Abstract
An understanding of the process by which tumor cells destroy the basement membrane of the surface epithelium, invade, and metastasize is essential to the development of novel treatment of head and neck squamous cell carcinoma (HNSCC). In recent years, there has been increased interest in the role of epithelial-mesenchymal transition (EMT) in invasion. EMT is a process that describes the development of motile, mesenchymal-like cells from non-motile parent epithelial cells. There are 3 known types of EMT that mediate development, wound healing, and carcinogenesis. This review summarizes studies of known EMT biomarkers in the context of HNSCC progression. The biomarkers discussed come from a wide range of proteins, including cell-surface proteins (E-cadherin, N-cadherin, and Integrins), cytoskeletal proteins (α-Smooth Muscle Actin, Vimentin, and β-catenin), extracellular matrix proteins (Collagens, Fibronectin, and Laminin), and transcription factors (SNAIL1, SNAIL2, TWIST, and LEF-1). Overall, the findings of these studies suggest that EMT mediates HNSCC progression. The mechanistic role of the EMT markers that have been associated with HNSCC should be more clearly defined if new anti-HNSCC therapies to block EMT progression are to be developed.
Keywords: disease progression, extracellular matrix, oral pathology, neoplasm invasiveness, biological markers, head and neck neoplasms
Introduction
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common cancer in the world (Leemans et al., 2011). In the United States, HNSCC accounts for more deaths each year than cervical cancer, melanoma, or Hodgkin’s lymphoma and costs more than 2 billion dollars to treat (Lee et al., 2004; Menzin et al., 2007). Since patients with HNSCC often present with late-stage tumors, the five-year survival rate is only 50%, which is poorer than that for breast cancer or melanoma (Siegel et al., 2011). Poor survival can be attributed to the high frequency of local recurrence, second primary tumors, and distant metastases. An understanding of the process by which tumor cells destroy the basement membrane, invade, and metastasize is essential for the advancement of HNSCC treatment strategies and improving survival.
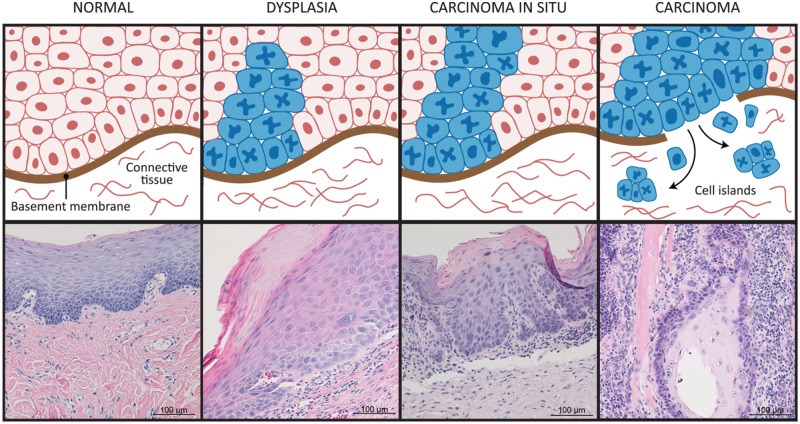
Destruction of the basement membrane and invasion of genetically and phenotypically altered cells into the underlying stroma are required for progression of epithelial dysplasia (pre-cancer) to HNSCC (Fig. 1). The basement membrane is the first and most robust structural barrier to invasion (Rowe and Weiss, 2008). In normal and pre-cancerous mucosa, the basement membrane separates surface-stratified squamous epithelium from the underlying connective tissue. Epithelial dysplasia exhibits less organization than normal oral epithelium (pre-cancer), due to accumulation of genetically altered cells above the basement membrane. After destruction of the basement membrane, tumor cells invade locally or metastasize to distant sites. Tumor spread contributes to the lethality of the disease.
Figure 1.
Head and neck squamous cell carcinoma (HNSCC) progression. Normal epithelium consists of cells with low mitotic activity and an intact basement membrane. In dysplasia, abnormal cells appear near the basement membrane, and in carcinoma in situ (full-thickness dysplasia), the abnormal cells are present throughout the full thickness of the epithelium. In carcinoma, the basement membrane is disrupted, and tumor cells invade the connective tissue.
Epithelial-to-mesenchymal transition (EMT) facilitates invasion. EMT describes the development of motile cells from non-motile parent epithelial cells (Fig. 2). EMT, which occurs in embryonic development, wound healing, and cancer (Fig. 3), is classified into 3 subtypes (Zeisberg and Neilson, 2009). Type 1 occurs in gastrulation and in migration of neural crest cells; some of the migrated cells undergo mesenchymal-to-epithelial transition (MET) to become epithelial cells in organs produced by the mesoderm and endoderm. This embryological EMT occurs in the orofacial region during palatogenesis. Type 2 occurs in wound healing and can result in fibrosis when there is persistent inflammation. Cytokines generated by tissue injury induce the fibroblast phenotype from epithelial or endothelial cells. Type 3 occurs in subsets of invasive cancer cells by using some of the Type 2 EMT program for migration and aggregation of epithelial cells in wound healing. After invading, tumor cells can transition back to the epithelial morphology (MET) to proliferate and generate tumors at distant sites.
Figure 2.
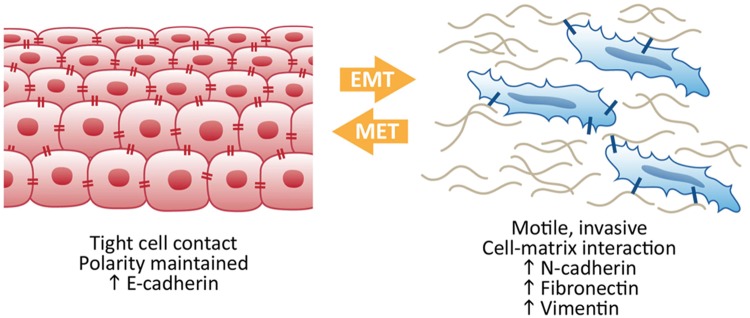
Epithelial-to-mesenchymal transition (EMT) to mesenchymal-to-epithelial transition (MET). Epithelial-like cells display tight cell-cell contacts and maintain polarity, whereas mesenchymal-like cells are more motile and display more contact with the extracellular matrix. Proteins associated with the epithelial-like or the mesenchymal-like states are referred to as biomarkers. As cells progress through EMT and MET, the levels of proteins associated with each state are altered, reflecting the phenotypic switch between the 2 states.
