Highlights
▸ In this review, neural substrates of episodic memory development are discussed. ▸ We argue that hippocampus-mediated binding continues to develop during middle childhood along with other cortical regions in the prefrontal and parietal cortex. ▸ We underscore the role of development of hippocampal projections to and from cortical regions.
Abbreviations: DLPFC, dorsolateral PFC; fMRI, functional magnetic resonance imaging; lPFC, lateral PFC; MTL, medial temporal lobes; PHG, parahippocampal gyrus; PFC, prefrontal cortex; PPC, posterior parietal cortex; DTI, diffusion tensor imaging
Keywords: Memory, Development, Hippocampus, Prefrontal cortex, Parietal cortex, White matter
Abstract
Episodic memory is central to the human experience. In typically developing children, episodic memory improves rapidly during middle childhood. While the developmental cognitive neuroscience of episodic memory remains largely uncharted, recent research has begun to provide important insights. It has long been assumed that hippocampus-dependent binding mechanisms are in place by early childhood, and that improvements in episodic memory observed during middle childhood result from the protracted development of the prefrontal cortex. We revisit the notion that binding mechanisms are age-invariant, and propose that changes in the hippocampus and its projections to cortical regions also contribute to the development of episodic memory. We further review the role of developmental changes in lateral prefrontal and parietal cortices in this development. Finally, we discuss changes in white matter tracts connecting brain regions that are critical for episodic memory. Overall, we argue that changes in episodic memory emerge from the concerted effort of a network of relevant brain structures.
1. Episodic memory is a fundamental component of human cognition
Episodic memory is the capacity to form and retrieve conscious memories of specific past events (Tulving, 1972). Specifically, episodic memory entails the capacity to encode, store, and retrieve an event, in conjunction with contextual content associated with that event (e.g., “I met Sophia last week at Jake's party”). Extant models of memory have demonstrated that episodic memory can be differentiated functionally (e.g., Yonelinas, 2002) and neurologically (Eichenbaum et al., 2007) from other forms of explicit memory. For example, recollecting an episode is different from recognizing a past event on the basis of its familiarity; indeed, familiarity enables us to quickly experience an event as being part of our past in the absence of memory for context (e.g., “I met this person before, but can’t remember when or where”). Episodic memory supports daily acts such as remembering where one has placed her keys or whether one has already taken a pill that day. Furthermore, it provides the foundation for autobiographical memory (Nelson and Fivush, 2004) and contributes to the sense of continuity of self over time (Buckner and Carroll, 2007).
The implications of healthy episodic memory development are far-reaching. Recent evidence indicates that episodic recollection of ideas, and not a generalized sense of familiarity for them, is preferentially involved in reading comprehension because it supports the ability to integrate ideas from the text during retrieval (Mirandola et al., 2011). Furthermore, measures of episodic memory are part of standardized assessments of intellectual ability (Woodcock and Johnson, 1989). Thus, episodic memory has broad implications for learning and associated positive outcomes.
Finally, the development of episodic memory is impaired following mild forms of neurological insult due to cerebral hypoxia or ischemia (e.g., De Haan, 2012, Ghetti et al., 2010c), or traumatic brain injury (e.g., Hanten et al., 2004). Episodic memory is also impaired in several disorders, including depression (Backman and Forsell, 1994, Whalley et al., 2009), post-traumatic stress disorder (PTSD; Moradi et al., 2008, Vasterling et al., 2009), anxiety (Airaksinen et al., 2005), schizophrenia (Heinrichs and Zakzanis, 1998, Ragland et al., 2003), and Fragile X Syndrome (Ornstein et al., 2008). In some cases, episodic memory deficits emerge in childhood and precede the onset of the disorder (e.g., schizophrenia; Erlenmeyer-Kimling et al., 2000), and may be thus considered an endophetotypic marker of the disorder (Gottesman and Gould, 2003).
Given the centrality of episodic memory for human cognition, as well as its susceptibility to impairment across an array of neurological and psychiatric disorders, it is critical to understand the fundamental mechanisms underlying its typical development. While the developmental cognitive neuroscience of episodic memory has been largely uncharted until recently, new research in this arena has begun to provide important insights. The present review will feature the most important findings to date on development during middle childhood (roughly, ages 6–11) and outline directions for future research.
2. Protracted behavioral development of episodic memory during middle childhood
Infants and young children exhibit impressive abilities to remember past events (Bauer, 2007, Howe et al., 2009) even after long delays (Bauer et al., 2000, Simcock and Hayne, 2003). The ability to retrieve specific episodes continues to improve during middle childhood (e.g., Brainerd et al., 2004, Ghetti and Angelini, 2008, Ghetti et al., 2011, Schneider et al., 2002). In this review we focus on middle childhood, which corresponds roughly to the elementary school years.
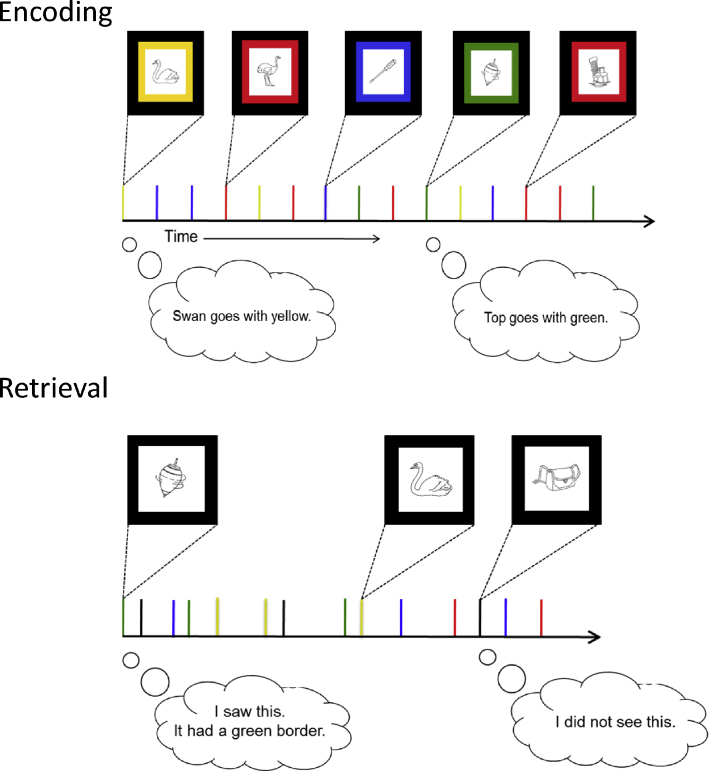
Children's episodic memory is assessed in a number of ways, for example with tasks that require participants to recall associations between events and the context in which they occurred (DeMaster and Ghetti, in press, Lloyd et al., 2009, Piolino et al., 2007). For example, children may be required to memorize objects presented with backgrounds of varying colors and they may be later asked to determine whether or not objects have been previous viewed and, if viewed, with what border color they were presented (Fig. 1). In some tasks, it is possible to assess the contribution of episodic recollection to performance, by either asking to participants to characterize their subjective memory experience as recollection or familiarity (Billingsley et al., 2002, Piolino et al., 2007) or by using estimation methods that tease apart the contribution of episodic recollection from that of familiarity (Brainerd et al., 2004, Ghetti and Angelini, 2008).
Fig. 1.
Example of task assessing recollection of episodic details. Participants are asked to remember the item as well as one contextual detail (in this case the color of the border surrounding the item). During the memory test their memory for the item as well as contextual details is probed. (For interpretation of the references to color in the figure caption and in the text, the reader is referred to the web version of the article.)
The robust improvement of episodic memory observed during middle childhood in these studies may be explained by a number of factors. There is extensive evidence of more frequent and efficient use of strategies based on semantic organization as well as increased sophistication of strategies used to regulate memory accuracy (e.g., Ghetti and Alexander, 2004, Ghetti et al., 2010a, Ornstein et al., 2006, Schwenk et al., 2007; for a review see Bjorklund et al., 2009). Furthermore, age-related differences are more pronounced when the testing situation imposes greater retrieval demands, suggesting improvement in the ability to conduct memory searches (e.g., Gee and Pipe, 1995, Hasselhorn, 1990, Paz-Alonso et al., 2009). Finally, there is a wealth of evidence indicating that memory improvements are in part explained by the development of metacognitive operations, which are involved in monitoring and controlling memory encoding and retrieval (DeMarie and Ferron, 2003, Ghetti, 2008, Ghetti et al., 2010a, Roebers et al., 2009). In this vein, it has been shown that, from age 6 to 17, subjective assessments of episodic memory quality are increasingly relied upon to make decisions, even though these assessments track actual episodic memory accurately across ages (Ghetti et al., 2011).
Importantly, it should be noted that improvements over middle childhood are not evident across all forms of explicit memory judgments. For example, several studies have shown age-invariance in the process of familiarity (i.e., ability to recognize past events without memory for specific detail) from age 8 onward (e.g., Billingsley et al., 2002, Brainerd et al., 2004, Ghetti and Angelini, 2008, Piolino et al., 2007) Thus, memory for isolated items or facts seems to reach adult levels before memory for items in context.
In summary, memory development in middle childhood predominantly involves increasingly skilled encoding and retention of complex event representations that make up our ability to encode and remember episodes (as opposed to, for example, quicker recognition of past events based on familiarity). The neural substrates of the development of episodic memory are germane to understanding the development of this important function. While the emphasis on strategies and metacognition highlight that the development of episodic memory fundamentally depends on changes in effortful control processes, our examination of the neural mechanisms will also underscore the role of changes in associative binding mechanisms.
3. The neural basis of the development of episodic memory
To recall the details of an event, our brains must process the specific features of the event and bind them in a way that specifies the spatiotemporal context in which these features were encountered. Adult episodic memory is supported by a distributed brain network that includes the hippocampus, and regions in the prefrontal cortex (PFC) and posterior parietal cortex (PPC).
The hippocampus, a structure in the medial temporal lobes, is critical for forming and retrieving representations that integrate the diverse aspects of an event – i.e., bound representations (Cansino et al., 2002, Davachi et al., 2003, Eichenbaum and Cohen, 2001, Eichenbaum et al., 2007, Konkel and Cohen, 2009). The perirhinal cortex and posterior parahippocampal gyrus (PHG), which surround the hippocampus along the anterior/posterior axis, are thought to send signals to the hippocampus to represent information about events (i.e., the perirhinal cortex) and context (i.e., the PHG) to be bound in the hippocampus (Diana et al., 2007, Ranganath, 2010). Functional magnetic resonance imaging (fMRI) research in adults suggests that the anterior hippocampus plays a key role in encoding and retrieval of flexibly bound representations (Chua et al., 2007, Chadwick et al., 2010, Giovanello et al., 2009, Prince et al., 2005). In contrast, the posterior hippocampus is proposed to be involved in establishing and retrieving a more fixed perceptual representation of the episode (Giovanello et al., 2009).
