Abstract
PURPOSE
This study was designed to investigate functional localization of both efflux (P-glycoprotein, P-gp) and influx (peptide) transporters in the mitochondrial membrane of cultured rabbit primary corneal epithelial cells (rPCECs).
METHODS
Isolation and purification of mitochondria was performed by optimized cell fractionation method. Mitochondrial integrity was measured by JC-1 uptake experiment. The efflux activity of P-gp was assessed by performing in vitro uptake studies on isolated mitochondria with Rhodamine 123 (Rho-123) alone and in the presence of P-gp inhibitors (quinidine and cyclosporine A) using fluorimetry and flow cytometry analysis. Functional activity of peptide transporter was assessed by performing in vitro uptake studies of [3H] Gly-sar on isolated mitochondria in the presence or absence of peptide transporter substrate (Val-Val). Molecular characterization of P-gp and peptide transporter was assessed by western blot and confocal analysis.
RESULTS
Enhanced JC-1 accumulation in the isolated fraction confirmed mitochondrial membrane integrity. Significantly higher uptake of Rho-123 on isolated mitochondria was observed in the presence of quinidine (75 and 100 μM) and cyclosporine A (10μM). Significantly lower uptake of [3H] Gly-sar was observed in the presence of val-val due to competitive inhibition of peptide transporter on isolated mitochondria. Western blot and confocal analysis further confirmed the presence of P-gp and peptide transporter on the mitochondrial membrane of rPCECs.
CONCLUSIONS
The present study demonstrates the functional and molecular characterization of P-gp and peptide transporters in the mitochondrial membranes of rPCECs. This knowledge of mitochondrial existence of P-gp and peptide transporter will aid in the development of subcellular ocular drug delivery strategies.
Keywords: Multidrug resistance (MDR), P-glycoprotein (P-gp), Peptide transporter, Mitochondria
1. INTRODUCTION
Multidrug resistance (MDR) is the major complication of cancer chemotherapy. It is a situation in which cancer cells become simultaneously resistant to structurally unrelated drugs with different mechanisms of action (Gottesman, 2002). Alterations in common drug targets, increased drug detoxification, drug efflux, DNA repair, and apoptosis defects have all been implicated under MDR mechanisms (Gillet and Gottesman, 2010; Gottesman et al., 2002; Hendrich and Michalak, 2003; Lage and Dietel, 1999; Liscovitch and Lavie, 2000; Pommier et al., 2004). Besides cancer chemotherapy, MDR also represents a major barrier to the success of ocular drug delivery. Since most drug targets are located within specific intracellular compartments, drug accumulation into these sites is a critical determinant of therapeutic response (Duvvuri and Krise, 2005). Drug resistance phenotype showing altered intracellular distribution of drugs has been observed in MDR cancer cell lines relative to drug sensitive lines (Hindenburg et al., 1989; Slapak et al., 1992). Such intracellular redistribution proceedings may decrease the opportunity for a drug molecule to invade into a drug targeting compartment and thus limit its therapeutic response (Duvvuri and Krise, 2005).
MDR associated transporter proteins such as P-glycoprotein (P-gp), breast cancer resistance protein (BCRP) and multidrug resistance protein 1 (MRP1) are also expressed at corneal cell membrane level for reducing intracellular accumulation of toxins and drugs (Barot et al., 2011; Karla et al., 2009; Karla et al., 2007). Nevertheless these proteins could be expressed in subcellular compartments and may actively sequester drugs and traffic away from their cellular targets (Duvvuri and Krise, 2005; Meschini et al., 2000; Munteanu et al., 2006; Rajagopal and Simon, 2003; Shapiro et al., 1998; Solazzo et al., 2009).
Mitochondria represent a vital intracellular organelle for ocular cell function and survival. It is an attractive target for drug-delivery because there is growing confirmation to support an association between mitochondrial dysfunction and a number of ocular diseases (such as age-related macular degeneration, diabetic retinopathy and glaucoma) (Barot et al., 2011; Kowluru et al., 2006; Liang and Godley, 2003). Several reports have indicated the mitochondrial localization and activity of P-gp efflux protein. Munteanu et al. have shown presence and functional activity of mitochondrial P-gp in MDR resistant human myeloid leukemia cells (K562). Authors have observed reversed orientation of mitochondrial P-gp relative to its outward localization on cell surface. This finding suggested that inward mitochondrial P-gp can offer higher drug accumulation inside the organelle and extracellular antibodies cannot affect such accumulations since they cannot reach mitochondrial binding site. However, mitochondrial membrane permeable small molecule inhibitors can reduce such intra-organelle drug concentrations. Therefore, inward localization of mitochondrial P-gp in MDR cells can protect the nucleus and prevent the therapeutic drug entry in its nuclear targets (Munteanu et al., 2006). Another study reports functional localization of P-gp in the mitochondrial membrane of MDR resistant hepatocellular carcinoma (HCC) cells. This finding suggested that mitochondrial membrane localized P-gp can works like a pump to efflux cytotoxic agents from mitochondria to the cytosol. Therefore, outward orientation of mitochondrial P-gp is likely to protect mitochondrial DNA from cytotoxic damage (Solazzo et al., 2006). Furthermore, Ling et al. have shown that overexpression and localization of P-gp on mitochondrial membrane of mitochondrial DNA depleted human hepatoma cells (SK-Hep1) confers resistance to chemotoxic drug-induced apoptosis (Ling et al., 2012). Recently, another study has reported mitochondrial localization of P-gp only on doxorubicin-resistant human breast cancer cells (MCF-7) but not in parent cell line (Shen et al., 2012). Overall, above two findings have further supported the hypothesis that mitochondrial membrane localized P-gp posses efflux function and facilitates MDR at the intracellular site by pumping chemotoxic drug out from mitochondria to protect mitochondrial functioning.
Expression of P-gp efflux and peptide influx transporters have been previously identified by our laboratory on corneal epithelial cell surface (Dey et al., 2003; Janoria et al., 2010). Therefore, the aim of this study was to elucidate the expression, localization, and functional activity of P-gp efflux and peptide (PepT-1) influx transporters in the mitochondria of cultured rabbit primary corneal epithelial cells (rPCECs). In this study, in vitro efflux activity of P-gp was measured by a model fluorescent P-gp substrate rhodamine-123 (Rho-123) and two specific inhibitors of P-gp (quinidine and cyclosporine A, CsA). In addition, two peptide transporter substrates [3H] Glycylsarcosine (Gly-Sar) and val-val were selected to examine the in vitro function of PepT-1 transporter. All in vitro uptake experiments were performed in isolated mitochondria from rPCECs. Furthermore, localization and protein expressions of both the transporters were confirmed by confocal microscopy, and western blot analysis.
