Abstract
Bone sialoprotein (BSP) is an extracellular matrix protein found in mineralized tissues of the skeleton and dentition. BSP is multifunctional, affecting cell attachment and signaling through an RGD integrin-binding region, and acting as a positive regulator for mineral precipitation by nucleating hydroxyapatite crystals. BSP is present in cementum, the hard tissue covering the tooth root that anchors periodontal ligament (PDL) attachment. To test our hypothesis that BSP plays an important role in cementogenesis, we analyzed tooth development in a Bsp null (-/-) mouse model. Developmental analysis by histology, histochemistry, and SEM revealed a significant reduction in acellular cementum formation on Bsp-/- mouse molar and incisor roots, and the cementum deposited appeared hypomineralized. Structural defects in cementum-PDL interfaces in Bsp-/- mice caused PDL detachment, likely contributing to the high incidence of incisor malocclusion. Loss of BSP caused progressively disorganized PDL and significantly increased epithelial down-growth with aging. Bsp-/- mice displayed extensive root and alveolar bone resorption, mediated by increased RANKL and the presence of osteoclasts. Results collected here suggest that BSP plays a non-redundant role in acellular cementum formation, likely involved in initiating mineralization on the root surface. Through its importance to cementum integrity, BSP is essential for periodontal function.
Keywords: bone sialoprotein, cementum, periodontal ligament, bone, alkaline phosphatase, extracellular matrix
Introduction
Bone sialoprotein (BSP, or integrin binding sialoprotein) is an extracellular matrix protein in the Small Integrin Binding Ligand N-linked Glycoprotein (SIBLING) family, associated with mineralized tissues of the skeleton and dentition (Ganss et al., 1999; Fisher and Fedarko, 2003). Like other SIBLING proteins, BSP is multifunctional, with roles in cell attachment and migration (through the integrin-binding RGD motif), cell signaling, collagen binding, and hydroxyapatite nucleation. The ability of BSP to act as a positive regulator of hydroxyapatite precipitation has been demonstrated in vitro (Hunter and Goldberg, 1993) and is of particular interest in terms of regulating mineralized tissue development. Mice null for the Bsp gene (Bsp-/-) feature delayed bone growth and mineralization (Malaval et al., 2008).
In addition to its inclusion in bone extracellular matrix, BSP is present in both acellular and cellular cementum (MacNeil et al., 1994; McKee et al., 1996). Acellular cementum is the thin, mineralized tissue covering the cervical portion of the tooth root, important for attachment of the periodontal ligament (PDL) to the root surface (Bosshardt, 2005; Foster et al., 2007). Cellular cementum is a more bone-like tissue covering apical portions of roots. Developmental factors directing cementogenesis remain poorly defined, hampering progress toward more effective therapies for cementum regeneration. The finding that BSP was present in cementum and strongly expressed by cementoblasts led to speculation that this protein might contribute to cementum formation and mineralization (Somerman et al., 1990; MacNeil et al., 1995); however, this hypothesis has remained untested in the 2 decades since these findings. Here, we analyzed the function of BSP in cementogenesis and periodontal stability by determining the effects of the lack of BSP on tooth formation.
Materials & Methods
Animals
Animal procedures were performed in accordance with guidelines of the Canadian Council on Animal Care and Animal Care and Veterinary Services, University of Western Ontario. Preparation and genotyping of Bsp-/- and wild-type (WT) mice have been described previously (Malaval et al., 2008). Mice were maintained on a mixed 129/CD1 background and fed a standard mouse diet (2018 Tekland Global 18% protein diet, Harlan Laboratories, Indianapolis, IN, USA). From 3 to 6 animals were analyzed per genotype at ages 14, 26, 30, and 60 post-natal days (dpn). Serum biochemistry was analyzed at the NIH Veterinary Services Clinical Chemistry (Bethesda, MD, USA).
Micro-computed Tomography (microCT) and Scanning Electron Microscopy (SEM)
MicroCT of formalin-fixed hemi-mandibles was performed with eXplore Locus SP (GE Healthcare, Mississauga, ON, Canada). 2D images were acquired at 80 kVP and 80 µA, with an integration time of 1,600 msec/frame, 4 frames/view, and a total of 900 views at increments of 0.4 degrees. Images were constructed at a spatial resolution of 13 µm and calibrated with a cortical bone phantom (SB3; Gammex RMI, Milwaukee, WI, USA) having a hydroxyapatite equivalent of 1,100 mg/cc (White, 1978). Data were analyzed with MicroView ABA version 2.2 (GE Healthcare).
For SEM analysis, extracted and cleansed molars were osmium-coated. For fracture analysis, an ultra-fine diamond burr (Brasseler DET6UF-31, Brasseler USA, Savannah, GA, USA) was used to drill through crowns, and teeth were fractured longitudinally. SEM images were analyzed in secondary electron mode at 1-10 kV on a Leo 1540 XB FIB/SEM (Carl Zeiss, Western Nanofabrication Facility, University of Western Ontario, London, ON, Canada).
