Abstract
Background: It has been suggested that dietary patterns are associated with future risk of depressive symptoms. However, there is a paucity of prospective data that have examined the temporality of this relation.
Objective: We examined whether adherence to a healthy diet, as defined by using the Alternative Healthy Eating Index (AHEI), was prospectively associated with depressive symptoms assessed over a 5-y period.
Design: Analyses were based on 4215 participants in the Whitehall II Study. AHEI scores were computed in 1991–1993 and 2003–2004. Recurrent depressive symptoms were defined as having a Center for Epidemiologic Studies Depression Scale score ≥16 or self-reported use of antidepressants in 2003–2004 and 2008–2009.
Results: After adjustment for potential confounders, the AHEI score was inversely associated with recurrent depressive symptoms in a dose-response fashion in women (P-trend < 0.001; for 1 SD in AHEI score; OR: 0.59; 95% CI: 0.47, 0.75) but not in men. Women who maintained high AHEI scores or improved their scores during the 10-y measurement period had 65% (OR: 0.35%; 95% CI: 0.19%, 0.64%) and 68% (OR: 0.32%; 95% CI: 0.13%, 0.78%) lower odds of subsequent recurrent depressive symptoms than did women who maintained low AHEI scores. Among AHEI components, vegetable, fruit, trans fat, and the ratio of polyunsaturated fat to saturated fat components were associated with recurrent depressive symptoms in women.
Conclusion: In the current study, there was a suggestion that poor diet is a risk factor for future depression in women.
INTRODUCTION
The potential effect of specific nutrients on physiologic pathways that lead to depression (1), which is allied to the observation that diet is a modifiable behavior, has prompted a series of studies that examined the potential etiologic role of dietary factors in the development of this mental health problem. However, studies that have examined the diet-depression relation have focused primarily on individual foods or nutrients. The most-frequently investigated dietary characteristics include long-chain n−3 PUFAs, fish, and nutrients involved in the homocysteine pathway (2), but the findings have been mixed, and a recent systematic review of observational studies reported a lack of consistent evidence that links these dietary factors with depression (2).
Some methodologic limitations may have contributed to inconsistencies in the evidence, including a reliance on cross-sectional studies, the lack of adjustment for potential confounding factors, or crude assessment of diet or depressive symptoms (DepSs)5 (2). An additional explanation is that, although a potential beneficial effect of some nutrients on the depression disease process may exist, the effect of single nutrients may be too small to be detected (3). Indeed, because people do not eat individual nutrients or individual foods but meals that consist of complex combinations of nutrients that interact with each other (3), it appears that the focus on individual nutrients or foods may provide an incomplete understanding of the relation between diet and DepSs. Accordingly, more emphasis needs to be given to the influence of dietary patterns on chronic diseases such as depression.
We have previously shown an association between a single baseline measurement of dietary pattern and future DepSs (4) assessed 5 y later. Reports from several other studies have since confirmed this finding in both adolescents (5, 6) and adults (7–11). However, the cross-sectional design of most of these studies (7–10) inevitably precludes any conclusion as to the direction of the association (12–14). To understand if poor diet constitutes a risk factor for depression and not only the reverse, studies with longitudinal design are needed.
With the use of updated data from the Whitehall II study, including DepSs measured twice over a 5-y period, we examined the association of the overall diet, which was assessed by using the Alternative Healthy Eating Index (AHEI), and 10-y change in diet with subsequent recurrent DepSs. The ability to examine dietary adherence repeatedly is particularly important: if diet is indeed associated with a new onset of DepSs, it would be expected that a change in diet would precipitate a change in risk of subsequent DepSs. These data are rare; to the best of our knowledge, no such study has previously been conducted.
SUBJECTS AND METHODS
Study population
Data were drawn from the Whitehall II Study, which is a large-scale, ongoing, prospective cohort study of 10,308 (3413 women) UK civil servants (government employees) aged 35–55 y at study induction (15). The baseline examination (phase 1) took place in 1985–1988 and involved a clinical examination and self-administered questionnaire. Subsequent phases of data collection have alternated between a postal questionnaire alone [phases 2 (1988–1990), 4 (1995–1996), 6 (2001), and 8 (2006)] and a postal questionnaire accompanied by a clinical examination [phases 3 (1991–1993; n = 8104), 5 (1997–1999; n = 7263), 7 (2003–2004; n = 6943), and 9 (2008-2009; n = 6354)]. After the study was described to the participants, written informed consent was obtained; the University College London ethics committee approved the study.
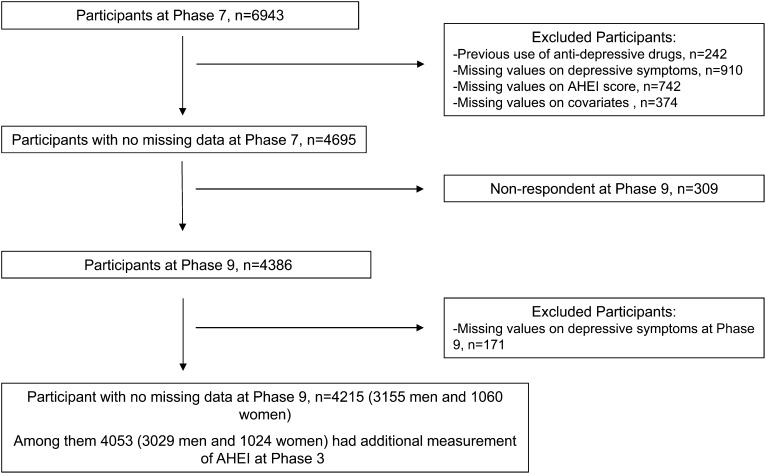
After the exclusion of subjects who were administered antidepressant treatment at phase 3 or 5, analyses were restricted to participants with dietary assessment and covariates at phase 7 and complete data on DepSs at phases 7 and 9, which resulted in a total of 4215 participants. Of these participants, 4053 subjects had complete data on the 10-y change in the AHEI between phases 3 and 7 (Figure 1).
FIGURE 1.