2. MATERIALS AND METHODS
2.1 Materials
Cell culture materials such as minimum essential medium (MEM), TripLE Express® solution and non-essential amino acids were obtained from Invitrogen (Carlsbad, CA). Fetal bovine serum (FBS) was procured from Atlanta biological (Lawrenceville, GA). Cell culture flasks (150 cm2 area) were purchased from Fisher Scientific (Houston, TX). Rho-123, CsA and quinidine were procured from Sigma-Aldrich (St. Louis, MO). [3H] Gly-Sar (specific radioactivity, 4 Ci/mmol) was obtained from Moravek Biochemicals (Brea, CA, USA).
2.2 Cell Culture
rPCECs were cultured according to our published procedure (Dey et al., 2003). Briefly, cells were grown with culture medium containing MEM, 10% FBS, HEPES, sodium bicarbonate, penicillin, streptomycin sulphate and 1% (v/v) non-essential amino acids, adjusted to pH 7.4. Cells were grown in 150 cm2 culture flasks and maintained at 37°C, in a humidified atmosphere of 5% CO2 and 90% relative humidity. The culture medium was replaced every other day.
2.3 Mitochondria Isolation
An isolation of mitochondria from the corneal cells was performed based on the principle of cell fractionation and differential centrifugation (Chaiyarit and Thongboonkerd, 2009; Munteanu et al., 2006; Bourgeron et al., 1992). Briefly, confluent rPCECs grown in 150 cm2 flask were harvested by trypsinization, washed twice with ice-cold phosphate buffered saline (PBS) and pelletized at 4°C (1000×g) for 10 minutes. Resulting pellet was re-suspended in 500 μL of ice-cold homogenization buffer (0.25 M sucrose, 1 mM EDTA, 10 mM HEPES; pH 7.4) and incubated on ice for 10 minutes. Following incubation, cells were homogenized with pre-chilled Dounce homogenizer (40-50 strokes) and cell lysis was ensured by LDH assay. The resulting homogenate was transferred into 10 mL centrifuge tube by making volume up to 5 mL with homogenization buffer and centrifuged at low speed (1000×g, 10 minutes, 4°C) to remove nuclei and unlysed cells. Resulting supernatant was again centrifuged at high speed (16,000×g, 40 minutes, 4°C) in order to remove lysosomal or peroxisomal contamination. The formed pellet (“crude mitochondria”) was resuspended in homogenization buffer containing 0.25 M sucrose and centrifuged at 16,000×g for 30 minutes at 4°C. The resulting mitochondrial pellet was re-suspended in mitochondrial suspension buffer (pH 7.0) containing sucrose (250 mmol/L), tris (10 mmol/L) and protease inhibitors for further studies.
2.4 Mitochondrial Membrane Integrity Evaluation by JC-1 Uptake
Mitochondrial membrane integrity was assessed by measuring the potential gradient (Δφ) across the membrane using the lipophilic, cationic JC-1 fluorescent dye as per the manufacturer’s instructions (Sigma). Generally in healthy cells with high mitochondrial φm, JC-1 concentrates in the mitochondrial matrix and forms red fluorescent aggregates (J-aggregates). Any incident that disperses the mitochondrial membrane potential also averts accumulation of the JC-1 dye in the mitochondria. As an outcome the dye is dispersed all over the cytoplasm leading to a shift from red (J-aggregates) to green fluorescence (JC-1 monomers) (Reers et al., 1991). Valinomycin is a antibiotic agent permeabilizes the mitochondrial membrane and therefore, dissipates the mitochondrial potential gradient. In this experiment, valinomycin (1 μL) has been used as a control that prevents JC-1 aggregation. Fluorescence of JC-1 stained mitochondrial aggregates was measured by fluorimeter at 490 nm (excitation) and 590 nm (emission) wavelengths respectively.
2.5 Mitochondrial Preparation for Transmission Electron Microscopy (TEM)
For morphological characterization, 100 μL of mitochondrial suspension was centrifuged at 7000×g for 10 minutes. The resulting pellet was fixed with glutaraldehyde (2.5%) in cacodylate buffer, post fixed with osmium tetroxide (2%) and dehydrated in ethanol. Fixed pellet was processed for thin sectioning using routine procedure. Ultrathin sections (0.2 μM) were examined by TEM.
2.6 Uptake Experiments on Isolated Mitochondria
In vitro efflux activity of P-gp was measured on isolated mitochondria with modifications. Briefly, isolated rPCECs mitochondria were washed with Dulbecco’s modified phosphate-buffered saline (DPBS) at 37°C. Rho-123 solution was prepared at a concentration of 5 μM in DPBS and was added to isolated mitochondria (0.5 mg/mL) for incubation in microfuge tube containing 1 mL of DPBS for 30 minutes at 37°C for control experiments or with 1 mL solution of appropriate P-gp inhibitor (quinidine, 75 μM and 100 μM) in DPBS. During incubation period tubes were rotated constantly. At the end of an experiment, samples were centrifuged at 2000×g for 5 minutes at 4°C. The resulting mitochondrial pellets were washed three times with ice cold stop solution (210 mM KCl, 2 mM HEPES) to arrest mitochondrial uptake. Mitochondria were then solubilized in 1 mL of lysis solution (0.3 M NaOH, 0.1% Triton X-100). The lysates were transferred to a 96-well plate and were assayed using a 96-well fluorescent microplate reader. Rho-123 fluorescence was measured at 485 nm (excitation) and 535 nm (emission) wavelengths, and quantified against a standard curve of Rho-123. The fluorescence of the mitochondrial lysates was corrected for auto-fluorescence of untreated mitochondria. The uptake was normalized to the protein content of mitochondria. Protein content of the mitochondrial samples was measured using BioRad protein estimation kit (BioRad, Hercules, CA). The results were calculated as the total Rho-123 uptake/milligram of protein. Nonspecific binding was deducted from the total uptake values obtained.
For identification of peptide transporter, uptake was initiated by adding 1 mL of solution containing 0.5 μCi/mL [3H] Gly-Sar in DPBS to isolated mitochondria (0.5 mg/mL) in the presence or absence of competing substrate (val-val, 2.5 mM and 5.0 mM). Uptake was then performed as described previously for Rho-123. For analysis, aliquots of 500 μL was withdrawn from each sample containing lysed mitochondria and transferred to scintillation vials containing 3 mL scintillation cocktail. Samples were then analyzed using Beckman Scintillation Counter (Model LS-6500, Beckman Instruments, Inc.). Uptake was normalized to the protein content of each sample and the amount of protein in the mitochondrial lysate was quantified as described previously.
