Abstract
Brain mechanisms underlying mastication have been studied in non-human mammals but less so in humans. We used functional magnetic resonance imaging (fMRI) to evaluate brain activity in humans during gum chewing. Chewing was associated with activations in the cerebellum, motor cortex and caudate, cingulate, and brainstem. We also divided the 25-second chew-blocks into 5 segments of equal 5-second durations and evaluated activations within and between each of the 5 segments. This analysis revealed activation clusters unique to the initial segment, which may indicate brain regions involved with initiating chewing. Several clusters were uniquely activated during the last segment as well, which may represent brain regions involved with anticipatory or motor events associated with the end of the chew-block. In conclusion, this study provided evidence for specific brain areas associated with chewing in humans and demonstrated that brain activation patterns may dynamically change over the course of chewing sequences.
Keywords: mastication, feeding behavior, eating, magnetic resonance imaging, neuroimaging, digestive system and oral physiological phenomena
Introduction
Mastication is critical to sustaining life. Major contributions to understanding the neural basis of mastication have come from animal studies over the past half century (for reviews, see Lund and Kolta, 2006a,b; Nakamura and Katakura, 1995). By contrast, the neural mechanisms underlying human mastication remain rarely studied.
Animal studies have identified the brainstem circuits responsible for generating the chewing rhythm and for coordinating orofaciopharyngeal muscle activity (Nakamura and Katakura, 1995; Lund, 2011). Animal studies have also shown that masticatory jaw movements are evocable via stimulation of specific cortical sites (Lund et al., 1984). Other contributions to producing or modulating jaw movements are made by the basal ganglia (Adachi et al., 2002) and other regions. The degree to which neural mechanisms of oral motor control compare between humans and non-human mammalian species is unclear. Since much of the animal work is done under the assumption that it will ultimately apply to the human condition, it seems critical to perform human studies to evaluate this assumption.
Most animal studies use invasive techniques such as neural ensemble recording, electrical stimulation, brain slice preparations, and lesion studies, among others (Lund and Kolta, 2006a). None of these protocols can be used in humans because of their invasiveness.
Functional magnetic resonance imaging (fMRI), which assesses cerebral blood flow associated with regional brain activity, has been used to demonstrate increased activation in the primary sensorimotor areas, supplementary motor areas, and cerebellum during gum chewing in humans (Onozuka et al., 2002, 2003). Similar results were found in studies of human chewing that used positron emission tomography (PET) (Momose et al., 1997). These and only a few other neuroimaging studies have provided some understanding of human brain activity related to oral function (Bracco et al., 2010; Kimoto et al., 2011) and parafunction (Tamura et al., 2003; Byrd et al., 2009; Jiang et al., 2010).
However, each of these neuroimaging studies has limitations associated with experimental designs, including small samples, lack of control regarding the side of the mouth on which study participants chewed, manipulation of the chewing rhythm with a metronome, etc. The present fMRI investigation used a relatively large number of human participants and allowed them to chew at their “natural” rates while chewing on the right side of the mouth only.
Materials & Methods
Study Population
Twenty-nine healthy, right-handed individuals (15 men, 14 women; mean age ± SD, 24.0 ± 3.5 yrs), with fully dentate Class I occlusions, were selected. The study was approved by the University of Michigan Medical Institutional Review Board; participants read and signed informed consents before the study began. The research diagnostic criteria for temporomandibular disorders (RDC-TMD) (Dworkin and LeResche, 1992) were used to exclude individuals with myogenous or arthrogenous conditions. Further exclusion criteria were: use of medications with known neuromotor effects, e.g., neuroleptics or antidepressants; use of over-the-counter medications within 3 days of the imaging session; presence of systemic, vascular, or central nervous system diseases; and use of medical devices or the presence of conditions that would be dangerous in or incompatible with the MRI environment.
fMRI Protocol
Participants were placed in the scanner (3T, GE Signa MRI, Milwaukee, WI, USA), and their heads were secured with Velcro straps and stabilized with wedge-shaped mini-pillows to decrease movement artifacts. T1 images (TR = 12.3 ms, TE = 5.2 ms, flip angle = 15 degrees, bandwidth = 15.63, field of view = 26 cm, number of slices = 144, and slice thickness = 1 mm) were performed and used for preprocessing of the anatomic and functional data (see fMRI data analysis). Participants used prismatic glasses to read commands that guided them to chew gum on the right side or to hold the gum next to the right cheek while remaining at rest. A 25-second rest block followed by a 25-second chew block design was repeated 10 times.
