Abstract
Dental caries, one of the most prevalent infectious diseases worldwide, affects approximately 80% of children and the majority of adults. Dental caries may result in endodontic disease, leading to dental pulp necrosis, periapical inflammation and bone resorption, severe pain, and tooth loss. Periapical inflammation may also increase inflammation in other parts of the body. Although many studies have attempted to develop therapies for this disease, there is still an urgent need for effective treatments. In this study, we applied a novel gene therapeutic approach using recombinant adeno-associated virus (AAV)-mediated RNAi knockdown of Cathepsin K (Ctsk) gene expression, to target osteoclasts and periapical bone resorption in a mouse model. We found that AAV-sh-Cathepsin K (AAV-sh-Ctsk) impaired osteoclast function in vivo and furthermore reduced bacterial infection-stimulated bone resorption by 88%. Reduced periapical lesion size was accompanied by decreases in mononuclear leukocyte infiltration and inflammatory cytokine expression. Our study shows that AAV-RNAi silencing of Cathepsin K in periapical tissues can significantly reduce endodontic disease development, bone destruction, and inflammation in the periapical lesion. This is the first demonstration that AAV-mediated RNAi knockdown gene therapy may significantly reduce the severity of endodontic disease.
Keywords: endodontic disease, inflammation, bone resorption, dental caries, osteoclast, gene therapy
Introduction
As infection in pulp tissue spreads throughout the root canal system toward the apical foramen and into the periodontal ligament (PDL), it inevitably leads to periapical bone resorption (Kakehashi et al., 1965; Nair, 1997; Stashenko, 1990). Endodontic therapy in the clinical setting removes necrotic infected pulp tissue and prevents this local inflammatory reaction. This treatment has a high degree of success (approximately 80%), but even when the highest standards are followed, endodontic failures still occur, and a complete bone healing or reduction of the apical lesion may not occur in all root-canal-treated teeth (Nair, 2004). It is known that osteoclasts function as the primary cell that mediates periapical bone resorption (Nair, 1997, 2004). It has also been established that receptor activator of nuclear factor-κβ ligand (RANKL), which stimulates osteoclast differentiation, is expressed by human dental pulp cells (Kojima et al., 2006; Yamaguchi et al., 2006; Uchiyama et al., 2009). In addition, T- and B-cells may express RANKL (Kawai et al., 2006; Lin et al., 2011), although the contributions of these cell types to the stimulation of osteoclastogenesis and activation of osteoclasts in periapical bone resorption have yet to be determined.
Cathepsin K (Ctsk) is a lysosomal cysteine protease of the peptidase C1 protein gene family. It is strongly expressed by osteoclasts and plays an essential role in osteoclast function and the degradation of protein components of the bone matrix. Cathepsin K has also been shown to be involved in the regulation of Toll-like receptor 9 signaling in dendritic cells (Asagiri et al., 2008). Mutations in Cathepsin K result in the human syndrome pycnodysostosis, in which bone resorption is impaired (Gelb et al., 1996).
The aim of the current investigation was to determine conclusively the therapeutic potential of silencing Cathepsin K in vivo using adeno-associated virus (AAV)-sh-Cathepsin K (AAV-sh-Ctsk), to reduce endodontic disease progression, bone resorption, and inflammation in periapical lesions in a well-established mouse model. In addition, the current study aimed to evaluate AAV as a viable strategy for successful RNAi gene delivery. The AAV silencing approach is a relatively new and effective tool that has been proven successful in humans in a clinical setting (Carter, 2005). In addition, this approach is safe and well-tolerated by patients with advanced Parkinson’s disease, suggesting that in vivo gene therapy is practical and causes only a very mild immune response to the AAV vector (Kaplitt et al., 2007). Therefore, in this study we used the AAV RNAi knockdown system to investigate the therapeutic potential of Cathepsin K silencing, due to its unique attributes as described.
Materials & Methods
For complete Materials & Methods, please see the online Appendix.
Animals
Eighty-four six-week-old female WT BALB/cJ mice, purchased from the Jackson Laboratory (Bar Harbor, ME, USA), were used in this study. All experimental protocols were approved by the University of Alabama at Birmingham (UAB) Institutional Animal Care and Use Committee (Animal Protocol Number 11090909236). This research conforms to the Animal Research: Reporting In Vivo Experiments (ARRIVE) guidelines. Animals were sacrificed by CO2 inhalation on day 42 after pulpal infection, and the mandibles were removed and prepared for analysis.