Histological Procedures
Procedures for histology, in situ hybridization (ISH), and immunohistochemistry (IHC) were previously described on Bouin’s fixed, decalcified, paraffin-embedded samples (Foster, 2012; Foster et al., 2012). Histomorphometry was used to quantify cementum, root-lining cells, and epithelial attachment, and statistical analysis was performed by independent-samples t test. ISH for Bsp mRNA used digoxigenin (DIG)-labeled cRNA probe (Marian Young, NIDCR, Bethesda, MD, USA) with NBT/BCIP substrate. IHC used biotinylated secondary antibodies and peroxidase substrate. Primary antibodies included rabbit anti-BSP (1:200; Renny Franceschi, University of Michigan, Ann Arbor, USA), rat anti-alkaline phosphatase (TNAP/ALPL, 1:200; R&D Systems, Minneapolis, MN, USA), LF-175 rabbit anti-osteopontin (OPN; 1:200) (Larry Fisher, NIDCR), rabbit anti-cytokeratin (Abcam, Cambridge, MA, USA), goat anti-RANKL (1:50; Santa Cruz Biotechnology, Santa Cruz, CA, USA), and rat anti-neutrophil antibody (1:200; NIMP; Abcam). Undecalcifed, glutaraldehyde/formalin-fixed tissues were LR white-embedded for ultramicrotome sectioning (1 µm) for von Kossa staining (Everts et al., 2012), or processed and methylmethacrylate-embedded for microtome sectioning (6 µm) for Goldner’s trichrome staining (Kacena et al., 2004).
Results
Bsp-/- Mice Exhibit Defective Acellular Cementum Formation
Bsp mRNA is highly expressed by cementoblasts during tooth root formation, as well as by osteoblasts of the alveolar bone (Fig. 1A). BSP protein is localized to alveolar bone and to acellular and cellular cementum covering the cervical and apical portions of the molar, respectively (Figs. 1B, 1C). The absence of BSP immunostaining was confirmed in Bsp-/- mice (Figs. 1D, 1E).
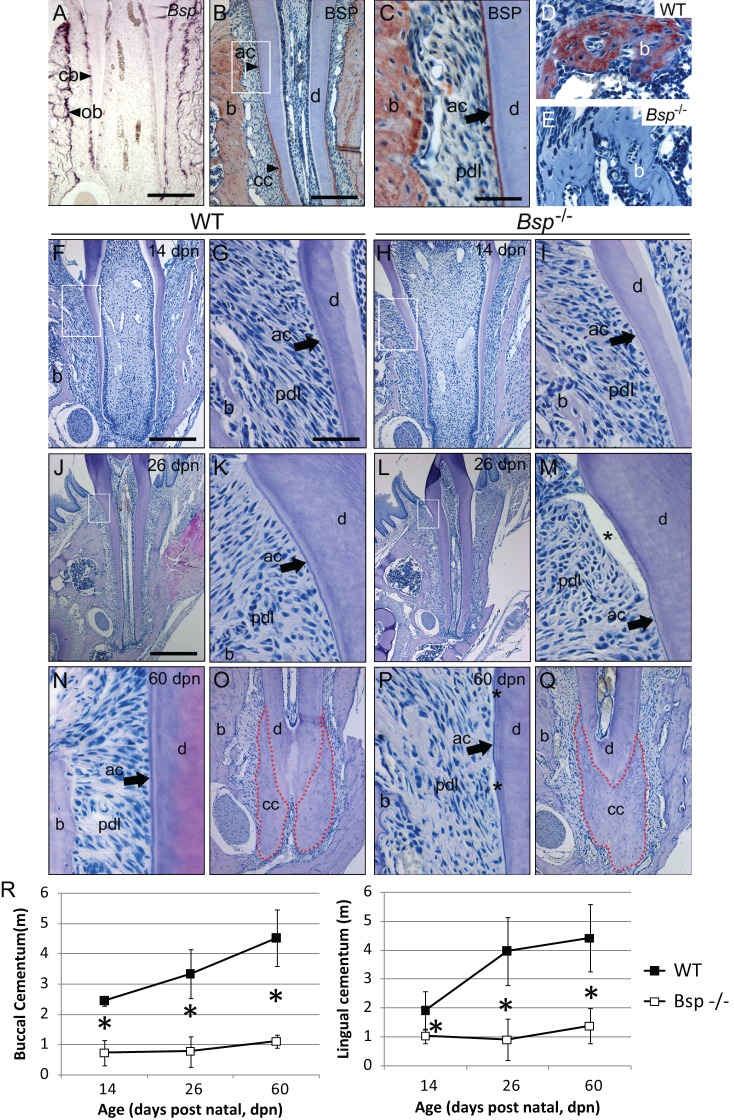
Figure 1.