Prior to MRI data collection, participants chewed the gum for 5 min to develop an even consistency. During the subsequent 10 chew-rest blocks, fMRI images were recorded (TR = 2,500 ms, TE = 30 ms, flip angle = 90 degrees, field of view = 22 cm, slice thickness = 3.0 mm, number of scans = 200, number of slices = 53, and voxel size = 3.44 x 3.44 x 3 mm). The TR we used was selected to allow for chew-block segmentation [see ‘Chewing Segment Contrasts’]. For each run, the first 5 images were discarded to account for signal stabilization.
fMRI Data Analysis
For data analysis, we used SPM 5 (http://www.fil.ion.ucl.ac.uk/spm/software/spm5/) executed under Matlab 7.1. Images were preprocessed with slice-time and motion correction to realign all the images with the first image and to remove movement artifacts associated with chew blocks and other movements. Anatomic images were normalized with a standard Montreal Neurological Institute (MNI) template. Thereafter, images were smoothed (6 mm Gaussian kernel) and fitted to hemodynamic response functions (HRF). In all analyses, a cluster-level- corrected p < 0.05 defined significance.
Chew-Rest Contrasts
A first-level analysis for each participant contrasted chew blocks with rest blocks. A second-level analysis was used to compare group-level differences between chew and rest blocks (Fig. 1).
Figure 1.
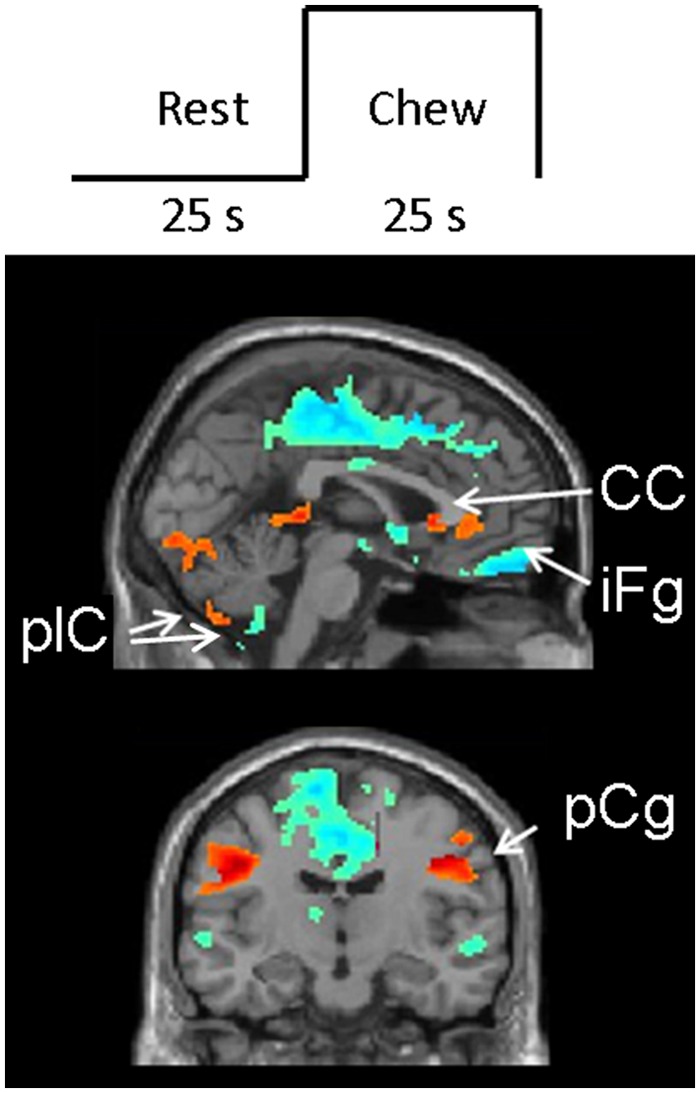
Block design and results. (top) Diagrammatic depiction of the block design. (bottom) Results of the contrast between chew blocks and rest blocks. Red/yellow areas show increased activations during chew-blocks relative to successive rest blocks; blue/green areas show decreased activations during chew-blocks relative to successive rest blocks. Note that both hyper- and hypo-activations occur in the posterior lobe of the left cerebellum; also see Table. CC = corpus callosum; plC = cerebellum posterior lobe; iFg = inferior frontal gyrus; pCg = pre-central gyrus.
Chewing Segment Contrasts
The 25-second chew blocks were divided into 5 non-overlapping 5-second segments. To evaluate brain activation differences between the segments, we performed a first-level analysis for each participant and a second-level analysis for the group, where each segment was contrasted to the rest block (Fig. 2). Another second-level analysis contrasted successive segments (Fig. 3).
Figure 2.