2.7 Efflux Assays for Mitochondrial P-gp by Flow Cytometry
Mitochondrial fractions, prepared as previously described, were suspended in PBS and kept on ice. A direct functional assay for the P-gp efflux pump on mitochondrial suspension in PBS was performed using flow cytometry analysis. Whole isolated mitochondria from rPCECs were divided in test tubes to evaluate mitochondrial auto-fluorescence as well as the accumulation of Rho-123 (500 nM) in the presence or absence of CsA (10μM) after 30 minutes. Fluorescence measurements of individual samples were performed using flowcytometer (Becton-Dickinson FACScanto II) equipped with an ultraviolet argon laser (excitation at 488 nm, emission at 530/30 band-pass filters). Analysis was gated to include single mitochondria on the basis of forward and side light-scatter and was based on an acquisition of data from the total events. Log fluorescence was collected and displayed as single parameter histograms.
Furthermore, isolated samples of mitochondria were equally divided into test tubes to conduct the uptake of Rho-123 (500 nM) at 37°C for 60 minutes. Following mitochondrial accumulation of Rho-123, samples were centrifuged at 200×g for 5 minutes. The mitochondrial pellets were re-suspended in PBS (pH 7.4) for 30 minutes in the presence or absence of CsA (10μM). Subsequently, the mitochondrial suspensions were centrifuged at 5000 rpm for 5 minutes and washed with PBS (pH 7.4). The mitochondrial accumulation of Rho-123 was determined using flow cytometry analysis as described previously.
2.8 Western Blot Analysis
Detection and analysis of P-gp and PepT-1 protein expressions from mitochondrial fractions of rPCECs were performed by immunoblotting. Preparation of mitochondrial protein lysate was performed similar to previously published protocol for whole cell protein lysate (Dey et al., 2003). Two concentrations (75 and 100 μg/μL) of mitochondrial protein were suspended in a denaturing buffer (Tris–HCl 10 mM, pH 8 containing 5% glycerol, 2.5% SDS, 5% b-mercaptoethanol and 0.005% bromophenol blue). Proteins from mitochondria were resolved by SDS-PAGE gel electrophoresis at 120 V and electroblotted at 15 V for 90 minutes onto polyvinylidene fluoride (PVDF) membrane (Immobilon-P, Millipore, IPVH00010). Blot was blocked for 2 hours with constant shaking in 2.5 % non-fat dry milk and 0.25 % BSA prepared in TBST (Tris-buffered saline + 0.1% Tween 20, pH 7.4). Resulting membrane was incubated with Mdr-1 goat polyclonal primary antibody (1:300 dilution, Santacruz Biotechnology, Catalog no. SC-1517) for overnight at 4°C. After five washes (4 minutes each) in TBST, the membranes were probed with secondary antibody in TBST (1:3000 HRP-conjugated goat anti-rabbit IgG antibody, Santacruz Biotechnology, Catalog No. SC-2030) for 2 hours. The blots were finally washed three times (15 minutes each) with TBST. SuperSignal West Pico Chemiluminescence Substrate (Thermo Scientific, Catalog No. 34077) was used to develop the blot, according to the manufacturer’s protocol.
Peptide transporter expression was measured by similar method using PepT-1 rabbit polyclonal primary antibody (1:3000 dilution, Santacruz Biotechnology, Catalog no. SC-20653) and HRP-conjugated goat anti-rabbit IgG secondary antibody (1:3000 dilution, Santacruz Biotechnology, Catalog No. SC-2030) respectively.
2.9 Confocal Immunofluorescence and Correlation Analysis
rPCECs grown on glass coverslips were incubated for 30 minutes at 37°C with 5 nm of MitoTracker® Red (molecular probe, Invitrogen), a cell-permeable mitochondria-selective dye. Cells were then rinsed with PBS for three times and then fixed in freshly prepared cold 4% paraformaldehyde in PBS for 30 minutes at 4°C. Cells were rinsed with cold PBS for four times and then incubated with a solution of 1% BSA and 4% non-fat dry milk in PBS for non-specific binding for 2 hours at room temperature (RT). Cells were rinsed with PBS again, and then incubated with mdr-1 mouse monoclonal and PepT-1 rabbit polyclonal antibodies (Santa Cruz) for P-gp and PepT-1 (diluted 1:500 as per manufacture’s instruction) separately for 2 hours at 37°C. Then cells were washed with PBST for three times (15 minutes each) at RT and exposed to Alexa Fluor® 488 goat anti-mouse (for P-gp, 1:50 as per Invitrogen’s instruction) and FITC labeled goat anti-rabbit monoclonal IgG (for PepT-1, 1:10,000 as per Sigma’s instruction) separately for 1 hour at 37°C. Cells were then washed with PBST for four times (15 minutes each) at RT. The glass coverslips were then placed on the glass slides, and covered with the cover glasses with mounting medium. Slides were observed under a confocal laser fluorescence microscope. A sequence of images was acquired in the z-series (0.2 μM) with Olympus FV300 confocal laser scanning unit coupled to an Olympus BX61 upright microscope. Finally, images were processed using ImageJ colocalization Plugin Software (NIH) to determine the Pearson’s correlation coefficient (Rr) and Overlap coefficient (R).
3. RESULTS
3.1 Integrity of the Isolated Mitochondria
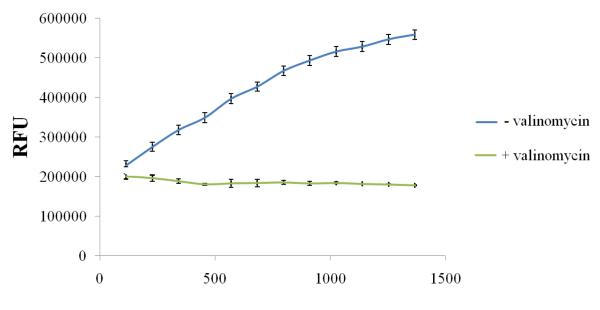
To confirm the integrity of the isolated mitochondria, we measured the mitochondrial membrane potential with JC-1 dye that can selectively enter into the mitochondria. Relative fluorescence intensity of JC-1 staining in the mitochondrial fractions was assessed by a fluorescence microplate assay. A 30-fold higher accumulation of JC-1 (fluorescent units) into the isolated mitochondria fraction over that obtained with the valinomycin treated control mitochondria has confirmed the presence of mitochondria-enriched fractions (Fig.1).
FIGURE 1.
Mitochondria staining using JC-1 stain in a Multiwell Plate Format. Mitochondria were isolated from rPCECs using the cell fractionation method and stained in a multiwell plate using the Isolated Mitochondria Staining Kit (Sigma). The upper line represents the JC-1 dye uptake of an intact mitochondrial sample. The lower line represents the dye uptake of the valinomycin treated mitochondrial control sample. RFU – Relative Fluorescence units.