Bone sialoprotein is required for proper acellular cementum formation. (A) Bsp mRNA is expressed by cementoblasts (cb) and osteoblasts (ob) during molar tooth root development at 14 dpn. (B, C) BSP protein is localized to alveolar bone (b), acellular cementum (ac), and cellular cementum (cc) at 26 dpn. White box in B is shown at higher magnification in C to highlight acellular cementum. BSP immunolabeling in (D) WT alveolar bone is absent in (E) Bsp-/- mice. (F, G) Newly formed acellular cementum anchors periodontal ligament (pdl) attachment in WT molars. (H, I) Acellular cementum in Bsp-/- molars is thin compared with that in WT mice. In contrast to the thickened acellular cementum in (J, K) WT mice at 26 dpn, (L, M) Bsp-/- molars feature stunted acellular cementum, and regions of PDL detachment from the tooth (*) were common, indicating defects in the cementum-PDL interface. The well-organized attachment of the acellular cementum, PDL, and bone in (N) WT molars at 60 dpn is severely degraded in (P) Bsp-/- molars, where stunted acellular cementum is associated with PDL disorganization. In contrast, cellular cementum in (O) WT and (Q) Bsp-/- mice at 60 dpn is not different in size. (R) Both buccal and lingual acellular cementum samples are significantly reduced in thickness in Bsp-/- mice compared with their WT counterparts (*indicates significant difference, p < 0.05). Scale bar is 200 µm in panels A, B, F, H, O, and Q (original, 100X), 50 µm in panels C, D, E, G, I, K, M, N, and P (original, 400X), and 400 µm in panels J and L (original, 50X).
Over the course of root development, Bsp-/- mouse molar teeth displayed deficiency in acellular cementum formation. At 14 post-natal days (dpn), a basophilic layer of acellular cementum covered the root dentin in WT mice (Figs. 1F, 1G), while in Bsp-/- molars, this layer was thinner (Figs. 1H, 1I). At 26 dpn, WT acellular cementum had grown thicker, and inserted PDL fibers radiated from the surface of the cervical root (Figs. 1J, 1K). Conversely, Bsp-/- acellular cementum showed no additional growth (Figs. 1L, 1M). It was common to find PDL disorganization in Bsp-/- molars, and a structural defect in the cementum-PDL interface was indicated by detachment in all Bsp-/- samples (possibly partly due to tissue processing, but not present in WT samples). By 60 dpn, acellular cementum remained stunted in Bsp-/- mice, and PDL detachment was present in all Bsp-/-, but not WT, samples (Figs. 1N vs. 1P). Histomorphometry confirmed that the apparent acellular cementum thickness was significantly reduced in Bsp-/- compared with WT molars (Fig. 1R). Alcian blue staining supported diminished acellular cementum, and Goldner’s trichrome staining indicated a lack of mineralized cementum matrix on Bsp-/- molar surfaces (Appendix Fig. 1). The continuously erupting mouse incisor features acellular cementum on the lingual root analog, and Bsp-/- incisors featured reduced cementum thickness and PDL disorganization (Appendix Fig. 2), contributing to a high rate of incisor malocclusion in these mice. The quantity of cellular cementum covering the apical molar root was unaffected in Bsp-/- compared with WT molars at 60 dpn (Figs. 1O vs. 1Q), though at 26 dpn mineralization was delayed in this layer (Appendix Fig. 3).
Failure of Acellular Cementum Mineralization in Bsp-/- Mice
SEM analysis supported histochemical observations that the thin layer of acellular cementum on Bsp-/- molar roots was not mineralized, because no mineralized cementum ultrastructure could be identified on the cervical root surfaces of Bsp-/- molars at 60 dpn, in contrast to the mineralized cementum layer on age-matched WT molars (Fig. 2).
Figure 2.