Figure 3.
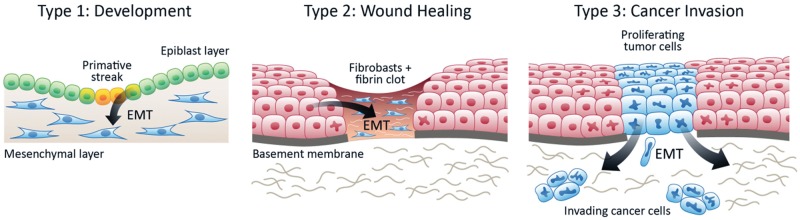
Three types of Epithelial-to-mesenchymal transition (EMT). Type 1 EMT occurs in development, for example, when gastrulation epithelial cells transition to motile mesenchymal cells. Type 2 EMT occurs when secondary epithelial or endothelial cells move to interstitial spaces in wound healing or chronic inflammation, resulting in fibrosis. Type 3 EMT occurs when epithelial tumor cells migrate beyond a primary tumor and metastasize.
The purpose of this review is to present the growing evidence that EMT plays a significant role in the invasion and metastasis of HNSCC. Many protein types, including cell-surface proteins, cytoskeletal proteins, extracellular matrix (ECM) components, and transcription factors, contribute to EMT (Fig. 4, Table).
Figure 4.
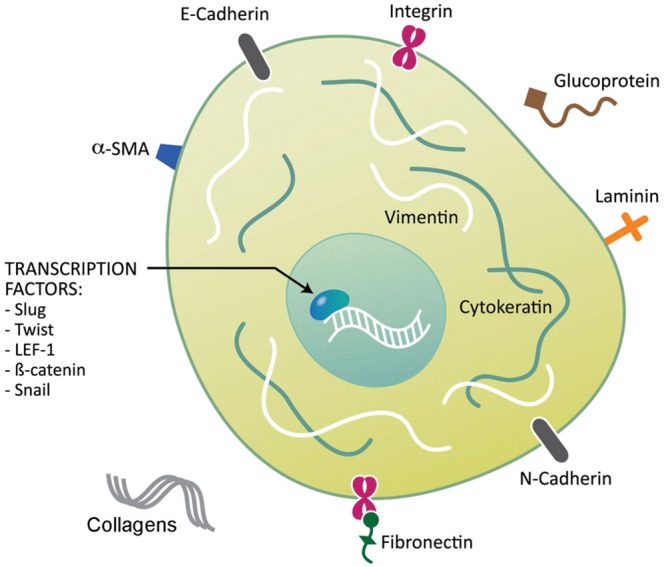
Proteins involved in Epithelial-to-mesenchymal transition (EMT). Several proteins have been identified as biomarkers of EMT. These proteins include cell-surface proteins, cytoskeletal proteins, extracellular matrix proteins, and transcription factors.
Table.
Known Biomarkers of EMT in HNSCC
| Biomarker Category | Proteins | References |
|---|---|---|
| Cell-surface proteins | E-Cadherin | Biddle et al., 2011 |
| N-Cadherin | Nguyen et al., 2011 | |
| Integrins | Dyce et al., 2002 | |
| Nakahara et al., 2003 | ||
| Koontongkaew et al., 2011 | ||
| Cytoskeletal proteins | α-SMA | Lim et al., 2011 |
| Vimentin | Yoon et al., 2007 | |
| Paccione et al., 2008 | ||
| Chen et al., 2011 | ||
| β-catenin | Chang et al., 2008 | |
| Tsai et al., 2009 | ||
| Goto et al., 2010 | ||
| Extracellular | Collagen (I) | Chen et al., 2008 |
| Matrix proteins | Koontongkaew et al., 2011 | |
| Collagen (III) | Kauppila et al., 1998 | |
| Tapper et al., 2001 | ||
| Collagen (IV) | Chen et al., 2008 | |
| Fibronectin | Dooley et al., 2003 | |
| Tijink et al., 2006 | ||
| Warawdekar et al., 2006 | ||
| Laminin 5 | Marinkovich, 2007 | |
| Chen et al., 2008 | ||
| Mendez et al., 2009 | ||
| Transcription factors | SNAIL1 | MH Yang et al., 2007 |
| Lyons et al., 2008 | ||
| Hayry et al., 2010 | ||
| Hsu et al., 2010 | ||
| Mendelsohn et al., 2012 | ||
| SNAIL2 | CH Huang et al., 2009 | |
| TWIST | Ou et al., 2008 | |
| LEF-1 | Segrelles et al., 2006 |
Abbreviations: EMT, epithelial-to-mesenchymal transition; HNSCC, head and neck squamous cell carcinoma.
Cell-Surface Proteins
Cell-surface proteins contributing to EMT in HNSCC include cadherins and integrins.
Cadherins
E-cadherin is the main protein of adherens junctions that anchor oral epithelial cells to each other. It is a calcium-dependent cell-surface protein that facilitates adhesion between and among epithelial cells. E-cadherin is characterized by long cytoplasmic and extracellular domains, which create homophilic interactions between adjacent cells to facilitate adhesion. The expression of E-cadherin is decreased during embryonic development, tumor fibrosis, and cancer progression (Zeisberg and Neilson, 2009). In oral epithelial cells, from which HNSCC develops, surface E-cadherin anchors cells to each other and links to the cytoskeleton via β-catenin. Loss or sequestration of E-cadherin in the nucleus impairs cell-cell adhesion and releases β-catenin, which translocates to the nucleus to induce transcription of EMT genes, such as TWIST.