Cells and Cell Culture
Pre-osteoclasts and mature osteoclasts in primary culture were generated from mouse bone marrow (MBM) as previously described (Yang and Li, 2007; Feng et al., 2009).
Design and Construction of shRNA
Using the Dharmacon siDESIGN centre (http://www.dharmacon.com) as described (Feng et al., 2009), we generated shRNA that would target Cathepsin K. As a control vector, we used the yellow fluorescent protein (YFP) expressing AAV-H1-shRNA-luc-YFP (gift from Dr. Sonoko Ogawa, University of Tsukuba, Japan), which contains a luciferase-specific shRNA and a YFP cassette (Musatov et al., 2006; Alexander et al., 2010).
AAV-shRNA Viral Production and Purification
We used the AAV2 serotype and subcloned shRNA targeting Cathepsin K into the AAV-H1 vector (Musatov et al., 2006). The AAV Helper-Free System (AAV Helper-Free System Catalog #240071, Stratagene, La Jolla, CA, USA) was used for viral production with a triple-transfection, helper-free method, and was purified according to a modified published protocol (Hommel et al., 2003).
Bacterial Infection and Transduction of AAV Vectors
Bacterial culture and infection procedure protocols were conducted as described (Sasaki et al., 2000). In brief, exposed dental pulps of mandibular first molars were infected with a mixture of 4 common human endodontic pathogens, including Prevotella intermedia [American Type Culture Collection (ATCC) 25611; Manassas, VA, USA], Fusobacterium nucleatum (ATCC 25586), Peptostreptococcus micros (ATCC 33270), and Streptococcus intermedius (ATCC 27335). The dental pulps of the mandibular first molars were exposed as described (Stashenko, 1990). Transduction consisted of injecting the viral vectors and PBS in a site-specific manner as described, with modifications (Musatov et al., 2006). Briefly, on day 1 and day 3 after pulpal infection, the mice were anesthetized via peritoneal injection of ketamine and xylazine. The right and left mandibular first molars were the sites of local injection of the vectors into the periapical tissues.
Data Quantification and Statistical Analyses
Experimental data are reported as mean ± SD of triplicate independent samples. The Figs. are representative of the data (n = 21 samples). Data were analyzed with the two-tailed Student’s t test. The p values < 0.05 were considered significant.
Results
Inhibitory Effect of AAV-sh-Cathepsin K Knockdown on Expression of Cathepsin K and Osteoclast-mediated Bone Resorption in vitro
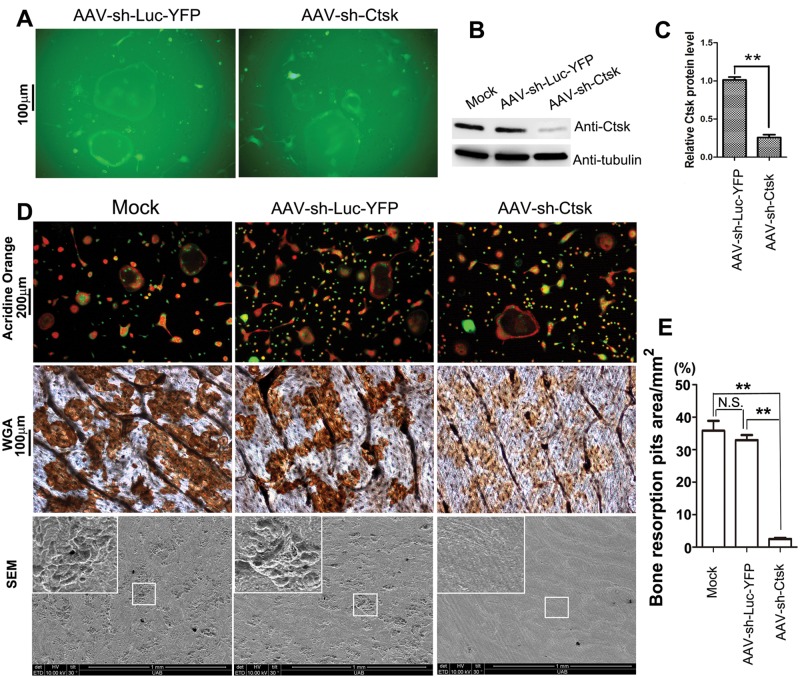
To evaluate the effect of inhibition of Cathepsin K on bone resorption, we generated shRNA that targeted the expression of this enzyme. Cell fluorescence indicated that efficient transduction of pre-osteoclasts and osteoclasts with AAV-sh-Cathepsin K (AAV-sh-Ctsk) or AAV-sh-luc-YFP was achieved with a titer of approximately 6×1011 DNase-resistant particles (DRP)/mL (Fig. 1A). To confirm the effect of AAV-sh-Cathepsin K on gene silencing, we performed Western blot analysis, and found that osteoclasts transduced with AAV-sh-Cathepsin K had an approximately 80% reduction in Cathepsin K expression compared with osteoclasts transduced with AAV-sh-luc-YFP (control for shRNA and AAV) (Figs. 1B, 1C). These results indicate that AAV-sh-Cathepsin K targets Cathepsin K mRNA efficiently and reduces Cathepsin K protein expression.