In contrast to the hippocampus, lateral PFC supports controlled processes that guide the encoding and monitor the retrieval of bound representations (e.g., Badre and Wagner, 2007, Blumenfeld and Ranganath, 2007, Gilboa et al., 2006). Finally, several regions in PPC have been implicated in episodic encoding and retrieval (e.g., Uncapher and Rugg, 2009, Uncapher and Wagner, 2009, Cabeza et al., 2008, Wagner et al., 2005). In the next sections we review extant literature on the development of the neural substrates of episodic memory, restricting our focus on studies using structural or functional neuroimaging (see Friedman, 2012 for a review on evidence gathered from event-related potentials).
3.1. Changes in medial-temporal lobes and the development of episodic representation
The medial temporal lobes, including the hippocampus and surrounding cortices, have long been linked to memory function (Cohen and Squire, 1980, Scoville and Milner, 1957). As noted earlier, the hippocampus is considered responsible for operations that bind representations, thereby forming and reinstating novel associations (Davachi, 2006, Konkel and Cohen, 2009, Moscovitch, 2008).
The hippocampus develops rapidly in the first years of life and more subtly thereafter. For example, hippocampal volumes have been found to double from birth to the first year, with further increases in the second year (Gilmore et al., 2011). After the first few years of life, volumetric changes are limited or absent (Gogtay et al., 2006) and the hippocampus has established the basic function of the tri-synaptic circuit (Seress, 2001; see also Seress and Ribak, 1995). This circuit is the major pathway within the hippocampus and feeds into efferent pathways which carry signals to the parietal and frontal lobes. This early development has been argued to support the emergence of episodic memory during infancy (Bauer, 2007). These finding have led to the hypothesis that hippocampal changes would be particularly important for episodic memory in infancy and during early childhood rather than later in childhood when, as discussed in later sections, cortical changes are robust. From this perspective, the behavioral episodic memory improvements observed during middle childhood would depend on cortical development, primarily in the prefrontal cortex (Newcombe et al., 2007).
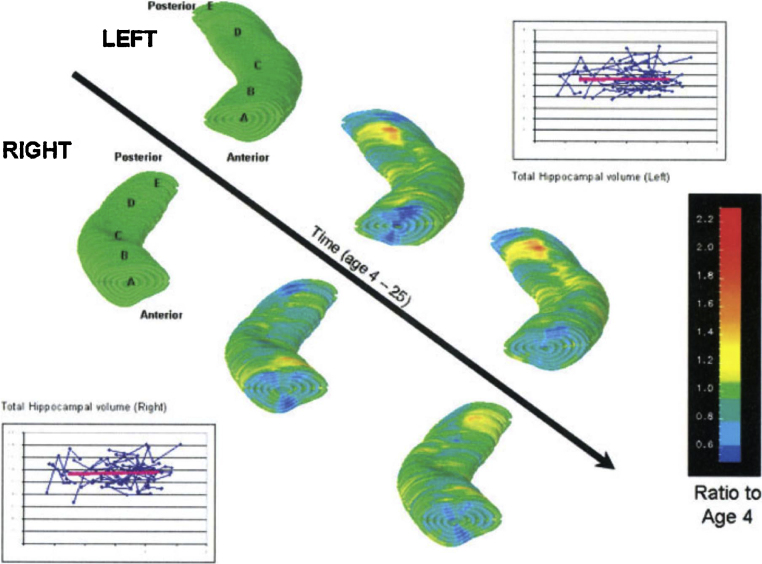
However, more fine-grained analyses have revealed that the microstructure of the hippocampus continues to mature well beyond early childhood. A longitudinal structural MRI study has now provided evidence that, while overall hippocampal volume is relatively stable after early childhood, the anterior hippocampus loses mass and the posterior hippocampus gains mass from age 4 to 25 (Gogtay et al., 2006) (Fig. 2). One could speculate that reductions in the size of the anterior hippocampus may reflect synaptic pruning (Johnson et al., 1996); in contrast, volume increases in the posterior hippocampus may reflect neurogenesis, synaptic elaboration (e.g., Eckenhoff and Rakic, 1991). Consistent with the results reported by Gogtay et al. (2006), a recent analysis of post mortem brains shows that the posterior hippocampal regions continue to grow into adolescence (Insausti et al., 2010). The functional significance of these developmental changes is unknown.
Fig. 2.
Dynamic sequence of typical left and right hippocampal development between age 4 and 25 years. The images are ratio maps at each age obtained by dividing the average hippocampal size at that age, at each hippocampal point by the size at the corresponding hippocampal point at age 4. The side bar provides a color representation of ratios where ratios >1 indicate that there is an increase in hippocampal volume at the point compared to age 4, while ratios <1 indicate a decrease in hippocampal volumes in later scans. The letters correspond to different hippocampal regions: A, head of the hippocampus; B, anterior half of the hippocampus; C, middle third of the hippocampus; D, posterior half of the hippocampus; E, posterior pole of the hippocampus.
Adapted with permission from Gogtay et al. (2006).
To our knowledge, only a handful of published studies have examined the relationship between hippocampal volume and episodic memory in typically developing children (e.g., Østby et al., 2011, Sowell et al., 2001, Yurgelun-Todd et al., 2003). In immediate memory tasks, several studies reported weakly negative correlations between overall volume and performance (Sowell et al., 2001, Yurgelun-Todd et al., 2003). In contrast, in a recall task occurring after a 1-week delay, a positive correlation was found (Østby et al., 2011). In this study, no correlation was found for immediate test performance, suggesting a connection between hippocampal volume and consolidation processes. These studies, however, did not distinguish between anterior and posterior hippocampus, which – as we have just discussed – show opposite trends in structural development (Gogtay et al., 2006). Further, these studies did not test for age differences in the relation between hippocampal volume and episodic memory; thus it is not clear whether the reported relationships hold at different points in development.
A recent study reported developmental differences in the relation between regional hippocampal volumes and episodic memory (DeMaster et al., in press). In adults, individuals with a smaller hippocampal head (i.e., anterior hippocampus) and larger hippocampal body (i.e., posterior hippocampus) exhibited stronger episodic memory performance; in children, these associations were not found, but positive associations were found with the tail of the hippocampus (see also Poppenk and Moscovitch, 2011 for similar hippocampal volume-episodic memory associations in adults). Consistent with Gogtay et al. (2006), age-related decreases in hippocampal volume were found in the head of the hippocampus, and age-related increases were found in the hippocampal body. These results lead to the hypothesis that age-related decreases in anterior hippocampal volume and increases in posterior hippocampus (Gogtay et al., 2006) may promote the regional specialization of hippocampal regions for episodic memory.
Furthermore, recent cross-sectional fMRI research provides initial evidence that changes in anterior regions of the hippocampus may be particularly relevant for episodic memory development (Ghetti et al., 2010b, Maril et al., 2010, Paz-Alonso et al., 2008). Based on these studies, we propose that an age-related reduction in the volume of the anterior hippocampus, which may reflect pruning of excess synapses, leads to greater selectivity of this region for episodic memory. Although the developmental relationship between structure and functional specialization has not been characterized, the idea that pruning as reflected in cortical thinning may underlie the emergence of functional specialization has been proposed (Casey et al., 2005, Lu et al., 2009). This hypothesis may be extended to guide investigations of changes in the anterior hippocampus.
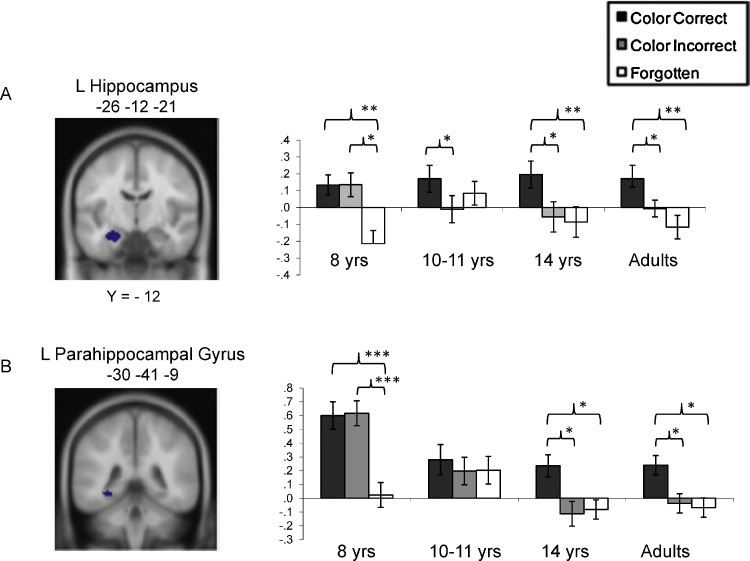
An fMRI study involving several age groups provides initial support for increased functional selectivity in the anterior hippocampus (Ghetti et al., 2010b). In this study, we examined age-related differences in hippocampal activation during stimulus encoding between 8-year-olds, 10–11-year-olds, 14-year-olds, and young adults. Participants engaged in incidental encoding of a series of object drawings that appeared either in green or red ink. They provided semantic judgments that differed based on the color of the item, thereby orienting participants to attend to associations between items and their color. Later, participants were asked to provide item recognition judgments on black-ink test items. For items that they reported recognizing, they were asked to recall the color of the drawing. We consider this task episodic because it tests an arbitrary association (between an object and the color in which it appears) formed during a single encoding exposure.
As shown in Fig. 3A, the adults’ activation profile in the left anterior hippocampus was consistent with selective involvement in episodic memory: increased activation was observed for memory for items and contextual information compared to recognized items without contextual information as well as to forgotten items, which did not differ from one another. The 8-year-olds’ anterior hippocampal profile was consistent with involvement in item recognition: greater activation for recognized versus unrecognized objects regardless of whether the contextual information (color) was remembered. The 10–11-year-olds’ profile seemed to reflect a phase of transition, as it showed neither a selective response to episodic memory nor a general response to item recognition. Finally, 14-year-olds exhibited the same selective pattern as adults. In the right hippocampus, these findings were largely replicated, though 10–11-year-olds exhibited a pattern of activation consistent with item recognition, as did 8-year-olds. These results suggest that the anterior hippocampus becomes functionally specialized for episodic memory around the transition to adolescence. More reliable selectivity in the anterior hippocampus in adults compared to 8–11-year-olds during episodic retrieval was also recently documented (DeMaster and Ghetti, in press).
