Abstract
Fully matured dental enamel is an architecturally and mechanically complex hydroxyapatite-based bioceramic devoid of most of the organic material that was essential in its making. Enamel formation is a staged process principally involving secretory and maturation stages, each associated with major changes in gene expression and cellular function. Cellular activities that define the maturation stage of amelogenesis include ion (e.g., calcium and phosphate) transport and storage, control of intracellular and extracellular pH (e.g., bicarbonate and hydrogen ion movements), and endocytosis. Recent studies on rodent amelogenesis have identified a multitude of gene products that appear to be linked to these cellular activities. This review describes the main cellular activities of these genes during the maturation stage of amelogenesis.
Keywords: amelogenesis, biomineralization, enamel, ion transport, pH homeostasis, endocytosis
Introduction
The mineralization of dental enamel is a unique and complex process involving the sequential passage of mineral ions across cell and fluid barriers. The 2 main stages recognized in the formation of enamel are the secretory and maturation stages, both regulated by the formative ameloblast cells. Secretory ameloblasts are tall, polarized cells that often reach up to 60 to 100 µm in length while maintaining a relatively narrow 5- to 8-µm diameter. The secretory stage provides an organic template form in which the enamel crystals grow under spatio-temporal guidelines dictated by the enamel-specific proteins secreted by the ameloblasts (Smith, 1998). The main goal at this stage is to build, in an organized manner, the full volume of enamel tissue, consisting of mineral crystals bundled into structures called prisms (rods) and other mineral crystals filling the spaces between the prisms (interrod). The patterns of enamel scaffolding thereby created are complex and largely species-specific; thus, this review will focus on rodents, one of the best-known enamel models. Enamel maturation is synonymous with calcification and mineralization, widely used terms in the literature (Glick, 1979). Maturation-stage ameloblasts re-organize and change morphology to become shorter and somewhat more ‘squat’ cells. These cells then orchestrate the mineralization of enamel, creating a biomaterial containing 95% mineral by weight (Smith and Nanci, 1996). They accomplish this by removing the organic content and water, originally provided by the cells, and by actively engaging in the organized passage of various ions required for the expansion of enamel mineral crystals (Smith, 1998; Hubbard, 2000). The intricate pathways of active movement of ions across ameloblasts and in between these cells are the primary focus of this review. Moreover, our research, as well as research conducted by other scientists, describes similarities between the physiology of ion transport by ameloblasts and epithelial-derived cells of other organs, allowing us to draw parallels between enamel formation and the formation of non-mineralizing tissues such as the pancreas and kidney, and because of this, the transport properties for these 2 soft-tissue organs will be briefly introduced.
Control of pH in Pancreatic Duct Cells and Kidney Proximal Tubules
Although all cells regulate their intracellular pH via a wide variety of hydrogen ion (H+) and bicarbonate (HCO3-) transport proteins, there are specific tissues that mediate vectorial transepithelial bicarbonate transport. For example, the kidney proximal tubule absorbs ~ 3,000 mmol of HCO3-per day, whereas the pancreatic duct secretes ~ 300 mmol of HCO3-per day. Salivary glands and intestine also mediate transepithelial bicarbonate transport. Vectorial transport in these epithelia is facilitated by the polarized expression of specific acid-base transporters. We will briefly discuss bicarbonate secretion in the pancreas and bicarbonate absorption in the proximal tubule as model systems. As evidenced by the research summarized in this section and in the following sections, many transporters identified in the pancreas and kidney are also associated with similar transport functions in the enamel organ.
In the pancreas, basolateral HCO3- uptake into pancreatic duct cells is mediated by the electrogenic Na+- HCO3- co-transporter NBCe1-B, which in turn is regulated by IRBIT (adenosylhomocysteinase-like 1) (Yang et al., 2011). The duct also expresses the sodium hydrogen exchanger NHE1 and the anion exchanger AE2 at the basolateral pole, although their contribution to transepithelial HCO3- transport is minimal (Yang et al., 2011). Secretion of apical HCO3- is mediated by the cystic fibrosis transmembrane conductance regulator (CFTR) and the solute carrier Slc26a6 (Singh et al., 2010). CFTR has limited permeability to HCO3-. The major apical HCO3- transporter is SLC26a6 (pendrin-like protein 1), functioning as a 2HCO3-/1Cl- exchanger. SLC26 transporters and CFTR are characterized by mutual regulation. The STAS domain of SLC26 transporters binds the R domain of CFTR and can activate both (Singh et al., 2010). The sodium hydrogen exchangers NHE2 and NHE3 are also expressed in the apical membrane and may function when bicarbonate secretion is decreased to salvage luminal HCO3- (Singh et al., 2010). NHE3 interacts with CFTR via PDZ-containing scaffolding proteins and is regulated by CFTR and IRBIT. Together, NBCe1-B, CFTR, and Slc26a6 achieve a luminal HCO3- concentration of ~ 140 mM (Singh et al., 2010; Yang et al., 2011). The carbonic anhydrase (CA) inhibitor acetazolamide significantly blocks ductal HCO3- secretion (Sener et al., 2007). CA2, CA4, CA9, and CA12 are expressed in the pancreatic duct (Lee and Muallem, 2008). The role of each CA isoform has not yet been clearly delineated.
In the renal proximal tubule, bicarbonate uptake across the apical membrane is indirect via the protonation of luminal HCO3-. Proton secretion is mediated by apical NHE3 (Girardi and Di Sole, 2012). Luminal HCO3- is converted CO2, which is passively absorbed across the luminal membrane. In proximal tubule cells, intracellular CO2 is re-converted to HCO3- and a proton (Girardi and Di Sole, 2012). The proton is recycled across the apical membrane via NHE3, and HCO3- is absorbed across the basolateral membrane via the electrogenic Na+- HCO3- co-transporter NBCe1-A. As in the pancreatic duct, various CA isoforms are expressed (CA2, CA4, CA9, CA12) (Purkerson and Schwartz, 2007).
