurrence or no recurrence as of February 2012. Death and recurrence data were obtained from medical records and the Social Security Death Index. Research assistants abstracted recurrence dates from medical records. Time to recurrence was calculated as the number of days from the date of diagnosis to the date of first recurrence. Patients with persistent disease were assigned a recurrence time of 1 d if they had completed treatment but were never determined by a medical doctor to be disease-free.
Statistical analysis
Descriptive statistics (means and frequencies) were generated for all demographic, epidemiologic and clinical variables. A Pearson's correlation analysis was conducted between self-reported weights and weights abstracted from medical records among a random sample of 27 participants, which showed excellent correlation between self-reported and clinical measures of weight (r = 0.98). Dietary intake data were assessed for missing values and energy outliers by using standard techniques (16). Food-consumption data derived from the FFQ were classified a priori into 39 foods and food groups by using methods similar to those described in previous studies of dietary patterns and disease (10, 22). Pretreatment dietary patterns present in the study population were derived by principal component analysis by using the orthogonal rotation procedure. For the establishment of the number of factors to retain in the final analysis, eigenvalues (≥3.0), Scree test, percentage of variability explained, and interpretability of factors were considered (23). Pattern factor scores were calculated for each study participant by summing reported intakes of the factor food variables weighted by factor loadings. Dietary pattern scores were categorized into quintiles for analysis with survival and recurrence.
Cox proportional hazards models were used to estimate HRs and 95% CIs for the associations of survival and recurrence with each retained dietary pattern and weight status. Previous evidence suggests differential associations of BMI and HNSCC mortality between smokers and nonsmokers (11). As such, we stratified the analyses by smoking status (current/former compared with never smokers). Covariates were assessed for collinearity, and exclusions of covariates in final models were made if they were highly correlated with other variables. The final multivariable models were fit by including age, sex, and cancer site in the model and using a forward selection strategy for the remaining potential covariates. Covariates considered for final models were age, sex, race, education, marital status, cancer site, stage, treatment, comorbidities, smoking, alcohol problem, multivitamin use, and depression. Final models for survival and recurrence included age, sex, site, stage, treatment, comorbidities, and smoking. HRs and CIs were estimated for each quintile of dietary pattern score compared with the lowest, quintile 1, and a test for trend across increasing quintiles of intake was performed. Dietary pattern and weight-status variables were included in the same multivariable models to investigate the independent association of each with survival and recurrence. All statistical analyses were performed in SAS 9.2 or 9.3 (SAS Institute Inc).
RESULTS
During longitudinal follow-up, there were 229 death events (42.2%) and 184 recurrence events (33.9%). Median follow-up time was 2199 d (∼6.0 y; range: 19 d to 8.3 y). Overall epidemiologic characteristics of the study population are shown in Table 1. The mean age of the study participants was 59 y. Most of the participants were white (92.6%), male (78.6%), and married (61.9%). Just more than half of the participants were college graduates. The most common tumor location was the pharynx (56.3%), and most participants presented to our clinics with late-stage (III or IV) cancers (65.1%). More than 75% of participants were current or former smokers, and ∼25% reported an alcohol problem (24% with an AUDIT score ≥8). Approximately 60% of patients were classified as overweight or obese at the time of diagnosis, slightly less than the proportion observed within the general US population (68%) from 1999 to 2008 (24).
TABLE 1.
Pretreatment characteristics of patients with newly diagnosed head and neck cancer1
| Characteristic | Patients (n = 542) |
| n (%) | |
| Follow-up time (d) | |
| Median | 2199 |
| Range | 19–3067 |
| Age (y) | |
| Mean ± SD | 59 ± 11 |
| Range | 21–92 |
| Sex | |
| Male | 426 (78.6) |
| Female | 116 (21.4) |
| Race | |
| Non-Hispanic white | 502 (92.6) |
| Non-white/Hispanic | 40 (7.4) |
| Education | |
| High school or less | 248 (45.9) |
| Some college or more | 292 (54.1) |
| Marital status | |
| Married | 335 (61.9) |
| Not married | 206 (38.1) |
| Site | |
| Oral cavity | 118 (21.8) |
| Pharynx | 305 (56.3) |
| Larynx | 119 (21.9) |
| Stage | |
| 1/2 | 112 (20.7) |
| 3 | 77 (14.2) |
| 4 | 353 (65.1) |
| Treatment | |
| Surgery only | 77 (14.2) |
| Radiation only | 47 (8.7) |
| Surgery + radiation | 66 (12.2) |
| Radiation + chemotherapy | 214 (39.5) |
| All treatment | 124 (22.9) |
| Unknown treatment or none | 14 (2.5) |
| ACE-27 comorbidity score | |
| None or mild | 364 (67.2) |
| Moderate or severe | 178 (32.8) |
| Smoking | |
| Current | 117 (21.4) |
| Former | 306 (56.1) |
| Never | 123 (22.5) |
| Alcohol problem, AUDIT ≥8 | |
| Yes | 130 (24.0) |
| No | 412 (76.0) |
| BMI | |
| Underweight, <18.5 kg/m2 | 19 (3.5) |
| Normal weight, 18.5–24.9 kg/m2 | 195 (36.0) |
| Overweight, 25–29.9 kg/m2 | 205 (37.8) |
| Obese, ≥30 kg/m2 | 123 (22.7) |
| Multivitamin use | |
| Current | 265 (48.9) |
| Past | 105 (19.4) |
| Never | 172 (31.7) |
| Depression status | |
| Depressed | 270 (49.8) |
| Not depressed | 257 (47.4) |
| Unknown | 15 (2.8) |
| Fruit and vegetable intake | |
| 0–1 serving/d | 43 (7.9) |
| >1–3 servings/d | 249 (45.9) |
| >3–5 servings/d | 149 (27.5) |
| >5 servings/d | 101 (18.6) |
ACE, Adult Comorbidity Evaluation; AUDIT, Alcohol Use Disorders Identification Test.