Fig. 3.
Age-related differences in patterns of activation during encoding in (A) the left anterior hippocampus and (B) left posterior parahippocampal gyrus as a function of subsequent memory performance.
Adapted with permission from Ghetti et al. (2010b).
Extrapolating from adult fMRI studies implicating anterior hippocampus in memory for flexibly bound associations and posterior hippocampus in memory for fixed perceptual representations of an episode (Chua et al., 2007, Chadwick et al., 2010, Giovanello et al., 2009, Prince et al., 2005), structural changes in the anterior hippocampus may lead to increasing ability to rapidly form and retrieve the flexible representations that are central to episodic memory, enabling retrieval across multiple cues. Synaptic pruning in the anterior hippocampus could increase the efficiency of the rapid binding mechanism, which could contribute to increased flexibility of memory retrieval over development. Changes in memory ability over middle childhood include increased access flexibility and reduced dependence on contextual cues to guide retrieval that has been linked to changes in PFC-mediated retrieval strategies (Ackerman, 1982, Paz-Alonso et al., 2009). Here, we hypothesize that hippocampal changes also contribute to improvements in episodic memory over middle childhood.
While the observed difference in the anterior hippocampus may be particularly critical for episodic memory development, it is possible that structural changes in the posterior hippocampus also play a role. Evidence of developmental differences in posterior hippocampal function has been reported (Ghetti et al., 2010b) and this region is closely connected with other regions in the MTL, such as the posterior PHG, which appear to exhibit developmental change. We put the posterior PHG findings into context below.
First, however, we should note that not all functional neuroimaging studies have reported developmental differences in hippocampal function between children and adults. A study by Ofen et al. (2007) involving participants aged 8–24 years found that hippocampus strongly predicted subsequent memory, but no age differences in hippocampal recruitment were found. In this study, participants were instructed to intentionally encode a series of photos of scenes. It is possible that age differences are less likely to emerge when individuals are oriented to process items globally as opposed to processing the relationship between an item and a specific contextual detail. Furthermore, scene stimuli strongly engage the hippocampus (Stern et al., 1996, Brewer et al., 1998); this strong engagement might have obscured subtle age differences. Reconciling these findings with others showing developmental differences in hippocampal function should be a goal for future investigations.
With regard to the posterior PHG, several models of episodic recollection identify this MTL region as supporting the encoding and retrieval of contextual information (e.g., Diana et al., 2007). Thus, in addition to changes to hippocampus proper, changes in the posterior PHG may contribute to development of episodic memory. Indeed, several studies, including ours, have shown patterns of activation in the PHG that are similar to those documented in the hippocampus (Ghetti et al., 2010b, Fig. 3B; see also Ranganath et al., 2004). In our study, it was not possible to disentangle the unique contribution of the hippocampus from that of the posterior PHG, because we did not assess memory for contextual information independent of memory for the association between items and contextual details.
Nonetheless, other developmental fMRI studies have provided evidence for robust development during childhood and adolescence in the posterior PHG associated with materials that are typically construed as contexts. For example, Golarai et al. (2007) found that age-related increases in size of an area in posterior PHG recruited during scene encoding that significantly predicted subsequent scene recognition memory but not face or object recognition (Golarai et al., 2007). In an additional study, age-related increases in recruitment of posterior PHG during encoding were observed for more visually complex scenes, but not for less visually complex scenes; this result was paralleled in behavioral performance, which improved with age for the former but not for the latter type of scene (Chai et al., 2010). Extrapolating from these findings, one may infer that the representation of contextual information changes during childhood, and that these changes may support the development of episodic memory.
3.2. Changes in prefrontal cortex and the development of controlled encoding and retrieval processes
The structural and functional development of the PFC has been long considered a candidate explanation for the development of episodic memory during middle childhood and beyond (Cycowicz et al., 2001, Ofen et al., 2007, Shing et al., 2008, Shing et al., 2010). This hypothesis is consistent with evidence that changes in PFC cortical thickness occur throughout adolescence and adulthood (e.g., Giedd, 2004, Paus, 1999, Sowell et al., 2004), and that these changes are related to episodic memory in cross-sectional samples (e.g., Sowell et al., 2001). Furthermore, this hypothesis is consistent with numerous behavioral studies showing that age-related improvements are largest in memory tasks that require PFC-dependent strategic processes (e.g., Bjorklund et al., 2009, Schneider and Pressley, 1997). The ability to monitor and manipulate information increases during middle childhood (e.g., Gathercole, 2004), and this behavioral change is associated with increased and more selective recruitment of PFC, particularly in the dorsolateral (DL) PFC (Crone et al., 2006).
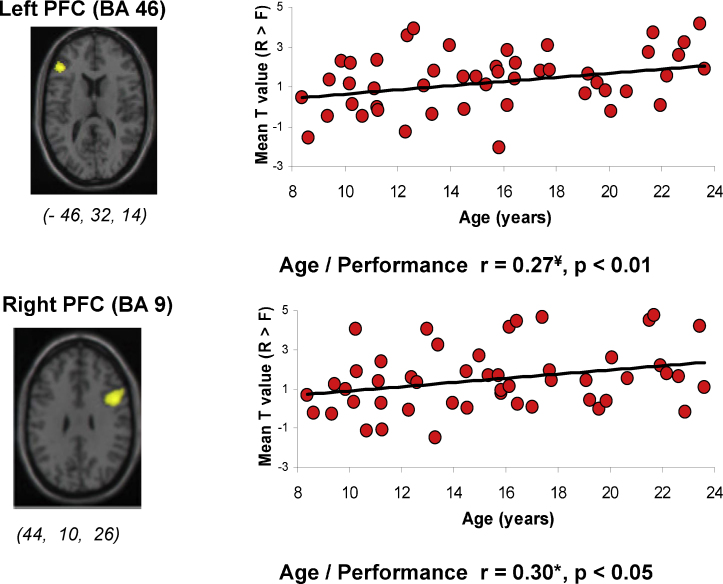
Consistent with the notion that PFC development might be the driving force of episodic memory development, the study by Ofen et al. (2007) discussed earlier found age-related increases in DL PFC recruitment during encoding which predicted subsequent subjective reports of episodic recollection (Fig. 4). Like Ofen et al. (2007), a second study confirms the importance of the development of lateral PFC in modulating controlled encoding mechanisms. Wendelken et al. (2011) investigated age-related differences in the neural correlates supporting selective encoding of relevant versus irrelevant items. They found a positive correlation between age, varying from 8 to 14 years, and level of recruitment of left DLPFC during selective encoding of scenes compared to passive viewing. This increase in activation was accompanied by an age-related increase in memory for the to-be-attended scenes.
Fig. 4.
Age-related differences in activation during encoding in bilateral dorsolateral prefrontal context as a function of subsequent memory performance. R refers to trials subsequently recognized and experienced as recollected (as opposed to known or evoking a general sense of familiarity); F refers to trials subsequently forgotten.
Adapted with permission from Ofen et al. (2007).
These findings are interesting in light of two other developmental fMRI studies of memory encoding. First, Ghetti et al. (2010b) found age-related increases in DLPFC recruitment between 8-year-olds and 10-year-olds, but no differences thereafter. Second, Maril et al. (2010), found no age-related differences in activity in lateral PFC between age 7 and 19. One difference between Ofen et al. (2007) and Wendelken et al. (2011), on the one hand, and Ghetti et al., 2010a, Ghetti et al., 2010b, Ghetti et al., 2010c and Maril et al. (2010) on the other, is the degree to which the tasks engage controlled processes. Ofen et al. (2007) explicitly instructed participants to attempt to remember the scenes; likewise, Wendelken et al. explicitly instructed participants to attend to a relevant category and ignore an irrelevant category of items. In contrast, Ghetti et al., 2010a, Ghetti et al., 2010b, Ghetti et al., 2010c and Maril et al. (2010) used less taxing, incidental encoding tasks. Thus, it is possible that age-related differences in PFC activation at encoding are most evident when the encoding task explicitly engages controlled processes involved in episodic encoding.
Age-related differences in PFC recruitment are also observed during retrieval. For example, in a study examining retrieval-related activity during true and false recognition in 8-year-olds, 12-year-olds, and adults, activity in left DLPFC, rostrolateral PFC, and ventrolateral PFC exhibit increasingly differentiated patterns of activation with age. These differences may reflect, respectively, developmental improvements in decision operations, judgments of relevance to the task goals, and specification of semantic retrieval cues (Paz-Alonso et al., 2008). Finally, a recent study examining the development of PFC-dependent processes regulating memory suppression revealed age-related increases in right DLPFC activity related to successful versus unsuccessful memory suppression (Paz-Alonso et al., 2010). Though the available evidence clearly points to developmental changes in controlled retrieval, many questions remain unanswered. For example, neuroscientists have yet to characterize the neurodevelopmental changes underlying the multiple controlled processes involved in episodic retrieval. The behavioral literature has traditionally distinguished among formal strategies and heuristics guiding memory retrieval: age-related improvements in the ability to remember episodes is supported by increasingly sophisticated use of strategies (Schwenk et al., 2007, Shing et al., 2010), flexible use of memory cues (Paz-Alonso et al., 2009) and tuned metacognitive monitoring of memory representations (Ghetti, 2008, Ghetti et al., 2008). While there is evidence that these processes recruit partially dissociable PFC regions (Mitchell and Johnson, 2009), the neurocognitive development of these processes has not been characterized. Nevertheless, the results to date already converge on the idea that development of the PFC plays a fundamental role in age-related improvements in strategic processes that support both episodic encoding and retrieval. An additional open question is how these processes influence the representations formed within the MTL. This question is addressed in the next section.