Enamel Maturation
Ameloblasts are highly polarized cells, acquiring this characteristic apical-basal polarity soon after differentiation. This polarity does not change until the enamel organ becomes reduced at the end of amelogenesis. During the post-secretory stage, enamel crystals experience large volumetric expansion within a mildly acidic environment, concurrent with rapid nucleation events and release of H+ (Smith and Nanci, 1995; Smith, 1998). A common mechanism to produce this volumetric expansion in mammals is associated with supersaturation of the enamel fluid, primarily with calcium and phosphate ions, thereby optimizing the driving force for mineralization and assisting to degrade and remove organic matrix to create space for crystal expansion, processes mediated by the ameloblasts (Nanci and Smith, 1992; Smith, 1998; Lacruz et al., 2012a).
Secretory-stage ameloblasts present a tight barrier for diffusion of mineral ions, as suggested by differential concentrations of various elements between the tissue and the enamel fluid (Takano, 1995; Hubbard, 2000). This is not the case in maturation-stage ameloblasts and associated cell layers. One of the most intriguing and as-yet-unexplained aspects of ameloblast maturation is the cyclical and rapid changes in morphology (within hours) from what has been described as “ruffle-ended” to “smooth-ended” cell phenotypes (Josephsen and Fejerskov, 1977; Smith, 1998). Smooth-ended ameloblasts (SA) are presumed to be impermeable to ion movement across the basal pole, whereas ruffle-ended ameloblasts (RA) have this barrier at the apical pole (Smith, 1998; Hubbard, 2000). This dynamic permeability pattern allows for bi-directional diffusion of small molecules into, and removal from, the enamel area (Smith, 1998; Josephsen et al., 2010). Prior to enamel maturation, the stratum intermedium (the cell layer in contact with the basal pole of ameloblasts), the stellate reticulum, and the outer dental epithelium undergo morphological transformations, resulting in the formation of the papillary layer (PL) (Garant and Nalbandian, 1968b; Sasaki et al., 1990). The paucity of protein-synthesizing organelles (rough ER and Golgi) suggests little protein-secretory function for the PL. However, the well-developed mitochondrial apparatus has been implicated as an important indicator of substantial energy-generating potential, permitting, for example, active ion transport function (Garant and Nalbandian, 1968a; Hubbard, 2000). The PL is deeply invaginated by numerous blood vessels surrounded by a basal membrane, thereby allowing for more rapid diffusion of ions and nutrients into the ameloblast layer (Decker, 1963). The proximity of capillary systems to ameloblasts ultimately permits rapid incorporation of ions into the enamel crystals (Takano, 1995) and improves the efficiency of local tissue fluid-buffering capacity. Extensive gap junctions connect the basal poles of maturation-stage ameloblasts to the PL, allowing for rapid intercellular communications (Sasaki et al., 1990).
Calcium Transport
Calcium regulation manifests as one of the more difficult challenges facing enamel-forming cells, since they produce the most highly calcified tissue of all. Recent studies support a new paradigm for calcium transport (termed “calcium transcytosis”) that now warrants further investigation in dental tissues. For over 20 years, enamel biologists have contemplated a perplexing question about the need for enamel-forming cells to supply calcium in sufficient bulk for mineralization, yet avoid the potentially cytotoxic effects of elevated cytosolic calcium (Hubbard, 2000). Cell viability depends on cytosolic-free calcium being maintained at low average concentrations (~ 0.2 µmol/L). Although calcium-signaling transients are well-tolerated, more extreme increases can be toxic and even lead to cell death (Hubbard, 2000). Building from anatomical evidence that the enamel epithelium acts as a semi-permeable barrier to calcium, previous biochemical studies revealed an exceptionally high abundance of calcium-handling proteins in enamel-forming rat and mouse cells. Moreover, various calcium-handling proteins were found to be up-regulated ~ 3-fold relative to the secretion phase, closely paralleling the increased calcium flux at maturation (Hubbard, 2000). However, contradicting the “calbindin ferry” dogma long held by the calcium-transport field (based largely on studies of gut and kidney), the major calbindin (Calb1/CB28) was found to be down-regulated during maturation, CB28-null mice lacked the dental phenotype, and the other 2 types of calbindin (Calb2 and S100g) were recently ruled out as alternative calcium transporters (Turnbull et al., 2004; Hubbard et al., 2011). Instead of a cytosolic transport mechanism, the biochemical data invoked an organellar route involving endoplasmic-reticulum-related calcium stores (ER/Ca stores). As such, calcium transcytosis is conceptually appealing, because it encompasses safety against calcium cytotoxicity as well as providing a high-flux transcellular pipeline (Hubbard, 2000; Hubbard et al., 2011).
Recent genome-wide transcript-profiling studies have strengthened this emerging paradigm, not only by illustrating that ameloblasts undergo a comprehensive molecular change at maturation, but also by invoking several new “key players” in calcium trancytosis (Lacruz et al., 2011a, 2012a). When one considers how calcium enters the putative transcellular pipeline, it is tantalizing that Stim1, a plasmalemmal calcium channel component associated with ER/Ca-stores, was expressed at exceptionally high levels during maturation (~ 8-fold increase over secretion at the protein level). At the other end of the putative pipeline, a recently discovered type of sodium-calcium exchanger (NCKX4; coded by Slc24a4) has been shown to be expressed at moderately high levels (Hu et al., 2012). Importantly, unlike the 5 other isoforms of NCKX and all 3 isoforms of the “classic” NCX sodium-calcium exchangers (Okumura et al., 2010), NCKX4 protein levels were up- regulated ~ 2-fold at maturation, producing a particularly strong candidate for mineralization-related calcium extrusion (Hu et al., 2012). In addition to novel machinery for calcium transport, the transcript studies identified S100a4 and Rcan1 as 2 calcium-signaling proteins up-regulated during maturation (Lacruz et al., 2012a). Analysis of these transcriptomics data, collectively, has broadened our theory about how enamel- forming cells might accomplish safe handling of bulk calcium, by balancing sequestration within organelles against the ongoing need for calcium-signaling dynamics in the cytosol. Further investigation of these newly suggested mechanisms appears justified, given the practical significance of calcium regulation with respect to dental bioengineering and prevention of enamel malformations. Furthermore, when one considers the significance for other biological research, it is noteworthy that recent calbindin-null studies failed to support the calbindin-ferry dogma in gut and kidney (although questions about compensation remain to be addressed) (Hubbard et al., 2011). Murine-enamel models now stand as particularly strong comparators for future investigations into transepithelial calcium transport and the functions of calbindins.