Two major dietary patterns were identified with principal component analysis. The first pattern, termed the whole-foods pattern, was characterized by high intakes of vegetables, fruit, legumes, fish, poultry, whole grains, fruit juice, olive oil, nuts, and garlic. The second pattern, termed the Western pattern, was characterized by high intakes of red and processed meats, refined grains, French fries, potatoes, condiments, high-fat dairy products, margarine, butter, eggs, coffee, desserts, snacks, mayonnaise, and regular beverages. The factor-loading matrix for the 2 dietary patterns is presented in Table 2.
TABLE 2.
Factor loading matrix
| Food group | Whole foods | Western |
| Green leafy vegetables | 0.741 | −0.05 |
| Other vegetables | 0.691 | 0.19 |
| Dark-yellow and orange vegetables | 0.661 | −0.01 |
| Fruit | 0.641 | −0.13 |
| Cruciferous vegetables | 0.631 | −0.05 |
| Legumes | 0.601 | 0.12 |
| Tomatoes | 0.521 | 0.09 |
| Fish | 0.471 | 0.10 |
| Poultry | 0.421 | 0.14 |
| Whole grains | 0.411 | 0.09 |
| Salad dressing | 0.371 | 0.13 |
| Olive oil | 0.371 | −0.13 |
| Fruit juice | 0.341 | −0.04 |
| Nuts | 0.331 | 0.09 |
| Garlic | 0.301 | 0.07 |
| Low-fat dairy products | 0.28 | −0.20 |
| Cereal | 0.28 | −0.03 |
| Wine | 0.24 | −0.12 |
| Tea | 0.21 | −0.05 |
| Beer | −0.22 | 0.17 |
| Red meats | 0.01 | 0.621 |
| Refined grains | −0.03 | 0.591 |
| Processed meats | −0.06 | 0.521 |
| French fries | −0.13 | 0.481 |
| Potatoes | 0.02 | 0.471 |
| Condiments | −0.03 | 0.431 |
| High-fat dairy products | 0.04 | 0.421 |
| Margarine | −0.08 | 0.381 |
| Butter | −0.15 | 0.361 |
| Eggs | 0.14 | 0.351 |
| Coffee | 0.07 | 0.331 |
| Desserts | −0.01 | 0.321 |
| Snacks | 0.16 | 0.311 |
| Mayonnaise | 0.14 | 0.301 |
| Regular beverages | −0.16 | 0.301 |
| Creamy soups and chowders | 0.06 | 0.25 |
| Pizza | 0.05 | 0.24 |
| Organ meats | 0.08 | 0.10 |
| Diet beverages | 0.07 | 0.09 |
| Liquor | −0.04 | 0.08 |
Factor loading ≥0.30 and considered to be a major contributor to the overall pattern.
Select characteristics of the study participants, according to quintile of factor score for each dietary pattern, are shown in Table 3. Patients in the highest quintile of whole-foods pattern score were more likely to have a higher BMI, be older, be female, be never smokers, and be more highly educated and were less likely to have an alcohol problem than those in the lowest quintile. Patients with the highest whole-foods pattern scores also consumed more servings per day of fruit, vegetables, whole grains, fish, and poultry and less total, saturated, and trans fat. Conversely, patients in the highest quintile of Western pattern score were more likely to be younger, male, less educated, and have an alcohol problem and were less likely to be never smokers than those in the lowest quintile. Patients in the highest quintile tended to consume more servings per day on average of red and processed meats, refined grains, potatoes, and French fries and more total fat than patients with low Western pattern scores.
TABLE 3.