Several studies have shown reduced E-cadherin in HNSCC, with lowest E-cadherin levels in poorly differentiated tumors (Wu et al., 2000). E-cadherin expression is similar between primary tumors and metastases, perhaps because of MET in metastatic tumors. The promoter region of the E-cadherin gene (CDH1) is hypermethylated in HNSCC, but hypermethylation is not correlated with advanced stage (Calmon et al., 2007), mortality, or second primary tumor (Dikshit et al., 2007). Meta-analysis of E-cadherin studies in HNSCC showed that abnormal E-cadherin expression is predictive of diminished disease- specific survival (Zhao et al., 2012).
The use of E-cadherin expression to personalize anti-HNSCC therapy has been explored (Eriksen et al., 2005; DH Huang et al., 2009). Higher E-cadherin expression is correlated with better sensitivity toward the EGFR-tyrosine kinase inhibitors. The ‘E-cadherin to N-cadherin’ switch, which occurs during embryonic development and cancer progression, is used to monitor EMT. E-cadherin is expressed in epithelial cells, and N-cadherin is up-regulated by TWIST in type 3 EMT in gastric cancer (Z Yang et al., 2007). In HNSCC, high expression of N-cadherin correlated with malignant behaviors such as a high-grade pattern of invasion and poorly differentiated cancer cells (Nguyen et al., 2011). Cadherin switching (high expression of N-cadherin and low expression of E-cadherin) was observed in 30 of the 80 cases and correlated with invasion and lymph node metastasis, as well as EMT features. Thus, cadherin switching may be a critical event in the progression of HNSCC through EMT.
Integrins
EMT facilitates relocation of cells from above the basement membrane into the ECM, which involves a change in expression of integrins (Zeisberg and Neilson, 2009). Integrins are heterodimeric adhesion receptors composed of α and β subunits. There are 18 α and 8 β subunits that variously combine into 24 different integrins. Integrins bind to ligands, including collagens, laminins, and fibronectin in the ECM. Ligand-bound integrins induce several signaling cascades that control cell polarity, motility, survival, shape, proliferation, and differentiation.
Integrins mediate interactions between cells and the surrounding ECM by making transmembrane connections between the cytoskeleton and the ECM. The interaction between the α5 integrin and fibronectin is necessary for metastasis of a melanoma cell line (Qian et al., 2005). Blocking the α5 subunit in HNSCC decreases adhesion to collagen IV and fibronectin (Dyce et al., 2002). The α5β1 integrin has been associated with cisplatin resistance and enhanced adhesion to fibronectin, which is abolished when the integrin is blocked by a neutralizing antibody (Nakahara et al., 2003). The β1 integrin increases invasion of HNSCC cells, which decreases significantly when the integrin is blocked (Koontongkaew et al., 2011).
Cytoskeletal Markers
Cytoskeletal proteins that contribute to EMT in HNSCC include alpha-smooth-muscle actin (α-SMA), vimentin, and β-catenin.
α-SMA
Cells expressing α-SMA contribute to EMT in embryogenesis and wound healing in normal epithelial cells (Zeisberg and Neilson, 2009). In squamous cell carcinoma (SCC), the tumor tissue is surrounded by reactive stroma, made up mostly of cancer-associated fibroblasts (CAFs), also known as myofibroblasts because they acquire characteristics of muscle fibers, including expression of α-SMA. Expression of α-SMA is controlled by growth factors and specialized ECM proteins. α-SMA is incorporated into stress fibers of fibroblasts, thereby augmenting their contractile ability, which is critical to tissue remodeling. CAFs are known to potentiate the development and progression of epithelial cancers. Fibroblasts can be distinguished based on the stage of tumor development by differences in α-SMA expression, which is expressed more highly in mature fibroblasts than newly transitioning cells. Fibroblasts from HNSCC tumors grow more slowly compared with normal fibroblasts from the oral cavity (Lim et al., 2011).
HNSCC characterized by extensive genetic copy number alterations, loss of heterozygosity, and inactivation of p53 and p16INK4A had higher α-SMA expression, which correlated with poor prognosis in an independent dataset of HNSCC samples when compared with tumors with less genetic instability (Lim et al., 2011). However, the mechanistic link between α-SMA expression and EMT and HNSCC is unknown.
Vimentin
Vimentin is an intermediate filament that is used as a marker of mesenchymal cells to distinguish them from epithelial cells (Zeisberg and Neilson, 2009). Vimentin is expressed at sites of cellular elongation and is associated with a migratory phenotype. Increased vimentin expression is frequently used as an EMT marker in cancer.
In HNSCC cell lines, Chen et al. isolated an ALDH1-rich subpopulation of cells and characterized their invasive potential and EMT phenotype (Chen et al., 2011). Spheroid-derived cells had increased vimentin when compared with monolayer-derived cells from different cell lines. Furthermore, vimentin expression decreased when cells were grown as a monolayer. Vimentin expression is higher in nodal metastatic cells than in the primary HNSCC tumors, and is enhanced by epidermal growth factor and TGF-β (Paccione et al., 2008). Reducing vimentin levels by RNA interference decreased the proliferation, migration, and invasion of metastatic cells compared with control cells (Paccione et al., 2008). Yoon et al. developed an orthotopic model of HNSCC metastasis and selected HNSCC cells through 4 rounds of serial metastasis to obtain a highly metastatic subpopulation (Yoon et al., 2007). The metastatic population acquired mesenchymal features, including increased vimentin and integrin β1, and reduced epithelial expression, including reduced E-cadherin and involucrin. In contrast, non-metastatic parental cells had low vimentin expression.
β-Catenin
The Wnt/β-catenin pathway has a critical role in invasion in HNSCC (Goto et al., 2010). E-cadherin is anchored to the cytoskeleton via β-catenin, a cytoplasmic plaque protein (Wheelock and Johnson, 2003). In loss of cell adhesion, as occurs in invasion, E-cadherin is endocytosed and β-catenin is released. In normal and non-invasive cells, β-catenin is usually localized to cell membranes. In cells undergoing EMT, β-catenin is located in the cytoplasm (reflective of its dissociation from E-cadherin). This cytosolic (free) β-catenin translocates to the nucleus to promote transcription of genes that induce EMT. Nuclear β-catenin is a transcriptional co-activator with T-cell factor (TCF)/Lymphoid enhancer-binding factor (LEF), which controls transcription of SNAIL1 (Zeisberg and Neilson, 2009).