3.3. Structural and functional changes in fronto-temporal networks
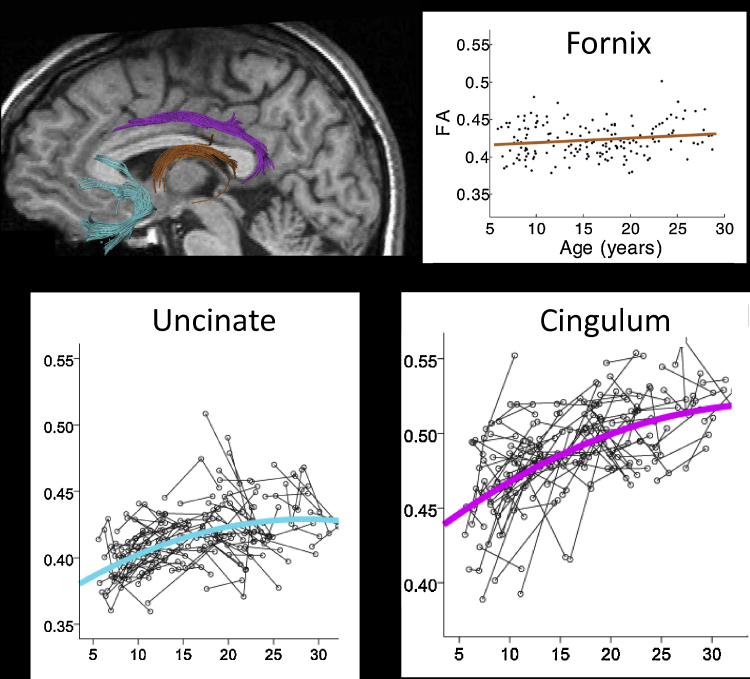
As reviewed above, regional changes in medial temporal and prefrontal regions play important roles in driving the development of episodic memory. In addition, changes in the way these and other regions communicate may be critical to current theories of episodic memory development. Three white matter tracts are particularly relevant to the exploration of changes in fronto-temporal interactions. First, the uncinate fasciculus connects the anterior hippocampus to lateral and orbitofrontal PFC (e.g., Kier et al., 2004, Petrides and Pandya, 1988, Schahmann and Pandya, 2006). Second, the cingulum bundle connects posterior hippocampus with the cingulate gyrus, as well as with parietal cortex, a region discussed further below (Mufson and Pandya, 1984, Nezamzadeh et al., 2010). Finally, the fornix connects the hippocampus with subcortical structures in the basal forebrain, thalamus, and mammillary bodies (Amaral and Insausti, 1990). Coherence in each of these three tracts has been associated with better episodic memory functioning in adults (Niogi et al., 2008, Tsivilis et al., 2008, Sepulcre et al., 2008, Villian et al., 2008).
A large cross-sectional diffusion tensor imaging (DTI) study of white matter tract development (N = 202) reports that the structural integrity of the fornix, as measured by both fractional anisotropy and mean diffusivity, is age-invariant between age 5 and young adulthood, whereas the uncinate fasciculus and the cingulum bundle continue to develop into adulthood (Lebel et al., 2008). The protracted development of these latter two tracts was recently replicated longitudinally by the same group (Lebel and Beaulieu, 2011; Fig. 5). This pattern of results suggests that changes in the latter two tracts would be particularly relevant for the development of episodic memory in middle and late childhood.
Fig. 5.
Probabilistic tractography image created by Catherine Lebel, showing the fornix, uncinate fasciculus, and cingulum bundle for one individual. Lebel, Beaulieu, and colleagues have conducted both cross-sectional and longitudinal research examining changes in DTI parameters in these and other tracts across 103 individuals ranging in age from 5 to 32. Shown here are a cross-sectional plot for fractional anisotropy (FA) as a function of age in the fornix and longitudinal plots for the uncinate fasciculus and cingulum bundle.
Adapted with permission from Lebel et al. (2008) and Lebel and Beaulieu (2011).
Thus far, there has been very little research linking developmental changes in these white matter tracts with episodic memory changes. However, one small cross-sectional study (N = 22) of 9–15-year-olds (Mabbott et al., 2009) reported that higher fractional anisotropy in the uncinate fasciculus is associated with better recall. Additionally, a small clinical study (Wu et al., 2010) reported that adolescents with traumatic brain injury exhibited lower fractional anisotropy in the uncinate fasciculus than age-matched controls, and that white matter integrity in this tract among the patients was correlated with episodic memory performance. Future research should investigate whether and how the maturation of the uncinate fasciculus, cingulum bundle, and fornix contributes to episodic memory development.
A structural change in these tracts may have direct implications for the reorganization of networks involving the hippocampus, consistent with shifts from short- to long-range connectivity reported in developmental work on other functional networks (e.g., Fair et al., 2007, Fair et al., 2009). Critically, although there is an emerging understanding of changes in functional connectivity over childhood and adolescence, there has been very little research on the consequences of these changes for cognition. Menon et al. (2005) conducted the only published study reporting functional connectivity analyses during episodic memory encoding in children (N = 25; ages 11–19 years). Using a blocked design fMRI paradigm, these authors found age-related increases in connectivity between MTL regions (i.e., the entorhinal cortex) and dorsolateral PFC.
Together, these results provide initial, indirect evidence that improvements in episodic memory depend in part on changes in long-range connectivity between the MTL and PFC. From this perspective, improvements in episodic memory would arise from increasingly coordinated activity among these regions, each of which is characterized by computational properties that are necessary to carry out one or more processes involved in episodic memory.
Of importance, strengthening of the network that supports episodic memory may drive and/or be driven by regional changes in the PFC, MTL, and/or parietal cortex. For example, development of controlled mechanisms implemented by lateral PFC might lead to enhanced connectivity with medial temporal regions resulting in developmental differences in hippocampal function. The use of longitudinal methods together with sophisticated statistical techniques such as dynamic causal modeling (DiQuattro and Geng, 2011, Friston et al., 2003) might help elucidate the nature of the developmental relationships among brain regions, the white matter tracts connecting them, and their influence on the development of episodic memory (see Wendelken et al., 2011).
3.4. Exploring the role of the parietal lobes in episodic memory development
Brain imaging research conducted over the last decade in adults has raised the possibility that parietal cortex plays a supporting role in episodic memory (for reviews, see Cabeza et al., 2008, Shimamura, 2010), despite the fact that memory deficits are not a core disturbance among patients with parietal lobe damage. fMRI studies of memory retrieval in adults have shown that lateral posterior parietal cortex (PPC; BA 7, 40), particularly in the left hemisphere, is more active when participants correctly indicate that they have seen a stimulus previously than when they correctly indicate that they have not (e.g., Cabeza et al., 2001, Shannon and Buckner, 2004, Slotnick and Schacter, 2004). A graded pattern of responses is observed in this region, as a function of the source and the amount of contextual information retrieved (Henson et al., 1999, McDermott et al., 2000) – or, at least, believed to be retrieved (Okado and Stark, 2003, Wheeler and Buckner, 2003).
Inspired by these findings, Berryhill et al. (2007) tested whether deficits in episodic retrieval would emerge if patients with bilateral PPC damage were tested with more sensitive measures. Indeed, they found that these patients provided fewer details when asked to engage in free recall of autobiographical memory.
Exactly what role(s) PPC plays in episodic memory is under active investigation (for review of various accounts, see Olson and Berryhill, 2009). It has been proposed that PPC serves as (1) an episodic buffer: a type of WM which maintains the episodic signal on-line for further assessment (Baddeley, 2000, Vilberg et al., 2006, Vilberg and Rugg, 2008), (2) a mnemonic accumulator which provides an assessment of memory signal strength (Wagner et al., 2005), (3) a center for the direction of attention to either bottom-up stimulus-driven memory signals, or top-down, internally driven memory states (Cabeza, 2008, Cabeza et al., 2008, Ciaramelli et al., 2008), or (4) a gauge of memory subjectivity or confidence (Ally et al., 2008, Simons et al., 2010). It has also been hypothesized that parietal cortex is a key intermediary in the modulation of the hippocampus by lPFC (Klostermann et al., 2008, Shimamura, 2010). Indeed, parietal cortex is anatomically connected both with posterior parahippocampal gyrus via the posterior cingulum bundle, and with PFC through the superior longitudinal fasciculus.
Complicating matters, the parietal lobes comprise multiple regions that are differentially engaged on memory tasks (Cabeza et al., 2008, Wheeler and Buckner, 2004, Vilberg and Rugg, 2008, Uncapher and Wagner, 2009). Nelson et al. (2010) have divided left lateral PPC into 6 subregions as a function of resting-state functional connectivity profiles, as well as episodic memory activation profiles. Among these regions, the left intraparietal sulcus area and the left anterior inferior parietal lobule exhibited retrieval success effects and were functionally connected with portions of the lPFC that have also been implicated in episodic memory (DLPFC and rostrolateral PFC, respectively).
A developmental approach could help to disambiguate various accounts of the role of PPC in episodic memory. Our fMRI research provides evidence for changes over childhood in PPC activation on episodic memory tasks (DeMaster and Ghetti, in press, Paz-Alonso et al., 2008, Paz-Alonso et al., 2010). In an fMRI study involving the Deese–Roediger–McDermott (DRM) paradigm (Paz-Alonso et al., 2008), we showed a retrieval success effect with a pattern of graded activation in left superior parietal cortex (BA 7) among 12-year olds and adults, but not among 8-year-olds. The absence in 8-year-olds of a graded PPC response that is observed by 12 years of age is consistent with the idea that during the course of childhood, regions associated with episodic retrieval become more specialized.
In future research, it will be important to keep in mind the fact that PPC is a functionally heterogeneous area. The initial parcellation of left PPC was conducted in adults (Nelson et al., 2010). However, the same research group has subsequently demonstrated that this parcellation can also be achieved in children between the ages of 7 and 10 years (Barnes et al., 2011). The next generation of research on episodic memory development should examine age-related changes in the functional profiles of each of these regions, as well as age-related changes in the strength of functional connectivity of each of these regions with lateral PFC and MTL.
4. Episodic memory beyond middle childhood
The development of episodic memory does not appear to be complete by the end of middle childhood. However, much less is known about the changes that take place during adolescence than during childhood. The possibility of gradual change is supported by a handful of studies showing improvements in strategy repertoire and selection during adolescence (Bray et al., 1985, Beuhring and Kee, 1987; for reviews see Bjorklund et al., 2009, Schneider and Pressley, 1997 for reviews). However, a handful of studies have provided a different picture, including evidence of no improvements – or even slight declines – during this period (e.g., Ghetti and Angelini, 2008, Waber et al., 2007). These findings raise new questions about the processes that might explain the occasionally counterintuitive results obtained for adolescents. Adolescence has been generally characterized as a period of substantial reorganization in brain and behavior leading to opportunity for further development as well as vulnerability (Dahl, 2004, Amso and Casey, 2006). Hormonal changes related to pubertal development may contribute to the reorganization of brain regions supporting episodic memory (e.g., Bramen et al., 2011, Neufang et al., 2009), which in turn may contribute to the observed changes in rate of change of episodic memory development during adolescence. Given large inter-individual differences in pubertal development, changes in episodic memory during adolescence could be studied most productively through longitudinal research tracking individual children from middle childhood into adolescence.