3.2 TEM Analysis of Isolated Mitochondria
TEM analysis of the isolated mitochondria pellet (Fig.2) has displayed a rather pure fraction of the mitochondria. These images have further confirmed the condensed conformation of the isolated mitochondria showing well visible cristae of the inner membrane along with the preserved outer mitochondrial membrane.
FIGURE 2.
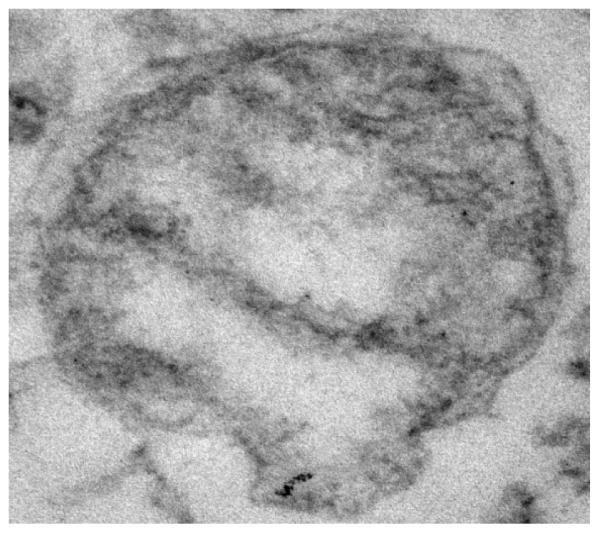
Transmission electron micrograph of mitochondria pellet isolated from rPCECs showing well visible cristae of the inner membrane along with the preserved outer mitochondrial membrane.
3.3 Uptake Experiments
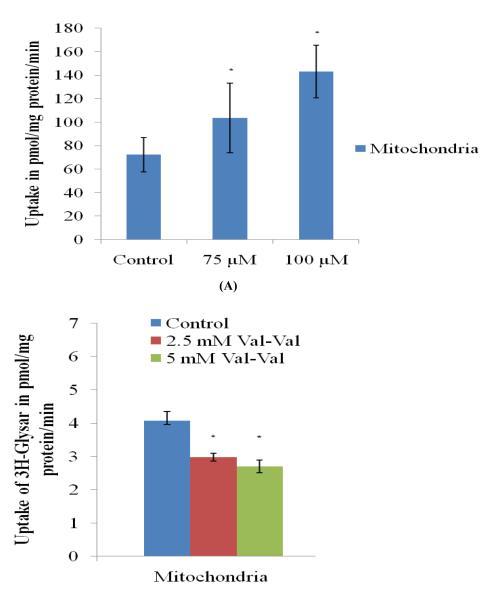
Accumulation of Rho-123 and [3H] Gly-Sar was individually investigated to explore the possibility of P-gp efflux pump and PepT-1 influx transporter on the mitochondrial fractions of rPCECs. Rho-123 (5 μM) uptake across isolated mitochondrial fraction of rPCECs was investigated at 30 minutes in the presence of 75 and 100 μM quinidine (Fig. 3A). An increase in Rho-123 uptake was observed after 30 minutes in the presence of quinidine. Control uptake of Rho-123 in mitochondrial fraction of rPCEC was found to be 72.35 ± 8.4 picomoles/mg protein, in 30 minutes. However, uptake was (p < 0.05) significantly higher (1.4 and 2.1 times) in the presence of 75 and 100 μM quinidine respectively, thereby suggesting that P-gp is significantly inhibited by 100 μM quinidine (Fig.3A).
FIGURE 3.
(A) Accumulation of Rhodamine 123 (5μM) alone and in the presence of quinidine (75 and 100 μM) in the isolated mitochondria fraction from rPCECs. (B) Accumulation of [3H] Gly-sar alone and in the presence of val-val (2.5 and 5.0 mM) in the isolated mitochondria fraction from rPCECs. Values are expressed as mean ± SD (n=3). *Data were considered statistically significant for P ≤ 0.05.
Uptake of [3H] Gly-Sar in the presence of 2.5 and 5.0 mM val-val was also investigated at 30 minutes across isolated mitochondrial fraction of rPCECs. The dipeptide val-val significantly inhibited (p < 0.05) the uptake of [3H] Gly-Sar in a competitive manner (Fig.3B).
3.4 Efflux Assays for Mitochondrial P-gp by Flow Cytometry
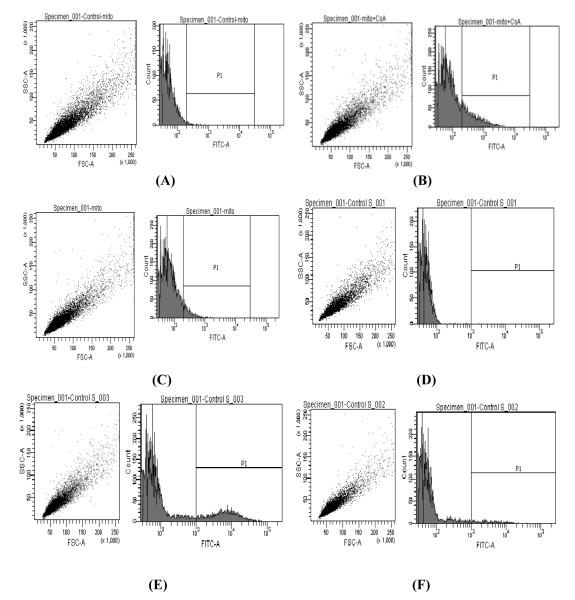
An accumulation and efflux of Rho-123 was evaluated in the isolated mitochondria of rPCECs. Flow cytometry observations suggested that Rho-123 accumulation in the isolated mitochondria was higher in the presence of CsA (Fig. 4B) but not in the absence of CsA (Fig. 4C). Figure 4A shows auto-fluorescence of untreated mitochondria. Reduction of Rho-123 fluorescence in CsA untreated mitochondria sample (Fig. 4C) after 30 minutes was probably due to P-gp mediated efflux of Rho-123. Above observation has been further confirmed by determining efflux of accumulated Rho-123 upon subsequent treatment with or without CsA (10 μM). The efflux of Rho-123 was significantly reduced (p ≤ 0.05) only upon subsequent treatment with CsA (Fig. 4E) whereas higher Rho123 efflux has been observed in the absence of CsA treatment (Fig. 4F). These results strongly suggested the presence of P-gp on the mitochondrial membrane of rPCECs. Quantitative accumulation of Rho-123 following above experiments has been listed in Table 1 and 2 respectively. Table 1 displays higher accumulation of Rho-123 in the mitochondria of rPCECs in the presence of CsA relative to untreated control samples whereas, table 2 shows lesser efflux of Rho-123 in the mitochondria subsequently treated with CsA compared to CsA untreated mitochondria.