Figure 1.
AAV-sh-Cathepsin K efficiently knocked down expression of Cathepsin K and impaired osteoclast-mediated bone resorption in vitro. (A) Immunofluorescent photomicrograph of AAV-sh-luc-YFP and AAV-sh-Cathepsin K treatment groups 7 days after transduction. The experiments were performed in triplicate. (B) Western blot of Cathepsin K expression in murine bone marrow cells stimulated with M-CSF/RANKL for 3 days to induce differentiation of osteoclasts, that were then transduced with no vector (mock), AAV-sh-luc-YFP (control vector), or AAV-sh-Cathepsin K. (C) Quantification of Western blot analysis demonstrates that the AAV-sh-Cathepsin K treatment group significantly reduced expression of Cathepsin K as compared with the AAV-sh-luc-YFP treatment group. (D) Untransduced osteoclasts (Mock) and osteoclasts transduced with AAV-sh-luc-YFP (control) or AAV-sh-luc-Cathepsin K stained with acridine orange to show extracellular acidification (top panel). Resorption pits on bone slices were visualized by WGA (middle panel) and scanning electron microscopy (SEM) (bottom panel). The experiment was performed in duplicate on 4 independent occasions. (E) Quantification of resorption pits on bone slices was significantly lower in the AAV-sh-Cathepsin K treatment group compared with the Mock and AAV-sh-luc-YFP groups. All assays were performed in triplicate; a representative photomicrograph from each assay is shown. N.S., Not Significant. **p < 0.01.
To establish the effect of AAV-mediated Cathepsin K knockdown on osteoclast function in vitro, we analyzed bone resorption using wheat germ agglutinin (WGA) and scanning electron microscopy (SEM) to visualize resorption pits on bone (Fig. 1D, middle and bottom panels). As shown, AAV-mediated knockdown of Cathepsin K was highly effective in impairing bone resorption compared with the control group (p < 0.005; Fig. 1E). We also performed acridine orange staining, which indicates extracellular matrix acidification. As expected, we found that osteoclasts transduced with AAV-sh-Cathepsin K showed no difference in extracellular acidification compared with osteoclasts transduced with no vector (mock group) or with AAV-sh-luc-YFP (Fig. 1D, top panel), demonstrating the specificity of the Cathepsin K inhibition.
AAV-sh-Cathepsin K Effectively Transduces Periapical Tissue in vivo and Protects Mice from Bone Loss Due to Endodontic Infection
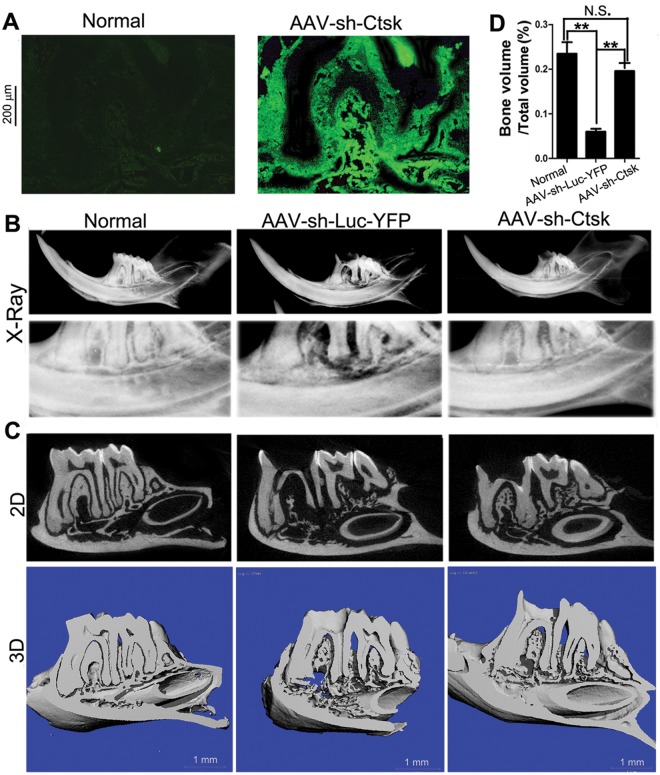
Fluorescent analysis of the infected mice treated with AAV-sh-Cathepsin K compared with that of the uninfected control (Normal) group demonstrated that the AAV vectors successfully infiltrated the periapical tissues (Fig. 2A). To determine the efficacy of AAV-sh-Cathepsin K in reducing the extent of endodontic disease, we used a model of periapical lesion induction as described (Sasaki et al., 2000). Exposed mandibular first molar dental pulps were infected with a mixture of 4 common endodontic pathogens: Prevotella intermedia, Fusobacterium nucleatum, Peptostreptococcus micros, and