Abstract
Mice carrying a knock-in mutation (Phe377del) in the Ank gene replicate many skeletal characteristics of human craniometaphyseal dysplasia, including hyperostotic mandibles. AnkKI/KI mice have normal morphology of erupted molars and incisors but excessive cementum deposition with increased numbers of Ibsp- and Dmp1-positive cells on root surfaces. The cervical loops of adult AnkKI/KI lower incisors are at the level of the third molars, while they are close to the mandibular foramen in Ank+/+ mice. Furthermore, AnkKI/KI incisors show decreased eruption rates, decreased proliferation of odontoblast precursors, and increased cell apoptosis in the stellate reticulum. However, their capability for continuous elongation is not compromised. Quantification of TRAP-positive cells in the apical ends of AnkKI/KI incisors revealed decreased osteoclast numbers and osteoclast surfaces. Bisphosphonate injections in Ank+/+ mice replicate the AnkKI/KI incisor phenotype. These results and a comparison with the dental phenotype of Ank loss-of-function mouse models suggest that increased cementum thickness may be caused by decreased extracellular PPi levels and that the incisor phenotype is likely due to hyperostosis of mandibles, which distinguishes AnkKI/KI mice from the other Ank mouse models.
Keywords: craniometaphyseal dysplasia; dental cementum; incisor; Ankh protein, mouse; hyperostosis; bone remodeling
Introduction
Craniometaphyseal dysplasia (CMD) is a genetic bone disorder characterized by hyperostosis of craniofacial bones and flared metaphyses of long bones. A few studies have reported dental anomalies in CMD patients, including delayed tooth eruption, enamel hypoplasia, and excess mineralization of the crowns of primary maxillary second molars (Hayashibara et al., 2000; Zajac et al., 2010; Zhang et al., 2007). It has been suggested that retention of primary teeth or slower eruption of permanent teeth is likely caused by hyperostosis of the jaw (Hayashibara et al., 2000). However, it has been unclear whether dental abnormalities in CMD patients are caused by direct effects of the CMD gene mutation or are secondary to abnormal bone remodeling.
Autosomal-dominant mutations in CMD patients have been identified in the ankylosis gene ANKH (Nurnberg et al., 2001; Reichenberger et al., 2001). ANK is a transmembrane protein known to transport intracellular pyrophosphate (PPi) into the extracellular matrix (Ho et al., 2000). PPi inhibits mineralization, while phosphate (Pi) promotes hydroxyapatite deposition. A balance of Pi and PPi is needed to regulate mineralization of bone and teeth.
We have generated a knock-in (KI) mouse model by introducing one of the most common CMD mutations, a Phe377 deletion into the Ank gene (Chen et al., 2009). AnkKI/KI mice develop many skeletal characteristics of human CMD, including hyperostotic mandibles and club-shaped femurs. Two Ank loss-of-function mouse models (Ankank/ank and Anknull/null mice) develop joint stiffness, cranial hyperostosis, and a narrowed foramen magnum (Ho et al., 2000; Gurley et al., 2006b), but miss certain CMD features like mandibular hyperostosis and flaring metaphyses of long bones. This phenotypic difference suggests that CMD mutations may cause more functional changes in ANK than purely loss of PPi transport and may indicate a novel role for ANK as a signaling protein.
Here we describe the dental abnormalities in AnkKI/KI mice and compare differences in tooth phenotype between AnkKI/KI mice and Anknull/null mice. We investigate whether the differences are due to reduced extracellular PPi (ePPi) levels or to CMD-specific effects of the Ank mutation on tooth development.
Materials & Methods
Mice
AnkKI/KI mice have previously been described (Chen et al., 2009). This CMD mouse model carries a deletion of TTC1130–1132 (phenylalanine 377) in exon 9 of Ank. AnkKI/KI mice were crossed with transgenic fluorescence reporter mice expressing Ibsp-Topaz/Dmp1-mCherry (Maye et al., 2009), a kind gift of Dr. Peter Maye. Anknull/null mice (Gurley et al., 2006a) were generously provided by Dr. David Kingsley and maintained in a mixed background of FVB and C57Bl/J6. Procedures were approved by the Animal Care Committee of the University of Connecticut Health Center.