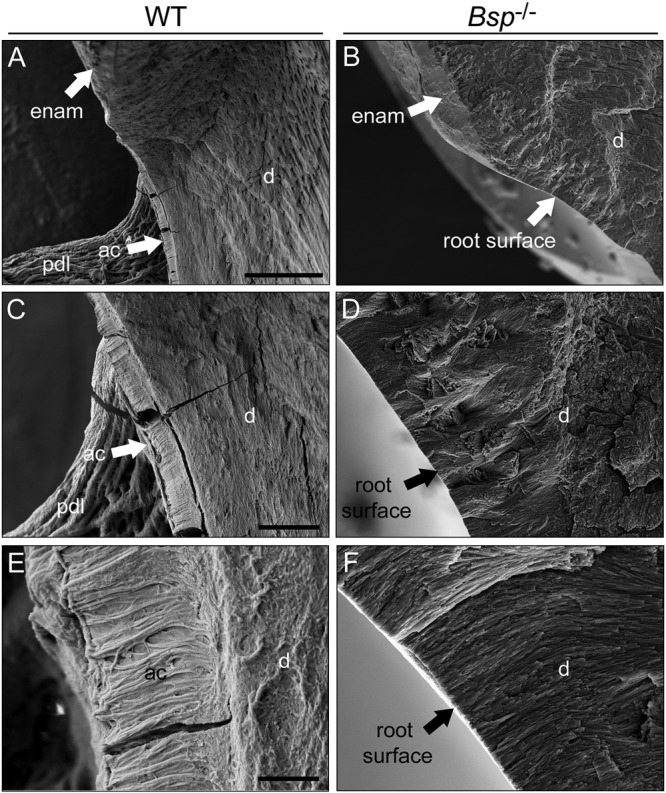
Lack of mineralized acellular cementum ultrastructure on Bsp-/- mouse molar teeth. Secondary electron SEM analysis on fractured first molars at 60 dpn indicated that, compared with the mineralized acellular cementum layer visible in WT molars at various magnifications (A, C, E), no mineralized cementum ultrastructure could be identified on the roots of Bsp-/- molars (B, D, F). Some cracking is evident as an artifact of sample preparation. Abbreviations: enam = enamel; ac = acellular cementum; pdl = periodontal ligament. Scale bar is 50 µm in panels A and B (original, 500X), 10 µm in panels C and D (original, 2,000X), and 2 µm in panels C and D (original, 10,000X).
We considered developmental possibilities predisposing to the inhibition of cementum formation in Bsp-/- mice. By histology and von Kossa staining, dentin, required as a foundation for cementum deposition, appeared sound and well-mineralized in Bsp-/- mice (Fig. 1; Appendix Figs. 4A, 4B). Acellular cementum formation requires migration of dental follicle/cementoblast precursors to the nascent root surface. Root-lining cells were present in similar numbers in Bsp-/- vs. WT first molar roots at 14 dpn (Appendix Fig. 4C). PDL fringe fibers at the root surface suggested that the proper acellular cementum matrix was present for cementogenesis (Appendix Figs. 4D, 4E). Immunostaining revealed the deposition of osteopontin (OPN) on the root surface, confirming functional cementoblasts in Bsp-/- mice (Appendix Figs. 4F-4I). Markers of local and systemic mineral metabolism were assayed. TNAP immunolabeling in Bsp-/- vs. WT molars (Appendix Figs. 4J-4M), and serum alkaline phosphatase, calcium, and phosphorus, were undiminished in Bsp-/- mice (Appendix Table), ruling out systemic mineral ion disturbance as an underlying cause of the cementum defect.
Periodontal Breakdown in Bsp-/- Mice
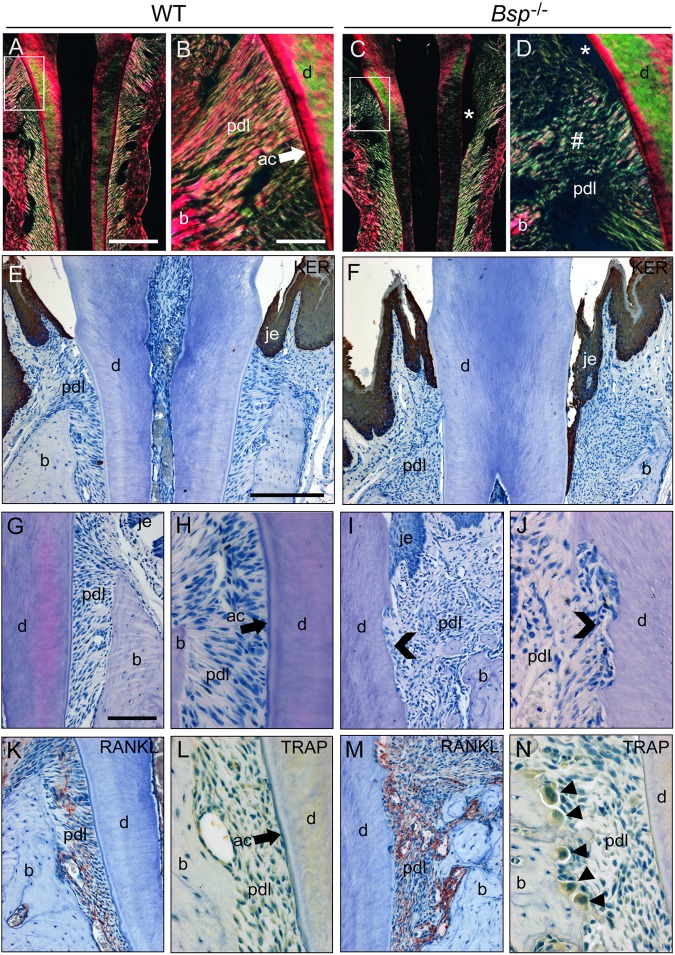
Defective acellular cementum in Bsp-/- mice preceded a progressive breakdown of periodontal organization at later ages. WT molars at 26 dpn displayed dense and highly organized collagen fiber insertions in the cervical root, visualized by picrosirius red staining under polarized light (Figs. 3A, 3B). Conversely, Bsp-/- molars lost PDL organization in the cervical root by this age (Figs. 3C, 3D), a condition that degenerated further over time (Appendix Fig. 5). Defective cementum-PDL attachment in Bsp-/- molars allowed for significant down-growth of gingival epithelial tissues into the periodontal region, initiating a long junctional epithelium (Figs. 3E, 3F; Appendix Fig. 6). Periodontal disarray in Bsp-/- mice was accompanied by increased incidence of root resorption by 60 dpn (Figs. 3G-3J) and increased numbers of osteoclast-like cells, RANKL immunostaining, and bone resorption in alveolar bone (Figs. 3K-3N). There was no indication that inflammation played a significant role in the periodontal breakdown, based on histology and the lack of neutrophil infiltration into the periodontia (Appendix Fig. 7).