Derivation of the analytic sample. Compared with participants excluded from the current analytic sample (n = 2728), included participants (n = 4215) were more likely to be men, white, younger, and with a high socioeconomic status (all P < 0.001) and less likely to report recurrent depressive symptoms (P < 0.001). Furthermore, a higher mean total energy intake (P < 0.001) and AHEI score (P < 0.001) were observed in included than excluded participants because of missing data on depressive symptoms or covariates. AHEI, Alternative Healthy Eating Index.
Data collection
Dietary pattern by using the AHEI at phases 3 and 7
Dietary intake at phases 3 and 7 was assessed by using a semiquantitative food-frequency questionnaire (FFQ) with 127 food items, as described previously (16, 17). The validity and reliability of the FFQ in terms of nutrient and food consumption have been documented in detail in our cohort and others (17, 18). The AHEI score (19) was created (see Table S1 under “Supplemental data” in the online issue) by summing its 9 component scores [1: fruit; 2: vegetable; 3: ratio of white meat (seafood and poultry) to red meat; 4: trans fat; 5: ratio of PUFA to SFA; 6: total fiber; 7: nuts and soy; 8: alcohol consumption; and 9: long-term multivitamin use]. Higher values corresponded to a healthier diet. At phase 3, the mean (±SD) score of the AHEI was 50.6 ± 11.9 points at phase 3 and 51.4 ± 12.3 points at phase 7. Pearson's correlation coefficient between the AHEI score at phase 3 and phase 7 was 0.59 (P < 10−4).
DepSs at phases 7 and 9
DepSs were assessed by using the Center for Epidemiologic Studies Depression Scale (CES-D) (20), which was first introduced at phase 7. At phases 7 and 9, DepSs were defined by a CES-D score ≥16 (20), self-reported use of antidepressant medications, or both. After the exclusion of participants who were administered antidepressant treatments before phase 7, recurrent DepS cases were defined as participants who had DepSs at both phases 7 and 9; participants with no recurrent DepS were defined as individuals with an absence of DepSs at phases 7 and 9 or who had DepSs in only one of the 2 phases.
Covariates assessed at phases 3 and 7
Sociodemographic variables included sex, age (y), ethnicity (white, South Asian, or black), living alone (yes or no), a 3-level measure of socioeconomic status (SES) (low, intermediate, or high) related to salary, social status, and level of responsibility, and retirement status (yes or no). Health behaviors were smoking habits (never, former, or current), total energy intake (kcal/d), and physical activity assessed by using a questionnaire that included 20 items on the frequency and duration of participation in different activities of different intensity (eg, walking, cycling, and sports). Participants were classified as active (>2.5 h/wk of moderate physical activity or >1 h/wk of vigorous physical activity), inactive (<1 h/wk of moderate physical activity and <1 h/wk of vigorous physical activity), or moderately active (neither active nor inactive) (21). Health status covariates included prevalent coronary artery disease (CAD) (denoted by clinically verified nonfatal myocardial infarction or definite angina), hypertension (defined by systolic or diastolic blood pressure ≥140 or ≥90 mm Hg, respectively, or the use of antihypertensive drugs), serum HDL cholesterol (in mmol/L), the use of lipid-lowering drugs, central obesity (waist circumference >102 cm in men and >88 cm in women), and cognitive impairment [≤27 score on the Mini-Mental State Examination] (22). Except for HDL cholesterol, all health status covariates were dichotomized as yes or no
Statistical analysis
Characteristics of men and women according to recurrent DepSs were compared by using the chi-square test for categorical covariates and ANOVA for continuous covariates. Logistic regression analyses were used to compute ORs with 95% CIs to assess the association between the AHEI score at phase 7 and recurrent DepSs. The AHEI score was first categorized into tertiles (lowest tertile as the reference) to assess whether the AHEI score was linearly associated with DepSs. We then derived a continuous standardized variable (mean ± SD z score: 0 ± 1) to compute the OR for recurrent DepSs per 1-SD increment in dietary score.
The effect modification of the association between the AHEI (z score) and recurrent DepSs by sociodemographic and health behaviors was examined. A significant interaction was shown with sex (P = 0.004), which led us to conduct analyses separately in men and women. No other significant interactions were shown. Models stratified by sex were first adjusted for age, ethnicity, and total energy intake (model 1) and, in addition, for SES, retirement, living alone, smoking behavior, physical activity, HDL cholesterol, type 2 diabetes, CAD, hypertension, use of lipid lowering drugs, central obesity, and cognitive impairment assessed at phase 7 (the time of exposure) (model 2).
Similar logistic regression models were performed to estimate the association of each AHEI-component z score with recurrent DepSs. To assess whether the AHEI-component–recurrent DepS association was independent of other components, a third model was also computed that incorporated all other AHEI components in addition to potential confounders (model 3). Additional analyses were performed to examine the contribution of AHEI components to the association between the AHEI and DepSs. For each component (component i), we computed a modified AHEI score on the basis of the total AHEI score without the component i as follows:
To analyze the 10-y change in the AHEI score, scores of the AHEI at phases 3 and 7 were categorized as high or low according to the median value of the AHEI score at phase 3 equal to 51.5 points. Four categories in the 10-y change of the AHEI were then defined as follows: participants who maintained a high score (phase 3 and 7 scores ≥51.5 points), participants who maintained a low score over the 10-y exposure period (phase 3 and 7 scores <51.5 points), participants who improved their AHEI score (phase 3 score <51.5 points and phase 7 score ≥51.5 points), and participants who decreased their AHEI score (phase 3 score ≥51.5 points and phase 7 score <51.5 points). Similar procedures were applied to categorize the 10-y change in AHEI components. Median values at phase 3 for AHEI components were 6 for vegetables, 6 for fruit, 3 for nuts and soy, 5for the ratio of white to red meat, 10 for fiber, 10 for trans fat, 5 for the ratio of PUFA to SFA, 2.5 for multivitamin use, and 5 for alcohol. Similar adjustments as in models 1 and 2 were made, but covariates assessed at phase 3 were includes in the models. The following covariates were not included: prevalent CAD, lipid-lowering drugs (because of the small number of cases in stratified analyses), and cognitive impairment (not assessed at phase 3).