Nuclear β-catenin is correlated with a poor prognosis in patients with metastasic HNSCC (Tsai et al., 2009). Rap1, a ras-like protein, stabilized β-catenin and increased its nuclear localization; more advanced N-stage lesions were associated with high free β-catenin and high active Rap1 (Goto et al., 2010).
ECM Proteins
The ECM proteins that promote EMT in HNSCC include collagen, fibronectin, and laminin.
Collagens
While migration of normal cells is strictly controlled by limited proteolysis of the ECM, in cancer, proteolytic remodeling of the ECM facilitates invasion (Egeblad et al., 2010). Collagens are the major structural components of the ECM. There are 28 types of collagen that have a triple-helical structure. Collagen I and II are fibrillary collagens, while collagen IV constitutes a sheet-like structure that is the major component of basement membranes. There is increased expression of collagen I (α1) and collagen III (α1) in type 1 and 3 EMT, while collagen IV (α1) is down-regulated in all 3 types of EMT (Zeisberg and Neilson, 2009).
Collagen type I is the most prevalent form in the interstitial matrix. In HNSCC, collagen type I RNA transcripts are more widely expressed in HNSCC than in pre-cancerous or normal tissue. Collagen I stimulated cytokine secretion in genetically matched primary and metastatic HNSCC cell lines, but cytokine secretion was significantly up-regulated in metastatic cells when seated on collagen I (α1) gel (Koontongkaew et al., 2011). The cytokines released (IL-1α, IL-1β, IL-6, TNF-α, and TNF-β) stimulated MMP activity and invasion of the HNSCC cells. IL-6 is overexpressed in HNSCC and is a biomarker of poor disease-specific survival (Van Tubergen et al., 2011). Moreover, collagen I enhanced MMP-2 and MMP-9 secretion in both primary and metastatic cell lines (Koontongkaew et al., 2011). MMP-9 is a biomarker that contributes to invasion of HNSCC and is correlated with poor disease-specific survival (Mitra et al., 2008).
Collagen III (α1) is an ECM component that promotes cancer progression in ovarian (Tapper et al., 2001) and breast cancers (Kauppila et al., 1998). In HNSCC, collagen III (α1) cDNA was found to be highly expressed in a Paclitaxel-resistant cell line (Schmidt et al., 2006). Altered collagen IV (α1) was linked to invasion and motility of cancer cells. Laminin in tumor cells binds collagen IV (α1) and then secretes gelatinases that break down collagen IV (α1) to facilitate migration of tumor cells (Zeisberg and Neilson, 2009). Surprisingly, Chen et al. found that collagen IV RNA is increased in HNSCC surgical specimens compared with dysplastic and normal tissues (Chen et al., 2008). Further investigation is necessary to determine the role of collagen IV in invasion of HNSCC.
Fibronectin
Fibronectin is a glycoprotein scaffold for fibrillar ECM (Zeisberg and Neilson, 2009). It is composed of a dimer of similar subunits of repetitive sequences covalently linked by 2 disulfide bonds at their C-termini. In normal cells, fibronectin mediates cellular interactions with the ECM and is important in migration, differentiation, growth, and adhesion of cells. Although fibronectin is up-regulated in all 3 types of EMT, its use as an EMT biomarker is limited because it is produced by many cell types, such as epithelial cells, fibroblasts, and mononuclear cells (Z Yang et al., 2007). Fibronectin can be up-regulated by SNAIL and TWIST in type 3 EMT.
In HNSCC, there are contradictory reports with respect to expression of fibronectin. Dooley et al. showed that fibronectin and its receptor are strongly up-regulated with α5β6 integrin in SCC cell lines and tissues (Dooley et al., 2003). Three fibronectin isoforms (extra domain A, extra domain B, and IIICS) are generated via alternative splicing, depending on cytokine and pH conditions. Fibronectin fragment ED-B is not expressed in normal tissues (except those undergoing wound healing), but expression is correlated with HNSCC and tumor cell aggressiveness (Tijink et al., 2006). The ED-A and IIICS isoforms are expressed in blood plasma of HNSCC patients, suggesting that hydrolytic enzyme-aided invasion leads to degradation of EMC components (Warawdekar et al., 2006).
Laminin
Similar to collagen, laminin is a major component of the basement membrane. Laminins are glycoproteins made up of one α chain, one β chain, and one γ chain. There are 15 known heterotrimers of laminin. Laminin 1 (α1β1γ1) is the laminin of greatest interest in EMT types I and II, where it is down-regulated or disrupted. Laminin 5 (α3β3γ2) has been linked to EMT in metaplastic carcinoma of the breast and hepatocarcinoma (Zeisberg and Neilson, 2009).
The role of laminin 5 in HNSCC is clearly significant. Laminin 5 has been shown to be a major component of the ECM, and laminin 5 expression has been correlated with invasion and patient prognosis. Interestingly, the laminin 332 G4 domain, a proteolytic product of laminin 5, promotes laminin 5 deposition, and may have a role in wound healing and SCC formation (Marinkovich, 2007). In a study comparing HNSCC, pre-cancer, and normal tissues, overexpression of transcripts of LAMC2 encoding laminin-γ2 chain and COL4A1 collagen type IV α1 distinguished HNSCC from pre-cancerous and normal tissues that had lower LAMC2 (Chen et al., 2008). In another study, high LAMC2 predicted poor HNSCC-specific survival (Mendez et al., 2009).
Transcription Factors
The transcription factors that promote EMT in HNSCC include SNAIL, TWIST, and LEF-1.
SNAIL Family
SNAIL proteins regulate various aspects of the EMT phenotype, including overexpression of mesenchymal markers fibronectin and vitronectin, and suppression of epithelial markers, including E-cadherin (Zeisberg and Neilson, 2009). In addition, SNAIL blocks cell-cycle progression and contributes to cell movement and survival. Other targets of SNAIL proteins include genes regulating cell polarity and apoptosis. SNAIL family proteins are evolutionarily conserved in vertebrates, where they have a conserved role in embryonic mesoderm formation.