Radiography, Micro-computed Tomography (micro-CT), and Scanning Electron Microscopy (SEM)
Radiographs of the mandibles were obtained with the use of a MX20 Radiography System (Faxitron X-Ray LLC, Lincolnshire, IL, USA). Micro-CT analysis was performed in mandibles of 1-, 2-, and 12-week-old mice at the Micro-CT facility at UCHC (mCT20; ScanCo Medical, Bassersdorf, Switzerland). For SEM imaging, mandibles of 10-week-old mice were sectioned at the sagittal plane, cleaned in 5% sodium hypochlorite, dehydrated in aqueous ethanol solutions, and observed under a TM1000 Tabletop Microscope (Hitachi High-Technologies Europe GmbH, Krefeld, Germany).
Eruption Rate of Incisors
A groove was made at the gingival margin of the lower incisors of 13-week-old Ank+/+, Ank+/KI, AnkKI/KI, and Anknull/null male mice, by means of a rotary tool (Lavelle, 1969). The distance between the groove and the gingival margin was measured 9 days later.
Dynamic Histomorphometry
Calcein (10 mg/kg body weight) and demeclocycline (50 mg/kg) were intraperitoneally injected into 13-week-old Ank+/+, Ank+/KI, AnkKI/KI, and Anknull/null male mice at seven-day intervals. Mice were sacrificed 2 days after the second injection. Newly mineralized dentin was examined in undecalcified frozen embedded sections. The distance between the apical end of the first label (calcein) and the second label (demeclocycline) in incisors was measured by AxioVision LE (Carl Zeiss MicroImaging, LLC, Thornwood, NY, USA).
Proliferation and Apoptosis Assay
Bromodeoxyuridine (BrdU; Sigma-Aldrich, St. Louis, MO, USA) was injected into seven-day-old Ank+/+, Ank+/KI, AnkKI/KI, and Anknull/null mice 1.5 hrs before their death. BrdU (Invitrogen Corporation, Carlsbad, CA, USA) and TUNEL staining (Terminal Transferase dUTP Nick End Labeling; Promega, Madison, WI, USA) was performed in sagittal sections of the lower incisors, and the number of labeled cells within dental epithelial and mesenchyme surrounding the cervical loop was quantified with Adobe Photoshop.
Incisor Organ Culture
Incisor tooth germs were dissected from the lower jaws of five-day-old mice. The incisors were then placed on Millicell culture plate inserts with a pore size of 0.4 µm (Millipore Corporation, Billerica, MA, USA). Incisors were cultured in DMEM with 10% FBS, 100 IU/mL penicillin, 100 mg/mL streptomycin, and 100 µg/mL ascorbic acid for 11 days. Images were taken on days 0, 3, 5, 7, and 11. Elongation of the dental epithelium was measured with ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Osteoclast Quantification
Sagittal paraffin sections of two-week-old Ank+/+, Ank+/KI, and AnkKI/KI mice were stained for tartrate-resistant acid phosphatase (TRAP) and analyzed with Osteomeasure software (OsteoMetrics, Decatur, GA, USA).
Bisphosphonate Treatment
Alendronate (Sigma-Aldrich, St. Louis, MO, USA) was injected intraperitoneally into Ank+/+ mice (1 µm/g of body weight). Injections were administered once a week for 5 wks starting in mice at the age of 1 wk. Mice were then sacrificed, and radiographs of the mandibles were taken.