Phosphate and Sodium Transport
Whereas some advances have developed from an understanding of calcium transport in the enamel organ as discussed above, phosphate and sodium transport remains poorly defined. Phosphate homeostasis is essential for cellular function, bone formation, and DNA replication (Murer et al., 2004). In enamel, phosphate is a fundamental element in the formation and growth of hydroxyapatite-based crystals (Smith, 1998). Most studies on phosphate transport in the enamel organ focus on observations of radiolabeled P (32P or 33P) injections in rodents to identify its incorporation into the enamel crystals or to identify phosphate concentrations at various developmental stages (Robinson et al., 1974; Wennberg and Bawden, 1978; McKee et al., 1989). The evidence described in these studies indicated that phosphate uptake increased in the maturation stage and might also be linked with the passive SA phase (Reith and Boyde, 1981). However, the mechanisms of phosphate transport remain obscure, and both transcellular and paracellular transport mechanisms may be involved. We have recently identified a member of the solute carrier 34 (SLC34) gene family as a possible candidate for phosphate transport in the enamel organ (Lacruz et al., 2012a). The SLC34 gene family is composed of 3 sodium-phosphate co-transporters, or Na/Pi’s, coded by genes Slc34a1, Slc34a2, and Slc34a3, and coded for the proteins NaPi-IIa, NaPi-IIb, and NaPi-IIc, respectively (Murer et al., 2004). A 64-fold increase in the expression of NaPi-IIb (Slc34a2) mRNA was found in maturation-stage enamel organ cells relative to secretory-stage cells (Lacruz et al., 2012a), providing a good candidate gene for major phosphate transport in the enamel organ. Alternative mechanisms for phosphate trafficking, from the cell to the enamel matrix, may play a role in amelogenesis, such as a possible storage and transport mechanism proposed for bone (Omelon and Grynpas, 2008). Here (Omelon and Grynpas, 2008), polyphosphates and/or calcium phosphates would be stored in the mitochondria and released to the extracellular matrix as needed through secretory vesicles. Polyphosphates would then be enzymatically cleaved into orthophosphates, allowing for hydroxyapatite (Hap) formation. For such an activity to occur, the expression of alkaline phosphatase would be required, and it has been shown that alkaline phosphatase activity is expressed at the apical pole of polarized ameloblasts, during both secretion and maturation (Kurahashi and Yoshiki, 1972).
Maintenance of basal cellular levels of sodium and potassium is typically achieved by the membrane-bound Na+,K+-ATPase pump (Glynn, 2002; Morth et al., 2011). The Na+,K+-ATPase pump is active as a dimer of 2 protein subunits, referred to as the α-subunit and the β-subunit (Glynn, 2002; Morth et al., 2011) and functions to move 3 Na+ out of cells in exchange for 2 K+ for every ATP that is hydrolyzed (Morth et al., 2011). Four unique genes code the α-subunit, and 4 unique genes code the β-subunit, while the α/β dimer combination appears to be somewhat tissue-specific (Morth et al., 2011). The presence of a Na+,K+-ATPase pump in enamel organ cells has previously been investigated by chemical and biochemical approaches suggesting that expression of such a pump localizes in the lateral plasma membranes of secretory ameloblasts and the stratum intermedium, and also in the PL cells of rat incisor enamel organs (Kashgarian et al., 1985; Garant et al., 1987). Analysis of recent immunolocalization data, however, suggests that a Na+,K+-ATPase pump, or at least one of the α subunits, localizes predominantly to the PL (Josephsen et al., 2010).
H+/Base Transport
Several review papers discussing pH regulation during amelogenesis are available (Smith et al., 1996; Smith, 1998; Lacruz et al., 2010a). During maturation, enamel organ cells express significant levels of carbonic anhydrases: Car2 and Car3 (intracellular CAs), Car6 (secreted), and Car12 (membrane-bound) (Lin et al., 1994; Toyosawa et al., 1996; Smith et al., 2006; Josephsen et al., 2010; Lacruz et al., 2012b). Enamel organ cells, including ameloblasts, also express at least 2 bicarbonate carrier proteins (NBCe1 and AE2) (Lyaruu et al., 2008; Paine et al., 2008; Bronckers et al., 2009). Based on the available antibodies and immunolocalizations, various models have been presented in recent years illustrating the spatial location of these bicarbonate transporters (Lyaruu et al., 2008; Paine et al., 2008; Bronckers et al., 2009; Josephsen et al., 2010) and an associated chloride channel CFTR (Bronckers et al., 2010) (Fig. 1). Analysis of our data indicated that NBCe1 is located at the basal pole of polarized (secretory)-stage ameloblasts, allowing for bicarbonate movement from the PL cells and/or blood circulation into ameloblasts, while AE2 and CFTR are located at the apical pole of ameloblasts, allowing for movement of bicarbonate from ameloblasts to the enamel matrix (Paine et al., 2008; Lacruz et al., 2010b). For AE2, our immunolocalization data were generated from frozen, unfixed sections of the mandibular incisors of 3-day-old mice (Paine et al., 2008), and, for NBCe1, 3-day-old mouse mandibles were fixed in Carnoy’s fixative (Paine et al., 2008; Lacruz et al., 2010b). Other investigators, working with formaldehyde, immersion-fixed, and paraffin-embedded mandibles from older mice (Lyaruu et al., 2008; Bronckers et al., 2009), or perfusion-fixed rats (Josephsen et al., 2010), have shown somewhat contradictory results related to AE2 and NBCe1 localization in ameloblasts, suggesting that AE2 is located to “cytoplasmic and in basolateral membranes” in maturation-stage ameloblasts (Bronckers et al., 2009), and NBCe1 expression is mostly, or exclusively, seen in the PL cells of the enamel organ (Josephsen et al., 2010). This would suggest that epitope presentation, antibody choice, and immunohistochemical methodologies have to be carefully and critically evaluated as data are gathered and presented. While there is still much to explore, it is clear, even at this time, that ameloblasts and the PL cells have an extraordinary capacity to buffer the enamel matrix against extreme pH changes arising from enamel Hap crystallite formation and growth (Smith et al., 1996; Smith, 1998; Lacruz et al., 2010a).