Figure 3.
Periodontal breakdown in Bsp-/- mice. (A, B) Picrosirius-red-stained sections viewed under polarized light reveal a high degree of organization and directionality (indicated by high-intensity yellowish fibers) of periodontal ligament (pdl) collagen fibrils between acellular cementum (ac) and bone (b) in WT molars at 26 dpn. (C, D) Bsp-/- molars at 26 dpn feature loss of organization of collagen fibers in the cervical PDL region (#), where detachment (*) is also present. (E) Pan-keratin immunohistochemistry (red-brown color) indicates a well-maintained junctional epithelium (je) in WT molars at 60 dpn, whereas (F) Bsp-/- molars feature down-growth and establishment of a long junctional epithelium in association with loss of PDL attachment. Compared with (G, H) WT molars at 60 dpn, (I, J) Bsp-/- molar teeth feature extensive root resorption (chevrons). Compared with (K, L) WT molars, Bsp-/- molars at 60 dpn feature (M) dramatically increased localization of receptor activator of nuclear factor kappa-B ligand (RANKL) near tooth and bone surfaces, and (N) numerous osteoclast-like cells [arrowheads; large, multinucleated, tartrate-resistant acid phosphatase (TRAP)- positive] on alveolar bone surfaces. Scale bar is 200 µm in panels A and C (original, 100X), 50 µm in panels B, D, H, J, L, and N (original, 400X), 100 µm in panels G, I, K, and M (original, 200X), and 100 µm in panels E and F (original, 100X).
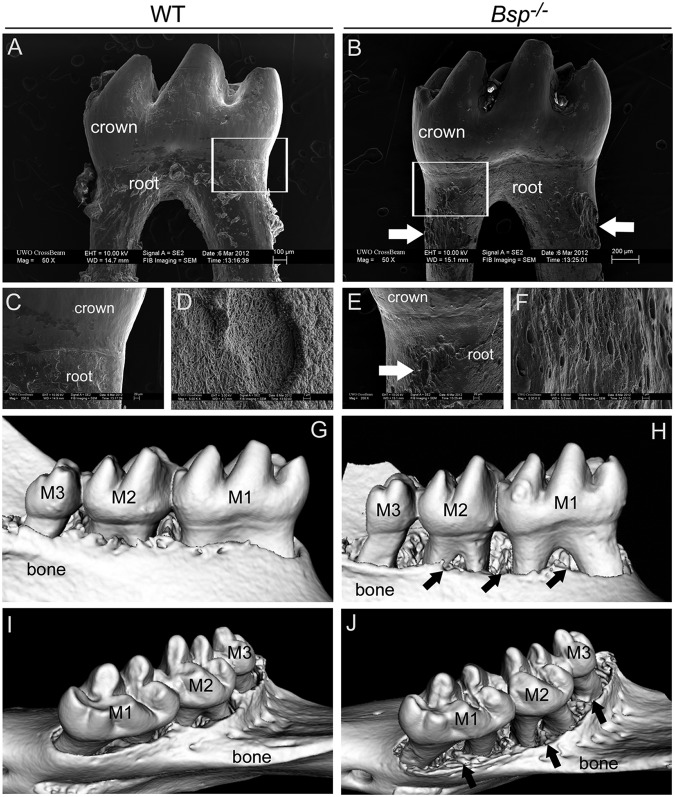
Tooth and bone resorption was documented further by SEM and microCT. SEM revealed extensive pitting in Bsp-/- vs. WT molars, concentrated in the cervical roots (Figs. 4A-4F). Lack of acellular cementum was indicated by clearly visible dentinal tubules on the external surfaces of Bsp-/- molar roots. MicroCT analysis revealed extensive reduction in alveolar bone height in Bsp-/- vs. WT mandibles, particularly along the lingual aspect (Figs. 4G-4J).