Results
AnkKI/KI Mice Present Excessive Cementum Deposition in Molars and Incisors
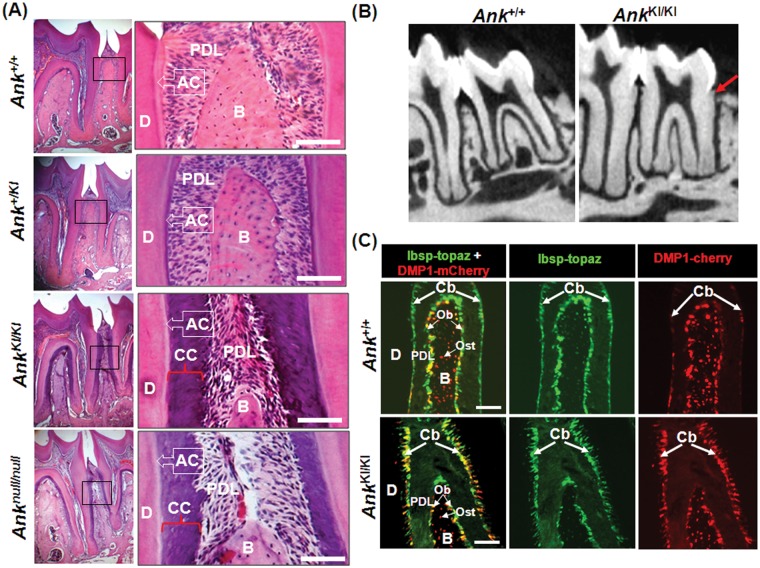
Histological evaluation by H&E staining shows excessive deposition of cementum in molars of AnkKI/KI mice as early as 2 wks of age with progressive thickening (Fig. 1A; Appendix Fig. 1). Excessive cementum is cellular (Appendix Fig. 2) in a region where a thin acellular cementum is normally found. Micro-CT images of AnkKI/KI molar roots indicate excessive mineralization, which overlaps the enamel at the cement-enamel junction (Fig. 1B). Incisors of AnkKI/KI mice show thicker cementum as well (Appendix Fig. 3). Heterozygous Ank+/KI molars present an intermediate phenotype of cementum thickness (Fig. 1A). Therefore, the cementum phenotype in AnkKI/KI mice is regulated by a time- and dose-dependent effect. Cementum deposition in AnkKI/KI mice resembles that of Anknull/null mice (Fig. 1A), suggesting that decreased ePPi levels may lead to this phenotype.
Figure 1.
Thicker cementum deposition in AnkKI/KI mice. (A) Representative sections of 1st and 2nd molars of 10-week-old Ank+/+, Ank+/KI, AnkKI/KI, and Anknull/null mice stained with H&E. (B) Micro-CT images of 12-week-old Ank+/+ and AnkKI/KI molars. Red arrow indicates excessive cementum deposition of molar roots of AnkKI/KI mice. (C) Increased Ibsp- and Dmp1-positive cells on root surfaces of AnkKI/KI molars (4-week-old Ank+/+ and AnkKI/KI /Ibsp-Topaz/Dmp1-mCherry mice). Dentin (D); acellular cementum (AC); periodontal ligament (PDL); alveolar bone (B); cellular cementum (CC); cementoblasts (Cb); osteoblasts (Ob); osteocytes (Ost). Scale bar = 100 µm.
It has been reported that BSP and DMP1 are markers for cementoblasts (D’Errico et al., 1997) and for cementocytes (Cao et al., 2012), respectively. Crossing of AnkKI/KI mice with reporter mice expressing Ibsp-Topaz/Dmp1-mCherry revealed increased numbers of Ibsp- and Dmp1-positive cells in the cementum of AnkKI/KI molars (Fig. 1C). This finding suggests that the Ank mutation may induce elevated DMP1 expression in cementoblasts, which is shown by increased numbers of BSP- and DMP1-double-positive cells on root surfaces and by increased numbers of cells with strong DMP1 label embedded in the cementum (Appendix Fig. 2).
Abnormal Incisor Development in AnkKI/KI Mice
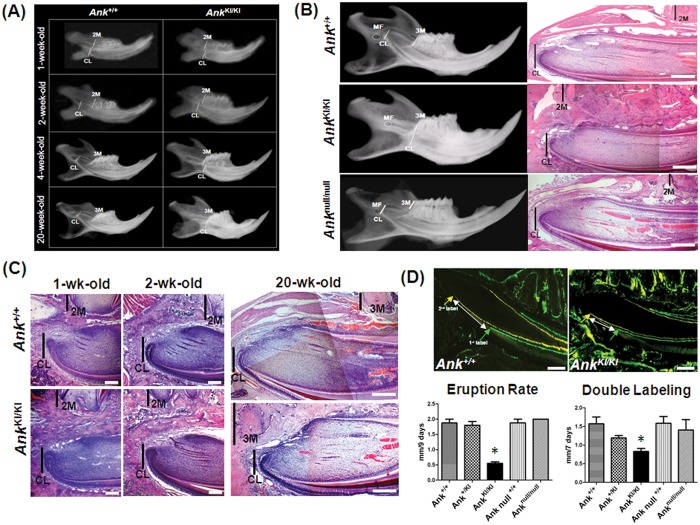
During normal mandibular incisor development, the apical end (cervical loop) moves backward from the anterior part of the mandible toward the mandibular foramen (Addison and Appleton, 1915). At post-natal day 5, the labial cervical loop of the lower incisor is located at the distal surface of the second molar in both Ank+/+ and AnkKI/KI mice shown by radiographs and histology (Appendix Fig. 4). However, in Ank+/+ mice, the apical end of the lower incisor begins to move posteriorly as early as post-natal day 7, while the cervical loop in AnkKI/KI mice remains close to the starting point (Figs. 2A, 2C). From 2 to 4 wks of age, the incisor in Ank+/+ mice elongates and the cervical loop almost reaches its final position anterior to the mandibular foramen, while the cervical loop fails to substantially change its position in AnkKI/KI mice (Fig. 2A). At 10 wks, the cervical loop in AnkKI/KI mice is still at the level of the third molar and does not move from this position as the mice get older (Figs. 2A-2C). The mandibular sizes of AnkKI/KI and Ank+/+ mice are comparable (Appendix Fig. 5). The development of upper incisors in AnkKI/KI mice is normal (data not shown).