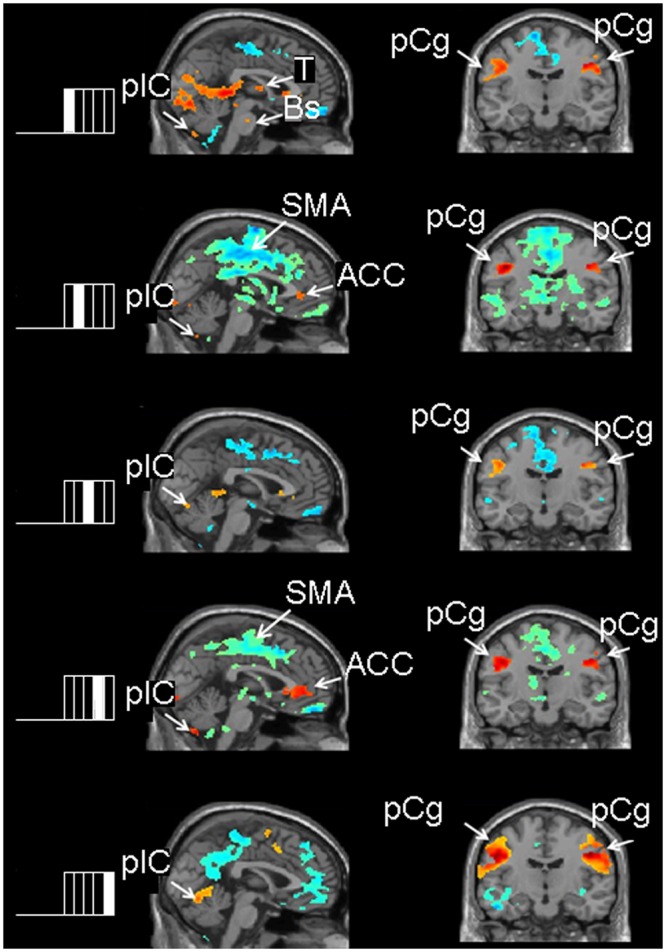
Images of the contrast between each of the 5 chew-block segments relative to rest blocks. The left side of the Fig. shows the chew block partitioned into 5 segments and indicates which chew-block segment results are depicted in the adjacent brain slices. Red/yellow areas show activations or increased brain activity during the respective chewing segment compared with rest; blue/green areas show areas of decreased brain activity during the respective chewing segment compared with rest. Sagittal images show activation in the cerebellum and hypo-activations in the supplementary motor area and cingulate cortex in all 5 contrasts. Coronal images show activations in the left and right pre-central gyrus in all 5 contrasts. ACC = anterior cingulate cortex; Bs = brainstem; pCg = pre-central gyrus; plC = posterior lobe of the cerebellum, SMA = supplementary motor area, T = thalamus.
Figure 3.
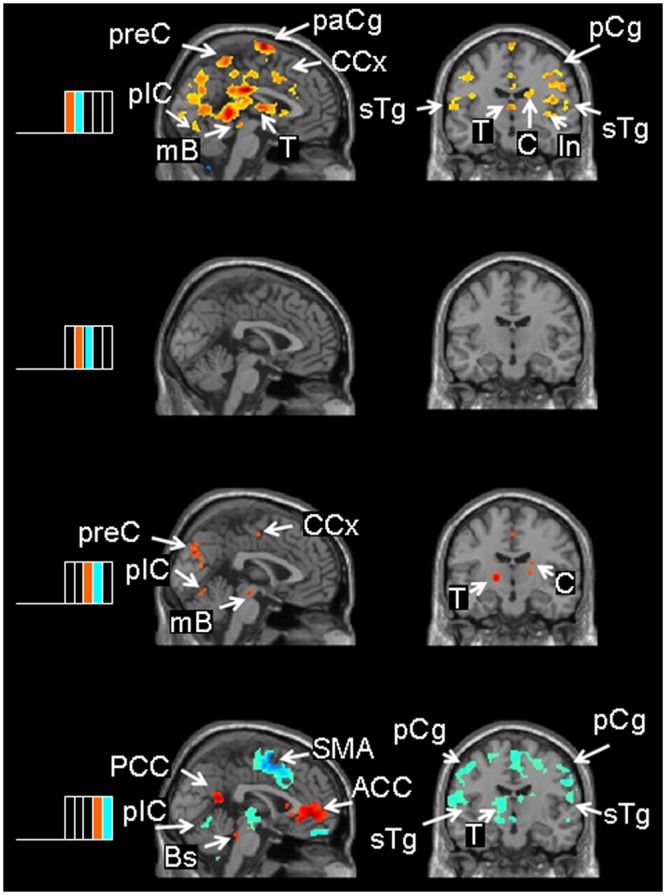
Contrasts between successive chew-block segments. The left side of the Fig. shows the 2 successive chew-block segments depicted in the adjacent brain slices. Note the color coding: Red/yellow areas show where brain activity was higher in a given segment relative to the ensuing segment; blue/green areas show areas where brain activity was higher in a given segment relative to the preceding segment. The first contrast (1/5 – 2/5) shows the brain activations during the initiation of the chew block. The other contrasts show brain activity during sustained chewing. ACC = anterior cingulate cortex; Bs = brainstem; C = caudate nucleus; CCx = cingulate cortex; In = insula; mB = midbrain; PCC = posterior cingulate cortex; pCg = pre-central gyrus; plC = cerebellum, posterior lobe; paCg = paracentral gyrus; preC = precuneus; sTg = superior temporal gyrus; T = thalamus.