Selected characteristics and dietary intakes of subjects by quintile of dietary pattern score (n = 542)1
| Whole-foods pattern |
Western pattern |
|||||||||
| Q1(n = 108) | Q2(n = 109) | Q3(n = 108) | Q4(n = 109) | Q5(n = 108) | Q1(n = 108) | Q2(n = 109) | Q3(n = 108) | Q4(n = 109) | Q5(n = 108) | |
| Clinical characteristic | ||||||||||
| Mean age (y) | 57.0 | 59.4 | 59.1 | 59.7 | 61.1 | 61.3 | 59.2 | 61.1 | 58.6 | 56.22 |
| Mean BMI (kg/m2) | 25.2 | 26.9 | 27.0 | 27.0 | 27.73 | 26.0 | 27.2 | 26.7 | 26.9 | 26.9 |
| Female (%) | 13.9 | 17.4 | 22.2 | 25.7 | 27.8 | 29.6 | 23.8 | 19.4 | 18.3 | 15.7 |
| Stage 4 (%) | 71.3 | 70.6 | 62.0 | 65.1 | 56.5 | 63.9 | 71.6 | 53.7 | 67.0 | 69.4 |
| ≤High school/GED education (%) | 67.3 | 45.4 | 40.7 | 45.9 | 30.62 | 30.6 | 47.7 | 38.7 | 60.5 | 51.82 |
| Never smokers (%) | 15.7 | 19.3 | 24.1 | 22.9 | 32.42 | 28.7 | 27.5 | 23.1 | 18.3 | 16.73 |
| Alcohol problem (%) | 45.4 | 24.8 | 21.3 | 18.3 | 10.22 | 14.8 | 23.8 | 23.1 | 26.6 | 31.5 |
| Current multivitamin use (%) | 35.2 | 49.5 | 51.8 | 53.2 | 54.6 | 64.8 | 55.0 | 41.7 | 43.1 | 39.82 |
| Depressed (%) | 50.9 | 45.9 | 51.8 | 53.2 | 47.2 | 53.7 | 50.5 | 52.8 | 55.0 | 37.0 |
| Mean food intake, energy-adjusted (servings/d) | ||||||||||
| Total fruit | 0.4 | 0.8 | 1.0 | 1.4 | 1.72 | 1.5 | 1.1 | 1.0 | 0.9 | 0.72 |
| Total vegetables | 1.2 | 1.6 | 2.1 | 2.7 | 4.32 | 2.9 | 2.5 | 2.3 | 2.0 | 2.12 |
| Whole grains | 0.5 | 0.9 | 1.1 | 1.2 | 1.32 | 1.2 | 1.1 | 1.2 | 0.9 | 0.82 |
| Fish | 0.1 | 0.2 | 0.2 | 0.2 | 0.32 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Poultry | 0.2 | 0.3 | 0.4 | 0.4 | 0.52 | 0.4 | 0.4 | 0.4 | 0.3 | 0.4 |
| Red and processed meats | 1.2 | 1.1 | 1.1 | 0.9 | 0.82 | 0.8 | 0.9 | 1.0 | 1.2 | 1.32 |
| Refined grains | 1.3 | 1.4 | 1.3 | 1.0 | 0.92 | 0.9 | 1.0 | 1.1 | 1.2 | 1.72 |
| Potatoes and French fries | 0.4 | 0.4 | 0.4 | 0.3 | 0.32 | 0.3 | 0.4 | 0.4 | 0.4 | 0.52 |
| Mean nutrient intake, energy-adjusted (g/d) | ||||||||||
| Total energy (kcal) | 1789 | 2020 | 2136 | 2357 | 25902 | 1414 | 1815 | 2155 | 2451 | 30572 |
| Total carbohydrate | 235 | 252 | 255 | 268 | 2682 | 267 | 266 | 264 | 244 | 2382 |
| Total protein | 77 | 83 | 89 | 86 | 962 | 87 | 85 | 85 | 86 | 892 |
| Total fat | 83 | 84 | 82 | 80 | 79 | 77 | 76 | 80 | 86 | 892 |
| SFAs | 31 | 30 | 29 | 27 | 252 | 26 | 27 | 27 | 31 | 312 |
| MUFAs | 31 | 32 | 31 | 30 | 31 | 31 | 29 | 30 | 33 | 342 |
| PUFAs | 13 | 14 | 14 | 15 | 142 | 13 | 13 | 14 | 14 | 152 |
| trans Fat | 3.4 | 3.5 | 3.4 | 3.1 | 2.62 | 2.8 | 3.0 | 3.3 | 3.6 | 3.42 |
P values were derived by using a chi-square test for categorical variables and by using the Kruskal-Wallis test for continuous variables. GED, General Education Development; Q, quintile.
P < 0.01.
P < 0.05.