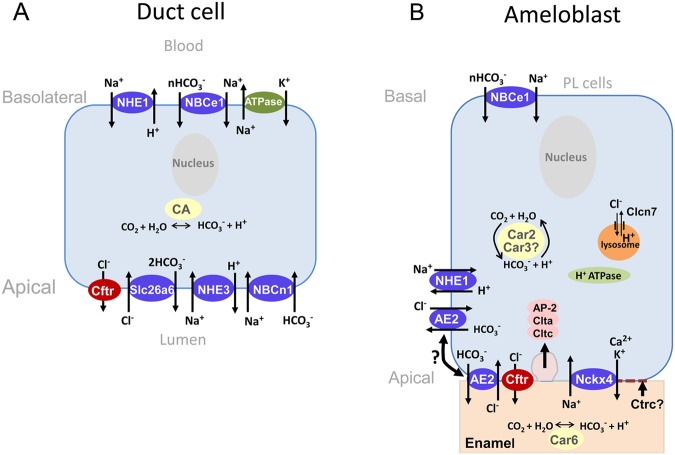
Figure 1.
Schematic representation of transepithelial transporters in pancreatic duct cells and ameloblasts. (A) Pancreatic duct cell. The main transepithelial transporters regulating pH via HCO3- movement, exhibited above, are modified from Park and Lee (2012). At the basolateral pole, HCO3- is regulated by NBCe1. Sodium uptake is mediated by NHE1, whereas extrusion involves Na+/K+ ATPases. Non-voltage-gated Na+ channels may also contribute to Na+ absorption at the basolateral pole (not shown). The apical pole of these cells expresses the Na+/HCO3- co-transporter (NBCn1) and the Na+/H+ exchanger (NHE3) that regulate Na+ intake, whereas Cl- is extruded by Cftr and exchanged by Slc26a6 for HCO3-. (B) Maturation-stage ameloblast. This model is not restricted to HCO3- transport but depicts our current knowledge of the proteins associated with the main activities of ameloblasts during maturation. Our model has been constructed based on previous reports (Bronckers et al., 2010; Josephsen et al., 2010) together with our own data. In this scheme, Ca2+ may be transported out of the cells at the apical pole by at least one of the NCKX members of sodium-calcium-potassium exchangers (NCKX4) (Hu et al., 2012). Cl- may be transported into the lysosomal system by Clcn7 to acidify luminal pH (Lacruz et al., 2013). We base our diagram on an osteoclast model (Jentsch, 2007) that commonly includes H+ATPases in the endosomal-lysosomal system. These pumps, as well as the function of a variety of CLC transporters, are ubiquitous features of endosomes-lysosomes required for luminal acidification (Jentsch, 2007). Chloride is also transported at the plasma membrane by Cftr and may also be linked to the anion exchanger AE2, as has been proposed (Bronckers et al., 2010). Two versions have been reported for the localization of AE2. In the schematic, AE2 localization at the apical pole was reported by Paine et al. (2008), whereas AE2 in the basolateral pole was described by Lyaruu et al. (2008) and Bronckers et al. (2009). Carbonic anhydrases 2 and 6 (Car2, Car6) remain important sources of localized bicarbonate production, as described elsewhere (Lacruz et al., 2012b). We hypothesize that, in addition, the isoform Car3 may also contribute to this function, which illustrates increased mRNA expression levels during maturation (Lacruz et al., 2012b). Intracellular passage of bicarbonate appears to be mediated by NBCe1 at the basal pole (Lacruz et al., 2010b). Such buffering may be required for ameliorating H+ release during Car2 and Car3 activities in the cytosol. Perhaps some of these H+ are shunted to the enamel area and others are used by the H+ATPase pumps of the endosome-lysosome. Extracellular bicarbonate transport to the enamel area may also be mediated by Cftr and AE2. In addition, as discussed in the text, if future research shows the presence of a Na+,K+-ATPase pump in ameloblasts, this would explain Na+ export to some extent. Endocytotic functions appear to be mediated, at least in part, by the AP-2/clathrin-dependent pathway in maturation-stage ameloblasts (Lacruz et al., 2013). It may also be the case that either the protease Ctrc functions in association with Klk4 in the processing of matrix breakdown which may subsequently undergo endocytosis, or it is perhaps directly associated with the lysosomal/endosomal apparatus. Note: The current diagram should be viewed as work in progress.
Future Directions
Enamel maturation and calcium transport were the subjects of 2 seminal papers published in the review series of this journal about 15 years ago (Smith, 1998; Hubbard, 2000). The resurgence of interest on the part of enamel biologists in gaining a broader understanding of the processes involved in the mineralization of enamel is reflected in the recent increase in publications on this subject. Our work has focused and will continue to focus on gaining a better understanding of chloride (Cl-) transport, endocytosis, and Ca2+ transport and on better defining proteolysis and associated processes. Regarding Cl- transport, we have shown abnormal mineralization in deciduous and permanent porcine teeth with cystic fibrosis transmembrane conductance regulator (CFTR) mutations (Lacruz et al., 2012b), highlighting the critical functions of this anion in amelogenesis. Chloride is the most abundant biological anion regulating intracellular pH, fluid secretion, cell volume, and changes in membrane excitability, functioning at the plasma membrane or in the membranes of intracellular organelles (al-Awqati et al., 1992; Jentsch, 2007). Our recent report on the expression of one chloride channel family (CLC) in maturation-stage ameloblasts associated with the lysosomal/endosomal pathway (Lacruz et al., 2013) prompted us to further investigate the possible expression of additional Cl- transporters in the enamel organ. Fig. 2 shows the expression levels of mRNA transcripts for the calcium-activated chloride channels (CLCA) and the chloride intracellular channels (CLIC), comparing secretory- and maturation-stage enamel organ cells from rats. These data add to the growing list of Cl- transporters with potential functions in enamel mineralization, either acting as Cl- exchangers, permitting entry into or exit from the cell or to be incorporated intracellularly into the lysosomal/endosomal system of ameloblasts. It remains to be elucidated whether the vast array of chloride channels detected in the enamel organ can function as alternative or complementary paths for the movement of this ion. What has become apparent is that the endocytosis of enamel matrix proteins (EMP) remains a challenging area of study. The resorptive functions of ameloblasts during maturation were recently clarified with the identification of several endocytosis-related gene products, such as clathrin and adaptor protein complex 2 (AP-2) subunits, in the enamel organ (Lacruz et al., 2013). The up-regulation of these mRNA transcripts and their cellular localization in the maturation-stage enamel organ prompted us to suggest that the endocytosis of enamel matrix protein debris involves ligand-receptor signaling activities (Lacruz et al., 2012a, 2013). Moreover, the identification in the enamel organ of the protease caldecrin (also known as chymotrypsin C or Ctrc) (Lacruz et al., 2011b, 2012a) may also be associated with endocytosis (see below). Originally, the similar expression pattern of Ctrc (Lacruz et al., 2011b) to that of the better known enamel protease kallikrein-4 (Klk4) (Bartlett and Simmer, 1999; Lu et al., 2008) led us to hypothesize that Ctrc may function to degrade enamel products; possibly smaller fragments generated by Klk4, although this was not tested. To help clarify the function of Ctrc in enamel, we have conducted an immunohistologic study of the cellular localization of Ctrc in enamel cells (Lacruz et al., 2011b). Fig. 3 shows the first molar of a 12-day-old mouse stained with Ctrc antibody, suggesting that Ctrc is localized to the apical pole of maturation-stage ameloblasts. Hence, it remains unclear whether Ctrc functions as an extracellular protease or at the plasma membrane/intracellular level, perhaps associated with the early endosomal apparatus.