Figure 2.
Short lower incisors, abnormal position of the cervical loop, and decreased eruption rate in AnkKI/KI mice. (A) Radiographs of mandibles from 1-, 2-, 4-, and 20-week-old Ank+/+ and AnkKI/KI mice. (B) Comparison of 10-week-old Ank+/+, AnkKI/KI, and Anknull/null incisors by x-ray imaging and histology (H&E). Scale bar = 200 µm. (C) Cervical loop sections of 1-, 2-, and 20-week-old Ank+/+ and AnkKI/KI mice (H&E). Scale bar = 200 µm. (D) Histomorphometry of incisors from 13-week-old Ank+/+ and AnkKI/KI mice. Arrows represent the beginning of the first (calcein; green) and second labels (demeclocycline; yellow), respectively. Histograms show eruption rates and distance between the first and second labels in Ank+/+ (n = 5), Ank+/KI (n = 5), AnkKI/KI (n = 5), and Anknull/null (n = 3) mice. *p < 0.005. Mandibular foramen (MF), distal root of the second molar (2M), root of the third molar (3M), position of the cervical loop (CL). Scale bar = 500 µm.
The cervical loop of the lower incisor in adult heterozygous Ank+/KI mice is positioned in an intermediate position between the cervical loop locations of Ank+/+ and AnkKI/KI mice (data not shown). Adult Anknull/null mice present normal cervical loop positions, suggesting that lack of PPi transport is not the primary cause of this incisor phenotype (Fig. 2C).
We next evaluated whether smaller incisors of the AnkKI/KI mouse differ in the rate of continuous eruption. We compared incisor eruption rate and dentin mineralization by double-labeling with calcein and demeclocycline of Ank+/+, Ank+/KI, AnkKI/KI, and Anknull/null mice. The eruption rate and the distance between the first and second labels were significantly decreased in AnkKI/KI mice in comparison with Ank+/+, Ank+/KI, and Anknull/null mice (Fig. 2D), suggesting a slower incisor growth rate in AnkKI/KI mice.