We also examined if the diet-DepS association was mediated by CAD. To do so, similar analyses were repeated after the exclusion of CAD cases. Three other sets of analyses were performed to assess the direction of the diet-DepS relation. First, we examined the association between the AHEI score at phase 3 and subsequent recurrent DepSs assessed 10 y later (phases 7 and 9) after adjustment for potential confounders assessed at phase 3. Second, the main analyses were repeated after the exclusion of participants who met the definition for General Health Questionnaire depression (GHQ-Dep) at phase 3 on the basis of the 4-item GHQ-Dep subscale (23). We also performed linear regression models that estimated the linear regression coefficient (β and its SE) of the diet score to assess whether GHQ-Dep cases at phase 3 showed significant differences in the subsequent AHEI score assessed at phase 7 or in the 10-y change in the AHEI score between phases 3 and 7 compared with GHQ-Dep noncases. All analyses were conducted with SAS software (version 9; SAS Institute).
RESULTS
Participant characteristics
In the 4215 participants, 260 subjects (6.2%) developed recurrent DepSs. In the 3955 participants without recurrent DepSs (93.8%), 575 subjects (13.6%) had DepSs in one measurement at phase 7 (n = 310) or 9 (n = 265) only and 3380 patients (80.2%) had no DepSs at either phase. Characteristics of participants according to recurrent DepS status separately in men and women are presented in Table 1.
TABLE 1.
Characteristics of participants without a history of depression according to DepSs over 5 y of follow-up (n = 4215)1
| Recurrent DepSs over 5 y of follow-up |
||||||
| Men (n = 3155) |
Women (n = 1060) |
|||||
| Characteristics at phase 7 | No (n = 2991) | Yes (n = 64) | P | No (n = 964) | Yes (n = 96) | P |
| Sociodemographic factors | ||||||
| Age (y) | 61.0 ± 5.92 | 59.7 ± 5.8 | 0.01 | 61.0 ± 5.91 | 60.8 ± 6.2 | 0.77 |
| Ethnicity (white) (%) | 96.7 | 89.0 | <0.001 | 93.1 | 86.5 | <0.001 |
| SES (low) (%) | 2.3 | 6.7 | <0.001 | 22.8 | 28.1 | 0.02 |
| Retired (yes) (%) | 51.1 | 48.8 | 0.56 | 54.7 | 57.3 | 0.62 |
| Living alone (yes) (%) | 15.4 | 26.2 | <0.001 | 39.7 | 52.1 | 0.02 |
| Health behavior factors | ||||||
| Smoking habits (current smokers) (%) | 6.1 | 10.4 | 0.09 | 8.6 | 11.5 | 0.40 |
| Physical activity (low) (%) | 20.7 | 32.9 | <0.001 | 26.8 | 38.5 | 0.03 |
| Total energy intake (kcal/d) | 2258 ± 634 | 2340 ± 788 | 0.19 | 1996 ± 594 | 2121 ± 732 | 0.11 |
| Health status factors | ||||||
| Type 2 diabetes (yes) (%) | 8.3 | 11.0 | 0.23 | 9.1 | 10.4 | 0.68 |
| Central obesity (yes) (%) | 22.2 | 29.3 | 0.03 | 43.9 | 46.9 | 0.57 |
| History of CAD (yes) (%) | 7.0 | 10.4 | 0.11 | 4.7 | 10.4 | 0.01 |
| Hypertension (yes) (%) | 35.7 | 39.0 | 0.33 | 36.4 | 37.5 | 0.83 |
| HDL cholesterol (mmol/L) | 1.49 ± 0.39 | 1.45 ± 0.38 | 0.23 | 1.86 ± 0.48 | 1.72 ± 0.46 | 0.009 |
| Use of lipid-lowering drugs (yes) (%) | 11.6 | 14.6 | 0.24 | 9.1 | 10.4 | 0.68 |
| Cognitive impairment (yes) (%) | 10.9 | 18.9 | 0.001 | 12.7 | 18.7 | 0.09 |
| AHEI scores (points) | ||||||
| At phase 7 | 50.5 ± 11.9 | 50.0 ± 14.1 | 0.66 | 54.6 ± 12.6 | 49.0 ± 11.9 | <0.001 |
| At phase 3 | 49.68 ± 11.5 | 49.3 ± 12.2 | 0.75 | 53.9 ± 12.9 | 51.4 ± 12.6 | 0.06 |
| Absolute 10-y change | 0.90 ± 10.9 | 0.60 ± 12.2 | 0.77 | −0.75 ± 11.3 | −3.0 ± 9.7 | 0.002 |
Recurrent DepS cases were defined as participants who had DepSs at both phases 7 and 9 and were compared with participants with no recurrent DepSs who were defined as individuals with an absence of DepSs at both phases 7 and 9 or who had DepSs in only one of the 2 phases (with DepSs cases defined as participants who had a CES-D score ≥16 or were using antidepressive drugs). Characteristics of participants included sociodemographic variables that consisted of sex, age (y), skin color (white, South Asian, and black) who were living alone (no compared with yes), SES (low, intermediate, or high), and retirement status (yes or no). Health behaviors considered were smoking habits (never, former, or current) and physical activity (inactive, moderately active, or active). Physical activity was assessed by using a questionnaire that included 20 items on the frequency and duration of participation in different physical activities (eg, walking, cycling, and sports) that were used to compute hours per week at each intensity level. Participants were classified as active (>2.5 h/wk of moderate physical activity or >1 h/wk of vigorous physical activity), inactive (<1 h/wk of moderate physical activity and <1 h/wk of vigorous physical activity), or moderately active (if not active or inactive) (21). Baseline health status was based on CAD (ie, clinically verified nonfatal myocardial infarction or definite angina); hypertension (systolic or diastolic blood pressure ≥40 or ≥90 mm Hg, respectively, or the use of antihypertensive drugs); HDL cholesterol, use of lipid-lowering drugs, central obesity (waist circumference >102 cm in men and >88 cm in women); and cognitive impairment (defined by a score ≤27 in the Mini-Mental State Examination) (22). Except for HDL cholesterol (mmol/L), all other health status covariates were dichotomized as yes or no. For P values, the chi-square test for categorical variables and ANOVA for quantitative variables were used to compare characteristics according to recurrent, nonrecurrent, and no DepSs. AHEI, Alternative Healthy Eating Index; CAD, coronary artery disease; DepS, depressive symptom; SES, socioeconomic status.