FIGURE 4.
Functional assay for the P-gp efflux pump on mitochondria by flow cytometry analysis. Untreated control mitochondria (A,D), Rho-123 accumulation in the isolated mitochondria of rPCECs with (B) and without (C) CsA treatment, Remaining amount of Rho-123 following subsequent treatment with (E) or without (F) CsA.
TABLE 1.
Functional analysis of P-gp in mitochondria isolated from rPCECs by flow cytometry. The analyses were performed with isolated mitochondria to evaluate the accumulation of Rho-123 alone and in presence of CsA. Mean fluorescence of Rho-123 (accumulation) was determined by flow cytometry after 30 minutes and was expressed as percentages of the mean fluorescence of the control mitochondria. The results are reported as a mean ± SD of three independent analyses.
| Sample | Rho-123 accumulation (mean fluorescence in percent of the control)> |
|---|---|
| (A) Untreated mitochondrial | - |
| (B) Rho-123 alone (control) | 100 |
| (C) Rho-123 + CsA (10μM) | 146.87±3.5 |
TABLE 2.
Functional analysis of P-gp in mitochondria isolated from rPCECs by flow cytometry. The analyses were performed with isolated mitochondria to evaluate the remaining amount of Rho-123 after subsequent treatment with or without CsA.
| Sample | % of Rho-123 remaining |
|---|---|
| (A) Untreated mitochondrial | 0.0 |
| (B) Rho-123 without CsA treatment | 2.6 |
| (C) Rho-123 after CsA treatment (10μM) | 14.6 |
3.5 Western Blot Analysis
To confirm the localization of P-gp and PepT-1 transporters, fractions of mitochondria isolated from rPCECs were studied to determine P-gp and PepT-1 protein expression of respective transporters by western blot analysis (Fig. 5). Results clearly show the expression of P-gp and PepT-1 transporters on the mitochondrial fraction of rPCECs, as identified by a dark band at 170 kDa (Fig. 5A) and 85 kDa (Fig. 5B) respectively.
FIGURE 5.
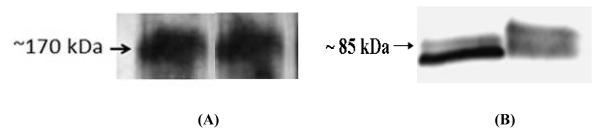
Western blot analysis of P-gp (A) and PepT-1 (B) expression in mitochondrial fraction of rPCECs. (A) The proteins were stained with MDR-1 goat polyclonal antibodies. Lanes 1 (left) and 2 (right): 75 and 100 μg/μL mitochondrial protein fractions from rPCECs respectively. (B) The proteins were stained with PepT-1 rabbit polyclonal antibodies. Lanes 1 (left) and 2 (right): 25 and 50 μg/μL mitochondrial protein fractions from rPCECs respectively.
3.6 Confocal Immunofluorescence and Correlation Analysis
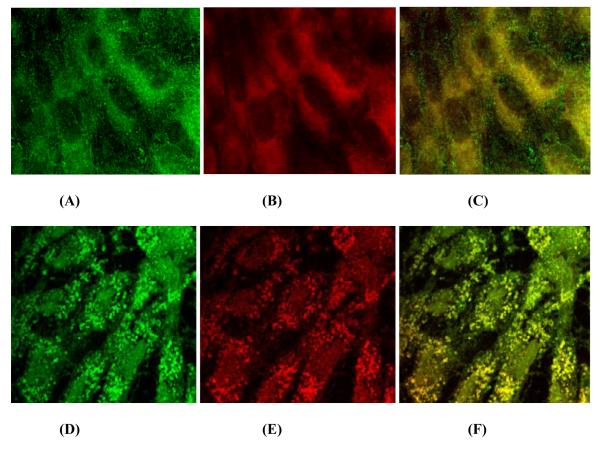
To determine the mitochondrial localization of P-gp, rPCECs were stained with mdr-1 mouse monoclonal antibody for P-gp (Fig. 6A, green fluorescence) and MitoTracker (Fig. 6B, red fluorescence), a specific fluorescent probe for mitochondria, and analyzed by confocal microscopy. As shown in Fig. 6C, the superimposition of the red mitochondria and the green P-gp labels shows the co-localization between the two signals (yellow).
FIGURE 6.
Confocal immunofluorescence analysis of mitochondrial localization of P-gp (A-C) and PepT-1 (D-F) proteins. rPCECs cultured on glass coverslips were incubated with 5 nm of MitoTracker® red to label mitochondria (red), fixed and processed for P-gp protein (green) using mdr-1 mouse monoclonal IgG or PepT-1 protein (green) using pepT-1 rabbit polyclonal IgG. (A) P-gp (green), (B) Mitochondria (red), (C) Image of colocalized points (yellow) between red (mitochondria) and green (P-gp), (D) PepT-1 (green), (E) Mitochondria (red), (F) Image of colocalized points (yellow) between red (mitochondria) and green (PepT-1). Fluorescence signals obtained using ImageJ colocalization Plugin Software (NIH). The images are representative of at least three independent experiments with similar results. Mean value of Pearson’s correlation coefficient (Rr) = 0.58 (A-C), 0.69 (D-F) and Overlap coefficient (R) = 0.99 (C, F).
Expression of PepT-1 was also studied in rPCECs by confocal microscopy with rabbit polyclonal antibody for PepT-1 (Fig. 6D, green fluorescence) and MitoTracker®Red (Fig. 6E, red fluorescence) for mitochondrial staining. Co-localization between PepT-1 and mitochondria is indicated by yellow fluorescence (Fig. 6F). Following confocal analysis, co-localization coefficients (Rr and R) were determined using ImageJ colocalization Plugin Software (NIH) for two independent experiments. Rr and R values greater than 0.5 and 0.6 were considered as a good colocalization respectively (Zinchuk and Zinchuk, 2008). For P-gp and mitochondria, the calculated value of mean Rr and R was 0.58 and 0.99 respectively. Whereas, for PepT-1 and mitochondria, the calculated value of mean Rr and R was 0.69 and 0.99 respectively.