Streptococcus intermedius. Radiographic imaging revealed that the infected group treated with the control AAV-sh-luc-YFP had significant periapical bone resorption surrounding the distal root of the mandibular first molar compared with the uninfected control (Normal) group. In contrast, the AAV-sh-Cathepsin K treatment group showed minimal bone resorption, which was comparable with that in the Normal group (Fig. 2B). This was confirmed by detailed microCT analysis of Bone Volume (BV)/Total Volume (TV) ratios of the periapical area surrounding the distal root of the first molars, which showed that infected mice treated with AAV-sh-Cathepsin K had an 88% reduction in infection-stimulated bone resorption (Fig. 2D), compared with infected mice treated with the AAV-sh-luc-YFP control vector (Fig. 2C).
Figure 2.
AAV effectively transduced endodontic tissue in vivo and protected mice from bone loss due to endodontic infection. (A) Fluorescent micrographs of eGFP expression by AAV-infected cells in uninfected (Normal) mice and infected mice treated with AAV-sh-Cathepsin K. The experiments were performed in triplicate. (B) Radiographic imaging of the crown and distal root of the mandibular first molar in uninfected mice (Normal), infected mice treated with AAV-sh-luc-YFP, and infected mice treated with AAV-sh-luc-Cathepsin K. Severe periapical bone loss is visible in the AAV-sh-luc-YFP image compared with the minimal bone loss in the AAV-sh-Cathepsin K image (n = 21). (C) µCT analysis of the mandibular first molar in uninfected mice (Normal), infected mice treated with AAV-sh-luc-YFP, and infected mice treated with AAV-sh-Cathepsin K. Severe periapical bone loss is visible in the AAV-sh-luc-YFP image compared with the minimal bone loss in the AAV-sh-Cathepsin K image (n = 21). (D) Quantification of Bone Volume (BV)/Total Volume (TV) from uninfected (Normal) mice, infected mice treated with AAV-sh-luc-YFP, and infected mice treated with AAV-sh-Cathepsin K. N.S., Not Significant. **p < 0.01 (n = 21).
AAV-sh-Cathepsin K Decreases Mononuclear Leukocytes and T-cells in Periapical Tissues
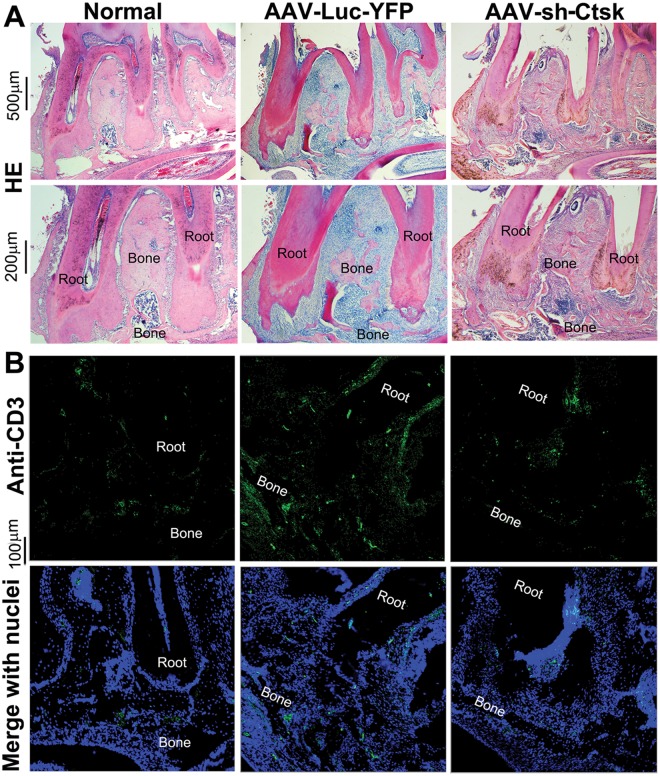
Hematoxylin and eosin (H&E) staining indicated that periapical tissue sections from infected mice treated with AAV-sh-Cathepsin K had significantly fewer mononuclear leukocytes, as indicated by nuclear morphology, compared with infected mice treated with AAV-sh-luc-YFP (34 ± 10 vs. 112 ± 8 per section, respectively; p < 0.001) or uninfected controls (17 ± 2) (Fig. 3A). Immunofluorescence staining revealed that the number of CD3+-expressing T-cells in periapical tissues showed a parallel reduction in the AAV-sh-Cathepsin K treatment group compared with the AAV-sh-luc-YFP group (39 ± 9 vs. 93 ± 8; p < 0.001) or uninfected controls (27 ± 2), indicating that AAV-sh-Cathepsin K also reduced T-cells in vivo (Fig. 3B). These results indicate that, in addition to inhibiting bone resorption, AAV-sh-Cathepsin K had the collateral effect of reducing inflammatory cell infiltration.