Decreased Proliferation of Odontoblast Precursors and Increased Cell Apoptosis in the Stellate Reticulum of the Incisor but Normal Elongation of the Dental Epithelium in AnkKI/KI Mice
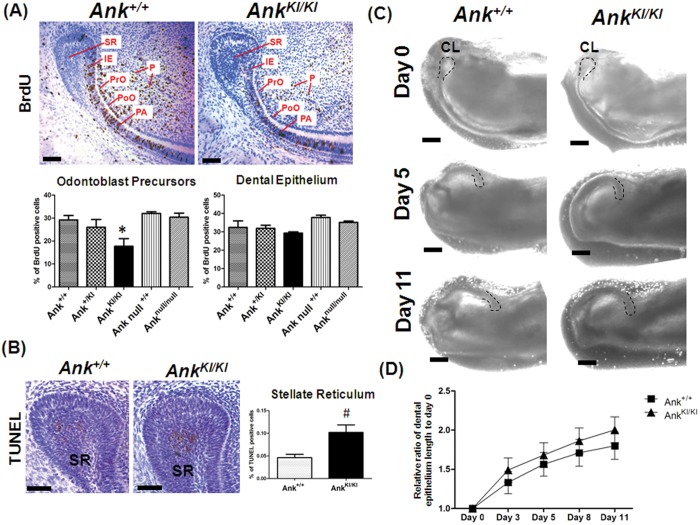
Tooth development involves proliferation of progenitor cells, cell differentiation, and matrix deposition. We hypothesized that CMD mutations in Ank may inhibit cell proliferation and therefore result in smaller incisors with cervical loops positioned anterior to the normal position. We examined cell proliferation by BrdU staining in post-natal day 7 Ank+/+, Ank+/KI, AnkKI/KI, and Anknull/null mice. We observed cells positive for BrdU in the epithelium (stellate reticulum, inner enamel epithelium, and pre-ameloblasts) and in the mesenchyme (pulp cells, pre-odontoblasts, and polarizing odontoblasts) (Fig. 3A). AnkKI/KI mice showed significantly decreased proliferation of odontoblast-lineage cells compared with Ank+/+, Ank+/KI, and Anknull/null mice. However, the number of proliferative cells of the epithelial lineage was not different (Fig. 3A). EdU (5-ethynyl-2′-deoxyuridine) quantification in 2-week-old Ank+/+ and AnkKI/KI mice showed reduced proliferation of odontoblast precursors and dental epithelial cells (Appendix Fig. 6). We detected apoptotic cells in the stellate reticulum in both Ank+/+ and AnkKI/KI mice, but not in other regions of the incisor (Fig. 3B). Quantification of TUNEL-positive cells revealed that AnkKI/KI mice have significantly increased cell apoptosis in the stellate reticulum compared with Ank+/+ mice (Fig. 3B).
Figure 3.
Decreased proliferation of odontoblast precursors and increased apoptosis in the stellate reticulum, but normal elongation of dental epithelium of AnkKI/KI lower incisors. (A) Sections of one-week-old Ank+/+ and AnkKI/KI cervical loops of incisors stained for BrdU. Graphs represent percentages of proliferative cells in the apical end of Ank+/+ (n = 3), Ank+/KI (n = 4), AnkKI/KI (n = 4), and Anknull/null (n = 3) mice. Odontoblast-lineage cells include pre-odontoblasts (PrO) and polarizing odontoblasts (PoO). Epithelial cells are stellate reticulum (SR), inner enamel epithelium (IE), and pre-ameloblasts (PA). *p < 0.05. Scale bar = 100 µm. (B) Sections of one-week-old Ank+/+ and AnkKI/KI cervical loops stained for TUNEL and percentage of apoptotic cells in the stellate reticulum (SR) of Ank+/+ (n = 5) and AnkKI/KI (n = 5) incisors. #p < 0.01. Scale bar = 50 µm. (C) Ank+/+ and AnkKI/KI incisor organ cultures. Dotted line indicates the location of cervical loop (CL). Scale bar = 200 µm. (D) Elongation rates of dental epithelium between Ank+/+ (n = 5) and AnkKI/KI (n = 5) incisor tooth germs are comparable.
We applied an organ culture system to determine whether the developmental defect is intrinsic to the cervical loop or secondary to defective bone resorption. Without the confinement of bone, the dental epithelium of Ank+/+ and AnkKI/KI cervical loops extended at a similar rate during 11 days of culture (Figs. 3C, 3D). Analysis of these data suggests that the ability for continuous elongation is not compromised in dental epithelium of AnkKI/KI incisors.