5. Brain development in context: factors that may influence the development of episodic memory
In reading the literature on age-related changes in the brain structures that support episodic memory, it would be easy to get the impression that these changes unfold in a prescribed manner as a child matures. However, there are important individual differences in episodic memory and underlying brain structures, and these differences are due in no small part to the influence of environmental factors. If we hope to understand why important individual differences in episodic memory exist during child development and beyond, we will have to take into consideration numerous environmental factors, from exposure to physical or psychosocial stressors to involvement in cognitive and physical activities. Below we outline some evidence for negative and positive influences on episodic memory, the MTL, and PFC.
On the negative side, there is evidence from multiple lines of research that hippocampal development is profoundly disrupted by negative environmental influences like maternal stress, chronic stress during childhood, and maltreatment (see Farah et al., 2008, Meaney, 2010, Tottenham and Sheridan, 2009). Thanks to extensive research in non-human animals, much is known about the underlying molecular and cellular mechanisms (e.g., Bagot and Meaney, 2010). There is also a growing body of literature indicating that PFC development is affected by chronic stress, intrauterine drug exposure, lead exposure during childhood, and other environmental factors (see Mackey et al., in press). These negative influences tend to cluster together as a function of socioeconomic status (SES), such that children growing up in a lower SES environment are at greater risk of being exposed to one or more of these negative experiences (Hackman et al., 2010).
On the positive side, there is also evidence that warm parental care during early childhood is associated with morphological changes in the hippocampus during later childhood (Luby et al., 2012, Rao et al., 2010). Furthermore, a growing literature is beginning to paint an encouraging picture about factors that might enhance the functioning of regions in the brain supporting episodic memory, with the promise for remediation. For example, cognitive training can lead to improved episodic memory (Brehmer et al., 2007, Schmiedek et al., 2010, Martensson and Lovden, 2011), and hippocampal structure and function can be modified by beneficial experiences (e.g., Draganski et al., 2006, Lövdén et al., 2011). Physical exercise also boosts episodic memory performance (e.g. Hötting et al., 2011) and hippocampal function (e.g. Griffin et al., 2011) in humans. In rodents, there is evidence that exercise induces neurogenesis (e.g. van Praag et al., 1999). These and other studies provide convincing evidence that the hippocampus changes as a function of experience, even in adulthood. Similarly, there is a growing body of evidence that PFC and PFC-dependent networks can be altered by cognitive training (Brem et al., 2010; for reviews see Klingberg, 2010, McCandliss, 2010).
6. Implications for education
The most profound changes in episodic memory take place during the elementary school years. Does this change influence how and how much a child can learn over this period? And, on the flipside, could the development of this cognitive skill itself be enhanced and/or accelerated by formal education? As noted in the introduction to a special issue of Developmental Cognitive Neuroscience examining the intersection of neuroscience and education, “to say that neuroscience is relevant to education is an understatement. By definition, education changes the brain; the brain changes every time a child – or an adult – learns something new” (Blakemore and Bunge, 2012). The emergent educational neuroscience literature has only just begun to intersect with research on memory development (see Sander et al., 2012), and the time is ripe to explore the reciprocal relationships between episodic memory development over middle childhood and elementary school education.
7. Conclusions
We have argued that the development of episodic memory emerges from the development of a brain network including at a minimum hippocampus, prefrontal cortex, and posterior parietal cortex. Local changes within a brain region as well as changes in long-range connectivity among these keys players have been documented. However, an assessment of the relationship between the development of the network as a whole and behavioral changes has yet to be undertaken. Furthermore, the many contextual factors affecting development underscore that it is of paramount importance that we include representative samples in our research, to ensure that our conclusions about ‘normative’ memory development are not based on a select subgroup of children.
Conflict of interest
The authors have no conflicts of interest to report.
Acknowledgments
The preparation of this manuscript was in part supported by R01MH091109 to S.G. and S.B. We thank Julia Ross from UC Davis for her technical assistance with the preparation of the manuscript, and Dr. Catherine Lebel from UCLA for providing the images for Fig. 5.
Contributor Information
Simona Ghetti, Email: sghetti@ucdavis.edu.
Silvia A. Bunge, Email: sbunge@berkeley.edu.
References
- Ackerman B.P. Retrieval variability: the inefficient use of retrieval cues by young children. Journal of Experimental Child Psychology. 1982;33:413–428. [Google Scholar]
- Airaksinen E., Larsson M., Forsell Y. Neuropsychological functions in anxiety disorders in population-based samples: evidence of episodic memory dysfunction. Journal of Psycholinguistic Research. 2005;39:207–214. doi: 10.1016/j.jpsychires.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Ally B.A., Simons J.S., McKeever J.D., Peers P.V., Budson A.E. Parietal contributions to recollection: electrophysiological evidence from aging and patients with parietal lesions. Neuropsychologia. 2008;46:1800–1812. doi: 10.1016/j.neuropsychologia.2008.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral D.G., Insausti R. The hippocampal formation. In: Paxinos G., editor. The Human Nervous System. Academic; San Diego: 1990. pp. 711–755. [Google Scholar]
- Amso D., Casey B.C. Beyond what develops when: neuroimaging may inform how cognition changes with development. Current Directions in Psychological Science. 2006;15:24–29. [Google Scholar]
- Backman L., Forsell Y. Episodic memory functioning in a community-based sample of old adults with major depression: utilization of cognitive support. Journal of Abnormal Psychology. 1994;103:361–370. doi: 10.1037//0021-843x.103.2.361. [DOI] [PubMed] [Google Scholar]
- Baddeley A. The episodic buffer: a new component of working memory? Trends in Cognitive Sciences. 2000;4:417–423. doi: 10.1016/s1364-6613(00)01538-2. [DOI] [PubMed] [Google Scholar]
- Badre D., Wagner A.D. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45:2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Bagot R.C., Meaney M.J. Epigenetics and the biological basis of gene × environment interactions. Journal of the American Academy of Child Psychiatry. 2010;49:752–771. doi: 10.1016/j.jaac.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Barnes K.A., Nelson S.M., Cohen A.L., Power J.D., Coalson R.S., Miezin F.M., Schlaggar B.L. Parcellation in left lateral parietal cortex is similar in adults and children. Cerebral Cortex. 2011 doi: 10.1093/cercor/bhr189. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer P.J. Recall in infancy: a neurodevelopmental account. Current Directions in Psychological Science. 2007;16:142–146. [Google Scholar]
- Bauer P.J., Wenner J.A., Dropik P.L., Wewerka S.S. Parameters of remembering and forgetting in the transition from infancy to early childhood. Monographs of the Society for Research in Child Development. 2000;65:1–204. [PubMed] [Google Scholar]
- Berryhill M.E., Phuong L., Picasso L., Cabeza R., Olson I.R. Parietal lobe and episodic memory: bilateral damage causes impaired free recall of autobiographical memory. Journal of Neuroscience. 2007;27:14415–14423. doi: 10.1523/JNEUROSCI.4163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuhring T., Kee D. Developmental relationships among metamemory, elaborative strategy use, and associative memory. Journal of Experimental Child Psychology. 1987;44:377–400. [Google Scholar]
- Billingsley R.L., Smith M.L., McAndrews M.P. Developmental patterns of priming and familiarity in explicit recollection. Journal of Experimental Child Psychology. 2002;82:251–277. doi: 10.1016/s0022-0965(02)00007-3. [DOI] [PubMed] [Google Scholar]
- Bjorklund D.F., Dukes C., Brown R.D. The development of memory strategies. In: Courage M., Cowan N., editors. The Development of Memory in Infancy and Childhood. Psychology Press; Hove East Sussex, UK: 2009. pp. 145–175. [Google Scholar]
- Blakemore S.J., Bunge S. At the nexus of neuroscience and education. Developmental Cognitive Neuroscience. 2012:2. doi: 10.1016/j.dcn.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld R.S., Ranganath C. Prefrontal cortex and long-term memory encoding: an integrative review of findings from neuropsychology and neuroimaging. Neuroscientist. 2007;13(3):280–291. doi: 10.1177/1073858407299290. [DOI] [PubMed] [Google Scholar]
- Brainerd C.J., Holliday R.E., Reyna V.F. Behavioral measurement of remembering phenomenologies: so simple a child can do it. Child Development. 2004;75:505–522. doi: 10.1111/j.1467-8624.2004.00689.x. [DOI] [PubMed] [Google Scholar]
- Bramen J.E., Hranilovich J.A., Dahl R.E., Forbes E.E., Chen J., Toga A.W., Sowell E.R. Puberty influences medial temporal lobe and cortical gray matter maturation differently in boys than girls matched for sexual maturity. Cerebral Cortex. 2011;21:636–646. doi: 10.1093/cercor/bhq137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray N.W., Turner L.A., Hersh R.E. Developmental progressions and regressions in the selective remembering states of EMR individuals. American Journal of Mental Deficiency. 1985;90:198–205. [PubMed] [Google Scholar]
- Brem S., Bach S., Kucian K., Guttorm T.K., Martin E., Lyytinen H., Brandeis D., Richardson U. Brain sensitivity to print emerges when children learn letter-speech sound correspondences. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:7939–7944. doi: 10.1073/pnas.0904402107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehmer Y., Li S.C., Muller V., Oertzen T.V., Lindenberger U. Memory plasticity across the life span: uncovering children's latent potential. Developmental Psychology. 2007;43:465–478. doi: 10.1037/0012-1649.43.2.465. [DOI] [PubMed] [Google Scholar]
- Brewer J.B., Zhao Z., Desmond J.E., Glover G.H., Gabrieli J.D.E. Making memories: brain activity that predicts how well visual experience will be remembered. Science. 1998;281:1185–1187. doi: 10.1126/science.281.5380.1185. [DOI] [PubMed] [Google Scholar]