Figure 3.
AAV-sh-Cathepsin K significantly decreased cell infiltration and bone resorption in periapical tissues. (A) Hematoxylin and eosin (H&E) staining of sections from untreated, uninfected mice (Normal), infected mice treated with AAV-sh-luc-YFP (Control), and infected mice treated with AAV-sh-Cathepsin K. Bone resorption and mononuclear cell infiltration was significantly decreased in the AAV-sh-Cathepsin K treatment group compared with the control AAV-sh-luc-YFP group (n = 21). (B) Immunofluorescence staining of periapical lesion sections indicates that uninfected mice (Normal) and infected mice treated with AAV-sh-Cathepsin K have fewer CD3-positive (green) T-cells compared with infected mice treated with AAV-sh-luc-YFP. Cell nuclei were labeled with DAPI DNA stain (blue). The experiments were performed in triplicate.
AAV-sh-Cathepsin K Reduces Expression of Inflammatory Mediators in Periapical Tissues
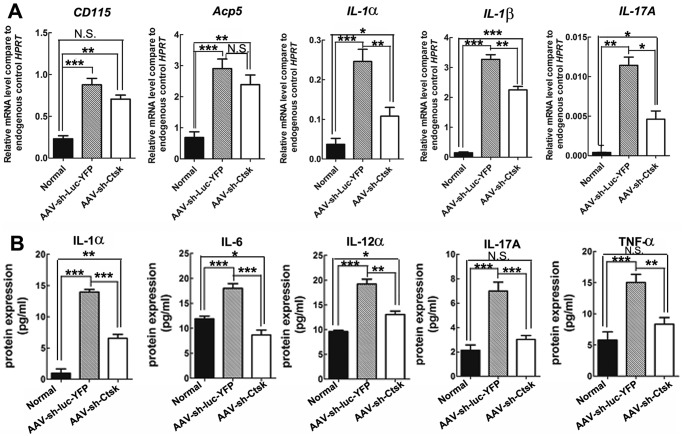
Investigation of the inflammation status of periapical tissues by qRT-PCR analysis revealed that the AAV-sh-Cathepsin K treatment group had significantly lower mRNA expression of the inflammatory mediators Interleukin-1α (IL-1α), Interleukin-1β (IL-1β), and Interleukin-17α (IL-17α) compared with the AAV-sh-luc-YFP group (p < 0.05) (Fig. 4A). The expression of tartrate-resistant acid phosphatase 5 (Acp5), which is an established marker of osteoclasts in vivo, and the macrophage marker CD115 (M-CSF receptor) was also significantly lower (p < 0.01 for Acp5; p < 0.05 for CD115) in the AAV-sh-Cathepsin K treatment group, albeit they were not as dramatically reduced, indicating that osteoclasts were present in lesions in all infected mice (Fig. 4A). To confirm the effect of Cathepsin K silencing on inflammatory cytokines at the protein level, we performed enzyme-linked immunosorbent assays (ELISA). The results showed that both infected groups had elevated levels of IL-1α, Interleukin-12α (IL-12α), IL-17α, and tumor necrosis factor-α (TNF-α), compared with uninfected controls. However, in all cases, the levels of these mediators were lower in the AAV-sh-Cathepsin K treatment group compared with the AAV-sh-luc-YFP group (Fig. 4B).
Figure 4.