Figure 4.
Increased tooth root and alveolar bone resorption in Bsp-/- mice. Compared with the smooth surfaces of (A, C) 10-week-old WT molars viewed by backscattered SEM, (B, E) Bsp-/- teeth exhibit abundant pitting (white arrows in B and E) of the root surface, consistent with external root resorption, concentrated on the cervical portion of the root surface. White boxes in A and B indicate regions shown at higher magnification in C and E, respectively. Compared with the (D) WT molar root surface at high magnification, (F) Bsp-/- molar roots display exposed dentinal tubules, indicating the absence of cementum in regions of root resorption. MicroCT revealed that, compared with (G, I) WT mandibles, (H, J) Bsp-/- mandibles display extensive reduction in alveolar bone height, especially on the lingual aspect (black arrows in H and J). Abbreviations: M1 = first molar; M2 = second molar; M3 = third molar.
Discussion
BSP is an extracellular matrix protein present in bone and cementum. Here we demonstrate for the first time that lack of BSP inhibited the formation and mineralization of acellular cementum. Further, reduction and loss-of-function of the acellular cementum in Bsp-/- mice led to structural defects in the cementum-PDL interface, PDL disorganization, down-growth of epithelium, and increased tooth and bone resorption. Results collected here demonstrated that BSP plays a non-redundant role in acellular cementum formation, likely involved in initiating mineralization and/or promoting appositional cementum growth. Because of its importance to cementum formation, BSP is essential for the formation of a functional periodontium.
Acellular Cementum as a Mineralization-sensitive Tissue
Lack of BSP led to a severe developmental inhibition of acellular cementum formation, shown here by histology, histochemistry, immunohistochemistry, and SEM. As a late stage in odontogenesis, cementum formation depends on a number of prior developmental stages. Our results suggested that the defect did not result from defective dentin, lack of migration/position of precursor cells, or root-surface collagen fringe fibers [considered the extracellular matrix proper for acellular cementum (Bosshardt and Schroeder, 1996)]. Local TNAP deficiency (by IHC), hypophosphatemia, and hypocalcemia were ruled out as causes of cementum inhibition in Bsp-/- mice. It is notable that acellular cementum formation is similarly inhibited in the Alpl-/- mouse model featuring loss of TNAP function (Beertsen et al., 1999; McKee et al., 2011; Foster et al., 2012). Alpl-/- mice harbor increased levels of the mineral inhibitor pyrophosphate (PPi) and serve as a model of hypophosphatasia (HPP), where lack of acellular cementum predisposes to tooth loss (van den Bos et al., 2005). We have demonstrated that reduction of PPi, in turn, promotes increased acellular cementum formation (Foster et al., 2011, 2012), and hypothesized that acellular cementum represents a tissue strongly regulated at the physicochemical level of mineralization, i.e., hydroxyapatite nucleation and growth. Cellular cementum size was undiminished by the loss of BSP, though mineralization was delayed at earlier ages; functional consequences of this are under study. Cellular cementum was unaffected in Alpl-/- mice (Beertsen et al., 1999) and in mice where acellular cementum increased via decreased PPi (Foster et al., 2012), supporting the view that these tissues are subject to different developmental influences.
Acellular cementum forms as a progressive mineralization of the PDL collagen fiber bundles inserting into the root surface. We have previously shown BSP binds collagen with high affinity (Tye et al., 2005) and mediates hydroxyapatite formation (Hunter and Goldberg, 1993; Harris et al., 2000) and that hydroxyapatite nucleation potency is increased 10-fold when BSP is incubated with collagen (Baht et al., 2010). Considering that BSP is localized across the width of the acellular cementum (McKee et al., 1996; Bosshardt et al., 1998), and based on our past and present findings, it is likely that BSP is critical for proper cementum formation and function.
Another SIBLING protein, OPN, was present at Bsp-/- root surfaces, as in WT controls. OPN functions as an inhibitor of hydroxyapatite crystal growth in vitro and in vivo (Hunter et al., 1994; Boskey et al., 2002). The presence of OPN in the absence of BSP may additionally tip the balance toward inhibition of cementum mineralization. It is also possible that another function of BSP (e.g., cell adhesion, signaling) may contribute to the cementum phenotype of the Bsp-/- mouse, and this is currently under study.
Loss of BSP Leads to Periodontal Breakdown
A functional periodontal complex depends on stable insertion of PDL collagen fibers into the acellular cementum, as well as ability for remodeling on the bone surface of the PDL space. We propose that loss of acellular cementum in Bsp-/- mice led to a progressive degradation of periodontal integrity over time. Lack of PDL-tooth anchorage was followed by PDL disorganization, epithelial down-growth, and tooth and alveolar bone resorption. These are hallmarks of periodontal breakdown associated with periodontal disease; however, this phenotype developed without an apparent inflammatory response in the periodontal compartment, suggesting failure of function/adaptation of the periodontium. Similar examples of periodontal degradation have resulted in transgenic mouse models with compromised periodontal function, e.g., Dmp1 null (Ye et al., 2008) and periostin null (Rios et al., 2008) mice. Substitution of a soft diet for pelletized chow is currently being studied to determine the contribution of occlusal stress to the incidence of periodontal breakdown and incisor malocclusion.