- Buckner R.L., Carroll D.C. Self-projection and the brain. Trends in Cognitive Sciences. 2007;11:49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Role of posterior parietal regions in episodic memory retrieval: the dual attentional processes hypothesis. Neuropsychologia. 2008;46:1813–1827. doi: 10.1016/j.neuropsychologia.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R., Ciaramelli E., Olson I.R., Moscovitch M. The parietal cortex and episodic memory: an attentional account. Nature Reviews Neuroscience. 2008;9:613–625. doi: 10.1038/nrn2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R., Rao S.M., Wagner A.D., Mayer A.R., Schacter D.L. Can medial temporal lobe regions distinguish true from false? An event-related functional MRI study of veridical and illusory recognition memory. PNAS. 2001;98:4805–4810. doi: 10.1073/pnas.081082698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cansino S., Maquet P., Dolan R.J., Rugg M.D. Brain activity underlying encoding and retrieval of source memory. Cerebral Cortex. 2002;12:1048–1056. doi: 10.1093/cercor/12.10.1048. [DOI] [PubMed] [Google Scholar]
- Casey B.J., Tottenham N., Liston C., Durston S. Imaging the developing brain: what have learned about cognitive development? Trends in Cognitive Sciences. 2005;9:104–110. doi: 10.1016/j.tics.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Chadwick M.J., Hassabis D., Weiskopf N., Maguire E. Decoding individual episodic memory traces in the human hippocampus. Current Biology. 2010;20:544–547. doi: 10.1016/j.cub.2010.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai X., Ofen N., Jacobs L., Gabrieli J.D.E. Scene complexity: influence on perception, memory, and development in the medial temporal lobe. Frontiers of Human Neuroscience. 2010;4:1–10. doi: 10.3389/fnhum.2010.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua E.F., Schacter D.L., Rand-Giovannetti E., Sperling R.A. Evidence for a specific role of the anterior hippocampal region in successful associative encoding. Hippocampus. 2007;17:1071–1080. doi: 10.1002/hipo.20340. [DOI] [PubMed] [Google Scholar]
- Ciaramelli E., Grady C.L., Moscovitch M. Top-down and bottom-up attention to memory: a hypothesis (AtoM) on the role of the posterior parietal cortex in memory retrieval. Neuropsychologia. 2008;46:1828–1851. doi: 10.1016/j.neuropsychologia.2008.03.022. [DOI] [PubMed] [Google Scholar]
- Cohen N.J., Squire L.R. Preserved learning and retention of pattern-analyzing skill in amnesia: dissociation of knowing and not knowing that. Science. 1980;210:207–210. doi: 10.1126/science.7414331. [DOI] [PubMed] [Google Scholar]
- Crone E.A., Wendelken C., Donohue S., van Leijenhorst L., Bunge S.A. Neurocognitive development of the ability to manipulate information in working memory. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:9315–9320. doi: 10.1073/pnas.0510088103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cycowicz Y., Friedman D., Snodgrass J.G., Duff M. Recognition and source memory for pictures in children and adults. Neuropsychologia. 2001;39(3):255–267. doi: 10.1016/s0028-3932(00)00108-1. [DOI] [PubMed] [Google Scholar]
- Dahl R.E. Adolescent brain development: a period of vulnerabilities and opportunities, keynote address. Annals of the New York Academy of Sciences. 2004;1021:1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- Davachi L. Item, context and relational episodic encoding in humans. Current Opinion in Neurobiology. 2006;16:693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Davachi L., Mitchell J.P., Wagner A.D. Multiple routes to memory: distinct medial temporal lobe processes build item and source memories. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:2157–2162. doi: 10.1073/pnas.0337195100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Haan M. Memory development following early medial temporal lobe injury. In: Ghetti S., Bauer P.J., editors. Origins and Development of Recollection. Oxford University Press; New York: 2012. pp. 265–285. [Google Scholar]
- DeMarie D., Ferron J. Capacity, strategies, and metamemory: tests of a three-factor model of memory development. Journal of Experimental Child Psychology. 2003;84:167–193. doi: 10.1016/s0022-0965(03)00004-3. [DOI] [PubMed] [Google Scholar]
- DeMaster, D., Ghetti, S. Developmental differences in hippocampal and cortical contributions to episodic retrieval. Cortex, in press. [DOI] [PubMed]
- DeMaster, D., Pathman, T., Ghetti, S. Structural development of the hippocampus and episodic memory: developmental dissociations along the anterior/posterior axis. [DOI] [PubMed]
- Diana R.A., Yonelinas A.P., Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends in Cognitive Sciences. 2007;11:379–386. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- DiQuattro N.E., Geng J.J. Contextual knowledge configures attentional control networks. Journal of Neuroscience. 2011;31:18026–18035. doi: 10.1523/JNEUROSCI.4040-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganski B., Gaser C., Kempermann G., Kuhn H.G., Winkler J., Buchel C., May A. Temporal and spatial dynamics of brain structure changes during extensive learning. Journal of Neuroscience. 2006;26:6314–6317. doi: 10.1523/JNEUROSCI.4628-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckenhoff M.F., Rakic P. A quantitative analysis of synaptogenesis in the molecular layer of the dentate gyrus in the rhesus monkey. Brain Research. 1991;64:129–135. doi: 10.1016/0165-3806(91)90216-6. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H., Cohen N.J. Oxford University Press; New York: 2001. From Conditioning to Conscious Recollection: Memory Systems of the Brain. [Google Scholar]
- Eichenbaum H., Yonelinas A.P., Ranganath C. The medial temporal lobe and recognition memory. Annual Review of Neuroscience. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlenmeyer-Kimling L., Rock D., Roberts S.A., Janal M., Kestenbaum C., Cornblatt B., Gottesman I.I. Attention, memory, and motor skills as childhood predictors of schizophrenia-related .psychoses: the New York High-Risk Project. American Journal of Psychiatry. 2000;157:1416–1422. doi: 10.1176/appi.ajp.157.9.1416. [DOI] [PubMed] [Google Scholar]
- Fair D.A., Cohen A.L., Power J.D., Dosenbach N.U., Church J.A., Miezin F.M., Petersen S.E. Functional brain networks develop from a local to distributed organization. PLoS Computational Biology. 2009;5:e1000381. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair D.A., Dosenbach N.U.F., Church J.A., Cohen A.L., Brahmbhatt S., Miezin F.M., Schlaggar B.L. Development of distinct control networks through segregation and integration. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:13507–13512. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah M.J., Betancourt L., Shera D.M., Savage J.H., Giannetta J.M., Brodsky N.L., Hurt H. Environmental stimulation, parental nurturance and cognitive development in humans. Developmental Science. 2008;11:793–801. doi: 10.1111/j.1467-7687.2008.00688.x. [DOI] [PubMed] [Google Scholar]
- Friedman D. The development of episodic memory: an event-related brain potential vintage point. In: Ghetti S., Bauer P.J., editors. Origins and Development of Recollection. Oxford University Press; New York: 2012. pp. 242–264. [Google Scholar]
- Friston K.J., Harrison L., Penny W. Dynamic casual modeling. NeuroImage. 2003;19:1273–1302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- Gathercole S.E. Working memory and learning during the school years. Proceedings of the British Academy. 2004;125:365–380. [Google Scholar]
- Gee S., Pipe M.E. Helping children to remember: the influence of object cues on children's accounts of a real event. Developmental Psychology. 1995;31:746–758. [Google Scholar]
- Giedd J.N. Structural magnetic resonance imaging of the adolescent brain. Annals of the New York Academy of Sciences. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Gilmore J.H., Shi F., Woolson S., Knickmeyer R.C., Short S.J., Lin W., Shen D. Longitudinal development of cortical and subcortical gray matter from birth to 2 years. Cerebral Cortex. 2011;22:1–8. doi: 10.1093/cercor/bhr327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanello K.S., Schnyer D., Verfaellie M. Distinct hippocampal regions make unique contributions to relational memory. Hippocampus. 2009;19:111–117. doi: 10.1002/hipo.20491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghetti S. Processes underlying developmental reversals in false-memory formation: comment on Brainerd, Reyna, and Ceci (2008) Psychological Bulletin. 2008;134:773–777. doi: 10.1037/0033-2909.134.5.764. [DOI] [PubMed] [Google Scholar]
- Ghetti S., Alexander K.W. If it happened, I would remember it: strategic use of event memorability in the rejection of false autobiographical events. Child Development. 2004;75:542–561. doi: 10.1111/j.1467-8624.2004.00692.x. [DOI] [PubMed] [Google Scholar]
- Ghetti S., Angelini L. The development of recollection and familiarity in childhood and adolescence: evidence from the dual-process signal detection model. Child Development. 2008;79:339–358. doi: 10.1111/j.1467-8624.2007.01129.x. [DOI] [PubMed] [Google Scholar]
- Ghetti S., Castelli P., Lyons K. Knowing about not remembering: developmental dissociations in lack-of-memory monitoring. Developmental Science. 2010;13:611–621. doi: 10.1111/j.1467-7687.2009.00908.x. [DOI] [PubMed] [Google Scholar]
- Ghetti S., DeMaster D.M., Yonelinas A.P., Bunge S.A. Developmental differences in medial temporal lobe function during memory encoding. Journal of Neuroscience. 2010;30:9548–9556. doi: 10.1523/JNEUROSCI.3500-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghetti S., Lee J., Sims C., DeMaster D., Glaser N. Diabetic ketoacidosis and memory dysfunction in children with type I diabetes. Journal of Pediatrics. 2010;156:109–114. doi: 10.1016/j.jpeds.2009.07.054. [DOI] [PubMed] [Google Scholar]
- Ghetti S., Lyons K.E., Lazzarin F., Cornoldi C. The development of metamemory monitoring during retrieval: the case of memory strength and memory absence. Journal of Experimental Child Psychology. 2008;99:157–181. doi: 10.1016/j.jecp.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Ghetti S., Mirandola C., Angelini L., Corndoli C., Ciaramelli E. Development of subjective recollection: understanding of and introspection on memory states. Child Development. 2011 doi: 10.1111/j.1467-8624.2011.01645.x. [DOI] [PubMed] [Google Scholar]
- Gilboa A., Alain C., Stuss D.T., Melo B., Miller S., Moscovitch M. Mechanisms of spontaneous confabulations: a strategic retrieval account. Brain. 2006;129:1399–1414. doi: 10.1093/brain/awl093. [DOI] [PubMed] [Google Scholar]
- Gogtay N., Nugent T.F., 3rd, Herman D.H., Ordonez A., Greenstein D., Hayashi K.M., Thompson P.M. Dynamic mapping of normal human hippocampal development. Hippocampus. 2006;16:664–672. doi: 10.1002/hipo.20193. [DOI] [PubMed] [Google Scholar]