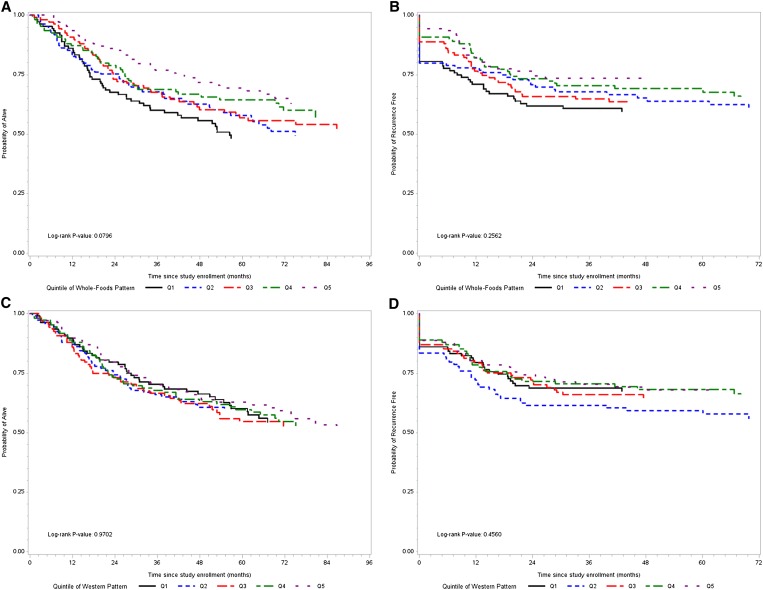
Kaplan-Meier curves for the association between each dietary pattern—categorized by quintiles, survival, and recurrence—are shown in Figure 1. HRs and 95% CIs for survival and recurrence in association with the whole-foods and Western dietary patterns are shown in Table 4. There was a significant and increasing trend toward a decreased risk of mortality across increasing quintiles of whole-foods pattern score in multivariable analyses. Although there appeared to be a trend toward a decreased risk of recurrence across increasing quintiles of whole-foods pattern scores, this association was not statistically significant in multivariable analysis. No significant associations were found between Western dietary pattern scores and survival or recurrence.
FIGURE 1.
Kaplan-Meier curves of survival and recurrence for dietary patterns. A: Survival by quintile of whole-foods pattern score. B: Recurrence by quintile of whole-foods pattern score. C: Survival by quintile of Western pattern score. D: Recurrence by quintile of Western pattern score.
TABLE 4.
Multivariate HRs and 95% CIs according to quintile of dietary pattern score for survival and recurrence events1
| Q1 (n = 108) | Q2 (n = 109) | Q3 (n = 108) | Q4 (n = 109) | Q5 (n = 108) | P2 | |
| Survival | ||||||
| Whole-foods pattern | ||||||
| Univariate | 1.0 | 0.88 (0.60, 1.3) | 0.81 (0.55, 1.19) | 0.70 (0.47, 1.05) | 0.56 (0.37, 0.86) | 0.004 |
| Multivariate3 | 1.0 | 0.81 (0.54, 1.23) | 0.88 (0.58, 1.35) | 0.65 (0.42, 1.03) | 0.56 (0.34, 0.92) | 0.01 |
| Western pattern3 | ||||||
| Univariate | 1.0 | 0.98 (0.65, 1.49) | 1.11 (0.74, 1.67) | 1.05 (0.70, 1.57) | 0.98 (0.65, 1.48) | 0.99 |
| Multivariate3 | 1.0 | 1.11 (0.71, 1.72) | 1.02 (0.65, 1.60) | 1.03 (0.62, 1.70) | 0.90 (0.49, 1.68) | 0.70 |
| Recurrence | ||||||
| Whole-foods pattern3 | ||||||
| Univariate | 1.0 | 0.88 (0.57, 1.36) | 0.87 (0.56, 1.30) | 0.75 (0.48, 1.17) | 0.60 (0.38, 0.97) | 0.03 |
| Multivariate3 | 1.0 | 0.87 (0.55, 1.38) | 0.98 (0.61, 1.57) | 0.78 (0.47, 1.29) | 0.66 (0.38, 1.16) | 0.13 |
| Western pattern3 | ||||||
| Univariate | 1.0 | 1.39 (0.89, 2.17) | 1.08 (0.68, 1.73) | 1.02 (0.64, 1.64) | 0.98 (0.61, 1.58) | 0.48 |
| Multivariate3 | 1.0 | 1.51 (0.95, 2.41) | 1.01 (0.60, 1.69) | 0.91 (0.52, 1.61) | 0.82 (0.42, 1.61) | 0.24 |
ACE, Adult Comorbidity Evaluation; Q, quintile.
P value derived from a trend test across quintiles of dietary pattern. Each individual's dietary pattern score was set to the median score for that quintile and treated as a continuous variable in the Cox regression models. P < 0.05 was considered significant.
Adjusted for age, sex, tumor site, cancer stage, treatment, ACE-27 comorbidities, smoking, BMI, and total energy intake.
In multivariable analyses, the participants classified as overweight or obese before treatment had a significantly lower risk of mortality and recurrence than did patients classified as normal or underweight. The results shown in Table 5 are from the multivariate model that includes the whole-foods dietary pattern as a covariate. The significant association between weight status and survival persisted in stratified analyses for both ever smokers and never smokers. The significant inverse association between recurrence and pretreatment BMI persisted for ever smokers, but was no longer significant for never smokers. Kaplan-Meier curves for the association between BMI and survival and BMI and recurrence for the total population, and stratified by smoking status, are shown elsewhere (see Supplemental Figure 1 under “Supplemental data” in the online issue).