There are 3 transcription factors in the SNAIL family, of which SNAIL1 and SNAIL2 are functionally equivalent (Zeisberg and Neilson, 2009). SNAIL1 is important in mediating invasion and inflammation through transcriptional regulation of cytokines, and also in preventing terminal differentiation of HNSCC cells (Lyons et al., 2008). EMT phenotypes and invasion in HNSCC are reduced with siRNA-mediated knockdown of SNAIL1 (MH Yang et al., 2007). SNAIL1 expression in primary tumors of HNSCC patients is correlated with metastasis and poor prognosis. SNAIL1 expression correlates with histopathologic grade and depth of invasion in HNSCC (Hayry et al., 2010) and is correlated with poor differentiation of the tumors, lymphovascular invasion, and regional metastasis (Mendelsohn et al., 2012). Knockdown of SNAIL1 in HNSCC cell lines attenuated cisplatin resistance by facilitating DNA excision repair by stabilizing ERCC1, which is necessary for nucleotide excision repair (Hsu et al., 2010). Lysyl Oxidase–Like 2, a marker of poor prognosis in SCC, regulates EMT in part by stabilizing SNAIL1 to facilitate tumor progression (Peinado et al., 2008).
Hypoxia contributes to tumor metastasis by initiating EMT through activation of SNAIL2 in HNSCC. SNAIL2 is critical for the induction of MT4-MMP in a hypoxic environment (CH Huang et al., 2009). Overall, there is strong evidence to suggest that SNAIL proteins play a role in EMT in HNSCC.
TWIST
TWIST is a basic helix-loop-helix protein that modulates many target genes through E-box-responsive elements. There are 2 TWIST genes (TWIST1 and TWIST 2) which are well-conserved in vertebrates. TWIST is activated in all 3 types of EMT and is up-regulated in cancer metastases (Zeisberg and Neilson, 2009). While mesoderm formation is controlled by SNAIL family proteins, TWIST1 is important in mesoderm differentiation. TWIST proteins can act as either transcriptional repressors (e.g., E-cadherin) or activators (e.g., N-cadherin and Fibronectin).
In HNSCC, TWIST expression is positively correlated with lymph node metastasis and clinical stage (Ou et al., 2008). Hypoxia-inducible factor-1α (HIF-1α) promotes EMT and metastatic phenotypes in HNSCC via up-regulation of TWIST expression. Repression of TWIST reverses the EMT and metastatic phenotypes. BMI1, a polycomb-group protein frequently overexpressed in cancers, is regulated by TWIST in HNSCC (Yang et al., 2010). BMI1 and TWIST expression lead to down-regulation of E-cadherin, which is associated with poor prognosis (Yang et al., 2010). In a study of 109 HNSCC patients, negative SNAIL1 and TWIST immunostaining was significantly correlated with improved five-year disease-specific survival (Jouppila-Matto et al., 2011).
LEF-1
LEF-1, a co-transcriptional activator with TCF, mediates WNT signaling. Through this regulation, LEF-1 plays a role in deciding the fate of cells in normal embryonic development (Zeisberg and Neilson, 2009). In EMT, the β-catenin/LEF-1 complex is localized to the nucleus, where it controls SNAIL gene expression, along with other markers associated with EMT. LEF-1 functions with β-catenin to promote cell survival and proliferation during mammary gland development and in breast cancer (Hatsell et al., 2003). LEF-1 and β-catenin are up-regulated and translocate to the nucleus in Akt-transformed keratinocytes (Segrelles et al., 2006).
Future Directions
In addition to proteins that have a role in HNSCC progression or a correlation with HNSCC, there are several potential biomarkers that should be explored in HNSCC. These proteins have been shown to have a role in EMT in other cancers, but to our knowledge have not been investigated in HNSCC.
Forkhead box protein c2 (FOXC2) is a transcription factor that is expressed in type I EMT and is important in angiogenesis, musculogenesis, and the development of the heart, kidney, and urinary tract. FOXC2 is expressed in ductal breast cancers and metastatic breast cancer (Zeisberg and Neilson, 2009). Overexpression of EMT transcription factors such as SNAIL and TWIST increase FOXC2 expression. Furthermore, the overexpression of FOXC2 can induce EMT, which suggests that FOXC2 may play a role in type 3 EMT.
Expression of zona-occludens 1 (ZO-1) occurs in all 3 types of EMT (Zeisberg and Neilson, 2009). ZO-1 is a tight junction protein that is usually located at cell-cell adhesion membrane complexes in normal epithelial cells. During EMT, ZO-1 relocates from the adhesion membrane complexes to the cytoplasm and then to the nucleus, depending on the degree of differentiation and migration of the cell (Polette et al., 2007). ZO-1 is involved in the EMT process in colorectal and bile duct cancers, but has not yet been linked to EMT in HNSCC (Hirakawa et al., 2009; Nemeth et al., 2009).
Zinc finger E-box binding homeobox 1 (ZEB1) is an E-cadherin transcriptional repressor that is down-regulated by miR-200 microRNAs in cancer cells that display an EMT phenotype (Zeisberg and Neilson, 2009). ZEB1 has been investigated in prostate cancer, non-small-cell lung carcinoma, and invasive ductal breast cancer, but has not been investigated in HNSCC (Drake et al., 2010; Gemmill et al., 2011; Montserrat et al., 2011).
Osteoblast cadherin (OB-cadherin) is a definitive marker for activated fibroblasts (Zeisberg and Neilson, 2009). While in cancer and embryonic development, an ‘E-cadherin to N-cadherin’ switch is used to monitor EMT progression, an ‘E-cadherin to OB-cadherin’ switch may indicate EMT progression in type II EMT specifically. In cancer, OB-cadherin has an association with prostate and breast cancer, where it is hypothesized to be involved in metastasis; its expression in HNSCC is unknown (Farina et al., 2009).
Cancer stem cells (CSC) are a small population of tumor cells that can both initiate a tumor and repopulate a tumor following treatment, contributing to treatment resistance. In HNSCC, a subpopulation of CD44+ cancer stem cells displays a phenotypic switch to become either proliferative or migratory (Biddle et al., 2011). The migratory population, designated CD44highESAlow, displays reduced E-cadherin and increased Vimentin, TWIST, SNAIL1, and SNAIL2, features of EMT cells. Given the striking similarities between EMT and this subpopulation of CSCs, it is likely that future research will elucidate the role of EMT in maintaining the CSC population.