Results
Chew Blocks vs. Rest Blocks
Areas of hyperactivation during chewing occurred in bilateral pre- and post-central gyri, extending over the primary motor cortex, in bilateral posterior cerebellar lobes (p < 0.001), and in the rostrum of the corpus callosum and anterior cingulate gyrus, extending into the head of the left caudate nucleus (p < 0.01) (Table, Fig. 1).
Table.
Neuro-anatomic Structures Manifesting Significantly Altered Activation during Chewing
| Coordinates |
|||||||
|---|---|---|---|---|---|---|---|
| Contrast | Anatomic Structure | Cluster Size | t | z | x | y | z |
| Chew-block > Rest-block | Left pre-central gyrus*** | 464 | 9.54 | 6.32 | -44 | -14 | 34 |
| Right pre-central gyrus*** | 390 | 9.72 | 6.38 | 48 | -14 | 40 | |
| Left cerebellum, posterior lobe*** | 1734 | 10.08 | 6.50 | -18 | -72 | -18 | |
| Right cerebellum, posterior lobe*** | 383 | 9.31 | 6.24 | 10 | -68 | -48 | |
| Corpus callosum & caudate** | 92 | 6.08 | 4.81 | -2 | 20 | 2 | |
| Right anterior cingulate cortex* | 77 | 4.57 | 3.92 | 2 | 38 | 0 | |
| Midbrain & pons* | 83 | 4.36 | 3.55 | -6 | -22 | -36 | |
| Rest-block > Chew-block | Right inferior frontal gyrus*** | 23897 | 9.87 | 6.43 | 32 | 30 | -14 |
| Right frontal inferior operculum*** | 259 | 7.23 | 5.39 | 40 | 10 | 26 | |
| Left cerebellum, posterior lobe** | 202 | 6.76 | 5.16 | -34 | -82 | -46 | |
| Segment 1/5 > Rest-block | Left pre-central gyrus (6) (4)*** | 310 | 7.94 | 5.70 | -44 | -16 | 34 |
| Right pre-central gyrus (6) (4)*** | 365 | 9.51 | 6.31 | 44 | -16 | 40 | |
| Left & right posterior cingulate cortex*** | 5521 | 9.21 | 6.23 | -6 | -44 | 4 | |
| Left cerebellum, posterior lobe* | 148 | 9.70 | 6.37 | 10 | -68 | -48 | |
| Right cerebellum, posterior lobe** | 203 | 6.88 | 5.22 | -6 | -68 | -50 | |
| Left brainstem** | 46 | 4.28 | 3.47 | -4 | -18 | -42 | |
| Right brainstem** | 49 | 5.04 | 4.24 | 8 | -24 | -40 | |
| Segment 2/5 > Rest-block | Left pre-central gyrus*** | 229 | 9.52 | 6.31 | -44 | -14 | 34 |
| Right pre-central gyrus*** | 161 | 6.90 | 5.23 | 40 | -12 | 34 | |
| Left cerebellum, posterior lobe*** | 223 | 10.01 | 6.47 | -16 | -64 | -18 | |
| Left cerebellum, inferior semilunar lobule*** | 242 | 9.56 | 6.32 | -8 | -70 | -50 | |
| Right cerebellum, posterior lobe*** | 352 | 7.21 | 5.38 | 20 | -58 | -22 | |
| Anterior cingulate cortex*** | 182 | 9.62 | 6.40 | 8 | -24 | -40 | |
| Segment 3/5 > Rest-block | Left pre-central gyrus** | 165 | 6.89 | 5.23 | -46 | -14 | 36 |
| Right pre-central gyrus** | 169 | 6.61 | 5.09 | 42 | -10 | 34 | |
| Left cerebellum, posterior lobe*** | 231 | 7.98 | 5.72 | -10 | -68 | -46 | |
| Right cerebellum, posterior lobe*** | 1303 | 10.88 | 6.75 | 12 | -60 | -16 | |
| Right cerebellum, inferior semilunar lobule** | 182 | 6.92 | 5.24 | 12 | -68 | -46 | |
| Segment 4/5 > Rest-block | Left pre-central gyrus*** | 281 | 8.56 | 5.95 | -42 | -16 | 32 |
| Right pre-central gyrus*** | 199 | 8.02 | 5.73 | 46 | -14 | 40 | |
| Left cerebellum, posterior lobe*** | 234 | 8.91 | 6.08 | -6 | -68 | -50 | |
| Right anterior cingulate cortex*** | 307 | 5.48 | 4.48 | 6 | 22 | 2 | |