Mean ± SD (all such values).
AHEI score at phase 7- and 5-y DepSs
The associations between the AHEI at phase 7 and recurrent DepSs are presented in Table 2. Irrespective of adjustments, analyses in which the AHEI was categorized in tertiles showed that this measure of dietary quality was associated with recurrent DepSs in a dose-response fashion in women (P-trend < 0.001) but not in men (P-trend = 0.81), (P-sex interaction = 0.004). In women, each 1-SD increase (12 points) in AHEI score was associated with a 40% lower odds of recurrent DepSs (OR: 0.59; 95% CI: 0.47, 0.75; P < 0.001) after adjustment for potential confounders. In men, such a relation was not shown (Table 2).
TABLE 2.
ORs (95% CIs) for the association between the AHEI score at phase 7 and subsequent recurrent DepSs over 5 y of follow-up in men and women1
| Men |
Women |
|||
| AHEI at phase 72 | OR (95% CI) | P | OR (95% CI) | P |
| Model 1 | ||||
| Tertile 1 | 1 (reference) | 1 (reference) | ||
| Tertile 2 | 0.80 (0.54, 1.19) | 0.28* | 0.56 (0.33, 0.94) | 0.02* |
| Tertile 3 | 0.85 (0.57, 1.26) | 0.42* | 0.31 (0.18, 0.54) | <0.001* |
| AHEI z score | 0.89 (0.75, 1.05) | 0.18 | 0.56 (0.44, 0.70) | <0.001 |
| Model 2 | ||||
| Tertile 1 | 1 (reference) | 1 (reference) | ||
| Tertile 2 | 0.89 (0.59, 1.32) | 0.55* | 0.61 (0.35, 1.04) | 0.07* |
| Tertile 3 | 0.95 (0.64, 1.42) | 0.81* | 0.36 (0.20, 0.64) | <0.001* |
| AHEI z score | 0.95 (0.80, 1.13) | 0.57 | 0.59 (0.47, 0.75) | <0.001 |
A significant interaction was shown with sex (P = 0.004), which led us to conduct analyses separately in men and women. Model 1 was adjusted for age, sex, ethnicity, and total energy intake at phase 7. Model 2 was adjusted as for model 1 and for SES, retirement, living alone, smoking, physical activity, coronary artery disease, type 2 diabetes, hypertension, HDL cholesterol, use of lipid-lowering drugs, central obesity, and cognitive impairment assessed at phase 7. *In men, P-trend = 0.41 in model 1 and P-trend = 0.81 in model 2; in women, P-trend < 0.001 in models 1 and 2. AHEI, Alternative Healthy Eating Index; DepS, depressive symptom.
Results of logistic regression of the estimation of odds of recurrent DepSs according to AHEI tertiles and by 1 SD of the total AHEI score (12 points). Median (range) scores were 39.5 (10.5–45.5) in tertile 1 (35.2% of men and 24.9% of women), 51.5 (46.5–56.5) in tertile 2 (33.7% of men and 31.2% of women), and 63.5 (57.5–87.5) in tertile 3 (31.1% of men and 43.9% of women).
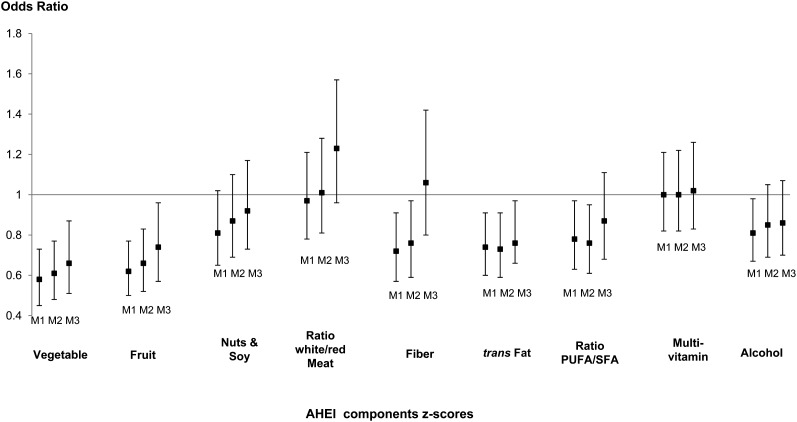
Additional analyses were performed in women to identify which of the AHEI components contributed most to the reduce odds of DepSs. As shown in Figure 2, a high consumption of vegetables and fruit, high intake of fiber, elevated PUFA:SFA ratio, and low intake of trans fat were all associated with lower odds of recurrent DepSs after adjustment for potential confounders (Figure 2, model 2). Of these AHEI components, only vegetable, fruit, and trans-fat components revealed independent effects (Figure 2, model 3). In addition, when the AHEI was computed without each AHEI component, the modified AHEI-recurrent–DepS association remained significant, which suggested that no single AHEI component was responsible for the generation of the total AHEI-DepS association (results not shown). In men, none of the AHEI components were shown to be significantly associated with recurrent DepSs after adjustment for potential confounders (see Figure S1 under “Supplemental data” in the online issue).
FIGURE 2.
Associations between AHEI-component scores assessed at phase 7 and onset of recurrent depressive symptoms over 5 y in women. ORs for the development of recurrent depression symptoms associated with an increase of 1 SD in AHEI-component scores at phase 7. M1 was adjusted for age, ethnicity, and total energy intake at phase 7. M2 was adjusted as for M1 and for socioeconomic status, retirement status, marital status, smoking, physical activity, coronary artery disease, type 2 diabetes, hypertension, HDL cholesterol, use of lipid-lowering drugs, central obesity, and cognitive impairment assessed at phase 7. M3 was adjusted as for M2 and for the 8 other AHEI-component scores. AHEI, Alternative Healthy Eating Index; M1, model 1; M2, model 2; M3, model 3.