4. DISCUSSION
The most recognized MDR mechanism correlates well with the expression of an adenosine triphosphate (ATP)–dependent drug efflux pump. This efflux pump is caused by an over-expression of several efflux proteins such as P-gp, MRP, and BCRP. A 170-kDa P-gp, with 1280 amino acids is a member of the ATP-binding cassette (ABC) protein super family and encoded by the MDR gene (Anand et al., 2002; Gottesman et al., 2002). Earlier our laboratory has reported peptide transporter mediated enhanced translocation of various altered compounds which were originally substrates of P-gp (Dey et al., 2003; Gunda et al., 2006; Janoria et al.; Katragadda et al., 2006). P-gp efflux transporter is generally considered to be cell surface localized. However, several intracellular compartments may also be potential site for P-gp functional activity. Mitochondria are attractive targets for ocular drug-delivery because of growing confirmation to support an association between mitochondrial dysfunction and a number of ocular diseases (Barot et al., 2011; Kowluru et al., 2006; Liang and Godley, 2003). Few studies have demonstrated the mitochondrial localization of P-gp efflux transporter as a possible reason for intracellular sub-therapeutic drug distribution (Meschini et al., 2000; Munteanu et al., 2006; Rajagopal and Simon, 2003; Shapiro et al., 1998; Solazzo et al., 2009; Solazzo et al., 2006).
A primary objective of this project was to identify functional localization of P-gp efflux and PepT-1 influx transporters on mitochondrial surface of corneal epithelial cells (rPCECs) by using in vitro uptake experiments, flow cytometry, western blot, and confocal microscopy. Mitochondrial isolation and purification using differential centrifugation principle is a widely used technique and similar to other reports (Chaiyarit and Thongboonkerd, 2009; Munteanu et al., 2006; Bourgeron et al., 1992) we have also obtained the high degree of mitochondrial purity and integrity. Using JC-1 staining, we first verified the integrity of isolated mitochondria by fluorescence microplate assay. Higher JC-1 accumulation in the isolated mitochondrial fraction, over that obtained with the valinomycin treated control mitochondria sample, indicated that the isolated fraction was enriched in intact mitochondria (Fig. 1). In addition, the purity of mitochondria was also confirmed by transmission electron microscopy (TEM). Figure 2 illustrates typical morphology of mitochondria obtained from cell fractionation method. TEM images clearly depict the purity and preservation of mitochondrial structure, both of which are important for functional and structural studies.
To investigate mitochondrial P-gp function, an accumulation and efflux of Rho-123 was evaluated in isolated mitochondria of rPCECs (Fig 3B) in presence or absence of two different P-gp inhibitors (quinidine and CsA). Rho 123 is a widely used mitochondrial fluorescent probe and has display strong analogy with anticancer agents. Rho-123 accumulation was significantly enhanced in the presence of different concentrations of quinidine in the isolated mitochondria compared to control mitochondria sample (Fig.3A). Reduced fluorescence of Rho-123 in control mitochondria was probably due to P-gp-mediated Rho-123 efflux. Quinidine mediated increased Rho-123 accumulation provides strong evidence that P-gp is expressed and functionally active in mitochondria of rPCEC. Efflux activity of P-gp on isolated mitochondria was further confirmed using another P-gp inhibitor (CsA) by flow cytometry . When Rho-123 (500 nM) diffuses into the mitochondria, P-gp actively pumps out Rho-123, and thereby showing lower fluorescence intensity (Fig. 4B). However, simultaneous treatment with CsA (10μM) enhances accumulation of Rho-123 in the isolated mitochondria, resulting in higher fluorescence intensity (Fig.4C, Table 1). In order to further confirm the functional activity of P-gp on mitochondria, isolated samples of mitochondria were first subjected to Rho-123 for 60 minutes to allow maximum accumulation of Rho-123 followed by subsequent treatment with or without CsA for 30 minutes to determine the quantitative efflux of Rho-123 in response to P-gp inhibitor (CsA). Significant reduction of Rho-123 efflux in response to CsA confirms the functional activity of P-gp on mitochondria of rPCECs (Fig 6, Table 2). Above results were found in complete agreement with the other report where reduced Rho 123 accumulation was observed in isolated mitochondria of MDR-positive cells (Solazzo et al., 2006).
Functional localization of PepT-1 transporter on mitochondria was also evaluated by performing radioactive uptake of [3H] Gly-sar in the presence of two different (2.5 and 5 mM) concentration of dipeptide substrate val-val. Significant inhibition of [3H] Gly-sar uptake in the presence of competing dipeptide substrate provides strong evidence of functional localization of PepT-1 transporter on mitochondria of rPCECs. Similar results showing peptide transporter mediated uptake of [3H] Gly-sar has already been observed in report showing characterization of peptide transporter across MDCKII-MDR1 cells (Agarwal et al., 2007).
Western blot was performed to confirm the expression of P-gp and PepT-1 in mitochondria of rPCECs. P-gp and PepT-1 bands detected approximately at 170 (Fig. 7) and 85 kDa (Fig. 8) respectively, clearly confirm the presence of both efflux and influx transporter in mitochondria of rPCECs. Dey et al previously reported the expression of P-gp in human and rabbit cornea and corneal epithelial cell membrane and detected P-gp with a molecular weight of approximately 170 kDa (Dey et al., 2003). Moreover, PepT-1 expression with a molecular weight of approximately 80 kDa has been reported in small intestine confirming PepT1-mediated epithelial transport of dipeptides and cephalexin (Buyse et al., 2001).
Dual fluorescence study allowed a positive approach of P-gp and PepT-1 localization on mitochondria of rPCECs. Simultaneous labeling of P-gp/PepT-1 and mitochondria provided the green fluorescence signal corresponding to P-gp/PepT-1 (Fig. 9A and 10A), red fluorescence signal corresponding to mitochondria (Fig. 9B and 10B) and yellow fluorescence signal corresponding to mitochondrial co-expression of P-gp/PepT-1 (Fig. 9C and 10C), confirming the colocalization observation of other reports (Munteanu et al., 2006; Solazzo et al., 2006). Co-localization coefficients (Rr and R) were used to estimate the level of co-localization between P-gp/Pept-1 and mitochondria marker MitoTracker® red. These coefficients represent the statistical relationship between fluorescence intensities. For P-gp and PepT-1 transporters, higher values of Rr > 0.5 (0.58 and 0.69) and R > 0.6 (0.99 and 0.99) have confirmed the strong co-localization of these transporters on mitochondria of rPCECs.