| Segment 5/5 > Rest-block | Left pre-central gyrus*** | 2184 | 12.26 | 7.15 | -50 | -12 | 44 |
| Right pre-central gyrus*** | 1535 | 13.50 | 7.46 | 46 | -10 | 34 | |
| Left cerebellum, posterior lobe*** | 1364 | 10.09 | 6.50 | -24 | -60 | -20 | |
| Right inferior parietal lobe** | 354 | 6.26 | 4.91 | 60 | -38 | 26 | |
| Right occipital pole* | 109 | 5.90 | 4.72 | 12 | -102 | -2 | |
| Right middle cingulate* | 110 | 5.80 | 4.66 | 8 | 10 | 44 | |
| Segment 1/5 > Segment 2/5 | Left pre-central gyrus** | 186 | 5.68 | 4.60 | -44 | -16 | 40 |
| Superior frontal gyrus (6) | 314 | 8.72 | 6.01 | 0 | -6 | 76 | |
| Left superior temporal gyrus*** | 964 | 8.75 | 6.02 | -40 | -34 | 16 | |
| Left superior temporal gyrus*** | 274 | 6.56 | 5.07 | -56 | 16 | -6 | |
| Right superior temporal gyrus** | 161 | 8.17 | 5.79 | 48 | 12 | -8 | |
| Right superior temporal gyrus (8)*** | 264 | 6.96 | 5.26 | 36 | 4 | -14 | |
| Left posterior insula* | 96 | 6.78 | 5.17 | -36 | -22 | -2 | |
| Left caudate | 249 | 6.76 | 5.16 | -14 | 22 | 0 | |
| Left putamen* | 98 | 6.10 | 4.83 | 32 | -18 | -2 | |
| Right amygdala** | 137 | 6.52 | 5.05 | 20 | 0 | -12 | |
| Right cingulate gyrus*** | 227 | 5.38 | 4342 | 2 | 8 | 44 | |
| Left precuneus, occipital and temporal lobes*** | 12436 | 9.87 | 6.43 | -4 | -42 | 4 | |
| Right cuneus* | 132 | 5.50 | 4.49 | 18 | -90 | 10 | |
| Left cerebellum, posterior lobe & tonsil* | 126 | 5.80 | 4.66 | -18 | -52 | -50 | |
| Brainstem** | 147 | 5.61 | 4.55 | -6 | -26 | -6 | |
| Segment 2/5 > Segment 3/5 | No significant results | ||||||
| Segment 3/5 > Segment 4/5 | Left posterior cingulate cortex | 178 | 5.10 | 4.25 | -14 | -62 | 6 |
| Right cuneus** | 146 | 5.01 | 4.20 | 2 | -78 | 30 | |
| Right superior temporal gyrus*** | 248 | 6.71 | 5.14 | 50 | -56 | 12 | |
| Right occipital lobe* | 97 | 6.46 | 5.01 | 16 | -88 | 10 | |
| Right cerebellum, anterior lobe*** | 254 | 6.79 | 5.18 | 28 | -50 | -22 | |
| Right cerebellum, posterior lobe*** | 279 | 5.39 | 4.43 | 12 | -62 | -14 | |
| Segment 4/5 > Segment 5/5 | Right anterior cingulate*** | 814 | 7.08 | 5.32 | 4 | 44 | 0 |
| Right posterior cingulate*** | 445 | 7.70 | 5.60 | 4 | -54 | 12 | |
p < 0.05, **p < 0.01, ***p < 0.001. Numbers in parentheses in the Anatomic Structure column refer to Brodmann’s areas. Cluster size is total voxels. t = t test score; z = z score. Coordinates are lateral (x), anteroposterior (y), and superior-inferior (z).
Areas of hypo-activation during chewing occurred in the right frontal lobe, especially in the inferior frontal gyrus (p < 0.001) and the inferior operculum (p < 0.001) and in the left posterior lobe of the cerebellum (p < 0.01) (Table, Fig. 1).
Chew-block Segments vs. Rest-blocks
The results of the contrasts between each 5-second chew-block segment vs. rest are shown in the Table and Fig. 2. Activations mirrored those observed in the block results [see ‘Chew-blocks vs. Rest-blocks’, above]. The main finding was the considerable variation in the regional volumes activated or deactivated from segment to segment (Fig. 2). Also, activations in the posterior lobe of the right cerebellum were present in the first 3 segments only.