Ten-year change in AHEI score and subsequent 5-y DepSs
As shown in Table 3, women who maintained a high AHEI score (≥51.5 points at phases 3 and 7) as well as those who improved their AHEI score (phase 3 score <51.5 points and phase 7 score ≥51.5 points) over the 10-y measurement period had a 65% (OR: 0.35; 95% CI: 0.19, 0.64) and 68% (OR: 0.32; 95% CI: 0.13, 0.18) lower odds of subsequent recurrent DepSs than participants did who maintained a low AHEI score at both phases 3 and 7 (score <51.5 points). Women whose AHEI score decreased over time (phase 3 score ≥51.5 points and phase 7 score <51.5 points) had a 2-fold increased odds of developing subsequent recurrent DepSs than women did who maintained a high AHEI score over the 10-y exposure period (OR: 2.15; 95% CI: 1.09, 4.22). In men, no evidence of an association between the 10-y change in total AHEI score and subsequent recurrent DepSs was observed (see Table S2 under “Supplemental data” in the online issue).
TABLE 3.
ORs (95% CIs) for the association between the 10-y change in AHEI score between phases 3 and 7 and subsequent recurrent DepSs over 5 y of follow-up in women1
| 10-y-change category in AHEI2 | n | OR (95% CI) | P |
| Model 1 (n = 1024) | |||
| Maintaining a high AHEI score (phase 3 and 7 scores ≥51.5 points) | 477 | 0.32 (0.18, 0.57) | 0.001 |
| Compared with a low score (phase 7 and 3 scores <51.5 points) | 251 | 1 (reference) | |
| Improved AHEI score (phase 3 score <51.5 points and phase 7 score ≥51.5 points) | 148 | 0.40 (0.18, 0.87) | 0.02 |
| Compared with maintaining a low score | 251 | 1 (reference) | |
| Decreased AHEI score (phase 3 score ≥51.5 points and phase 7 score <51.5 points) | 148 | 2.34 (1.24, 4.42) | 0.009 |
| Compared with maintaining a high score | 477 | 1 (reference) | |
| Model 2 (n = 968) | |||
| Maintaining a high AHEI score (phases 3 and 7 scores ≥51.5 points) | 449 | 0.35 (0.19, 0.64) | <0.001 |
| Compared with a low score (phase 7 and 3 scores <51.5 points) | 233 | 1 (reference) | |
| Improved AHEI score (phase 3 score <51.5 points and phase 7 score ≥51.5 points) | 142 | 0.32 (0.13, 0.78) | 0.01 |
| Compared with maintaining a low score | 233 | 1 (reference) | |
| Decreased AHEI score (phase 3 score ≥51.5 points and phase 7 score <51.5 points) | 144 | 2.15 (1.09, 4.22) | 0.03 |
| Compared with maintaining a high score | 449 | 1 (reference) |
Results of logistic regression of the estimation of odds of recurrent DepSs according to the 10-y change in AHEI score are shown. To analyze the 10-y change in the AHEI score, scores of AHEI at phases 3 and 7 were categorized as high or low according to the median value of the AHEI score at phase 3 equal to 51.5 points. Model 1 was adjusted for age, ethnicity, and total energy intake at phase 3. Model 2 was adjusted as for model 1 and for SES, retirement, marital status, smoking, physical activity, hypertension, coronary artery disease, HDL cholesterol, and central obesity at phase 3. AHEI, Alternative Healthy Eating Index; DepS, depressive symptom; SES, socioeconomic status.
Four categories in 10-y change of AHEI were defined as follows: participants who maintained a high score (phase 3 and 7 scores ≥51.5 points), participants who maintained a low score over the 10-y exposure period (phase 3 and 7 scores <51.5 points), participants who improved their AHEI score (phase 3 score <51.5 points and phase 7 score ≥51.5 points), and participants who decreased their score (phase 3 score ≥51.5 points and phase 7 score <51.5 points).
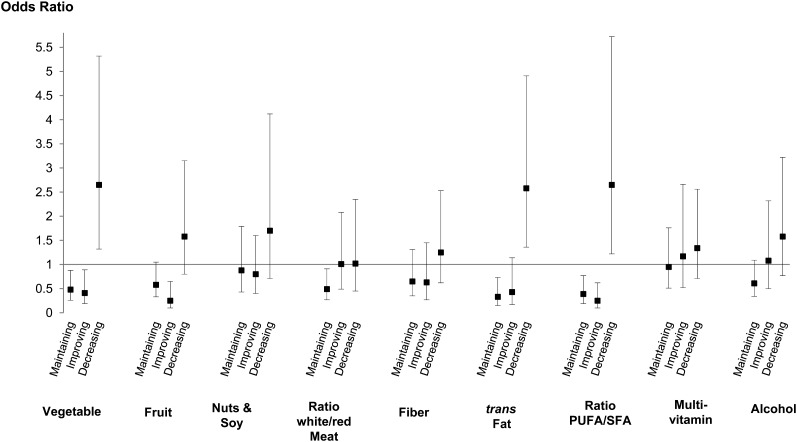
Similar analyses were performed for the 10-y change in each AHEI component. In women (Figure 3) with improvements in scores for vegetables, fruit, trans fat, and the PUFA:SFA ratio, there was an association with lower odds of subsequent recurrent DepSs compared with in women who maintained low scores in the respective components over the 10-y measurement period. Women whose scores for these components decreased over time had higher odds of recurrent DepSs (this was not the case for the fruit component). Their 10-y changes of other components were not related to recurrent DepSs. See Figure S2 under “Supplemental data” in the online issue for results of associations in men between the 10-y change for each AHEI-component score and recurrent DepSs. Except for an association between maintained high scores in nuts and soy and multivitamin use components and higher odds of DepSs, no improvement or decrease in AHEI-component score over the 10-y period was associated with DepSs in men.