- Golarai G., Ghahremani D.G., Whitfield-Gabrieli S., Reiss A., Eberhardt J.L., Gabrieli J.D. Differential development of high-level visual cortex correlates with category-specific recognition memory. Nature Neuroscience. 2007;10:512–522. doi: 10.1038/nn1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman H., Gould T.D. The endophenotype concept in psychiatry: etymology and strategic intentions. American Journal of Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Griffin E.W., Mullally S., Foley C., Warmington S.A., O’Mara S.M., Kelly A.M. Aerobic exercise improves hippocampal function and increases BDNF in the serum of young adult males. Physiology and Behavior. 2011;104:934–941. doi: 10.1016/j.physbeh.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Hackman D.A., Farah M.J., Meaney M.J. Socioeconomic status and the brain: mechanistic insights from human and animal research. Nature Reviews. 2010;11:651–659. doi: 10.1038/nrn2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanten G., Dennis M., Zhang L., Barnes M., Roberson G., Archibald J., Levin H.S. Childhood head injury and metacognitive processes in language and memory. Developmental Neuropsychology. 2004;25:85–106. doi: 10.1080/87565641.2004.9651923. [DOI] [PubMed] [Google Scholar]
- Hasselhorn M. The emergence of strategic knowledge activation in categorical clustering during retrieval. Journal of Experimental Child Psychology. 1990;50:59–80. doi: 10.1016/0022-0965(90)90032-4. [DOI] [PubMed] [Google Scholar]
- Heinrichs R.W., Zakzanis K.K. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- Henson R.N., Rugg M.D., Shallice T., Josephs O., Dolan R.J. Recollection and familiarity in recognition memory: an event-related functional magnetic resonance imaging study. Journal of Neuroscience. 1999;19:3962–3972. doi: 10.1523/JNEUROSCI.19-10-03962.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hötting K., Reich B., Holzschneider K., Kauschke K., Schmidt T., Reer R., Röder B. Differential cognitive effects of cycling versus stretching/coordination training in middle-age adults. Health Psychology. 2011 doi: 10.1037/a0025371. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Howe M.L., Wimmer M.C., Blease K. The role of associative strength in children's false memory illusions. Memory. 2009;17:8–16. doi: 10.1080/09658210802438474. [DOI] [PubMed] [Google Scholar]
- Insausti R., Cebada-Sanchez S., Marcos P. Postnatal development of the human hippocampal formation. Advances in Anatomy Embryology and Cell Biology. 2010;206:1–86. [PubMed] [Google Scholar]
- Johnson M., Perry R.H., Piggott M.A., Court J.A., Spurden D., Lloyd S., Perry E.K. Glutamate receptor binding in the human hippocampus and adjacent cortex during development and aging. Neurobiology of Aging. 1996;17:639–651. doi: 10.1016/0197-4580(96)00064-4. [DOI] [PubMed] [Google Scholar]
- Kier E.L., Staib L.H., Davis L.M., Bronen R.A. MRI imaging of the temporal stem: Anatomic dissection tractography of the uncinate fasciculus, inferior occipitofrontal fasciculus, and Meyer's loop of the optic radiation. American Journal of Neuroradiology. 2004;25:677–691. [PMC free article] [PubMed] [Google Scholar]
- Klingberg T. Training and plasticity of working memory. Trends in Cognitive Sciences. 2010;14:317–324. doi: 10.1016/j.tics.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Klostermann E.C., Kane A.J., Shimamura A.P. Parietal activation during retrieval of abstract and concrete auditory information. NeuroImage. 2008;40:896–901. doi: 10.1016/j.neuroimage.2007.10.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkel A., Cohen N.J. Relational memory and the hippocampus: representations and methods. Frontiers of Human Neuroscience. 2009;2:166–174. doi: 10.3389/neuro.01.023.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C., Walker L., Leemans A., Phillips L., Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. NeuroImage. 2008;40:1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Lebel C., Beaulieu C. Longitudinal development of human brain wiring continues from childhood into adulthood. Journal of Neuroscience. 2011;31:10937–10947. doi: 10.1523/JNEUROSCI.5302-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd M.E., Doydum A.O., Newcombe N.S. Memory binding in early childhood; evidence for a retrieval deficit. Child Development. 2009;80:1321–1328. doi: 10.1111/j.1467-8624.2009.01353.x. [DOI] [PubMed] [Google Scholar]
- Lövdén M., Schaefer S., Noack H., Bodammer N.C., Kühn S., Heinze H.J., Lindenberger U. Spatial navigation training protects the hippocampus against age-related changed during early and late adulthood. Neurobiology of Aging. 2011 doi: 10.1016/j.neurobiolaging.2011.02.013. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Lu L., Dapretto M., O’Hare M., Kan E., McCourt S., Thompson P., Sowell E.R. Relationships between brain activation and brain structure in normally developing children. Cerebral Cortex. 2009;19:2595–2604. doi: 10.1093/cercor/bhp011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby J.L., Barch D.M., Belden A., Gaffrey M.S., Tillman R., Babb C., Nishino T., Suzuki H., Botteron K.N. Maternal support in early childhood predicts larger hippocampal volumes at school age. PNAS. 2012;109:2854–2859. doi: 10.1073/pnas.1118003109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabbott D.J., Rovet J., Noseworthy M.D., Smith M.L., Rockel C. The relations between white matter and declarative memory in older children and adolescents. Brain Research. 2009;1294:80–90. doi: 10.1016/j.brainres.2009.07.046. [DOI] [PubMed] [Google Scholar]
- Mackey, A.P., Raizada, R.D.S., Bunge, S.A. Environmental influences on prefrontal development. In: Stuss, D., Knight, R. (Eds.), Principles of Frontal Lobe Function. 2nd edition. Oxford University Press, Oxford, in press.
- Maril A., Davis P.E., Koo J.J., Reggev N., Zuckerman M., Ehrenfeld L., Rivkin M.J. Developmental fMRI study of episodic verbal memory encoding in children. Neurology. 2010;75:2110–2116. doi: 10.1212/WNL.0b013e318201526e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martensson J., Lovden M. Do intensive studies of foreign languages improve associative memory performance? Frontiers in Psychology. 2011;2:1–7. doi: 10.3389/fpsyg.2011.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCandliss B. Educational neuroscience: the early years. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:8049–8050. doi: 10.1073/pnas.1003431107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott K.B., Jones T.C., Petersen S.E., Lageman S.K., Roediger H.L. Retrieval success if accompanied by enhanced activation in anterior prefrontal cortex during recognition memory: an event-related fMRI study. Journal of Cognitive Neuroscience. 2000;12:956–976. doi: 10.1162/08989290051137503. [DOI] [PubMed] [Google Scholar]
- Meaney M. Epigenetics and the biological definition of gene × environment interactions. Child Development. 2010;81:41–79. doi: 10.1111/j.1467-8624.2009.01381.x. [DOI] [PubMed] [Google Scholar]
- Menon V., Boyett-Anderson J.M., Reiss A.L. Maturation of medial temporal lobe response and connectivity during memory encoding. Brain Research. Cognitive. 2005;25:379–385. doi: 10.1016/j.cogbrainres.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Mirandola C., Del Prete F., Ghetti S., Cornoldi C. Recollection but not familiarity differentiates memory for text in students with and without learning difficulties. Learning and Individual Differences. 2011;21:206–209. [Google Scholar]
- Mitchell K.J., Johnson M.K. Source monitoring 15 years later: what have we learned from fMRI about the neural mechanisms of source memory? Psychological Bulletin. 2009;135:638–677. doi: 10.1037/a0015849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moradi A.R., Herlihy J., Yasseri G., Shahraray M., Turner S., Dalgleish T. Specificity of episodic and semantic aspects of autobiographical memory in relation to symptoms of posttraumatic stress disorder (PTSD) Acta Psychologica. 2008;127:645–653. doi: 10.1016/j.actpsy.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Moscovitch M. The hippocampus as a stupid domain-specific module: implications for theories of remote memory and of imagination. Canadian Journal of Experimental Psychology. 2008;62:62–79. doi: 10.1037/1196-1961.62.1.62. [DOI] [PubMed] [Google Scholar]
- Mufson E., Pandya D. Some observations on the course and composition of the cingulum bundle in the rhesus monkey. Journal of Comparative Neurology. 1984;225:31–43. doi: 10.1002/cne.902250105. [DOI] [PubMed] [Google Scholar]
- Nelson S.M., Cohen A., Power J., Wig G., Miezin F.M., Wheeler M.E., Petersen S.E. A parcellation scheme for human left lateral parietal cortex. Neuron. 2010;67(1):156–170. doi: 10.1016/j.neuron.2010.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson K., Fivush R. The emergence of autobiographical memory: a social cultural developmental theory. Psychological Review. 2004;111:486–511. doi: 10.1037/0033-295X.111.2.486. [DOI] [PubMed] [Google Scholar]
- Neufang S., Specht K., Hausmann M., Gunturkun O., Herpertz-Dahlmann B., Fink G.R., Konrad K. Sex differences and the impact of steroid hormones on the developing human brain. Cerebral Cortex. 2009;19:464–473. doi: 10.1093/cercor/bhn100. [DOI] [PubMed] [Google Scholar]
- Newcombe N.S., Lloyd M.E., Ratliff K.R. Development of episodic and autobiographical memory: a cognitive neuroscience perspective. In: Kail R.V., editor. Advances in Child Development and Behavior. Elsevier; San Diego: 2007. pp. 37–85. [DOI] [PubMed] [Google Scholar]
- Nezamzadeh M., Weeden V.J., Wang R., Zhang Y., Zhan W., Young K., Schuff N. In vivo investigation of the human cingulum bundle using the optimization of MR diffusion spectrum imaging. European Journal of Radiology. 2010;75:e29–e36. doi: 10.1016/j.ejrad.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niogi S.N., Mukherjee P., Ghajar J., Johnson C.E., Kolster R., Lee H., McCandliss B.D. Structural dissociation of attentional control and memory in adults with and without mild traumatic brain injury. Brain. 2008;131:3209–3221. doi: 10.1093/brain/awn247. [DOI] [PubMed] [Google Scholar]
- Ofen N., Kao Y.C., Sokol-Hessner P., Kim H., Whitfield-Gabrieli S., Gabrieli J.D. Development of the declarative memory system in the human brain. Nature Neuroscience. 2007;10:1198–1205. doi: 10.1038/nn1950. [DOI] [PubMed] [Google Scholar]
- Okado Y., Stark C.E.L. Neural processing associated with true and false memory retrieval. Cognitive, Affective & Behavioral Neuroscience. 2003;3:323–334. doi: 10.3758/cabn.3.4.323. [DOI] [PubMed] [Google Scholar]
- Olson I.R., Berryhill M.E. Some surprising findings on the involvement of the parietal lobe in human memory. Neurobiology of Learning and Memory. 2009;91:155–165. doi: 10.1016/j.nlm.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornstein P.A., Baker-Ward L., Gordon B.N., Pelphrey K.A., Tyler C.S., Gramzow E. The influence of prior knowledge and repeated questioning on children's long-term retention of the details of a pediatric examination. Developmental Psychology. 2006;42:332–344. doi: 10.1037/0012-1649.42.2.332. [DOI] [PubMed] [Google Scholar]