Contrasts between Successive Chew-block Segments
The results of the contrasts between successive chew-block segments are shown in the Table and Fig. 3. Hyperactivations in segment 1/5 relative to segment 2/5 occurred in the left superior frontal gyrus, extending to the supplementary motor cortex and the middle frontal cortex, the pre-central gyrus, and the caudate and putamen. No significant changes were noted between segments 2/5 and 3/5. Hyperactivations were observed in the anterior lobe of the right cerebellum, superior temporal gyrus, and cuneus in segment 3/5 vs. segment 4/5. Finally, increased activity in the right anterior and posterior cingulate cortices was observed during segment 4/5 relative to segment 5/5 (Table, Fig. 3), with hyperactivations in the cingulate cortex, superior temporal gyrus, and pre-central gyrus in segment 5/5 relative to 4/5 (not shown in the Table; however, see blue colors, Fig. 3, bottom panel).
Discussion
The chew-block vs. rest-block results were similar to the results observed in animal and human studies, with corroborating findings of activity in the primary motor cortex and supplementary motor cortex (Onozuka et al., 2003; Tamura et al., 2003; Lund and Kolta, 2006a). Activations in the middle section of the pre-central gyrus (Fig. 2) approximate those in orofacial motor regions and may represent the human analogs of the cortical masticatory areas (CMA) from which masticatory movements can be evoked in animals (Nakamura and Katakura, 1995).
Different sites within the CMA appear to control variation in jaw movement kinematics in mammals (Nakamura and Katakura, 1995), suggesting that the broader the areas activated, the more diverse the chewing movements during a chewing sequence. We would expect a gum-chewing task to involve relatively little kinematic variation, except during gum manipulation by the tongue, lips, cheeks, and teeth at the beginning and end of a chew block. Inspection of Figs. 2 and 3 reveals that the activations of these motor cortical areas do indeed increase in size near the beginning (segment 1/5) and end (segment 5/5) of a chew block.
There is evidence from animal studies that the CMA is responsible for initiating chewing (Sessle, 2011). In our results, activity in the motor cortices was highest during segment 1/5 of the chew-block (Figs. 2, 3), corroborating this evidence from former animal studies.
Circuits formed among the motor cortex, pons, and cerebellum are involved in motor coordination (Glickstein and Doron, 2008). A previous fMRI investigation reported bilateral motor cortical and cerebellar activations during a gum-chewing task (Onozuka et al., 2002). By contrast, our investigation reported a switch from bilateral to contralateral cerebellar activation as chew blocks progressed through the 5 segments (see Table, contrasts between chew segments and rest blocks). The Onozuka et al. study allowed participants to chew on the left and/or right side, whereas our study asked participants to perform right-sided chewing only. Also, the Onozuka et al. study had participants chew to a metronome, whereas our participants chewed at self-determined paces. The cerebellum is involved in the coordination and rhythmicity of oral functions (Bryant et al., 2010); hence, the observed differences in cerebellar activity could reflect differences in how the masticatory movements were controlled in the 2 studies.
Additionally, the switch from bilateral to contralateral cerebellar activation was seen only in the 4th and 5th chewing segments of the chew block, whereas the traditional chew-block analysis revealed bilateral cerebellar activations (compare the top of the Table with contrasts between chewing segments and rest blocks in the Table). In this regard, our traditional analysis corroborates the findings from the Onozuka et al. study. Hence, the switch from bilateral to contralateral cerebellar activity likely reflects an important shift in central masticatory control that occurs during relatively long chewing bouts.
The supplementary motor cortex, premotor cortex, and superior temporal gyrus are involved with modulating chewing movements associated with changes in bolus hardness (Takahashi et al., 2007). However, another investigation showed that many of these regions are activated during tooth clenching, suggesting that relatively high bite forces are sufficient to activate them (Iida et al., 2010). Because these regions were more activated at the beginning or end of our chew blocks (Fig. 3), we hypothesize that they may have been involved in modulating the type of muscle activity, which would be associated with moving the gum from the cheek to the occlusal table and vice versa.
We observed a brainstem activation cluster in the contrast between chew blocks and rest blocks; however, fMRI does not provide adequate spatial resolution to identify specific brainstem sites. Based on the position of the clusters, they likely included brainstem regions known to be involved with masticatory rhythm and muscle activity pattern generation in animals (Dellow and Lund, 1971; Nakamura et al., 2004; Lund and Kolta, 2006a). Thus, our results support the hypothesis that these brainstem regions are involved as central pattern generators and central timing networks in humans as well.