FIGURE 3.
Associations [ORs (95% CIs)] between changes in AHEI components over the 10-y exposure period and subsequent recurrent depressive symptoms over 5 y in women. To analyze the 10-y change in AHEI-component scores, the AHEI-component scores were categorized as high or low according to the median value of AHEI-component scores at phase 3. Median values at phase 3 for AHEI-component scores were, respectively, 6, 6, 3, 5, 10, 10, 5, 2.5, and 5 for vegetables, fruit, nuts and soy, the ratio of white to red meat, fiber, trans fat, the ratio of PUFA to saturated fat, multivitamin use, and alcohol. Four categories in the 10-y change in AHEI components were defined as follows: participants who maintained a high AHEI-component score [phase 3 and 7 scores of at least the median value at phase 3 (eg, 6 for vegetables)], participants who maintained a low AHEI-component score (phase 3 and 7 scores <6), participants who improved their AHEI-component score (phase 3 score <6 and phase 7 score ≥6), and participants who decreased their AHEI-component score (phase 3 score ≥6 and phase 7 score <6). Odds of 5-y recurrent depressive symptoms were estimated for 1) participants who maintained a high AHEI-component score (compared with individuals who maintained a low score), 2) participants who improved their AHEI-component score (compared with individuals who maintained a low score), and 3) participants who decreased their AHEI-component score (compared with individuals who maintained a high score). This procedure was applied to the 9 AHEI components. ORs were adjusted for age, ethnicity, total energy intake, SES, retirement status, marital status, smoking, physical activity, HDL cholesterol, coronary artery disease, hypertension, and central obesity assessed at phase 3. AHEI, Alternative Healthy Eating Index; SES, socioeconomic status.
Sensitivity analyses
With an association only observed in women, sensitivity analyses were confined to this group. First, in a subgroup in which women with prevalent CAD (n = 55) were excluded, both AHEI at phase 7 and the 10-y change in AHEI remained significantly associated with recurrent DepSs (AHEI z score at phase 7, OR: 0.56; 95% CI: 0.43, 0.73; z score of the 10-y change, OR: 0.75; 95% CI: 0.59, 0.96). This result suggested that the association was not driven by the effects of CAD on diet and DepSs. Second, the association between the AHEI score assessed at phase 3 and recurrent DepSs 10 y later was examined. We showed that, after adjustment for sociodemographics, health behaviors, hypertension, HDL cholesterol, and central obesity assessed at phase 3, adherence to the AHEI at phase 3 in participants who were not taking antidepressant treatment at phase 3 was associated with lower odds of developing recurrent DepSs 10 y later (per 1 SD of the AHEI score at phase 3, OR: 0.77; 95% CI: 0.60, 0.97). Third, although participants treated with antidepressive drugs before phase 7 were already excluded from the current analyses, we conducted additional sensitivity analyses to explore the effects of existing mental health problems on dietary intake (reverse causality). Thus, a supplementary analysis in which women who met the definition for GHQ-Dep (n = 134) and women with missing values on the GHQ-Dep (n = 35) at phase 3 were additionally excluded. Similar trends between the AHEI score at phase 7 (OR: 0.57; 95% CI: 0.42, 0.78), 10-y change in the AHEI score (OR: 0.77; 95% CI: 0.58, 1.03; P = 0.075), and subsequent recurrent DepSs were shown in this subsample of 891 women. Fourth, an additional test to assess the direction of the association included 1494 women for whom data on the GHQ-Dep at phase3, use of antidepressive drugs, and AHEI score at phases 3 and 7 were available. Results from linear regression models provided no evidence of differences between General Health Questionnaire cases (n = 230) and noncases (n = 1264) in the subsequent AHEI score assessed at phase 7 (β ± SE: −0.84 ± 0.83; P = 0.31) or in the 10-y change in AHEI score between phases 3 and 7 (β ± SE: −0.92 ± 0.90; P = 0.31) after adjustment for age, ethnicity, and SES. These results suggested that reverse causation could not fully explain our findings.
DISCUSSION
This study sought to examine the longitudinal association between overall diet and the development of recurrent DepSs. To do so, the association between adherence to the AHEI, its 10-y change, and recurrent DepSs at 2 consecutive follow-ups over 5 y was examined. We showed that that adherence to healthy dietary recommendations such as those provided by the AHEI reduced the likelihood of developing recurrent DepSs in women but not in men. These effects were independent of sociodemographic, behavioral, metabolic, and health status factors such as cognitive performance and vascular diseases. Women who maintained or improved their AHEI score over the 10-y measurement period had 65% lower odds of subsequent recurrent DepSs than did women who maintained low AHEI scores, whereas women whose AHEI scores decreased had twice the odds of recurrent DepSs. These prospective results add to the modest evidence base concerning the temporality of the diet-DepS association in middle-aged people.
The prospective association between adherence in the AHEI and lower risk of recurrent DepSs reported here accords with the results of studies that have investigated the cross-sectional relation between overall diet and depression in adults (7–11), including the current study (4). One type of these studies assessed diet by using dietary patterns (4, 7, 8, 10). Across diverse samples, there is a suggestion that patterns that characterize healthier eating behaviors were inversely associated with DepSs. Because dietary patterns are derived through statistical modeling of dietary data without an a priori hypothesis, dietary patterns closely matched those of the population samples considered but not necessarily those of other populations. Another group of studies assessed overall diet by building dietary indexes on the basis of dietary recommendations (7–9, 11), such as the AHEI as used in the current study. Poor adherence to several healthy dietary recommendations, including the Healthy Eating Index 2005 (24) and the Diet Quality Score (7), has been associated with DepSs in cross-sectional data (7, 9). Only one study further assessed the temporal direction of the diet-depression association. Investigators in a large Spanish cohort study showed, consistent with our findings, that a high Mediterranean diet (Med-Diet) score was associated with 30% reduced risk of self-reported depression (11). The Med-Diet index shares several components with the AHEI, but in contrast to the AHEI, thresholds used for computing the Med-Diet score are defined according to the median of the food and/nutrient intakes in the specific population studied. Although the Med-Diet score has been associated with improvements in health (25, 26), the use of a dietary index with population-specific cutoffs may be problematic for quantitative diet recommendations and comparisons of results between Mediterranean and non-Mediterranean countries.