TABLE 5.
Multivariate HRs and 95% CIs of pretreatment BMI >25 kg/m2 (overweight/obese) for recurrence and survival events1
| Survival |
Recurrence |
|||||
| HR | 95% CI | P2 | HR | 95% CI | P2 | |
| Total population3 | 0.65 | 0.49, 0.85 | 0.001 | 0.7 | 0.52, 0.95 | 0.02 |
| Ever smokers4 | 0.60 | 0.45, 0.81 | <0.001 | 0.66 | 0.48, 0.92 | 0.01 |
| Never smokers4 | 0.35 | 0.13, 0.91 | 0.03 | 0.54 | 0.22, 1.36 | 0.19 |
ACE, Adult Comorbidity Evaluation.
P < 0.05 was considered significant.
Adjusted for age, sex, tumor site, cancer stage, treatment, ACE-27 comorbidities, smoking, whole-foods dietary pattern, and total energy intake.
Adjusted for age, sex, tumor site, cancer stage, treatment, ACE-27 comorbidities, whole-foods dietary pattern, and total energy intake.
DISCUSSION
This study showed that a high whole-foods dietary pattern score before treatment was associated with a lower risk of recurrence and enhanced survival among HNSCC patients, independent of other factors known to influence prognosis. Being overweight or obese at the time of diagnosis was also associated with better prognosis, independent of diet. These results support and build on the work of prior studies. A small prospective cohort study in Spain reported a significantly reduced risk of recurrence, overall mortality, and cancer mortality in participants consuming high amounts of vegetables before and after diagnosis of oral cancer (5). Similarly, our prior work showed a trend of increased risk of death among HNSCC patients with a low fruit intake, although this was not statistically significant in multivariable analysis. Other studies have reported significant inverse associations between plasma carotenoids, specifically lutein, α-carotene, and β-carotene (6), and lycopene (7) and HNSCC survival. Our findings support the results of these studies, because higher plasma carotenoid concentrations may reflect a higher fruit and vegetable intake consistent with the whole-foods pattern.
The foods that characterize the whole-foods pattern are rich sources of vitamins, carotenoids, and polyphenols with known anticancer functions. Individually, many of the nutritive compounds abundant in these foods have been shown to reduce inflammation and oxidative stress in the body; maintain normal cellular processes such as cell growth, proliferation, and apoptosis; inhibit angiogenesis; and stimulate phase II detoxification enzymes to promote the excretion of carcinogenic compounds from the body (25, 26). All of these functions are shown to inhibit tumor growth, which may explain the associations between higher whole-foods pattern score and reduced HNSCC mortality and recurrence. In a subset of this same HNSCC population, our group recently reported an association between hypomethylation of tumor suppressor genes and increased intakes of folate, vitamin B-12, vitamin A, and cruciferous vegetables (27). It is possible that a high consumption of foods characterizing the whole-foods dietary pattern can alter the tumor DNA methylation profile in these patients, which allows tumor suppressor genes to continue being expressed and thus contributing to survival.
No association was found between HNSCC prognosis and Western dietary pattern score. This result was unexpected, because a high consumption of red and processed meats was previously shown to be associated with cancer development (28) and mortality (29). It has been hypothesized that high consumption of these foods is involved in tumor development and progression due to the carcinogenic actions of heterocyclic amines and aromatic hydrocarbons produced during the cooking of red meat and of nitrates and nitrites present in processed meats (30). One possible explanation for the lack of association between the Western pattern and prognosis is that many patients with high baseline Western pattern scores may have changed their dietary intake after diagnosis with HNSCC. Evidence suggests that many people attempt to make positive lifestyle changes, including changes in diet, after a cancer diagnosis (31, 32). If patients decreased their consumption of foods included in the Western pattern and increased their consumption of foods included in the whole-foods pattern, the potential adverse effect of a Western diet may have been countervailed by the protective effect of the foods with anticarcinogenic (and antimetastatic) activities. Future studies should address whether patients’ dietary patterns change after diagnosis of HNSCC, throughout treatment and in the years following, and how these potential changes affect prognosis.
The finding that overweight and obese pretreatment BMI was associated with a decreased risk of mortality and recurrence in the total study population supports the findings of data collected from the larger Cancer Prevention Study-II cohort and the Nutrition cohort case-control study (11) and evidence from 2 smaller cohort studies of HNSCC patients (12, 13). The mechanisms underlying the association of higher BMI with lower recurrence and mortality are unclear. It is estimated that >50% of patients with advanced HNSCC experience significant weight loss and cachexia during the course of disease (33). Marked weight loss and cachexia are associated with an increased susceptibility to infection and treatment-related toxicity and with a reduction in quality of life and the likelihood of survival (34). The increased fat and energy stores of overweight and obese patients may help them to withstand deterioration in nutritional status resulting from cachexia and rigorous treatment regimens (13).