AAV-sh-Cathepsin K reduced the expression of inflammatory mediators in periapical tissues. (A) qRT-PCR results for osteoclast differentiation marker (CD115 Acp5) and inflammatory mediator (IL-1α, IL-1β, IL-17α) gene expression, in the periapical tissues of normal uninfected mice and infected mice treated with AAV-sh-luc-YFP or with AAV-sh-Cathepsin K. Expression levels were normalized to the housekeeping gene hypoxanthine-guanine phosphoribosyl transferase (Hprt) (n = 21). (B) Protein levels of IL-1α, IL-6, IL-12α, IL-17α, and TNF-α in periapical lesions as detected by ELISA. N.S., Not Significant. *p < 0.05; **p < 0.01; ***p < 0.005 (n = 21).
Discussion
Endodontic therapy in the clinical setting is directed toward the removal of necrotic, infected pulp tissue, to inhibit the local inflammatory reaction in periapical tissues, thereby permitting the formation of reparative bone. Bone healing, even in cases of successful treatment, takes 6 mos or longer; hence an earlier reversal of the resorptive process would be important for the resolution of the periapical lesion. In this study, we tested a novel gene therapy approach to this problem using AAV2 as a vector to knock down the osteoclast-expressed enzyme Cathepsin K, to reduce bone resorption induced by pulpal infection. Our results demonstrated that the AAV vector efficiently suppressed Cathepsin K and reduced osteoclast bone resorption in vitro, but did not affect extracellular acidification by these cells. Remarkably, transduction of periapical lesions with AAV-sh-Cathepsin K profoundly reduced infection-stimulated bone resorption by 88% in vivo. The reduction in bone resorption and lesion size resulted in fewer infiltrating mononuclear cells, including T-cells, and lowered expression of inflammatory mediators. This study is the first to report the successful use of AAV-mediated gene knockdown as a gene therapy strategy for periapical inflammatory disease. Since patients with endodontic disease suffer from both inflammation-induced tissue damage and bone loss, gene therapy against a single target that can dramatically inhibit both disease manifestations may have potential as a therapeutic approach in humans.
Gene therapy with AAV vectors has previously been reported in models of periodontal disease, which, like endodontic disease, have a microbial etiology. Systemic transduction with TIMP-4 was reported to reduce both adjuvant-induced arthritis and periodontitis in rats (Ramamurthy et al., 2005). Inhibition of periodontal bone loss was also reported following AAV gene transfer of mitogen-activated protein kinase phosphatase-1 (MKP-1), which dephosphorylates MAPKs and inhibits immune responses (Yu et al., 2011). Transduction of gingival tissues with AAV/2/1-TNF Receptor: Fc reduced P. gingivalis-induced periodontal bone loss by approximately 50% in a mouse model, with parallel reductions in pro-inflammatory cytokines and osteoclasts (Cirelli et al., 2009). In the latter study, the AAV-TNFR:Fc vector was delivered 3 times per wk for 8 wks, compared with 2 injections of AAV-sh-Cathepsin K in our protocol. The greater efficiency of cathepsin K inhibition (88%) may reflect the critical importance of this enzyme for osteoclast function, given that humans and mice with cathepsin K deficiency are osteopetrotic (‘pycnodysostosis’), with highly compromised resorption due to an inability to degrade the collagen matrix of bone (Gelb et al., 1996; Chen et al., 2007). Thus, the direct targeting of osteoclasts may be the most effective strategy for gene therapy aimed at inhibiting bone loss. Another factor in the efficiency in the endodontic vs. periodontal models may be the better localization of the AAV vector to the confines of the periapical lesion vs. the gingiva, which furthermore requires delivery at multiple sites. No systemic effects were observed in mice following treatment with AAV-sh-Cathepsin K. Furthermore, osteoclasts transduced with AAV-sh-Cathepsin K showed no difference in extracellular acidification compared with osteoclasts transduced with no vector (mock group) or with AAV-sh-luc-YFP, demonstrating the specificity of the Cathepsin K inhibition.
In a non-oral infection model, AAV-mediated gene transfer of IL-10 reduced airway inflammation in mice infected with Pseudomonas aeruginosa, the major pathogen in cystic fibrosis (Buff et al., 2010). In bone resorption models, osteoprotegerin (OPG) gene transfer attenuated osteoclast number and activity in debris-induced osteolysis (Ulrich-Vinther, 2007) and reduced aseptic joint prosthesis loosening (Zhang et al., 2010). Collagen-induced arthritis and inflammatory responses were attenuated by AAV-IL-4 (Cottard et al., 2000). In general, the direct targeting of osteoclasts by AAV-sh-Cathepsin K appears to be both more specific and more efficient in reducing inflammatory bone resorption, compared with indirect approaches that target inflammatory mediators and indirectly regulate osteoclasts.