Figure 2.
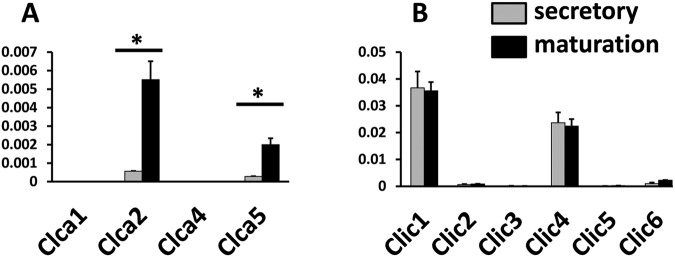
Relative expression of Clca and Clic mRNA transcripts in rat mandibular incisor enamel organ by qPCR. Following methods described in Lacruz et al. (2012a,b), we investigated the expression levels in the enamel organ of CLCA and CLIC gene families involved in Cl- transport. CLCAs are Cl- channels activated by cytosolic Ca2+ concentrations showing a strong outward rectification current (Begenisich and Melvin, 1998). The family is comprised of 5 different members (CLCA1-5), illustrating wide tissue distribution (Kidd and Thorn, 2000; Hartzell et al., 2005). Note that the Clca3 gene does not appear to be part of the rat genome and was not investigated. The CLIC family is comprised of 6 members (CLIC1-6), also exhibiting wide tissue distribution, but their physiological roles remain poorly described (Cromer et al., 2007). (A) Real-time PCR shows that levels of Clca2 and Clca5 significantly increased (one-way ANOVA; p < 0.05) in the maturation stage relative to the secretory stage. For the CLIC genes, differences in expression were observed only for Clic6 (2.3-fold increase in maturation). Levels of Clic1 and Clic4 were high in secretory and maturation stages, but no changes in transcript abundance were identified between stages. Values were normalized to β-actin (Y-axis) for each stage and calculated by the ΔΔCT method (Livak and Schmittgen, 2001). Real-time PCR primers used are listed in the Appendix Table.
Figure 3.
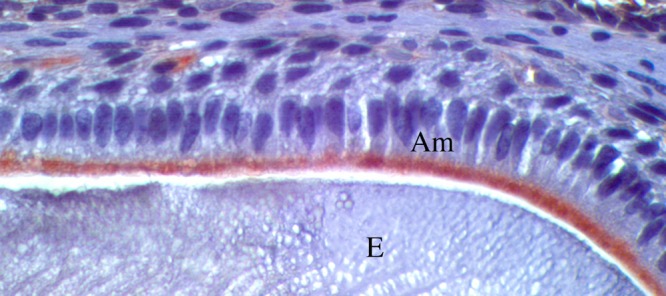
Immunostaining for Ctrc in mouse molars. Twelve-day-old mouse mandibles were fixed in 10% formalin, demineralized for 7 days in 10% EDTA, embedded in paraffin, and sectioned. Following imunohistochemistry protocols as described (Lacruz et al., 2013), sagittal sections of molars were immunostained with Ctrc antibody (ab35694, Abcam, Cambridge, UK). Ctrc is expressed at the apical pole of maturation-stage ameloblasts (Am) but not in the enamel matrix (E). Secretory ameloblasts did not stain for Ctrc (data not shown).
Many of these ion channels/transporters described in the enamel organ are already well-known in studies of the physiology of other soft-tissue organs, such as the kidney and pancreas, permitting the construction of a model for the mechanistic physiology of transport in enamel. Fig. 1 (see also the Table for gene products associated with the mature enamel organ cells that have been discussed in this review) is a schematic for interpreting intra- and extracellular transport in pancreatic duct cells and maturation-stage ameloblasts. From recent works (Bronckers et al., 2009; Josephsen et al., 2010; Okumura et al., 2010; Lacruz et al., 2012a), we understand that the solute carrier (SLC) gene family is highly represented in amelogenesis, indicating that there is an enormous void of knowledge as to the functions and relevance of other ions (i.e., sodium, potassium, zinc, iron) in enamel maturation (McKee et al., 1987; Lacruz et al., 2013). Similarities of ion transport with kidney and pancreas physiology indicate that enamel development should not be viewed as an isolated occurrence within mammalian development, but rather, ion transport biology must be integrated within overall organismic phenomena and thus is equally affected by physiological anomalies.
Table.