Reduced Mandibular Bone Remodeling in AnkKI/KI Mice
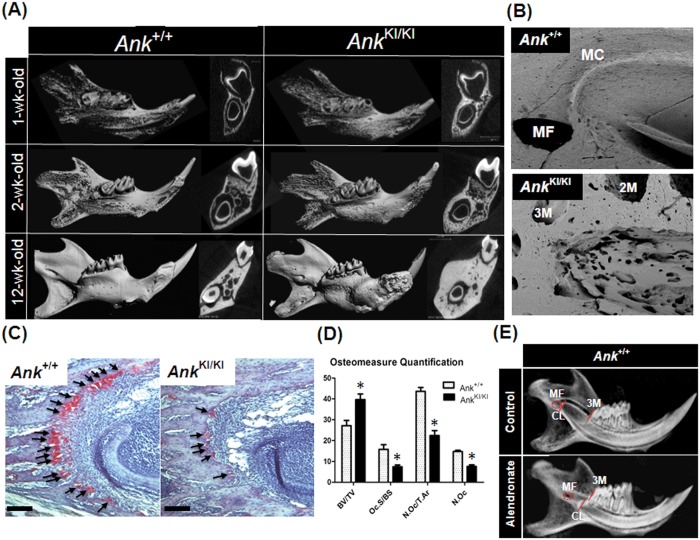
The remodeling of jawbones is harmonized with tooth development (Marks and Schroeder, 1996). We hypothesized that hyperostotic mandibles in AnkKI/KI mice can inhibit the apical elongation of mandibular incisors. We analyzed 1-, 2-, and 12-week-old Ank+/+ and AnkKI/KI mandibles by micro-CT and observed increased bone volume in AnkKI/KI mice as early as 1 wk of age, especially in the body of the mandible (Fig. 4A). Bone around the incisor progressively thickens, and bone marrow spaces in the mandible are markedly reduced as AnkKI/KI mice age (Fig. 4A). We next examined bone at the apical end of the incisor using scanning SEM. Ank+/+ mice had a smooth bone surface and well-defined delimitation between the mandibular canal and the apical end of the incisor (Fig. 4B). In contrast, SEM images of AnkKI/KI mice showed a rough and disorganized bone surface, suggesting abnormal bone remodeling of this region (Fig. 4B).
Figure 4.
Abnormal bone remodeling in mandibles of AnkKI/KI mice. (A) Micro-CT images show excessive bone volume and decreased bone marrow spaces in AnkKI/KI mandibles at 1, 2, and 12 wks. (B) SEM images of bone at the apical end of the incisor (10-week-old Ank+/+ and AnkKI/KI mice). (C) TRAP staining of two-week-old Ank+/+ and AnkKI/KI mandibles. Arrows indicate TRAP-positive cells. Scale bar = 100 µm. (D) Bone volume is increased and osteoclast number decreased in AnkKI/KI mice. [BV/TV (%): bone volume/total volume. Oc.S/BS (%): osteoclast surface/bone surface. N.Oc/T.Ar (n/mm2): number of osteoclasts/tissue area. N.Oc: number of osteoclasts.] Ank+/+ (n = 5), AnkKI/KI (n = 6). *p < 0.01. (E) Alendronate injections in Ank+/+ mice replicate the failure of cervical loop migration similar to the AnkKI/KI phenotype. Six-week-old control (no injection) and alendronate-treated Ank+/+ mice. Mandibular foramen (MF), mandibular canal (MC), root of the third molar (3M), distal root of the second molar (2M), position of the cervical loop (CL).
Previous studies demonstrated that osteoclasts from AnkKI/KI mice are smaller and resorb less bone in vitro (Chen et al., 2009). To study whether bone resorption was reduced and negatively affects the extension of cervical loops in AnkKI/KI mice, we analyzed the osteoclast numbers and surfaces in the apical region of lower incisors. Analysis of histological data from two-week-old mice showed TRAP-positive cells in the alveolar bone surrounding the cervical loop of Ank+/+ and AnkKI/KI incisors (Fig. 4C). However, osteoclast numbers and surfaces were decreased and the bone volume increased in the apical ends of the AnkKI/KI incisors (Fig. 4D).
To further assess the association between bone resorption and cervical loop positioning of the lower incisor, we inhibited the activity of osteoclasts in Ank+/+ mice by bisphosphonate (alendronate) injections. Treatment of Ank+/+ mice resulted in short lower incisors with incomplete cervical loop migration toward the mandibular foramen, similarly to AnkKI/KI mice (Fig. 4E). Analysis of these data suggests that abnormal positioning of cervical loops of AnkKI/KI mouse incisors is primarily due to decreased bone resorption in this region.
Discussion
We observed that a CMD-causing mutation in Ank results in excessive cementum deposition of molars and shorter lower incisors with abnormal positioning of the cervical loops. The cementum phenotype is shared by Ank loss-of-function mouse models (Ankank/ank and Anknull/null mice), but the lower incisor phenotype is unique to AnkKI/KI mice.