Osteoclast markers including Acp5 were reduced by AAV-sh-Cathepsin K, as was CD115, a marker for macrophages and osteoclast precursors. Similarly, all cytokines examined showed reduced levels in periapical tissues in AAV-sh-Cathepsin K-treated animals. These mediators (IL-1α, IL-1β, IL-17α) have all been previously identified in periapical lesion tissues (Stashenko, 1990; Wang and Stashenko, 1993; Kamolmatyakul et al., 2004; Oseko et al., 2009). Since the block sections taken for analysis included bone, tooth roots, and periapical tissues, it is likely that the reduced lesion size in the AAV-sh-Cathepsin K group accounts for the observed reductions in both osteoclasts and cytokines. Alternatively, recent evidence suggests that cathepsin K may regulate immune and inflammatory responses, in part via Toll-like receptor 9 signaling (Asagiri et al., 2008). Additional studies are needed to distinguish between these possibilities.
In summary, our findings demonstrate that inhibition of osteoclast function via AAV-mediated gene therapy may be a viable therapeutic approach to the resolution of inflammatory bone loss in endodontic diseases.
Supplementary Material
Acknowledgments
We thank Ms. Christie Paulson and Mr. Zach Nolen for their excellent assistance with the manuscript. We also thank Drs. Sergei Musatov and Sonoko Ogawa for kindly providing the AAV-H1-shRNA-luc-YFP and AAV-H1 vectors and for helpful suggestions. We appreciate the assistance of the Center for Metabolic Bone Disease at the University of Alabama at Birmingham (P30 AR046031). We are also grateful for the assistance of the Small Animal Phenotyping Core, Metabolism Core, and Neuroscience Molecular Detection Core Laboratory at the University of Alabama at Birmingham (P30 NS0474666).
Footnotes
This work was supported by NIH grant RC1-DE-020533-01 (Y.P.L.).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Alexander B, Warner-Schmidt J, Eriksson T, Tamminga C, Arango-Lievano M, Ghose S, et al. (2010). Reversal of depressed behaviors in mice by p11 gene therapy in the nucleus accumbens. Sci Transl Med 2:54ra76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asagiri M, Hirai T, Kunigami T, Kamano S, Gober HJ, Okamoto K, et al. (2008). Cathepsin K-dependent Toll-like receptor 9 signaling revealed in experimental arthritis. Science 319:624-627. [DOI] [PubMed] [Google Scholar]
- Buff SM, Yu H, McCall JN, Caldwell SM, Ferkol TW, Flotte TR, et al. (2010). IL-10 delivery by AAV5 vector attenuates inflammation in mice with Pseudomonas pneumonia. Gene Ther 17:567-576. [DOI] [PubMed] [Google Scholar]
- Carter BJ. (2005). Adeno-associated virus vectors in clinical trials. Hum Gene Ther 16:541-550. [DOI] [PubMed] [Google Scholar]
- Chen W, Yang S, Abe Y, Li M, Wang Y, Shao J, et al. (2007). Novel pycnodysostosis mouse model uncovers cathepsin K function as a potential regulator of osteoclast apoptosis and senescence. Hum Mol Genet 16:410-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirelli JA, Park CH, MacKool K, Taba M, Lustig KH, Burstein H, et al. (2009). AAV2/1-TNFR:Fc gene delivery prevents periodontal disease progression. Gene Ther 16:426-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottard V, Mulleman D, Bouille P, Mezzina M, Boissier MC, Bessis N. (2000). Adeno-associated virus-mediated delivery of IL-4 prevents collagen-induced arthritis. Gene Ther 7:1930-1939. [DOI] [PubMed] [Google Scholar]
- Feng S, Deng L, Chen W, Shao J, Xu G, Li YP. (2009). Atp6v1c1 is an essential component of the osteoclast proton pump and in F-actin ring formation in osteoclasts. Biochem J 417:195-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelb BD, Shi GP, Chapman HA, Desnick RJ. (1996). Pycnodysostosis, a lysosomal disease caused by cathepsin K deficiency. Science 273:1236-1238. [DOI] [PubMed] [Google Scholar]
- Hommel JD, Sears RM, Georgescu D, Simmons DL, DiLeone RJ. (2003). Local gene knockdown in the brain using viral-mediated RNA interference. Nat Med 9:1539-1544. [DOI] [PubMed] [Google Scholar]
- Kakehashi S, Stanley HR, Fitzgerald RJ. (1965). The effects of surgical exposures of dental pulps in germ-free and conventional laboratory rats. Oral Surg Oral Med Oral Pathol 20:340-349. [DOI] [PubMed] [Google Scholar]