Summary of Rodent Genes/Proteins Associated with Transport Functions in Maturation-stage Enamel Organ
| Gene Symbol | Gene Name | Protein | Function | Localization in Enamel Organ | References |
|---|---|---|---|---|---|
| Ap2a1 | adaptor protein complex 2, alpha 1 subunit | Ap2a1 | endocytosis | apical pole Am | Lacruz et al., 2013 |
| Ap2a2 | adaptor protein complex 2, alpha 2 subunit | Ap2a2 | endocytosis | apical pole Am | Lacruz et al., 2013 |
| Ap2b1 | adaptor protein complex-2, beta 1 subunit | Ap2b1 | endocytosis | apical pole Am | Lacruz et al., 2013 |
| Ap2m1 | adaptor protein complex 2, mu 1 subunit | Ap2m1 | endocytosis | apical pole Am | Lacruz et al., 2013 |
| Ap2s1 | adaptor protein complex 2, sigma 1 subunit | Ap2s1 | endocytosis | apical pole Am | Lacruz et al., 2013 |
| Atp6v0a1 | ATPase, H+ transporting, lysosomal V0 subunit A1 | Atp6v0a1 | vacuolar proton pump | apical in RA and SA Am | Josephsen et al., 2010 |
| Atp6v0d2 | ATPase, H+ transporting, lysosomal V0 subunit D2 | Atp6v0d2 | vacuolar proton pump | Am | Lacruz et al., 2013 |
| Calb1 | calbindin 1 | Calb1 | calcium handling | cytoplasmic | Hubbard, 2000; Turnbull et al., 2004; Hubbard et al., 2011 |
| Calb2 | calbindin 2 | Calb2 | calcium handling | cytoplasmic | Hubbard, 2000; Turnbull et al., 2004; Hubbard et al., 2011 |
| Car12 | carbonic anhydrase 12 | Car12 | generate bicarbonate, H+, CO2 | membrane-bound | Lacruz et al., 2012b |
| Car2 | carbonic anhydrase 2 | Car2 | generate bicarbonate, H+, CO2 | intracellular | Lin et al., 1994; Toyosawa et al., 1996; Josephsen et al., 2010 |
| Car3 | carbonic anhydrase 3 | Car2 | generate bicarbonate, H+, CO2 | cytoplasmic | Lacruz et al., 2012b |
| Car6 | carbonic anhydrase 6 | Car6 | generate bicarbonate, H+, CO2 | extracellular (enamel matrix) | Smith et al., 2006 |
| Cftr | cystic fibrosis transmenbrane conductance regulator | Cftr | Cl- transport | apical pole of Am | Bronckers et al., 2010 |
| Clcn7 | chloride channel voltage sensitive 7 | Clc7 | Cl- transport (H+/Cl- exchanger?) | intracellular (lysosomes?) | Lacruz et al., 2013 |
| Clta | clathrin light-chain | Clta | endocytosis | apical pole Am | Lacruz et al., 2013 |
| Cltc | clathrin heavy-chain | Cltc | endocytosis | apical pole Am and PL | Lacruz et al., 2013 |
| Ctrc | caldecrin (chymotrypsin c) | Ctrc | enzyme | apical pole Am | Lacruz et al., 2011 |
| Klk4 | kallikrein-related peptidase 4 | Klk4 | enzyme | secreted to enamel matrix | Bartlett and Simmer, 1999; Lu et al., 2008 |
| Mcoln1 | lysosomal membrane-bound transient receptor potential cation channel | Mcoln1 | endocytosis | Am and PL | Lacruz et al., 2012a, 2013 |
| Nhe1 | Na+/H+ exchanger 1 | NHE1 | sodium/hydrogen exchanger | basolateral pole of Am | Josephsen et al., 2010 |
| Rab10 | member RAS oncogene family 10 | Rab10 | endocytosis | Am and PL | Lacruz et al., 2013 |
| Rab24 | member RAS oncogene family 24 | Rab24 | endocytosis | Am and PL | Lacruz et al., 2013 |
| Rcan1 | regulator of calcineurin 1 | Rcan1 | calcium handling | cytoplasmic | Lacruz et al., 2012a |
| S100a4 | S100 calcium-binding protein A4 | S100a4 | calcium handling | cytoplasmic | Hubbard et al., 2011; Lacruz et al., 2012a |
| S100g | S100 calcium-binding protein G | S100g | calcium handling | cytoplasmic | Lacruz et al., 2012a |
| Slc4a2 | anion-exchanger 2 | AE2 | possibly exchange Cl- | apical pole of Am | Lyaruu et al., 2008; Paine et al., 2008 |
| Slc4a4 | electrogenic sodium bicarbonate co-transporter | NBCe1 | import of bicarbonate | basolateral pole of Am and also in PL | Paine et al., 2008; Josephsen et al., 2010; Lacruz et al., 2010 |
| Slc24a4 | solute carrier family 24 | NCKX4 | sodium/potassium/calcium exchanger | apical pole of Am | Hu et al., 2012 |
| Slc34a2 | solute carrier family 34 (sodium phosphate), member 2 | NaPi-2b | membrane-bound | unknown | Lacruz et al., 2012a |
| Tfrc | transferrin receptor | Tfrc | ion transport | Am and PL/ membrane-bound | McKee et al., 1987; Lacruz et al., 2013 |
| unknown* | ATPase, Na+/K+ transporting-alpha subunit | ? | Na+/K+ transporting ATPase | PL, Am, Si | Kashgarian et al., 1985; Garant et al., 1987; Josephsen et al., 2010 |
Am = ameloblasts; PL = papillary layer; Si = stratum intermedium. *Note that the antibody used in the study by Josephsen and co-workers (2010) was an ATPase, Na+/K+ transporting-alpha subunit, of which there are 4 unique genes. It is unclear which of the 4 gene products this particular antibody recognizes.
Supplementary Material
Acknowledgments
The authors also thank the anonymous reviewers for their comments and critique.