EMT progression involves many signaling pathways that may be targeted in the clinical setting (Foroni et al., 2012; Prud’homme, 2012). Fig. 5 summarizes some of the pathways and anti-EMT therapies. The mechanistic role of the EMT markers associated with HNSCC should be clearly defined in the development of new anti-HNSCC therapies to block HNSCC progression. An understanding of the role of EMT in HNSCC will allow clinicians to personalize treatment for patients with particularly aggressive tumors and improve treatment outcomes.
Figure 5.
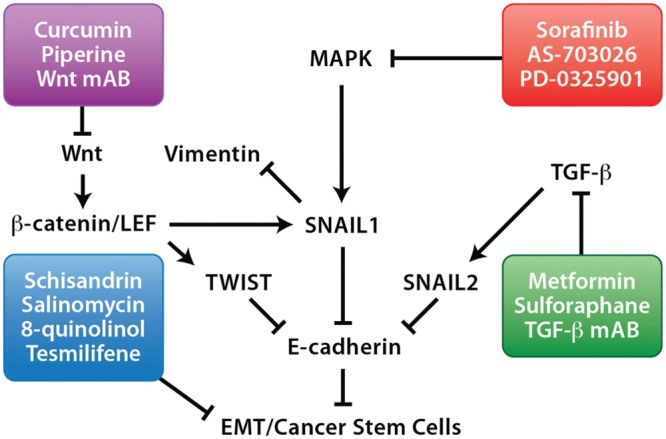
Targeted therapies against epithelial-to-mesenchymal transition (EMT) pathways. EMT progression involves many signaling pathways that may be targeted in the clinical setting, which include monoclonal antibodies (mAB) and small molecule inhibitors (boxed).
Acknowledgments
The authors thank Chris Jung and Kenneth Rieger for their artistic assistance with illustrations.
Footnotes
This work was supported by National Institute of Dental and Craniofacial Research grants DE018512, DE019513, and DE017977 (NJD), F30 DEO21293 (CSS), and F32 DE213052 (EVT).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Biddle A, Liang X, Gammon L, Fazil B, Harper LJ, Emich H, et al. (2011). Cancer stem cells in squamous cell carcinoma switch between two distinct phenotypes that are preferentially migratory or proliferative. Cancer Res 71:5317-5326. [DOI] [PubMed] [Google Scholar]
- Calmon MF, Colombo J, Carvalho F, Souza FP, Filho JF, Fukuyama EE, et al. (2007). Methylation profile of genes CDKN2A (p14 and p16), DAPK1, CDH1, and ADAM23 in head and neck cancer. Cancer Genet Cytogenet 173:31-37. [DOI] [PubMed] [Google Scholar]
- Chang HW, Roh JL, Jeong EJ, Lee SW, Kim SW, Choi SH, et al. (2008). Wnt signaling controls radiosensitivity via cyclooxygenase-2-mediated Ku expression in head and neck cancer. Int J Cancer 122:100-107. [DOI] [PubMed] [Google Scholar]
- Chen C, Mendez E, Houck J, Fan W, Lohavanichbutr P, Doody D, et al. (2008). Gene expression profiling identifies genes predictive of oral squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev 17:2152-2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Wei Y, Hummel M, Hoffmann TK, Gross M, Kaufmann AM, et al. (2011). Evidence for epithelial-mesenchymal transition in cancer stem cells of head and neck squamous cell carcinoma. PLoS One 6:e16466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikshit RP, Gillio-Tos A, Brennan P, De Marco L, Fiano V, Martinez-Penuela JM, et al. (2007). Hypermethylation, risk factors, clinical characteristics, and survival in 235 patients with laryngeal and hypopharyngeal cancers. Cancer 110:1745-1751. [DOI] [PubMed] [Google Scholar]
- Dooley TP, Reddy SP, Wilborn TW, Davis RL. (2003). Biomarkers of human cutaneous squamous cell carcinoma from tissues and cell lines identified by DNA microarrays and qRT-PCR. Biochem Biophys Res Commun 306:1026-1036. [DOI] [PubMed] [Google Scholar]
- Drake JM, Barnes JM, Madsen JM, Domann FE, Stipp CS, Henry MD. (2010). ZEB1 coordinately regulates laminin-332 and {beta}4 integrin expression altering the invasive phenotype of prostate cancer cells. J Biol Chem 285:33940-33948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyce OH, Ziober AF, Weber RS, Miyazaki K, Khariwala SS, Feldman M, et al. (2002). Integrins in head and neck squamous cell carcinoma invasion. Laryngoscope 112:2025-2032. [DOI] [PubMed] [Google Scholar]
- Egeblad M, Rasch MG, Weaver VM. (2010). Dynamic interplay between the collagen scaffold and tumor evolution. Curr Opin Cell Biol 22:697-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen JG, Steiniche T, Overgaard J. (2005). The role of epidermal growth factor receptor and E-cadherin for the outcome of reduction in the overall treatment time of radiotherapy of supraglottic larynx squamous cell carcinoma. Acta Oncol 44:50-58. [DOI] [PubMed] [Google Scholar]
- Farina AK, Bong YS, Feltes CM, Byers SW. (2009). Post-transcriptional regulation of cadherin-11 expression by GSK-3 and beta-catenin in prostate and breast cancer cells. PLoS One 4:e4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foroni C, Broggini M, Generali D, Damia G. (2012). Epithelial-mesenchymal transition and breast cancer: role, molecular mechanisms and clinical impact. Cancer Treat Rev 38:689-697. [DOI] [PubMed] [Google Scholar]
- Gemmill RM, Roche J, Potiron VA, Nasarre P, Mitas M, Coldren CD, et al. (2011). ZEB1-responsive genes in non-small cell lung cancer. Cancer Lett 300:66-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto M, Mitra RS, Liu M, Lee J, Henson BS, Carey T, et al. (2010). Rap1 stabilizes beta-catenin and enhances beta-catenin-dependent transcription and invasion in squamous cell carcinoma of the head and neck. Clin Cancer Res 16:65-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsell S, Rowlands T, Hiremath M, Cowin P. (2003). Beta-catenin and Tcfs in mammary development and cancer. J Mammary Gland Biol Neoplasia 8:145-158. [DOI] [PubMed] [Google Scholar]