Increased cementum deposition has been described in mouse models with mutations in genes that lead to increased ratios of Pi/PPi in Ankank/ank and Anknull/null mice and Enpp1 (PC-1) null mice (PC-1 plasma cell membrane glycoprotein 1, an enzyme that generates extracellular and intracellular PPi by ATP hydrolysis). They present with a cementum phenotype similar to that of the AnkKI/KI mice, with a more than 10-fold cellular cementum increase compared with wild-type mice (Nociti et al., 2002; Popowics et al., 2005; Foster et al., 2011, 2012). In contrast, TNAP knock-out mice (TNAP hydrolyzes PPi to generate Pi) have a thinner acellular cementum (Beertsen et al., 1999; Foster et al., 2012). In vitro experiments suggested that cementoblasts, the cells that produce cementum, are sensitive to Pi (Foster et al., 2006; Rutherford et al., 2006). The similarity between AnkKI/KI mice and mouse models with decreased transport or generation of ePPi (Ankank/ank, Anknull/null, and PC-1null/null mice) suggests that the increased cementum formation is likely caused by a decrease of ePPi. Excessive cementum deposition has not been described in CMD patients, but excessive mineralization of the crowns of some primary teeth has been reported (Zhang et al., 2007). We are currently investigating tooth mineralization in a group of CMD patients.
Delayed tooth eruption has been reported in CMD patients (Hayashibara et al., 2000; Kornak et al., 2010). Rodent incisors are continuously growing, while molars have limited eruption, similar to human teeth. Both molars and incisors in AnkKI/KI mice can erupt comparably with those in Ank+/+ mice, but with a slower eruption rate of incisors. Sustained growth of incisors is maintained by proliferation of progenitor cells at the cervical loop. Our BrdU assay showed decreased proliferation of odontoblast precursors in one-week-old AnkKI/KI mice, but no difference in the proliferation of epithelial cells (cells that differentiate into ameloblasts) compared with Ank+/+ and Anknull/null mice. In addition, we observed increased cell apoptosis in the stellate reticulum of the incisors. The stellate reticulum is considered to be the stem cell niche of incisors (Harada et al., 1999). Interestingly, incisor organ cultures demonstrated that the capacity for continuous elongation is not compromised in AnkKI/KI dental epithelium. Analysis of our data, taken together, suggests that the ratio of some cell populations in the cervical loop of the AnkKI/KI incisors is altered due to abnormal proliferation and apoptosis rates and other extrinsic factors, such as insufficient bone resorption at the apical end of the incisor, which may contribute to the lack of incisor elongation.
AnkKI/KI bone-marrow-derived monocytes (BMM) cultured with M-CSF and RANKL show less osteoclast formation and reduced resorption on bone chips as well as on calcium-phosphate-coated plates (Chen et al., 2009). Therefore, insufficient bone resorption in AnkKI/KI mice may play a major role in the abnormal dental phenotype, specifically in preventing migration of the cervical loop and slowing tooth eruption. Bisphosphonate-treated Ank+/+ mice also had shorter lower incisors, suggesting a strong correlation between reduced bone resorption and abnormal position of the cervical loop. The mechanism for reduced bone resorption in AnkKI/KI mice still needs to be investigated.
Supplementary Material
Footnotes
This work was supported by R01-DE019458 (NIH/NIDCR) to EJR and T32-DE007302 (NIH/NIDCR) to EHD.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Addison WH, Appleton JL. (1915). The structure and growth of the incisor teeth of the albino rat. J Morphol 26:43-96. [Google Scholar]
- Beertsen W, VandenBos T, Everts V. (1999). Root development in mice lacking functional tissue non-specific alkaline phosphatase gene: inhibition of acellular cementum formation. J Dent Res 78:1221-1229. [DOI] [PubMed] [Google Scholar]
- Cao Z, Zhang H, Zhou X, Han X, Ren Y, Gao T, et al. (2012). Genetic evidence for the vital function of osterix in cementogenesis. J Bone Miner Res 27:1080-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen IP, Wang CJ, Strecker S, Koczon-Jaremko B, Boskey A, Reichenberger EJ. (2009). Introduction of a Phe377del mutation in ANK creates a mouse model for craniometaphyseal dysplasia. J Bone Miner Res 24:1206-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Errico JA, Macneil RL, Takata T, Berry J, Strayhorn C, Somerman MJ. (1997). Expression of bone associated markers by tooth root lining cells, in situ and in vitro. Bone 20:117-126. [DOI] [PubMed] [Google Scholar]