To our knowledge, this was the largest study to date examining the association between diet and HNSCC prognosis and the first to relate overall dietary patterns to HNSCC outcomes. The long-term follow-up time and ability to control for multiple confounding factors are strengths of this study. Whereas the observational study design allows for the determination of an association between dietary patterns and weight status with disease prognosis, it does not demonstrate causality. Our work previously suggested significant differences in survival by cancer site, in which patients with laryngeal cancer had the best survival (4, 35); however, in this study we report no significant difference in survival by cancer site, perhaps because of the longer follow-up time as we continue this longitudinal study.
The heterogeneous nature of the study population with regard to tumor site was a limitation; however, we were able to control for site in the final models, and the large numbers of patients allowed for good statistical power. Whereas we did not have biochemical verification for smoking status, our work (36) and the work of others (37, 38) have shown >90% sensitivity and specificity for self-reported compared with biochemical validation of smoking status, with low misclassification rates; thus, self-reported smoking status was thought to be sufficient, especially because it is a covariate and not the main variable of interest. Although BMI was based on self-reported measures of height and weight, several studies have shown a strong correlation between self-reported and clinical measures of these variables (39–41). A random sampling of 27 of our own participants showed excellent (r = 0.98) agreement between self-reported and clinically measured weights.
There was insufficient statistical power to examine human papilloma virus as a confounding or effect modifying factor, but controlling for site could be considered a reasonable surrogate for human papilloma virus status, because it is likely that up to 80% of oropharyngeal cases were positive for human papilloma virus (42). Although the FFQ used to measure diet is susceptible to measurement error, which can lead to misclassification bias, this type of bias is likely to attenuate associations toward the null (16). Because patients reported their usual prediagnosis dietary intake after their cancer diagnosis, recall bias related to social desirability, stress of the diagnosis, or concerns about contributing to their own disease could have occurred. Whereas recall bias is a concern, previous studies of diet and breast cancer indicate either little evidence of recall bias (43, 44) or that the measures of association are attenuated when recall bias occurs (45). Future research in HNSCC populations should be conducted to determine whether dietary recall bias exists and how it may influence the study results. Despite recruitment efforts at an inner city and Veterans Affairs hospital, the study population consisted predominantly of white males; thus, the results may not be generalizable to racially diverse HNSCC populations.
In conclusion, consuming a diet high in vegetables, fruit, fish, poultry, and whole grains and an overweight or obese status at the time of HNSCC diagnosis is associated with reduced mortality and recurrence. Patients who consume low amounts of foods included in the whole-foods dietary pattern or with normal or underweight status may benefit from more aggressive surveillance throughout the disease trajectory. The results of this study highlight the need for randomized controlled trials aimed at optimizing the likelihood of a favorable disease prognosis via early nutritional intervention.
Acknowledgments
We thank the patients, clinicians, and principal investigators of the individual projects at the UM Head and Neck SPORE program, who provided access to the longitudinal clinical database and were responsible for the recruitment, treatment, and follow-up of patients included in this report. These investigators included Avraham Eisbruch, Theodore Lawrence, Mark Prince, Jeffrey Terrell, Shaomeng Wang, and Frank Worden.
The authors’ responsibilities were as follows—AEA, KEP, SAD, and LSR: designed the research and had primary responsibility for the final content; AEA, KEP, SAD, LSR, GTW, JET, JMGT, and DBC: conducted the research; AEA, EL, JMGT, KEP, SAD, and LSR: analyzed the data; and AEA, KEP, SAD, LSR, JRH, and GTW: wrote the manuscript. None of the authors had a conflict of interest to declare.
Footnotes
Abbreviations used: AUDIT, Alcohol Use Disorders Identification Test; FFQ, food-frequency questionnaire; HNSCC, head and neck squamous cell carcinoma; UM HN-SPORE, University of Michigan Head and Neck Specialized Program of Research Excellence.