- Hayry V, Makinen LK, Atula T, Sariola H, Makitie A, Leivo I, et al. (2010). Bmi-1 expression predicts prognosis in squamous cell carcinoma of the tongue. Br J Cancer 102:892-897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirakawa H, Shibata K, Nakayama T. (2009). Localization of cortactin is associated with colorectal cancer development. Int J Oncol 35:1271-1276. [DOI] [PubMed] [Google Scholar]
- Hsu DS, Lan HY, Huang CH, Tai SK, Chang SY, Tsai TL, et al. (2010). Regulation of excision repair cross-complementation group 1 by Snail contributes to cisplatin resistance in head and neck cancer. Clin Cancer Res 16:4561-4571. [DOI] [PubMed] [Google Scholar]
- Huang CH, Yang WH, Chang SY, Tai SK, Tzeng CH, Kao JY, et al. (2009). Regulation of membrane-type 4 matrix metalloproteinase by SLUG contributes to hypoxia-mediated metastasis. Neoplasia 11:1371-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang DH, Su L, Peng XH, Zhang H, Khuri FR, Shin DM, et al. (2009). Quantum dot-based quantification revealed differences in subcellular localization of EGFR and E-cadherin between EGFR-TKI sensitive and insensitive cancer cells. Nanotechnology 20:225102. [DOI] [PubMed] [Google Scholar]
- Jouppila-Matto A, Narkio-Makela M, Soini Y, Pukkila M, Sironen R, Tuhkanen H, et al. (2011). Twist and snai1 expression in pharyngeal squamous cell carcinoma stroma is related to cancer progression. BMC Cancer 11:350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauppila S, Stenback F, Risteli J, Jukkola A, Risteli L. (1998). Aberrant type I and type III collagen gene expression in human breast cancer in vivo. J Pathol 186:262-268. [DOI] [PubMed] [Google Scholar]
- Koontongkaew S, Amornphimoltham P, Monthanpisut P, Saensuk T, Leelakriangsak M. (2011). Fibroblasts and extracellular matrix differently modulate MMP activation by primary and metastatic head and neck cancer cells. Med Oncol 29:690-703. [DOI] [PubMed] [Google Scholar]
- Lee JM, Turini M, Botteman MF, Stephens JM, Pashos CL. (2004). Economic burden of head and neck cancer. A literature review. Eur J Health Econ 5:70-80. [DOI] [PubMed] [Google Scholar]
- Leemans CR, Braakhuis BJ, Brakenhoff RH. (2011). The molecular biology of head and neck cancer. Nat Rev Cancer 11:9-22. [DOI] [PubMed] [Google Scholar]
- Lim KP, Cirillo N, Hassona Y, Wei W, Thurlow JK, Cheong SC, et al. (2011). Fibroblast gene expression profile reflects the stage of tumour progression in oral squamous cell carcinoma. J Pathol 223:459-469. [DOI] [PubMed] [Google Scholar]
- Lyons JG, Patel V, Roue NC, Fok SY, Soon LL, Halliday GM, et al. (2008). Snail up-regulates proinflammatory mediators and inhibits differentiation in oral keratinocytes. Cancer Res 68:4525-4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovich MP. (2007). Tumour microenvironment: laminin 332 in squamous-cell carcinoma. Nat Rev Cancer 7:370-380. [DOI] [PubMed] [Google Scholar]
- Mendelsohn AH, Lai CK, Shintaku IP, Fishbein MC, Brugman K, Elashoff DA, et al. (2012). Snail as a novel marker for regional metastasis in head and neck squamous cell carcinoma. Am J Otolaryngol 33:6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez E, Houck JR, Doody DR, Fan W, Lohavanichbutr P, Rue TC, et al. (2009). A genetic expression profile associated with oral cancer identifies a group of patients at high risk of poor survival. Clin Cancer Res 15:1353-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzin J, Lines LM, Manning LN. (2007). The economics of squamous cell carcinoma of the head and neck. Curr Opin Otolaryngol Head Neck Surg 15:68-73. [DOI] [PubMed] [Google Scholar]
- Mitra RS, Goto M, Lee JS, Maldonado D, Taylor JM, Pan Q, et al. (2008). Rap1GAP promotes invasion via induction of matrix metalloproteinase 9 secretion, which is associated with poor survival in low N-stage squamous cell carcinoma. Cancer Res 68:3959-3969. [DOI] [PubMed] [Google Scholar]
- Montserrat N, Gallardo A, Escuin D, Catasus L, Prat J, Gutierrez-Avigno FJ, et al. (2011). Repression of E-cadherin by SNAIL, ZEB1, and TWIST in invasive ductal carcinomas of the breast: a cooperative effort? Hum Pathol 42:103-110. [DOI] [PubMed] [Google Scholar]
- Nakahara S, Miyoshi E, Noda K, Ihara S, Gu J, Honke K, et al. (2003). Involvement of oligosaccharide changes in alpha5beta1 integrin in a cisplatin-resistant human squamous cell carcinoma cell line. Mol Cancer Ther 2:1207-1214. [PubMed] [Google Scholar]
- Nemeth Z, Szasz AM, Somoracz A, Tatrai P, Nemeth J, Gyorffy H, et al. (2009). Zonula occludens-1, occludin, and E-cadherin protein expression in biliary tract cancers. Pathol Oncol Res 15:533-539. [DOI] [PubMed] [Google Scholar]
- Nguyen PT, Kudo Y, Yoshida M, Kamata N, Ogawa I, Takata T. (2011). N-cadherin expression is involved in malignant behavior of head and neck cancer in relation to epithelial-mesenchymal transition. Histol Histopathol 26:147-156. [DOI] [PubMed] [Google Scholar]