An unexpected finding was the increased incidence of tooth and alveolar bone resorption in Bsp-/- mice. Previous studies in Bsp-/- mice showed that BSP deficiency impairs osteoclastogenesis and bone resorption in vivo and in vitro (Malaval et al., 2008; Boudiffa et al., 2010). Conversely, BSP over-expression increased osteoclastic activity (Valverde et al., 2008). We propose that increased resorption in the periodontal compartment of Bsp-/- mice is the result of local loss of integrity and function in a tissue that functions under high mechanical stress and depends on rapid remodeling for proper maintenance. While increased presence of RANKL and TRAP-positive osteoclast-like cells was noted in periodontia of Bsp-/- mice, the cause for increased tooth and bone resorption is being explored further in this model.
In summary, results from these studies show, for the first time, that BSP is critical for acellular cementum formation and periodontal attachment. The role of BSP in acellular cementogenesis is likely to be in promoting mineralization at the root surface to anchor PDL fibers and provide strong attachment of the tooth to the alveolar bone. Loss-of-function of BSP may predispose to periodontal disease through defective cementum-PDL attachment and inability to prevent establishment of biofilm in the periodontal space. Ongoing studies will further elucidate potential roles of BSP in cementum formation and regeneration, and in wider periodontal biology.
Supplementary Material
Acknowledgments
We thank Jirawan Wade and Daisy Matsa-Dunn (UW, Seattle, WA, USA) for histological procedures, Simrandeep Sidhu (NIAMS) for IHC assistance, Frank Beier and Vasek Pitelka (UWO) for assistance with animal studies, Cristiane Salmon (UNICAMP, Piracicaba, SP, Brazil) for mouse ligature-periodontitis tissues, and Marc McKee (McGill University, Montreal, Quebec, Canada) for technical advice on sectioning undecalcified tissues.
Footnotes
This research was supported by the Intramural Research Program of the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) of the National Institutes of Health (NIH), and by the Canadian Institutes of Health Research (FRN83704-JEA, FRN93598-HAG/GKH). YS is supported by The Joint Motion Program, a CIHR Training Program and the AO Foundation (Switzerland).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Baht GS, O’Young J, Borovina A, Chen H, Tye CE, Karttunen M, et al. (2010). Phosphorylation of Ser136 is critical for potent bone sialoprotein-mediated nucleation of hydroxyapatite crystals. Biochem J 428:385-395. [DOI] [PubMed] [Google Scholar]
- Beertsen W, VandenBos T, Everts V. (1999). Root development in mice lacking functional tissue non-specific alkaline phosphatase gene: inhibition of acellular cementum formation. J Dent Res 78:1221-1229. [DOI] [PubMed] [Google Scholar]
- Boskey AL, Spevak L, Paschalis E, Doty SB, McKee MD. (2002). Osteopontin deficiency increases mineral content and mineral crystallinity in mouse bone. Calcif Tissue Int 71:145-154. [DOI] [PubMed] [Google Scholar]
- Bosshardt DD. (2005). Are cementoblasts a subpopulation of osteoblasts or a unique phenotype? J Dent Res 84:390-406. [DOI] [PubMed] [Google Scholar]
- Bosshardt D, Schroeder H. (1996). Cementogenesis reviewed: a comparison between human premolars and rodent molars. Anat Rec 245:267-292. [DOI] [PubMed] [Google Scholar]
- Bosshardt D, Zalzal S, McKee M, Nanci A. (1998). Developmental appearance and distribution of bone sialoprotein and osteopontin in human and rat cementum. Anat Rec 250:13-33. [DOI] [PubMed] [Google Scholar]
- Boudiffa M, Wade-Gueye NM, Guignandon A, Vanden-Bossche A, Sabido O, Aubin JE, et al. (2010). Bone sialoprotein deficiency impairs osteoclastogenesis and mineral resorption in vitro. J Bone Miner Res 25:2669-2679. [DOI] [PubMed] [Google Scholar]
- Everts V, Niehof A, Tigchelaar-Gutter W, Beertsen W. (2012). Transmission electron microscopy of bone. Methods Mol Biol 816:351-363. [DOI] [PubMed] [Google Scholar]
- Fisher L, Fedarko N. (2003). Six genes expressed in bones and teeth encode the current members of the SIBLING family of proteins. Connect Tissue Res 44(Suppl 1):33-40. [PubMed] [Google Scholar]
- Foster BL. (2012). Methods for studying tooth root cementum by light microscopy. Int J Oral Sci 4:119-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster BL, Popowics TE, Fong HK, Somerman MJ. (2007). Advances in defining regulators of cementum development and periodontal regeneration. Curr Top Dev Biol 78:47-126. [DOI] [PubMed] [Google Scholar]