- Foster BL, Nociti FH, Swanson EC, Matsa-Dunn D, Berry JE, Cupp CJ, et al. (2006). Regulation of cementoblast gene expression by inorganic phosphate in vitro. Calcif Tissue Int 78:103-112. [DOI] [PubMed] [Google Scholar]
- Foster BL, Nagatomo KJ, Bamashmous SO, Tompkins KA, Fong H, Dunn D, et al. (2011). The progressive ankylosis protein regulates cementum apposition and extracellular matrix composition. Cells Tissues Organs 194:382-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster BL, Nagatomo KJ, Nociti FH, Fong H, Dunn D, Tran AB, et al. (2012). Central role of pyrophosphate in acellular cementum formation. PLoS ONE 7:e38393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurley KA, Chen H, Guenther C, Nguyen ET, Rountree RB, Schoor M, et al. (2006a). Mineral formation in joints caused by complete or joint-specific loss of ANK function. J Bone Miner Res 21:1238-1247. [DOI] [PubMed] [Google Scholar]
- Gurley KA, Reimer RJ, Kingsley DM. (2006b). Biochemical and genetic analysis of ANK in arthritis and bone disease. Am J Hum Genet 79:1017-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada H, Kettunen PI, Jung HS, Mustonen T, Wang YA, Thesleff I. (1999). Localization of putative stem cells in dental epithelium and their association with Notch and Fgf signaling. J Cell Biol 147:105-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashibara T, Komura T, Sobue S, Ooshima T. (2000). Tooth eruption in a patient with craniometaphyseal dysplasia: case report: J Oral Pathol Med 29:460-462. [DOI] [PubMed] [Google Scholar]
- Ho AM, Johnson MD, Kingsley DM. (2000). Role of the mouse ank gene in control of tissue calcification and arthritis. Science 289:265-270. [DOI] [PubMed] [Google Scholar]
- Kornak U, Brancati F, Merrer ML, Lichtenbelt K, Höhne W, Tinschert S, et al. (2010). Three novel mutations in the ANK membrane protein cause craniometaphyseal dysplasia with variable conductive hearing loss. Am J Med Genet Part A 152A:870-874. [DOI] [PubMed] [Google Scholar]
- Lavelle CL. (1969). The effect of age on the eruption rate of the incisor teeth of the rat (Rattus norvegicus). J Anat 104(Pt 1):109-116. [PMC free article] [PubMed] [Google Scholar]
- Marks SC, Schroeder HE. (1996). Tooth eruption: theories and facts. Anat Rec 245:374-393. [DOI] [PubMed] [Google Scholar]
- Maye P, Stover ML, Liu Y, Rowe DW, Gong S, Lichtler AC. (2009). A BAC-bacterial recombination method to generate physically linked multiple gene reporter DNA constructs. BMC Biotechnol 9:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nociti FH, Berry JE, Foster BL, Gurley KA, Kingsley DM, Takata T, et al. (2002). Cementum: a phosphate-sensitive tissue. J Dent Res 81:817-821. [DOI] [PubMed] [Google Scholar]
- Nurnberg P, Thiele H, Chandler D, Hohne W, Cunningham ML, Ritter H, et al. (2001). Heterozygous mutations in ANKH, the human ortholog of the mouse progressive ankylosis gene, result in craniometaphyseal dysplasia. Nat Genet 28:37-41. [DOI] [PubMed] [Google Scholar]
- Popowics T, Foster BL, Swanson EC, Fong H, Somerman MJ. (2005). Defining the roots of cementum formation. Cells Tissues Organs 181:248-257. [DOI] [PubMed] [Google Scholar]
- Reichenberger E, Tiziani V, Watanabe S, Park L, Ueki Y, Santanna C, et al. (2001). Autosomal dominant craniometaphyseal dysplasia is caused by mutations in the transmembrane protein ANK. Am J Hum Genet 68:1321-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford RB, Foster BL, Bammler T, Beyer RP, Sato S, Somerman MJ. (2006). Extracellular phosphate alters cementoblast gene expression. J Dent Res 85:505-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajac A, Baek SH, Salhab I, Radecki MA, Kim S, Hakonarson H, et al. (2010). Novel ANKH mutation in a patient with sporadic craniometaphyseal dysplasia. Am J Med Genet Part A 152A:770-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Somerman MJ, Berg J, Cunningham ML, Williams B. (2007). Dental anomalies in a child with craniometaphysial dysplasia. Pediatr Dent 29:415-419. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.