Due to its critical functioning, mitochondrial dysfunctioning is severely associated with a number of cancerous as well as ocular diseases. Therefore, functional localization of P-gp in mitochondria can play a crucial role in intracellular drug trafficking and intrinsic drug resistance (Meschini et al., 2000; Munteanu et al., 2006; Solazzo et al., 2006; Ling et al., 2012; Shen et al., 2012). MDR modulators blocking P-gp function have been widely utilized presumably to evade cell membrane based efflux mechanisms. However, there is a need to explore alternative strategies which can also address P-gp mediated intracellular drug sequestration and resistance. It has been shown that P-gp expression inhibits cytochrome c release from mitochondria into cytosol following apoptotic stimuli, which otherwise is an important step to initiate cell apoptosis. This process ultimately hampers the anticancer drug induced apoptosis. It has been hypothesized that inhibition or cleavage of P-gp can facilitates the cytochrome c release from mitochondria by sensitizing drug resistant cells for anticancer agents (Solazzo et al., 2006; Mantovani et al., 2006). Moreover, several reports have shown that prodrug derivatization of therapeutic drugs that are P-gp substrates can evade P-gp mediated drug efflux (Gunda et al., 2006; Janoria et al., 2010). Therefore, identification and utilization of influx transporters such as Pept-1 on the mitochondria can be utilized as a promising strategy to evade mitochondrial P-gp mediated drug efflux event.
Intracellular drug targeting is a potential area of research and therefore, continued research in the area of cell organelles (nucleus, mitochondria, golgi apparatus etc.) based transporter localization will undoubtedly provide a greater understanding of how these transporters can contribute to the intracellular drug trafficking and intrinsic drug resistance. Furthermore, it would be exciting to explore any possible differential expression of organelles localized transporters in diseased versus non-diseased state so that it can be utilize to develop intracellular drug targeting strategies.
5. CONCLUSION
This report provides evidence that P-gp and PepT-1 are functionally expressed in the mitochondria of rPCECs. Mitochondrial dysfunctioning is associated with ocular diseases and have been considered as a potential target for drug delivery. According to most probable hypothesis, P-gp may be involved in protecting mitochondria by effluxing therapeutic drug molecule out from the intra-mitochondrial target site. Ultimate outcome of which could be lesser opportunity for ocular drug molecules to enter into a desired mitochondrial site and thereby limiting its therapeutic effect. On the contrary, localization of peptide transporter on the mitochondria can be utilized for the evasion of mitochondria localized P-gp mediated drug efflux event. This process may result in higher drug accumulation at the mitochondrial target site to produce optimal therapeutic effect against targeted ocular diseases.
We have determined influx (peptide) and efflux (P-glycoprotein) transporters localization on mitochondria of corneal cells.
Transporters localization was confirmed by in vitro uptake experiments, confocal and western blot analysis.
Both, peptide and P-glycoprotein transporters were identified functionally active on mitochondria of corneal cells.
Acknowledgements
The authors are thankful to Dr. Vladimir Dusevich, School of Dentistry, for assisting with the operation of transmission electron micrograph, Dr. Michael Ferrari, School of Biological Sciences, for helping in confocal studies, and Dr. Pranjali Dalvi for technical assistance in western blot studies. This work was supported by NIH grants RO1 EY 09171-16, RO1 EY 10659-14 and UMKC women’s council graduate assistance fund award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agarwal S, Jain R, Pal D, Mitra AK. Functional characterization of peptide transporters in MDCKII-MDR1 cell line as a model for oral absorption studies. Int J Pharm. 2007;332:147–52. doi: 10.1016/j.ijpharm.2006.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand BS, Dey S, Mitra AK. Current prodrug strategies via membrane transporters/receptors. Expert Opin Biol Ther. 2002;2:607–620. doi: 10.1517/14712598.2.6.607. [DOI] [PubMed] [Google Scholar]
- Barot M, Gokulgandhi MR, Haghnegahdar M, Dalvi P, Mitra AK. Effect of emergence of fluoroquinolone resistance on intrinsic expression of P-glycoprotein phenotype in corneal epithelial cells. J Ocul Pharmacol Ther. 2011;27:553–559. doi: 10.1089/jop.2011.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barot M, Gokulgandhi MR, Mitra AK. Mitochondrial dysfunction in retinal diseases. Curr Eye Res. 2011;36:1069–1077. doi: 10.3109/02713683.2011.607536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeron T, Chretien D, Rötig A, Munnich A, Rustin P. Isolation and characterization of mitochondria from human B lymphoblastoid cell lines. Biochem Biophys Res Commun. 1992;186:16–23. doi: 10.1016/s0006-291x(05)80769-7. [DOI] [PubMed] [Google Scholar]
- Buyse M, Berlioz F, Guilmeau S, Tsocas A, Voisin T, Peranzi G, Merlin D, Laburthe M, Lewin MJ, Roze C, Bado A. PepT1-mediated epithelial transport of dipeptides and cephalexin is enhanced by luminal leptin in the small intestine. J Clin Invest. 2001;108:1483–1494. doi: 10.1172/JCI13219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaiyarit S, Thongboonkerd V. Comparative analyses of cell disruption methods for mitochondrial isolation in high-throughput proteomics study. Anal Biochem. 2009;394:249–258. doi: 10.1016/j.ab.2009.07.026. [DOI] [PubMed] [Google Scholar]
- Dey S, Patel J, Anand BS, Jain-Vakkalagadda B, Kaliki P, Pal D, Ganapathy V, Mitra AK. Molecular evidence and functional expression of P-glycoprotein (MDR1) in human and rabbit cornea and corneal epithelial cell lines. Invest Ophthalmol Vis Sci. 2003;44:2909–2918. doi: 10.1167/iovs.02-1142. [DOI] [PubMed] [Google Scholar]
- Duvvuri M, Krise JP. Intracellular drug sequestration events associated with the emergence of multidrug resistance: a mechanistic review. Front Biosci. 2005;10:1499–1509. doi: 10.2741/1634. [DOI] [PubMed] [Google Scholar]
- Gillet JP, Gottesman MM. Mechanisms of multidrug resistance in cancer. Methods Mol Biol. 2010;596:47–76. doi: 10.1007/978-1-60761-416-6_4. [DOI] [PubMed] [Google Scholar]