- Foster BL, Nagatomo KJ, Bamashmous SO, Tompkins KA, Fong H, Dunn D, et al. (2011). The progressive ankylosis protein regulates cementum apposition and extracellular matrix composition. Cells Tissues Organs 194:382-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster BL, Nagatomo KJ, Nociti FH, Fong H, Dunn D, Tran AB, et al. (2012). Central role of pyrophosphate in acellular cementum formation. PLoS One 7:e38393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganss B, Kim R, Sodek J. (1999). Bone sialoprotein. Crit Rev Oral Biol Med 10:79-98. [DOI] [PubMed] [Google Scholar]
- Harris NL, Rattray KR, Tye CE, Underhill TM, Somerman MJ, D’Errico JA, et al. (2000). Functional analysis of bone sialoprotein: identification of the hydroxyapatite-nucleating and cell-binding domains by recombinant peptide expression and site-directed mutagenesis. Bone 27:795-802. [DOI] [PubMed] [Google Scholar]
- Hunter GK, Goldberg HA. (1993). Nucleation of hydroxyapatite by bone sialoprotein. Proc Natl Acad Sci USA 90:8562-8565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter GK, Kyle CL, Goldberg HA. (1994). Modulation of crystal formation by bone phosphoproteins: structural specificity of the osteopontin-mediated inhibition of hydroxyapatite formation. Biochem J 300(Pt 3):723-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacena MA, Troiano NW, Wilson KM, Coady CE, Horowitz MC. (2004). Evaluation of two different methylmethacrylate processing, infiltration, and embedding techniques on the histological, histochemical, and immunohistochemical analysis of murine bone samples. J Histotechnol 27:119-130. [Google Scholar]
- MacNeil R, Sheng N, Strayhorn C, Fisher L, Somerman M. (1994). Bone sialoprotein is localized to the root surface during cementogenesis. J Bone Miner Res 9:1597-1606. [DOI] [PubMed] [Google Scholar]
- MacNeil R, Berry J, D’Errico J, Strayhorn C, Piotrowski B, Somerman M. (1995). Role of two mineral-associated adhesion molecules, osteopontin and bone sialoprotein, during cementogenesis. Connect Tissue Res 33:1-7. [DOI] [PubMed] [Google Scholar]
- Malaval L, Wade-Guéye NM, Boudiffa M, Fei J, Zirngibl R, Chen F, et al. (2008). Bone sialoprotein plays a functional role in bone formation and osteoclastogenesis. J Exp Med 205:1145-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee MD, Zalzal S, Nanci A. (1996). Extracellular matrix in tooth cementum and mantle dentin: localization of osteopontin and other noncollagenous proteins, plasma proteins, and glycoconjugates by electron microscopy. Anat Rec 245:293-312. [DOI] [PubMed] [Google Scholar]
- McKee MD, Nakano Y, Masica DL, Gray JJ, Lemire I, Heft R, et al. (2011). Enzyme replacement therapy prevents dental defects in a model of hypophosphatasia. J Dent Res 90:470-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios H, Ma D, Xie Y, Giannobile W, Bonewald L, Conway S, et al. (2008). Periostin is essential for the integrity and function of the periodontal ligament during occlusal loading in mice. J Periodontol 79:1480-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerman M, Shroff B, Agraves W, Morrison G, Craig A, Denhardt D, et al. (1990). Expression of attachment proteins during cementogenesis. J Biol Buccale 18:207-214. [PubMed] [Google Scholar]
- Tye CE, Hunter GK, Goldberg HA. (2005). Identification of the type I collagen-binding domain of bone sialoprotein and characterization of the mechanism of interaction. J Biol Chem 280:13487-13492. [DOI] [PubMed] [Google Scholar]
- Valverde P, Zhang J, Fix A, Zhu J, Ma W, Tu Q, et al. (2008). Overexpression of bone sialoprotein leads to an uncoupling of bone formation and bone resorption in mice. J Bone Miner Res 23:1775-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bos T, Handoko G, Niehof A, Ryan L, Coburn S, Whyte M, et al. (2005). Cementum and dentin in hypophosphatasia. J Dent Res 84:1021-1025. [DOI] [PubMed] [Google Scholar]
- White DR. (1978). Tissue substitutes in experimental radiation physics. Med Phys 5:467-479. [DOI] [PubMed] [Google Scholar]
- Ye L, Zhang S, Ke H, Bonewald LF, Feng JQ. (2008). Periodontal breakdown in the Dmp1 null mouse model of hypophosphatemic rickets. J Dent Res 87:624-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.