- Kamolmatyakul S, Chen W, Yang S, Abe Y, Moroi R, Ashique AM, et al. (2004). IL-1alpha stimulates cathepsin K expression in osteoclasts via the tyrosine kinase-NF-kappaB pathway. J Dent Res 83:791-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplitt MG, Feigin A, Tang C, Fitzsimons HL, Mattis P, Lawlor PA, et al. (2007). Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson’s disease: an open label, phase I trial. Lancet 369:2097-2105. [DOI] [PubMed] [Google Scholar]
- Kawai T, Matsuyama T, Hosokawa Y, Makihira S, Seki M, Karimbux NY, et al. (2006). B and T lymphocytes are the primary sources of RANKL in the bone resorptive lesion of periodontal disease. Am J Pathol 169:987-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima T, Yamaguchi M, Kasai K. (2006). Substance P stimulates release of RANKL via COX-2 expression in human dental pulp cells. Inflamm Res 55:78-84. [DOI] [PubMed] [Google Scholar]
- Lin X, Han X, Kawai T, Taubman MA. (2011). Antibody to receptor activator of NF-kappaB ligand ameliorates T cell-mediated periodontal bone resorption. Infect Immun 79:911-917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musatov S, Chen W, Pfaff DW, Kaplitt MG, Ogawa S. (2006). RNAi-mediated silencing of estrogen receptor A in the ventromedial nucleus of hypothalamus abolishes female sexual behaviors. Proc Natl Acad Sci USA 103:10456-10460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair PN. (1997). Apical periodontitis: a dynamic encounter between root canal infection and host response. Periodontol 2000 13: 121-148. [DOI] [PubMed] [Google Scholar]
- Nair PN. (2004). Pathogenesis of apical periodontitis and the causes of endodontic failures. Crit Rev Oral Biol Med 15:348-381. [DOI] [PubMed] [Google Scholar]
- Oseko F, Yamamoto T, Akamatsu Y, Kanamura N, Iwakura Y, Imanishi J, et al. (2009). IL-17 is involved in bone resorption in mouse periapical lesions. Microbiol Immunol 53:287-294. [DOI] [PubMed] [Google Scholar]
- Ramamurthy NS, Greenwald RA, Celiker MY, Shi EY. (2005). Experimental arthritis in rats induces biomarkers of periodontitis which are ameliorated by gene therapy with tissue inhibitor of matrix metalloproteinases. J Periodontol 76:229-233. [DOI] [PubMed] [Google Scholar]
- Sasaki H, Hou L, Belani A, Wang CY, Uchiyama T, Muller R, et al. (2000). IL-10, but not IL-4, suppresses infection-stimulated bone resorption in vivo. J Immunol 165:3626-3630. [DOI] [PubMed] [Google Scholar]
- Stashenko P. (1990). Role of immune cytokines in the pathogenesis of periapical lesions. Endod Dent Traumatol 6:89-96. [DOI] [PubMed] [Google Scholar]
- Uchiyama M, Nakamichi Y, Nakamura M, Kinugawa S, Yamada H, Udagawa N, et al. (2009). Dental pulp and periodontal ligament cells support osteoclastic differentiation. J Dent Res 88:609-614. [DOI] [PubMed] [Google Scholar]
- Ulrich-Vinther M. (2007). Gene therapy methods in bone and joint disorders. Evaluation of the adeno-associated virus vector in experimental models of articular cartilage disorders, periprosthetic osteolysis and bone healing. Acta Orthop Suppl 78:1-64. [PubMed] [Google Scholar]
- Wang CY, Stashenko P. (1993). Characterization of bone-resorbing activity in human periapical lesions. J Endod 19:107-111. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Aihara N, Kojima T, Kasai K. (2006). RANKL increase in compressed periodontal ligament cells from root resorption. J Dent Res 85:751-756. [DOI] [PubMed] [Google Scholar]
- Yang S, Li YP. (2007). RGS10-null mutation impairs osteoclast differentiation resulting from the loss of [Ca2+]i oscillation regulation. Genes Dev 21:1803-1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Li Q, Herbert B, Zinna R, Martin K, Junior CR, et al. (2011). Anti-inflammatory effect of MAPK phosphatase-1 local gene transfer in inflammatory bone loss. Gene Ther 18:344-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Jia TH, Chong AC, Bai L, Yu H, Gong W, et al. (2010). Cell-based osteoprotegerin therapy for debris-induced aseptic prosthetic loosening on a murine model. Gene Ther 17:1262-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.