To our knowledge, the association of a repeat assessment of diet with recurrent DepSs in women constitutes a novel finding. Because we showed that maintaining a high AHEI score or improving AHEI score over the 10-y exposure period was associated with lower odds of recurrent DepSs compared with maintaining a low AHEI score, our findings are consistent with the hypothesized temporal association between diet on DepSs in women.
The reason that this gradient was apparent in women but not men was unclear. One possible explanation, although nontestable with the current data set, was that the instrument we used to assess DepSs was less sensitive to male depression; some CES-D items have been shown to produce biased responses in comparisons of male and female respondents (27). Therefore, additional prospective studies that use a clinical interview or other sensitive measures to detect depression both in men and women are needed.
Our study highlighted the importance of specific dietary components in the AHEI-DepS association. We showed that, in women, vegetables, fruit, trans fat, and an elevated ratio of PUFA:SFA were independently associated with reduced odds of recurrent DepSs over 5 y. Our study also showed that the maintenance or improvement of scores for these components over adult life was associated with recurrent DepSs. Whether these results were related to the high amounts of folate and other vitamin B and antioxidants provided by vegetables and fruit or the vascular protective and antiinflammatory properties of PUFAs (conversely to trans-fat properties), they all proved compatible with the hypothesis of a long-term beneficial impact of adherence to the AHEI to prevent DepSs (see Table S1 under “Supplemental data” in the online issue). Because we showed that the AHEI-DepS association was not completely generated by any individual AHEI components, our study suggested that the effects were likely due to the cumulative and synergic effect of nutrients from different sources of foods rather than from the effect of a single nutrient. However, at this stage, additional investigations are needed to identify the potential underlying mechanisms by which the overall diet may act on depression processes.
Limitations of the current findings included, first, the use of the CES-D to assess DepSs. Even if CES-D scale has been shown to be a reliable and valid measurement tool that indicates the presence of DepSs (28), the 2 repeated measurements of the CES-D did not capture the severity or chronicity of DepSs. We sought to take into account this limitation by considering recurrent DepSs defined as participants who met CES-D criteria at 2 consecutive measurements. However, our results, which were based on DepSs, cannot be extended to major depression. Second, our study was limited by the assessment of dietary intake. We used a semiquantitative FFQ that only covered specific foods and is recognized to be less precise than dietary assessment by diary records. However, we have previously shown that nutrient intake estimated by the FFQ method is well correlated with biomarker concentrations and intake estimates from the generally more accurate 7-d diary in this study (17). Third, the extent to which our results are generalizable is an important consideration. Study participants in Whitehall II are mainly white, office-based civil servants and not fully representative of the British general population (15). Finally, even with the prospective design, the possibility remains that a low AHEI score could be the consequence, rather than the cause, of DepSs. Actually, participants who self-reported CES-D DepSs at phases 7 and 9 may already have been depressed before phase 7 and, then, adopted a less healthy diet. Because we showed that adherence to the AHEI at phase 3 (10 y before the assessment of CES-D DepSs) was associated with DepSs assessed at phase 7, this result makes this possibility, as the sole explanation for our findings, unlikely.
In conclusion, our report provides evidence of an association between adherence to healthy recommendations as provided by the AHEI and lower risk of recurrent DepSs assessed over 5 y in women but not men. Because we showed that long- term adherence to AHEI over adult life was associated with lower odds of DepSs in late middle-aged individuals, the current study is, to our knowledge, unique in expanding the evidence on the temporality of the diet-DepS association. Our findings suggest that, at least in women, existing healthy eating policies might generate additional benefits for depression.
Acknowledgments
We thank all participating men and women in the Whitehall II study; all participating civil service departments and their welfare, personnel, and establishment officers, the Occupational Health and Safety Agency, and the Council of Civil Service Unions. The Whitehall II study team comprises research scientists, statisticians, study coordinators, nurses, data managers, administrative assistants and data entry staff who make the study possible.
The authors’ responsibilities were as follows—TNA, SS, MJS, GDB, and MK: developed the research question; TNA and MK: conducted research; TNA: analyzed the data and had primary responsibility for the final content of the manuscript; TNA and MK: drafted the manuscript; and SS, MJS, GDB, and MK: made critical revisions to the manuscript for important intellectual content. None of the authors had a conflict of interest.
Footnotes
Abbreviations used: AHEI, Alternative Healthy Eating Index; CAD, coronary artery disease; CES-D, Center for Epidemiologic Studies Depression Scale; DepS, depressive symptom; FFQ, food-frequency questionnaire; GHQ-Dep, General Health Questionnaire depression; Med-Diet, Mediterranean diet; SES, socioeconomic status.