- Gottesman MM. Mechanisms of cancer drug resistance. Annu Rev Med. 2002;53:615–627. doi: 10.1146/annurev.med.53.082901.103929. [DOI] [PubMed] [Google Scholar]
- Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- Gunda S, Hariharan S, Mitra AK. Corneal absorption and anterior chamber pharmacokinetics of dipeptide monoester prodrugs of ganciclovir (GCV): in vivo comparative evaluation of these prodrugs with Val-GCV and GCV in rabbits. J Ocul Pharmacol Ther. 2006;22:465–476. doi: 10.1089/jop.2006.22.465. [DOI] [PubMed] [Google Scholar]
- Hendrich AB, Michalak K. Lipids as a target for drugs modulating multidrug resistance of cancer cells. Curr Drug Targets. 2003;4:23–30. doi: 10.2174/1389450033347172. [DOI] [PubMed] [Google Scholar]
- Hindenburg AA, Gervasoni JE, Jr., Krishna S, Stewart VJ, Rosado M, Lutzky J, Bhalla K, Baker MA, Taub RN. Intracellular distribution and pharmacokinetics of daunorubicin in anthracycline-sensitive and -resistant HL-60 cells. Cancer Res. 1989;49:4607–4614. [PubMed] [Google Scholar]
- Janoria KG, Boddu SH, Natesan S, Mitra AK. Vitreal pharmacokinetics of peptide-transporter targeted prodrugs of ganciclovir in conscious animals. J Ocul Pharmacol Ther. 2010;26:265–271. doi: 10.1089/jop.2009.0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karla PK, Earla R, Boddu SH, Johnston TP, Pal D, Mitra A. Molecular expression and functional evidence of a drug efflux pump (BCRP) in human corneal epithelial cells. Curr Eye Res. 2009;34:1–9. doi: 10.1080/02713680802518251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karla PK, Pal D, Mitra AK. Molecular evidence and functional expression of multidrug resistance associated protein (MRP) in rabbit corneal epithelial cells. Exp Eye Res. 2007;84:53–60. doi: 10.1016/j.exer.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Katragadda S, Talluri RS, Mitra AK. Modulation of P-glycoprotein-mediated efflux by prodrug derivatization: an approach involving peptide transporter-mediated influx across rabbit cornea. J Ocul Pharmacol Ther. 2006;22:110–120. doi: 10.1089/jop.2006.22.110. [DOI] [PubMed] [Google Scholar]
- Kowluru RA, Atasi L, Ho YS. Role of mitochondrial superoxide dismutase in the development of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2006;47:1594–1599. doi: 10.1167/iovs.05-1276. [DOI] [PubMed] [Google Scholar]
- Lage H, Dietel M. Involvement of the DNA mismatch repair system in antineoplastic drug resistance. J Cancer Res Clin Oncol. 1999;125:156–165. doi: 10.1007/s004320050258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang FQ, Godley BF. Oxidative stress-induced mitochondrial DNA damage in human retinal pigment epithelial cells: a possible mechanism for RPE aging and age-related macular degeneration. Exp Eye Res. 2003;76:397–403. doi: 10.1016/s0014-4835(03)00023-x. [DOI] [PubMed] [Google Scholar]
- Ling X, He Y, Zhang G, Zhou Y, Yan B. Increased P-glycoprotein expression in mitochondria is related to acquired multidrug resistance in human hepatoma cells depleted of mitochondrial DNA. Int J Oncol. 2012;40:109–18. doi: 10.3892/ijo.2011.1181. [DOI] [PubMed] [Google Scholar]
- Liscovitch M, Lavie Y. Multidrug resistance: a role for cholesterol efflux pathways? Trends Biochem Sci. 2000;25:530–534. doi: 10.1016/s0968-0004(00)01668-6. [DOI] [PubMed] [Google Scholar]
- Mantovani I, Cappellini A, Tazzari PL, Papa V, Cocco L, Martelli AM. Caspase-dependent cleavage of 170-kDa P-glycoprotein during apoptosis of human T-lymphoblastoid CEM cells. J Cell Physiol. 2006;207:836–44. doi: 10.1002/jcp.20628. [DOI] [PubMed] [Google Scholar]
- Meschini S, Calcabrini A, Monti E, Del Bufalo D, Stringaro A, Dolfini E, Arancia G. Intracellular P-glycoprotein expression is associated with the intrinsic multidrug resistance phenotype in human colon adenocarcinoma cells. Int J Cancer. 2000;87:615–628. [PubMed] [Google Scholar]
- Munteanu E, Verdier M, Grandjean-Forestier F, Stenger C, Jayat-Vignoles C, Huet S, Robert J, Ratinaud MH. Mitochondrial localization and activity of P-glycoprotein in doxorubicin-resistant K562 cells. Biochem Pharmacol. 2006;71:1162–1174. doi: 10.1016/j.bcp.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Pommier Y, Sordet O, Antony S, Hayward RL, Kohn KW. Apoptosis defects and chemotherapy resistance: molecular interaction maps and networks. Oncogene. 2004;23:2934–2949. doi: 10.1038/sj.onc.1207515. [DOI] [PubMed] [Google Scholar]
- Rajagopal A, Simon SM. Subcellular localization and activity of multidrug resistance proteins. Mol Biol Cell. 2003;14:3389–3399. doi: 10.1091/mbc.E02-11-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reers M, Smith TW, Chen LB. J-aggregate formation of a carbocyanine as a quantitative fluorescent indicator of membrane potential. Biochemistry. 1991;30:4480–4486. doi: 10.1021/bi00232a015. [DOI] [PubMed] [Google Scholar]
- Shapiro AB, Fox K, Lee P, Yang YD, Ling V. Functional intracellular P-glycoprotein. Int J Cancer. 1998;76:857–864. doi: 10.1002/(sici)1097-0215(19980610)76:6<857::aid-ijc15>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Shen Y, Chu Y, Yang Y, Wang Z. Mitochondrial localization of P-glycoprotein in the human breast cancer cell line MCF-7/ADM and its functional characterization. Oncol Rep. 2012;27:1535–40. doi: 10.3892/or.2012.1671. [DOI] [PubMed] [Google Scholar]
- Slapak CA, Lecerf JM, Daniel JC, Levy SB. Energy-dependent accumulation of daunorubicin into subcellular compartments of human leukemia cells and cytoplasts. J Biol Chem. 1992;267:10638–10644. [PubMed] [Google Scholar]
- Solazzo M, Fantappie O, D’Amico M, Sassoli C, Tani A, Cipriani G, Bogani C, Formigli L, Mazzanti R. Mitochondrial expression and functional activity of breast cancer resistance protein in different multiple drug-resistant cell lines. Cancer Res. 2009;69:7235–7242. doi: 10.1158/0008-5472.CAN-08-4315. [DOI] [PubMed] [Google Scholar]
- Solazzo M, Fantappie O, Lasagna N, Sassoli C, Nosi D, Mazzanti R. P-gp localization in mitochondria and its functional characterization in multiple drug-resistant cell lines. Exp Cell Res. 2006;312:4070–4078. doi: 10.1016/j.yexcr.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Zinchuk V, Zinchuk O. Quantitative colocalization analysis of confocal fluorescence microscopy images. Curr Protoc Cell Biol. 2008 doi: 10.1002/0471143030.cb0419s39. Chapter 4, Unit 4 19. [DOI] [PubMed] [Google Scholar]