Footnotes
This work was supported by grants DE013404 and DE019629 (MLP), DE022799 (RSL), and DK058563 and DK077162 (IK) from the US National Institutes of Health, as well as by the Melbourne Research Unit for Facial Disorders (MJH).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- al-Awqati Q, Barasch J, Landry D. (1992). Chloride channels of intracellular organelles and their potential role in cystic fibrosis. J Exp Biol 172:245-266. [DOI] [PubMed] [Google Scholar]
- Bartlett JD, Simmer JP. (1999). Proteinases in developing dental enamel. Crit Rev Oral Biol Med 10:425-441. [DOI] [PubMed] [Google Scholar]
- Begenisich T, Melvin JE. (1998). Regulation of chloride channels in secretory epithelia. J Membr Biol 163:77-85. [DOI] [PubMed] [Google Scholar]
- Bronckers AL, Lyaruu DM, Jansen ID, Medina JF, Kellokumpu S, Hoeben KA, et al. (2009). Localization and function of the anion exchanger Ae2 in developing teeth and orofacial bone in rodents. J Exp Zool B Mol Dev Evol 312(B):375-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronckers A, Kalogeraki L, Jorna HJ, Wilke M, Bervoets TJ, Lyaruu DM, et al. (2010). The cystic fibrosis transmembrane conductance regulator (CFTR) is expressed in maturation stage ameloblasts, odontoblasts and bone cells. Bone 46:1188-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromer BA, Gorman MA, Hansen G, Adams JJ, Coggan M, Littler DR, et al. (2007). Structure of the Janus protein human CLIC2. J Mol Biol 374:719-731. [DOI] [PubMed] [Google Scholar]
- Decker JD. (1963). A light and electron microscope study of the rat molar enamel organ. Arch Oral Biol 8:301-310. [DOI] [PubMed] [Google Scholar]
- Garant PR, Nalbandian J. (1968a). Observations on the ultrastructure of ameloblasts with special reference to the golgi complex and related components. J Ultrastruct Res 23:427-443. [DOI] [PubMed] [Google Scholar]
- Garant PR, Nalbandian J. (1968b). The fine structure of the papillary region of the mouse enamel organ. Arch Oral Biol 13:1167-1185. [DOI] [PubMed] [Google Scholar]
- Garant PR, Sasaki T, Colflesh PE. (1987). Na-K-ATPase in the enamel organ: localization and possible roles in enamel formation. Adv Dent Res 1:267-275. [DOI] [PubMed] [Google Scholar]
- Girardi AC, Di Sole F. (2012). Deciphering the mechanisms of the Na+/H+ exchanger-3 regulation in organ dysfunction. Am J Physiol Cell Physiol 302:C1569-C1587. [DOI] [PubMed] [Google Scholar]
- Glick PL. (1979). Patterns of enamel maturation. J Dent Res 58(Spec Iss B):883-895. [DOI] [PubMed] [Google Scholar]
- Glynn IM. (2002). A hundred years of sodium pumping. Annu Rev Physiol 64:1-18. [DOI] [PubMed] [Google Scholar]
- Hartzell C, Putzier I, Arreola J. (2005). Calcium-activated chloride channels. Annu Rev Physiol 67:719-758. [DOI] [PubMed] [Google Scholar]
- Hu P, Lacruz RS, Smith CE, Smith SM, Kurtz I, Paine ML. (2012). Expression of the sodium/calcium/potassium exchanger, NCKX4, in ameloblasts. Cells Tissues Organs 196:501-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard MJ. (2000). Calcium transport across the dental enamel epithelium. Crit Rev Oral Biol Med 11:437-466. [DOI] [PubMed] [Google Scholar]
- Hubbard MJ, McHugh NJ, Mangum JE. (2011). Exclusion of all three calbindins from a calcium-ferry role in rat enamel cells. Eur J Oral Sci 119(Suppl 1):112-119. [DOI] [PubMed] [Google Scholar]
- Jentsch TJ. (2007). Chloride and the endosomal-lysosomal pathway: emerging roles of CLC chloride transporters. J Physiol 578(Pt 3):633-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephsen K, Fejerskov O. (1977). Ameloblast modulation in the maturation zone of the rat incisor enamel organ. A light and electron microscopic study. J Anat 124(Pt 1):45-70. [PMC free article] [PubMed] [Google Scholar]
- Josephsen K, Takano Y, Frische S, Praetorius J, Nielsen S, Aoba T, et al. (2010). Ion transporters in secretory and cyclically modulating ameloblasts. A new hypothesis for cellular control of preeruptive enamel maturation. Am J Physiol Cell Physiol 299:C1299-C1307. [DOI] [PubMed] [Google Scholar]
- Kashgarian M, Biemesderfer D, Caplan M, Forbush B. (1985). Monoclonal antibody to Na,K-ATPase: immunocytochemical localization along nephron segments. Kidney Int 28:899-913. [DOI] [PubMed] [Google Scholar]
- Kidd JF, Thorn P. (2000). Intracellular Ca2+ and Cl- channel activation in secretory cells. Annu Rev Physiol 62:493-513. [DOI] [PubMed] [Google Scholar]
- Kurahashi Y, Yoshiki S. (1972). Electron microscopic localization of alkaline phosphatase in the enamel organ of the young rat. Arch Oral Biol 17:155-163. [DOI] [PubMed] [Google Scholar]
- Lacruz RS, Nanci A, Kurtz I, Wright JT, Paine ML. (2010a). Regulation of pH during amelogenesis. Calcif Tissue Int 86:91-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacruz RS, Nanci A, White SN, Wen X, Wang H, Zalzal SF, et al. (2010b). The sodium bicarbonate cotransporter (NBCe1) is essential for normal development of mouse dentition. J Biol Chem 285:24432-24438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacruz RS, Smith CE, Chen YB, Hubbard MJ, Hacia JG, Paine ML. (2011a). Gene-expression analysis of early- and late-maturation-stage rat enamel organ. Eur J Oral Sci 119(Suppl 1):149-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacruz RS, Smith CE, Smith SM, Hu P, Bringas P, Sahin-Tóth M, et al. (2011b). Chymotrypsin C (caldecrin) is associated with enamel development. J Dent Res 90:1228-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacruz RS, Smith CE, Moffatt P, Chang EH, Bromage TG, Bringas P, et al. (2012a). Requirements for ion and solute transport, and pH regulation during enamel maturation. J Cell Physiol 227:1776-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacruz RS, Smith CE, Bringas P, Chen YB, Smith SM, Snead ML, et al. (2012b). Identification of novel candidate genes involved in mineralization of dental enamel by genome-wide transcript profiling. J Cell Physiol 227:2264-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacruz RS, Brookes SJ, Wen X, Jimenex JM, Vikman S, Hu P, et al. (2013). Adaptor protein complex 2 (AP-2) mediated, clathrin dependent endocytosis, and related gene activities, are a prominent feature during maturation stage amelogenesis. J Bone Miner Res (Epub ahead of print 10/8/2012) (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MG, Muallem S. (2008). Physiology of duct cell secretion. In: Pancreas: an integrated textbook of basic science, medicine, and surgery. Beger H, Buchler M, Kozarek R, editors. Oxford, UK: Blackwell Publishing, pp. 78-90. [Google Scholar]