The basal ganglia and amygdala are implicated in oral movements, including chewing, dyskinesias, and parafunctions (Furata and Murakami, 1989; Nishino et al., 1991; Masuda et al., 2001; McCormick and Stoessl, 2002; Andreassen et al., 2003; Lee et al., 2004; Jiang et al., 2010). The present study demonstrated activations in the head of the caudate nucleus, putamen, and amygdala (Table, Fig. 2) during gum chewing, suggesting that these regions may be associated with routine chewing. The activations appeared most pronounced near the onset of chew-blocks (Table), suggesting a role in chewing initiation.
Since gum chewing does not require relatively large kinematic changes from chew to chew, it might be expected that brain activity throughout the chew block would remain stable. However, the contrasts between successive chew-block segments showed considerable variation in the volumes activated in motor and supplementary motor cortices and the cerebellum, suggesting dynamic local changes in brain activity while participants chewed. Future studies will need to investigate the nature and function of these dynamic changes.
Several study limitations should be mentioned. First, brain activation patterns reported here may not reflect those that would be observed under routine conditions. The involvement of cerebral centers likely differs between natural chewing and chewing that is controlled by experimental protocols. Also, we did not monitor chewing kinematics during chewing; consequently, the effects due to variation in masticatory muscle activity could not be evaluated. We did not assess chewing side preference beforehand, and this may also have affected findings.
Additionally, the use of a fixed 25-second block design likely leads to brain activations associated with anticipating the next commands, and this may affect results near the beginnings and ends of blocks. It will be important to develop studies that will mimic routine ‘spontaneous’ chewing. Toward that end, because our participants were less likely to be thinking about the commands to start or stop chewing during the middle of chew blocks, perhaps the observed activations in segments 2/5, 3/5, and 4/5 better approximate those that would occur during routine chewing (Fig. 2). Other factors to consider in future studies will be food tastes, evolution of food bolus consistency and size, chewing in a supine vs. upright position, and repeating the experiments on the same individuals to study reliability.
Finally, this study’s segmental analyses were novel, and study designs, e.g., block duration vs. repetition time, were used to minimize physiological and statistical artifacts. Non-parametric analysis methods exist, e.g., finite impulse response; however, there are limitations to these methods as well. Whatever the case, studies of the time-varying aspects of brain activity during ongoing tasks are a compelling avenue for future study.
Acknowledgments
This work is based on a thesis submitted by Dr. Andres Quintero to the graduate faculty, University of Michigan, in partial fulfillment of the requirements for the PhD degree. We acknowledge Keith Newnham’s technical assistance with the MRI scanner and discussions with Scott Peltier and Tobias Schmidt-Wilcke on study design and analysis issues.
Footnotes
This project was supported by USPHS Research Grant (DE-018528 to GEG) from the NIDCR, NIH.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Adachi K, Hasegawa M, Fujita S, Sato M, Miwa Y, Ikeda H, et al. (2002). Dopaminergic and cholinergic stimulation of the ventrolateral striatum elicit rat jaw movements that are funneled via distinct efferents. Eur J Pharmacol 442:81-92. [DOI] [PubMed] [Google Scholar]
- Andreassen OA, Ferrante RJ, Aamo TO, Beal MF, Jorgensen HA. (2003). Oral dyskinesias and histopathological alterations in substantia nigra after long-term haloperidol treatment of old rats. Neuroscience 122:717-725. [DOI] [PubMed] [Google Scholar]
- Bracco P, Anastasi G, Piancino MG, Frongia G, Milardi D, Favaloro A, et al. (2010). Hemispheric prevalence during chewing in normal right-handed and left-handed subjects: a functional magnetic resonance imaging preliminary study. Cranio 28:114-121. [DOI] [PubMed] [Google Scholar]
- Bryant JL, Boughter JD, Gong S, LeDoux MS, Heck DH. (2010). Cerebellar cortical output encodes temporal aspects of rhythmic licking movements and is necessary for normal licking frequency. Eur J Neurosci 32:41-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd KE, Romito LM, Dzemidzic M, Wong D, Talavage TM. (2009). fMRI study of brain activity elicited by oral parafunctional movements. J Oral Rehabil 36:346-361. [DOI] [PubMed] [Google Scholar]