REFERENCES
- 1.Gómez-Pinilla F. Brain foods: the effects of nutrients on brain function. Nat Rev Neurosci 2008;9:568–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murakami K, Sasaki S. Dietary intake and depressive symptoms: a systematic review of observational studies. Mol Nutr Food Res 2010;54:471–88 [DOI] [PubMed] [Google Scholar]
- 3.Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol 2002;13:3–9 [DOI] [PubMed] [Google Scholar]
- 4.Akbaraly TN, Brunner EJ, Ferrie JE, Marmot MG, Kivimaki M, Singh-Manoux A. Dietary pattern and depressive symptoms in middle age. Br J Psychiatry 2009;195:408–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacka FN, Kremer PJ, Berk M, de Silva-Sanigorski AM, Moodie M, Leslie ER, Pasco JA, Swinburn BA. A prospective study of diet quality and mental health in adolescents. PLoS ONE 2011;6:e24805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oddy WH, Robinson M, Ambrosini GL, O'Sullivan TA, de Klerk NH, Beilin LJ, Silburn SR, Zubrick SR, Stanley FJ. The association between dietary patterns and mental health in early adolescence. Prev Med 2009;49:39–44 [DOI] [PubMed] [Google Scholar]
- 7.Jacka FN, Mykletun A, Berk M, Bjelland I, Tell GS. The association between habitual diet quality and the common mental disorders in community-dwelling adults: the Hordaland Health study. Psychosom Med 2011;73:483–90 [DOI] [PubMed] [Google Scholar]
- 8.Jacka FN, Pasco JA, Mykletun A, Williams LJ, Hodge AM, O'Reilly SL, Nicholson GC, Kotowicz MA, Berk M. Association of Western and traditional diets with depression and anxiety in women. Am J Psychiatry 2010;167:305–11 [DOI] [PubMed] [Google Scholar]
- 9.Kuczmarski MF, Cremer Sees A, Hotchkiss L, Cotugna N, Evans MK, Zonderman AB. Higher Healthy Eating Index-2005 scores associated with reduced symptoms of depression in an urban population: findings from the Healthy Aging in Neighborhoods of Diversity Across the Life Span (HANDLS) study. J Am Diet Assoc 2010;110:383–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nanri A, Kimura Y, Matsushita Y, Ohta M, Sato M, Mishima N, Sasaki S, Mizoue T. Dietary patterns and depressive symptoms among Japanese men and women. Eur J Clin Nutr 2010;64:832–9 [DOI] [PubMed] [Google Scholar]
- 11.Sánchez-Villegas A, Delgado-Rodriguez M, Alonso A, Schlatter J, Lahortiga F, Serra Majem L, Martinez-Gonzalez MA. Association of the Mediterranean dietary pattern with the incidence of depression: the Seguimiento Universidad de Navarra/University of Navarra follow-up (SUN) cohort. Arch Gen Psychiatry 2009;66:1090–8 [DOI] [PubMed] [Google Scholar]
- 12.Konttinen H, Mannisto S, Sarlio-Lähteenkorva S, Silventoinen K, Haukkala A. Emotional eating, depressive symptoms and self-reported food consumption. A population-based study. Appetite 2010;54:473–9 [DOI] [PubMed] [Google Scholar]
- 13.Sarlio-Lähteenkorva S, Lahelma E, Roos E. Mental health and food habits among employed women and men. Appetite 2004;42:151–6 [DOI] [PubMed] [Google Scholar]
- 14.Jeffery RW, Linde JA, Simon GE, Ludman EJ, Rohde P, Ichikawa LE, Finch EA. Reported food choices in older women in relation to body mass index and depressive symptoms. Appetite 2009;52:238–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marmot M, Brunner E. Cohort profile: the Whitehall II study. Int J Epidemiol 2005;34:251–6 [DOI] [PubMed] [Google Scholar]
- 16.Akbaraly TN, Ferrie JE, Berr C, Brunner EJ, Head J, Marmot MG, Singh-Manoux A, Ritchie K, Shipley MJ, Kivimaki M. Alternative Healthy Eating Index and mortality over 18 y of follow-up: results from the Whitehall II cohort. Am J Clin Nutr 2011;94:247–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brunner E, Stallone D, Juneja M, Bingham S, Marmot M. Dietary assessment in Whitehall II: comparison of 7 d diet diary and food-frequency questionnaire and validity against biomarkers. Br J Nutr 2001;86:405–14 [DOI] [PubMed] [Google Scholar]
- 18.Bingham SA, Gill C, Welch A, Cassidy A, Runswick SA, Oakes S, Lubin R, Thurnham DI, Key TJ, Roe L, et al. Validation of dietary assessment methods in the UK arm of EPIC using weighed records, and 24-hour urinary nitrogen and potassium and serum vitamin C and carotenoids as biomarkers. Int J Epidemiol 1997;26(suppl 1):S137–51 [DOI] [PubMed] [Google Scholar]
- 19.McCullough ML, Feskanich D, Stampfer MJ, Giovannucci EL, Rimm EB, Hu FB, Spiegelman D, Hunter DJ, Colditz GA, Willett WC. Diet quality and major chronic disease risk in men and women: moving toward improved dietary guidance. Am J Clin Nutr 2002;76:1261–71 [DOI] [PubMed] [Google Scholar]
- 20.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas 1977;1:385–401 [Google Scholar]
- 21.Sabia S, Dugravot A, Kivimaki M, Brunner E, Shipley MJ, Singh-Manoux A. Effect of intensity and type of physical activity on mortality: results from the Whitehall II cohort study. Am J Public Health 2012;102:698–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crum RM, Anthony JC, Bassett SS, Folstein MF. Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA 1993;269:2386–91 [PubMed] [Google Scholar]
- 23.Stansfeld SA, Head J, Marmot MG. Explaining social class differences in depression and well-being. Soc Psychiatry Psychiatr Epidemiol 1998;33:1–9 [DOI] [PubMed] [Google Scholar]
- 24.Guenther PM, Reedy J, Krebs-Smith SM. Development of the Healthy Eating Index-2005. J Am Diet Assoc 2008;108:1896–901 [DOI] [PubMed] [Google Scholar]
- 25.Sofi F, Abbate R, Gensini GF, Casini A. Accruing evidence on benefits of adherence to the Mediterranean diet on health: an updated systematic review and meta-analysis. Am J Clin Nutr 2010;92:1189–96 [DOI] [PubMed] [Google Scholar]
- 26.Sofi F, Cesari F, Abbate R, Gensini GF, Casini A. Adherence to Mediterranean diet and health status: meta-analysis. BMJ 2008;337:a1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stommel M, Given BA, Given CW, Kalaian HA, Schulz R, McCorkle R. Gender bias in the measurement properties of the Center for Epidemiologic Studies Depression Scale (CES-D). Psychiatry Res 1993;49:239–50 [DOI] [PubMed] [Google Scholar]
- 28.St John PD, Blandford AA, Strain LA. Depressive symptoms among older adults in urban and rural areas. Int J Geriatr Psychiatry 2006;21:1175–80 [DOI] [PubMed] [Google Scholar]