- Ou DL, Chien HF, Chen CL, Lin TC, Lin LI. (2008). Role of Twist in head and neck carcinoma with lymph node metastasis. Anticancer Res 28:1355-1359. [PubMed] [Google Scholar]
- Paccione RJ, Miyazaki H, Patel V, Waseem A, Gutkind JS, Zehner ZE, et al. (2008). Keratin down-regulation in vimentin-positive cancer cells is reversible by vimentin RNA interference, which inhibits growth and motility. Mol Cancer Ther 7:2894-2903. [DOI] [PubMed] [Google Scholar]
- Peinado H, Moreno-Bueno G, Hardisson D, Perez-Gomez E, Santos V, Mendiola M, et al. (2008). Lysyl oxidase-like 2 as a new poor prognosis marker of squamous cell carcinomas. Cancer Res 68:4541-4550. [DOI] [PubMed] [Google Scholar]
- Polette M, Mestdagt M, Bindels S, Nawrocki-Raby B, Hunziker W, Foidart JM, et al. (2007). Beta-catenin and ZO-1: shuttle molecules involved in tumor invasion-associated epithelial-mesenchymal transition processes. Cells Tissues Organs 185:61-65. [DOI] [PubMed] [Google Scholar]
- Prud’homme GJ. (2012). Cancer stem cells and novel targets for antitumor strategies. Curr Pharm Des 18:2838-2849. [DOI] [PubMed] [Google Scholar]
- Qian F, Zhang ZC, Wu XF, Li YP, Xu Q. (2005). Interaction between integrin alpha(5) and fibronectin is required for metastasis of B16F10 melanoma cells. Biochem Biophys Res Commun 333:1269-1275. [DOI] [PubMed] [Google Scholar]
- Rowe RG, Weiss SJ. (2008). Breaching the basement membrane: who, when and how? Trends Cell Biol 18:560-574. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Schler G, Gruensfelder P, Hoppe F. (2006). Differential gene expression in a paclitaxel-resistant clone of a head and neck cancer cell line. Eur Arch Otorhinolaryngol 263:127-134. [DOI] [PubMed] [Google Scholar]
- Segrelles C, Moral M, Lara MF, Ruiz S, Santos M, Leis H, et al. (2006). Molecular determinants of Akt-induced keratinocyte transformation. Oncogene 25:1174-1185. [DOI] [PubMed] [Google Scholar]
- Siegel R, Ward E, Brawley O, Jemal A. (2011). Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin 61:212-236. [DOI] [PubMed] [Google Scholar]
- Tapper J, Kettunen E, El-Rifai W, Seppala M, Andersson LC, Knuutila S. (2001). Changes in gene expression during progression of ovarian carcinoma. Cancer Genet Cytogenet 128:1-6. [DOI] [PubMed] [Google Scholar]
- Tijink BM, Neri D, Leemans CR, Budde M, Dinkelborg LM, Stigter-van Walsum M, et al. (2006). Radioimmunotherapy of head and neck cancer xenografts using 131I-labeled antibody L19-SIP for selective targeting of tumor vasculature. J Nucl Med 47:1127-1135. [PubMed] [Google Scholar]
- Tsai YP, Yang MH, Huang CH, Chang SY, Chen PM, Liu CJ, et al. (2009). Interaction between HSP60 and beta-catenin promotes metastasis. Carcinogenesis 30:1049-1057. [DOI] [PubMed] [Google Scholar]
- Van Tubergen E, Vander Broek R, Lee J, Wolf G, Carey T, Bradford C, et al. (2011). Tristetraprolin regulates interleukin-6, which is correlated with tumor progression in patients with head and neck squamous cell carcinoma. Cancer 117:2677-2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warawdekar UM, Zingde SM, Iyer KS, Jagannath P, Mehta AR, Mehta NG. (2006). Elevated levels and fragmented nature of cellular fibronectin in the plasma of gastrointestinal and head and neck cancer patients. Clin Chim Acta 372:83-93. [DOI] [PubMed] [Google Scholar]
- Wheelock MJ, Johnson KR. (2003). Cadherins as modulators of cellular phenotype. Annu Rev Cell Dev Biol 19:207-235. [DOI] [PubMed] [Google Scholar]
- Wu H, Lotan R, Menter D, Lippman SM, Xu XC. (2000). Expression of E-cadherin is associated with squamous differentiation in squamous cell carcinomas. Anticancer Res 20:1385-1390. [PubMed] [Google Scholar]
- Yang MH, Chang SY, Chiou SH, Liu CJ, Chi CW, Chen PM, et al. (2007). Overexpression of NBS1 induces epithelial-mesenchymal transition and co-expression of NBS1 and Snail predicts metastasis of head and neck cancer. Oncogene 26:1459-1467. [DOI] [PubMed] [Google Scholar]
- Yang MH, Hsu DS, Wang HW, Wang HJ, Lan HY, Yang WH, et al. (2010). Bmi1 is essential in Twist1-induced epithelial-mesenchymal transition. Nat Cell Biol 12:982-992. [DOI] [PubMed] [Google Scholar]
- Yang Z, Zhang X, Gang H, Li X, Li Z, Wang T, et al. (2007). Up-regulation of gastric cancer cell invasion by Twist is accompanied by N-cadherin and fibronectin expression. Biochem Biophys Res Commun 358:925-930. [DOI] [PubMed] [Google Scholar]
- Yoon Y, Liang Z, Zhang X, Choe M, Zhu A, Cho HT, et al. (2007). CXC chemokine receptor-4 antagonist blocks both growth of primary tumor and metastasis of head and neck cancer in xenograft mouse models. Cancer Res 67:7518-7524. [DOI] [PubMed] [Google Scholar]
- Zeisberg M, Neilson EG. (2009). Biomarkers for epithelial-mesenchymal transitions. J Clin Invest 119:1429-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Ge J, Sun Y, Tian L, Lu J, Liu M, et al. (2012). Is E-cadherin immunoexpression a prognostic factor for head and neck squamous cell carcinoma (HNSCC)? A systematic review and meta-analysis. Oral Oncol 48:761-767. [DOI] [PubMed] [Google Scholar]