REFERENCES
- 1.Argiris A, Karamouzis MV, Raben D, Ferris RL. Head and neck cancer. Lancet 2008;371(9625):1695–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pfister DG, Ang KK, Brizel DM, Burtness BA, Cmelak AJ, Colevas AD, Dunphy F, Eisele DW, Gilbert J, Gillison ML, et al. Head and neck cancers. J Natl Compr Canc Netw 2011;9(6):596–650 [DOI] [PubMed] [Google Scholar]
- 3.Gillison ML. Current topics in the epidemiology of oral cavity and oropharyngeal cancers. Head Neck 2007;29:779–92 [DOI] [PubMed] [Google Scholar]
- 4.Duffy SA, Ronis DL, McLean S, Fowler KE, Gruber SB, Wolf GT, Terrell JE. Pretreatment health behaviors predict survival among patients with head and neck squamous cell carcinoma. J Clin Oncol 2009;27(12):1969–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sandoval M, Font R, Manos M, Dicenta M, Quintana MJ, Bosch FX, Castellsague X. The role of vegetable and fruit consumption and other habits on survival following the diagnosis of oral cancer: a prospective study in Spain. Int J Oral Maxillofac Surg 2009;38:31–9 [DOI] [PubMed] [Google Scholar]
- 6.Sakhi AK, Bohn SK, Smeland S, Thoresen M, Smedshaug GB, Tausjo J, Svilaas A, Karlsen A, Russnes KM, Svilaas T, et al. Postradiotherapy plasma lutein, alpha-carotene, and beta-carotene are positively associated with survival in patients with head and neck squamous cell carcinoma. Nutr Cancer 2010;62:322–8 [DOI] [PubMed] [Google Scholar]
- 7.Mayne ST, Cartmel B, Lin H, Zheng T, Goodwin WJ., Jr Low plasma lycopene concentration is associated with increased mortality in a cohort of patients with prior oral, pharynx or larynx cancers. J Am Coll Nutr 2004;23:34–42 [DOI] [PubMed] [Google Scholar]
- 8.Gugatschka M, Kiesler K, Obermayer-Pietsch B, Groselj-Strele A, Griesbacher A, Friedrich G. Vitamin D status is associated with disease-free survival and overall survival time in patients with squamous cell carcinoma of the upper aerodigestive tract. Eur Arch Otorhinolaryngol 2011;268:1201–4 [DOI] [PubMed] [Google Scholar]
- 9.Meyer F, Liu G, Douville P, Samson E, Xu W, Adjei A, Bairati I. Dietary vitamin D intake and serum 25-hydroxyvitamin D level in relation to disease outcomes in head and neck cancer patients. Int J Cancer 2011;128(7):1741–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu FB, Rimm EB, Stampfer MJ, Ascherio A, Spiegelman D, Willett WC. Prospective study of major dietary patterns and risk of coronary heart disease in men. Am J Clin Nutr 2000;72:912–21 [DOI] [PubMed] [Google Scholar]
- 11.Gaudet MM, Patel AV, Sun J, Hildebrand JS, McCullough ML, Chen AY, Gapstur SM. Prospective studies of body mass index with head and neck cancer incidence and mortality. Cancer Epidemiol Biomarkers Prev 2012;21(3):497–503 [DOI] [PubMed] [Google Scholar]
- 12.Mell LK, Dignam JJ, Salama JK, Cohen EE, Polite BN, Dandekar V, Bhate AD, Witt ME, Haraf DJ, Mittal BB, et al. Predictors of competing mortality in advanced head and neck cancer. J Clin Oncol 2010;28(1):15–20 [DOI] [PubMed] [Google Scholar]
- 13.McRackan TR, Watkins JM, Herrin AE, Garrett-Mayer EM, Sharma AK, Day TA, Gillespie MB. Effect of body mass index on chemoradiation outcomes in head and neck cancer. Laryngoscope 2008;118:1180–5 [DOI] [PubMed] [Google Scholar]
- 14.Pednekar MS, Hakama M, Hebert JR, Gupta PC. Association of body mass index with all-cause and cause-specific mortality: findings from a prospective cohort study in Mumbai (Bombay), India. Int J Epidemiol 2008;37:524–35 [DOI] [PubMed] [Google Scholar]
- 15.Rosner BA. Percentage points for a generalized ESD many-outlier procedure. Technometrics 1983;25:8 [Google Scholar]
- 16.Willett W. Nutritional epidemiology. 2nd ed. New York, NY: Oxford University Press, 1998 [Google Scholar]
- 17.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 1985;122:51–65 [DOI] [PubMed] [Google Scholar]
- 18.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol 1992;135:1114–26, discussion 27–36 [DOI] [PubMed] [Google Scholar]
- 19.Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption–II. Addiction 1993;88:791–804 [DOI] [PubMed] [Google Scholar]
- 20.Piccirillo JF, Tierney RM, Costas I, Grove L, Spitznagel EL., Jr Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA 2004;291:2441–7 [DOI] [PubMed] [Google Scholar]
- 21.Lewinsohn PM, Seeley JR, Roberts RE, Allen NB. Center for Epidemiologic Studies Depression Scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychol Aging 1997;12:277–87 [DOI] [PubMed] [Google Scholar]
- 22.Heidemann C, Schulze MB, Franco OH, van Dam RM, Mantzoros CS, Hu FB. Dietary patterns and risk of mortality from cardiovascular disease, cancer, and all causes in a prospective cohort of women. Circulation 2008;118(3):230–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldberg R. PROC FACTOR: how to interpret the output of a real-world example. Available from: http://www2.sas.com/proceedings/sugi22/STATS/PAPER268.PDF (cited 15 August 2011)