- Dellow PG, Lund JP. (1971). Evidence for central timing of rhythmical mastication. J Physiol 215:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin SF, LeResche L. (1992). Research diagnostic criteria for temporomandibular disorders: review, criteria, examinations and specifications, critique. J Craniomandib Disord 6:301-355. [PubMed] [Google Scholar]
- Furuta A, Murakami T. (1989). Modulation of the jaw muscle activity during the rhythmical jaw movement by stimulation of the cortical masticatory area and amygdala in the rabbit. Shigaku 77:607-617. [PubMed] [Google Scholar]
- Glickstein M, Doron K. (2008). Cerebellum: connections and functions. Cerebellum 7:589-594. [DOI] [PubMed] [Google Scholar]
- Iida T, Kato M, Komiyama O, Suzuki H, Asano T, Kuroki T, et al. (2010). Comparison of cerebral activity during teeth clenching and fist clenching: a functional magnetic resonance imaging study. Eur J Oral Sci 118:635-641. [DOI] [PubMed] [Google Scholar]
- Jiang H, Liu H, Liu G, Jin Z, Liu X. (2010). The effects of chewing-side preference on human brain activity during tooth clenching: an fMRI study. J Oral Rehabil 37:877-883. [DOI] [PubMed] [Google Scholar]
- Kimoto K, Ono Y, Tachibana A, Hirano Y, Otsuka T, Ohno A, et al. (2011). Chewing-induced regional brain activity in edentulous patients who received mandibular implant-supported overdentures: a preliminary report. J Prosthodont Res 55:89-97. [DOI] [PubMed] [Google Scholar]
- Lee J, Adachi K, Gionhaku N, Fujita S, Uchida T, Gerstner GE, et al. (2004). Evidence that angiotensin II enhances apomorphine-induced jaw movements via AT1 receptors in the ventrolateral striatum: studies by magnet-sensing system in freely moving rats. Methods Find Exp Clin Pharmacol 26:195-199. [DOI] [PubMed] [Google Scholar]
- Lund JP. (2011). Chapter 15—chew before you swallow. Prog Brain Res 188:219-228. [DOI] [PubMed] [Google Scholar]
- Lund JP, Kolta A. (2006a). Generation of the central masticatory pattern and its modification by sensory feedback. Dysphagia 21:167-174. [DOI] [PubMed] [Google Scholar]
- Lund JP, Kolta A. (2006b). Brainstem circuits that control mastication: do they have anything to say during speech? J Commun Disord 39:381-390. [DOI] [PubMed] [Google Scholar]
- Lund JP, Sasamoto K, Murakami T, Olsson KA. (1984). Analysis of rhythmical jaw movements produced by electrical stimulation of motor-sensory cortex of rabbits. J Neurophysiol 52:1014-1029. [DOI] [PubMed] [Google Scholar]
- Masuda Y, Kato T, Hidaka O, Matsuo R, Inoue T, Iwata K, Morimoto T. (2001). Neuronal activity in the putamen and the globus pallidus of rabbit during mastication. Neurosci Res 39:11-19. [DOI] [PubMed] [Google Scholar]
- McCormick SE, Stoessl AJ. (2002). Blockade of nigral and pallidal opioid receptors suppresses vacuous chewing movements in a rodent model of tardive dyskinesia. Neuroscience 112:851-859. [DOI] [PubMed] [Google Scholar]
- Momose I, Nishikawa J, Watanabe T, Sasaki Y, Senda M, Kubota K, et al. (1997). Effect of mastication on regional cerebral blood flow in humans examined by positron-emission tomography with 15O-labelled water and magnetic resonance imaging. Arch Oral Biol 42:57-61. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Katakura N. (1995). Generation of masticatory rhythm in the brainstem. Neurosci Res 23:1-19. [PubMed] [Google Scholar]
- Nakamura Y, Katakura N, Nakajima M, Liu J. (2004). Rhythm generation for food-ingestive movements. Prog Brain Res 143:97-103. [DOI] [PubMed] [Google Scholar]
- Nishino H, Hattori S, Muramoto K, Ono T. (1991). Basal ganglia neural activity during operant feeding behavior in the monkey: relation to sensory integration and motor execution. Brain Res Bull 27:463-468. [DOI] [PubMed] [Google Scholar]
- Onozuka M, Fujita M, Watanabe K, Hirano Y, Niwa M, Nishiyama K, et al. (2002). Mapping brain region activity during chewing: a functional magnetic resonance imaging study. J Dent Res 81:743-746. [DOI] [PubMed] [Google Scholar]
- Onozuka M, Fujita M, Watanabe K, Hirano Y, Niwa M, Nishiyama K, et al. (2003). Age-related changes in brain regional activity during chewing: a functional magnetic resonance imaging study. J Dent Res 82:657-660. [DOI] [PubMed] [Google Scholar]
- Sessle BJ. (2011). Chapter 5—Face sensorimotor cortex: its role and neuroplasticity in the control of orofacial movements. Prog Brain Res 188:71-82. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Miyamoto T, Terao A, Yokoyama A. (2007). Cerebral activation related to the control of mastication during changes in food hardness. Neuroscience 145:791-794. [DOI] [PubMed] [Google Scholar]
- Tamura T, Kanayama T, Yoshida S, Kawasaki T. (2003). Functional magnetic resonance imaging of human jaw movements. J Oral Rehabil 30:614-622. [DOI] [PubMed] [Google Scholar]