- Lin HM, Nakamura H, Noda T, Ozawa H. (1994). Localization of H(+)-ATPase and carbonic anhydrase II in ameloblasts at maturation. Calcif Tissue Int 55:38-45. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402-408. [DOI] [PubMed] [Google Scholar]
- Lu Y, Papagerakis P, Yamakoshi Y, Hu JC, Bartlett JD, Simmer JP. (2008). Functions of KLK4 and MMP-20 in dental enamel formation. Biol Chem 389:695-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyaruu DM, Bronckers AL, Mulder L, Mardones P, Medina JF, Kellokumpu S, et al. (2008). The anion exchanger Ae2 is required for enamel maturation in mouse teeth. Matrix Biol 27:119-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee MD, Zerounian C, Martineau-Doize B, Warshawsky H. (1987). Specific binding sites for transferrin on ameloblasts of the enamel maturation zone in the rat incisor. Anat Rec 218:123-127. [DOI] [PubMed] [Google Scholar]
- McKee MD, Warshawsky H, Nanci A. (1989). Cyclical incorporation of 33P into rat incisor enamel in vivo as visualized by whole-mount radioautography. Arch Oral Biol 34:989-993. [DOI] [PubMed] [Google Scholar]
- Morth JP, Pedersen BP, Buch-Pedersen MJ, Andersen JP, Vilsen B, Palmgren MG, et al. (2011). A structural overview of the plasma membrane Na+,K+-ATPase and H+-ATPase ion pumps. Nat Rev Mol Cell Biol 12:60-70. [DOI] [PubMed] [Google Scholar]
- Murer H, Forster I, Biber J. (2004). The sodium phosphate cotransporter family SLC34. Pflugers Arch 447:763-767. [DOI] [PubMed] [Google Scholar]
- Nanci A, Smith CE. (1992). Development and calcification of enamel. In: Calcification in biological systems. Bonucci E, editor. Boca Raton, FL: CRC Press, pp. 313-343. [Google Scholar]
- Okumura R, Shibukawa Y, Muramatsu T, Hashimoto S, Nakagawa K, Tazaki M, et al. (2010). Sodium-calcium exchangers in rat ameloblasts. J Pharmacol Sci 112:223-230. [DOI] [PubMed] [Google Scholar]
- Omelon SJ, Grynpas MD. (2008). Relationships between polyphosphate chemistry, biochemistry and apatite biomineralization. Chem Rev 108:4694-4715. [DOI] [PubMed] [Google Scholar]
- Paine ML, Snead ML, Wang HJ, Abuladze N, Pushkin A, Liu W, et al. (2008). Role of NBCe1 and AE2 in secretory ameloblasts. J Dent Res 87:391-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HW, Lee MG. (2012). Transepithelial bicarbonate secretion: lessons from the pancreas. Cold Spring Harb Perspect Med 2:a009571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purkerson JM, Schwartz GJ. (2007). The role of carbonic anhydrases in renal physiology. Kidney Int 71:103-115. [DOI] [PubMed] [Google Scholar]
- Reith EJ, Boyde A. (1981). Autoradiographic evidence of cyclical entry of calcium into maturing enamel of the rat incisor tooth. Arch Oral Biol 26:983-987. [DOI] [PubMed] [Google Scholar]
- Robinson C, Hiller CR, Weatherell JA. (1974). Uptake of 32P-labelled phosphate into developing rat incisor enamel. Calcif Tissue Res 15:143-152. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Goldberg M, Takuma S, Garant PR. (1990). Cell biology of tooth enamel formation. Functional electron microscopic monographs. Monogr Oral Sci 14:1-199. [PubMed] [Google Scholar]
- Sener A, Jijakli H, Zahedi Asl S, Courtois P, Yates AP, Meuris S, et al. (2007). Possible role of carbonic anhydrase in rat pancreatic islets: enzymatic, secretory, metabolic, ionic, and electrical aspects. Am J Physiol Endocrinol Metab 292:E1624-E1630. [DOI] [PubMed] [Google Scholar]
- Singh AK, Riederer B, Chen M, Xiao F, Krabbenhoft A, Engelhardt R, et al. (2010). The switch of intestinal Slc26 exchangers from anion absorptive to HCOFormula secretory mode is dependent on CFTR anion channel function. Am J Physiol Cell Physiol 298:C1057-C1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CE. (1998). Cellular and chemical events during enamel maturation. Crit Rev Oral Biol Med 9:128-161. [DOI] [PubMed] [Google Scholar]
- Smith CE, Nanci A. (1995). Overview of morphological changes in enamel organ cells associated with major events in amelogenesis. Int J Dev Biol 39:153-161. [PubMed] [Google Scholar]
- Smith CE, Nanci A. (1996). Protein dynamics of amelogenesis. Anat Rec 245:186-207. [DOI] [PubMed] [Google Scholar]
- Smith CE, Issid M, Margolis HC, Moreno EC. (1996). Developmental changes in the pH of enamel fluid and its effects on matrix-resident proteinases. Adv Dent Res 10:159-169. [DOI] [PubMed] [Google Scholar]
- Smith CE, Nanci A, Moffatt P. (2006). Evidence by signal peptide trap technology for the expression of carbonic anhydrase 6 in rat incisor enamel organs. Eur J Oral Sci 114(Suppl 1):147-153. [DOI] [PubMed] [Google Scholar]
- Takano Y. (1995). Enamel mineralization and the role of ameloblasts in calcium transport. Connect Tissue Res 33:127-137. [DOI] [PubMed] [Google Scholar]
- Toyosawa S, Ogawa Y, Inagaki T, Ijuhin N. (1996). Immunohistochemical localization of carbonic anhydrase isozyme II in rat incisor epithelial cells at various stages of amelogenesis. Cell Tissue Res 285:217-225. [DOI] [PubMed] [Google Scholar]
- Turnbull CI, Looi K, Mangum JE, Meyer M, Sayer RJ, Hubbard MJ. (2004). Calbindin independence of calcium transport in developing teeth contradicts the calcium ferry dogma. J Biol Chem 279:55850-55854. [DOI] [PubMed] [Google Scholar]
- Wennberg A, Bawden JW. (1978). Comparison of 33P with 45Ca distribution in developing rat molar enamel in vivo and in vitro. J Dent Res 57:111-117. [DOI] [PubMed] [Google Scholar]
- Yang D, Li Q, So I, Huang CL, Ando H, Mizutani A, et al. (2011). IRBIT governs epithelial secretion in mice by antagonizing the WNK/SPAK kinase pathway. J Clin Invest 121:956-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.