- 24.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA 2010;303:235–41 [DOI] [PubMed] [Google Scholar]
- 25.Hu ML. Dietary polyphenols as antioxidants and anticancer agents: more questions than answers. Chang Gung Med J 2011;34:449–60 [PubMed] [Google Scholar]
- 26.Yao H, Xu W, Shi X, Zhang Z. Dietary flavonoids as cancer prevention agents. J Environ Sci Health Part C Environ Carcinog Ecotoxicol Rev 2011;29:1–31 [DOI] [PubMed] [Google Scholar]
- 27.Colacino JA, Arthur AE, Dolinoy DC, Sartor MA, Duffy SA, Chepeha DB, Bradford CR, Walline HM, McHugh JB, D'Silva N, et al. Pretreatment dietary intake is associated with tumor suppressor DNA methylation in head and neck squamous cell carcinomas. Epigenetics 2012;7(8):883–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng W, Lee SA. Well-done meat intake, heterocyclic amine exposure, and cancer risk. Nutr Cancer 2009;61:437–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pan A, Sun Q, Bernstein AM, Schulze MB, Manson JE, Stampfer MJ, Willett WC, Hu FB. Red meat consumption and mortality: results from 2 prospective cohort studies. Arch Intern Med 2012;172(7):555–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferguson LR. Meat and cancer. Meat Sci 2010;84:308–13 [DOI] [PubMed] [Google Scholar]
- 31.Wayne SJ, Lopez ST, Butler LM, Baumgartner KB, Baumgartner RN, Ballard-Barbash R. Changes in dietary intake after diagnosis of breast cancer. J Am Diet Assoc 2004;104:1561–8 [DOI] [PubMed] [Google Scholar]
- 32.Alfano CM, Day JM, Katz ML, Herndon JE, 2nd, Bittoni MA, Oliveri JM, Donohue K, Paskett ED. Exercise and dietary change after diagnosis and cancer-related symptoms in long-term survivors of breast cancer: CALGB 79804. Psychooncology 2009;18:128–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Couch M, Lai V, Cannon T, Guttridge D, Zanation A, George J, Hayes DN, Zeisel S, Shores C. Cancer cachexia syndrome in head and neck cancer patients: part I. Diagnosis, impact on quality of life and survival, and treatment. Head Neck 2007;29:401–11 [DOI] [PubMed] [Google Scholar]
- 34.Nitenberg G, Raynard B. Nutritional support of the cancer patient: issues and dilemmas. Crit Rev Oncol Hematol 2000;34(3):137–68 [DOI] [PubMed] [Google Scholar]
- 35.Duffy SA, Taylor JM, Terrell JE, Islam M, Li Y, Fowler KE, Wolf GT, Teknos TN. Interleukin-6 predicts recurrence and survival among head and neck cancer patients. Cancer 2008;113:750–7 [DOI] [PubMed] [Google Scholar]
- 36.Noonan D JY, Duffy SA. Utility of biochemical verification of tobacco cessation in the Department of Veterans Affairs. Addict Behav 2013;38(3):1792–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Studts JL, Ghate SR, Gill JL, Studts CR, Barnes CN, LaJoie AS, Andrykowski MA, LaRocca RV. Validity of self-reported smoking status among participants in a lung cancer screening trial. Cancer Epidemiol Biomarkers Prev 2006;15(10):1825–8 [DOI] [PubMed] [Google Scholar]
- 38.Heck JD. Smokers of menthol and nonmenthol cigarettes exhibit similar levels of biomarkers of smoke exposure. Cancer Epidemiol Biomarkers Prev 2009;18(2):622–9 [DOI] [PubMed] [Google Scholar]
- 39.Finardi P, Nickel CH, Koller MT, Bingisser R. Accuracy of self-reported weight in a high risk geriatric population in the emergency department. Swiss Med Wkly 2012;142:w13585. [DOI] [PubMed] [Google Scholar]
- 40.Haverkort EB, de Haan RJ, Binnekade JM. van Bokhorst-de van der Schueren MA. Self-reporting of height and weight: valid and reliable identification of malnutrition in preoperative patients. Am J Surg 2012;203:700–7 [DOI] [PubMed] [Google Scholar]
- 41.Bes-Rastrollo M, Sabate J, Jaceldo-Siegl K, Fraser GE. Validation of self-reported anthropometrics in the Adventist Health Study 2. BMC Public Health 2011;11:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, Jiang B, Goodman MT, Sibug-Saber M, Cozen W, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol 2011;29(32):4294–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Friedenreich CM, Howe GR, Miller AB. The effect of recall bias on the association of calorie-providing nutrients and breast cancer. Epidemiology 1991;2:424–9 [DOI] [PubMed] [Google Scholar]
- 44.Holmberg L, Ohlander EM, Byers T, Zack M, Wolk A, Bruce A, Bergstrom R, Bergkvist L, Adami HO. A search for recall bias in a case-control study of diet and breast cancer. Int J Epidemiol 1996;25:235–44 [DOI] [PubMed] [Google Scholar]
- 45.Giovannucci E, Stampfer MJ, Colditz GA, Manson JE, Rosner BA, Longnecker MP, Speizer FE, Willett WC. Recall and selection bias in reporting past alcohol consumption among breast cancer cases. Cancer Causes Control 1993;4:441–8 [DOI] [PubMed] [Google Scholar]