- Ornstein P.A., Schaaf J.M., Hooper S.R., Hatton D.D., Mirrett P., Bailey D.B., Jr. Memory skills of boys with fragile X syndrome. American Journal of Mental Retardation. 2008;113:453–465. doi: 10.1352/2008.113:453-465. [DOI] [PubMed] [Google Scholar]
- Østby Y., Tamnes C.K., Fjell A.M., Walhovd K.B. Dissociating memory processes in the developing brain: the role of hippocampal volume and cortical thickness in recall after minutes versus days. Cerebral Cortex. 2011 doi: 10.1093/cercor/bhr116. (E-pub ahead of print) [DOI] [PubMed] [Google Scholar]
- Paus T. Imaging the brain before, during, and after transcranial magnetic stimulation. Neuropsychologia. 1999;37:219–224. doi: 10.1016/s0028-3932(98)00096-7. [DOI] [PubMed] [Google Scholar]
- Paz-Alonso P.M., Ghetti S., Donohue S.E., Goodman G.S., Bunge S.A. Neurodevelopmental correlates of true and false recognition. Cerebral Cortex. 2008;18:2208–2216. doi: 10.1093/cercor/bhm246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz-Alonso P.M., Ghetti S., Matlen B.J., Anderson M.C., Bunge S.A. Memory suppression is an active process that develops during middle childhood. Frontiers in Human Neuroscience. 2009;3:24. doi: 10.3389/neuro.09.024.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz-Alonso P.M., Ghetti S., Anderson M.C., Bunge S.A. Memory suppression develops over childhood. Poster at the Frontal Lobes 20th annual Rotman Research Institute conference; Toronto, Canada ; 2010, March. [Google Scholar]
- Petrides M., Pandya D.N. Association fiber pathways to the frontal cortex from the superior temporal region in the rhesus monkey. Journal of Comparative Neurology. 1988;273:52–66. doi: 10.1002/cne.902730106. [DOI] [PubMed] [Google Scholar]
- Piolino P., Hisland M., Ruffeveille I., Matuszewski V., Jambaqué I., Eustache F. Do school-age children remember or know the personal past? Consciousness and Cognition. 2007;16(1):84–101. doi: 10.1016/j.concog.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Poppenk J., Moscovitch M. A hippocampal marker of recollection memory ability among healthy young adults: contributions of posterior and anterior segments. Neuron. 2011;72:931–937. doi: 10.1016/j.neuron.2011.10.014. [DOI] [PubMed] [Google Scholar]
- Prince S.E., Daselaar S.M., Cabeza R. Neural correlates of relational memory: successful encoding and retrieval of semantic and perceptual associations. Journal of Neuroscience. 2005;25:1203–1210. doi: 10.1523/JNEUROSCI.2540-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland J.D., Moelter S.T., McGrath C., Hill S.K., Gur R.E., Bilker W.B., Gur R.C. Levels-of-processing effect on word recognition in schizophrenia. Biological Psychiatry. 2003;54:1154–1161. doi: 10.1016/s0006-3223(03)00235-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C. A unified framework for the functional organization of the medial temporal lobes and the phenomenology of episodic memory. Hippocampus. 2010;20:1263–1290. doi: 10.1002/hipo.20852. [DOI] [PubMed] [Google Scholar]
- Ranganath C., Yonelinas A.P., Cohen M.X., Dy C.J., Tom S.M., D’Esposito M. Dissociable correlates of recollection and familiarity within the medial temporal lobes. Neuropsychologia. 2004;42:2–13. doi: 10.1016/j.neuropsychologia.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Rao H., Betancourt L.M., Giannetta J.M., Brodsky N.L., Korczykowski M., Avants B.B., Farah M.J. Early parental care is important for hippocampal maturation: evidence from brain morphology in humans. NeuroImage. 2010;49:1144–1150. doi: 10.1016/j.neuroimage.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roebers C.M., Schmid C., Roderer T. Metacognitive monitoring and control processes involved in primary school children's test performance. British Journal of Educational Psychology. 2009;79:749–767. doi: 10.1348/978185409X429842. [DOI] [PubMed] [Google Scholar]
- Sander M.C., Werkle-Bergner M., Gerjets P., Shing Y.L., Lindenberger U. The two-component model of memory development and its potential implications for educational settings. Developmental Cognitive Neuroscience. 2012:2. doi: 10.1016/j.dcn.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schahmann J.D., Pandya D.N. Oxford University Press; New York: 2006. Fiber Pathways in the Brain. [Google Scholar]
- Schmiedek F., Lovden M., Lindenberger U. Hundred days of cognitive training enhance broad cognitive abilities in adulthood: findings from the cognitive study. Frontiers in Aging Neuroscience. 2010;13 doi: 10.3389/fnagi.2010.00027. ii-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider W., Knopf M., Stefanek J. The development of verbal memory in childhood and adolescence: findings from the Munich Longitudinal Study. Journal of Educational Psychology. 2002;94:751–761. [Google Scholar]
- Schneider W., Pressley M. Erlbaum; Mahwah, NJ: 1997. Memory Development Between Two and Twenty. [Google Scholar]
- Schwenk C., Bjorklund D.F., Schneider W. Factors influencing the incident of utilization deficiencies and other patterns of recall/strategy-use relations in a strategic memory task. Child Development. 2007;78:1771–1787. doi: 10.1111/j.1467-8624.2007.01090.x. [DOI] [PubMed] [Google Scholar]
- Scoville W.B., Milner B. Loss of recent memory after bilateral hippocampal lesions. Journal of Neurology, Neurosurgery and Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepulcre J., Masdeu J.C., Sastre-Garriga J., Goni J., Velez-de-Mendizabal N., Duque B., Villoslada P. Mapping the brain pathways of declarative verbal memory: evidence from white matter lesions in the living human brain. NeuroImage. 2008;42:1237–1243. doi: 10.1016/j.neuroimage.2008.05.038. [DOI] [PubMed] [Google Scholar]
- Seress L. Morphological changes of the human hippocampal formation from midgestation to early childhood. In: Nelson C.A., Luciana M., editors. Handbook of Cognitive Developmental Neuroscience. Massachusetts Institute of Technology; Boston: 2001. pp. 45–58. [Google Scholar]
- Seress L., Ribak C.E. Postnatal development and synaptic connections of hilar mossy cells in the hippocampal dentate gyrus of rhesus monkeys. Journal of Comparative Neurology. 1995;355:93–110. doi: 10.1002/cne.903550111. [DOI] [PubMed] [Google Scholar]
- Shannon B.J., Buckner R.L. Functional–anatomic correlates of memory retrieval that suggest nontraditional processing roles for multiple distinct regions within posterior parietal cortex. Journal of Neuroscience. 2004;24:10084–10092. doi: 10.1523/JNEUROSCI.2625-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamura A.P. Hierarchical relational binding in the medial temporal lobe: the strong get stronger. Hippocampus. 2010;20:1206–1216. doi: 10.1002/hipo.20856. [DOI] [PubMed] [Google Scholar]
- Shing Y.L., Werkle-Bergner M., Li S.-C., Lindenberger U. Associative and strategic components of episodic memory: a lifespan dissociation. Journal of Experimental Child Psychology. 2008;137(3):495–513. doi: 10.1037/0096-3445.137.3.495. [DOI] [PubMed] [Google Scholar]
- Shing Y.L., Werkie-Bergner M., Brehmer Y., Müller V., Li S., Lindenberger U. Episodic memory across the lifespan: the contributions of associative and strategic components. Neuroscience and Biobehavioral Reviews. 2010;34:1080–1091. doi: 10.1016/j.neubiorev.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Simcock G., Hayne H. Age-related changes in verbal and nonverbal memory during early childhood. Developmental Psychology. 2003;39:805–814. doi: 10.1037/0012-1649.39.5.805. [DOI] [PubMed] [Google Scholar]
- Simons J.S., Peers P.V., Mazuz Y.S., Berryhill M.E., Olson I.R. Dissociation between memory accuracy and memory confidence following bilateral parietal lesions. Cerebral Cortex. 2010;20:479–485. doi: 10.1093/cercor/bhp116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotnick S.D., Schacter D.L. A sensory signature that distinguishes true from false memories. Nature Neuroscience. 2004;7:664–672. doi: 10.1038/nn1252. [DOI] [PubMed] [Google Scholar]
- Sowell E.R., Delis D., Stiles J., Jernigan T.L. Improved memory functioning and frontal lobe maturation between childhood and adolescence: a structural MRI study. Journal of the International Neuropsychological Society. 2001;7:312–322. doi: 10.1017/s135561770173305x. [DOI] [PubMed] [Google Scholar]
- Sowell E.R., Thompson P.M., Toga A.W. Mapping changes in the human cortex throughout the span of life. Neuroscientist. 2004;10:372–392. doi: 10.1177/1073858404263960. [DOI] [PubMed] [Google Scholar]
- Stern C.E., Corkin S., Gonzalez R.G., Guimaraes A.R., Baker J.R., Jennings P.J., Rosen B.R. The hippocampal formation participates in novel picture encoding: evidence from functional magnetic resonance imaging. PNAS. 1996;93:8660–8665. doi: 10.1073/pnas.93.16.8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N., Sheridan M.A. A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Frontiers in Human Neuroscience. 2009;3:1–18. doi: 10.3389/neuro.09.068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsivilis D., Vann S.D., Denby C., Roberts N., Mayes A.R., Montaldi D., Aggleton J.P. A disproportionate role for the fornix and mammillary bodies in recall versus recognition memory. Nature Neuroscience. 2008;11:834–842. doi: 10.1038/nn.2149. [DOI] [PubMed] [Google Scholar]
- Tulving E. Episodic and semantic memory. In: Tulving E., Donaldson W., editors. Organization of Memory. Academic Press; New York: 1972. pp. 381–402. [Google Scholar]
- Uncapher M.R., Rugg M.D. Selecting for memory? The influence of selective attention on the mnemonic binding of contextual information. Journal of Neuroscience. 2009;29:8270–8279. doi: 10.1523/JNEUROSCI.1043-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uncapher M.R., Wagner A.D. Posterior parietal cortex and episodic encoding: insights from fMRI subsequent memory effects and dual-attention theory. Neurobiology of Learning and Memory. 2009;91:139–154. doi: 10.1016/j.nlm.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H., Kempermann G., Gage F.H. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nature Neuroscience. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- Vasterling J.J., Verfaellie M., Sullivan K.D. Mild traumatic brain injury and posttraumatic stress disorder in returning veterans: perspectives from cognitive neuroscience. Clinical Psychology Review. 2009;29:674–864. doi: 10.1016/j.cpr.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Vilberg K.L., Moosavi R.F., Rugg M.D. The relationship between electrophysiological correlates of recollection and amount of information retrieved. Brain Research. 2006;1122:161–170. doi: 10.1016/j.brainres.2006.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilberg K.L., Rugg M.D. Memory retrieval and the parietal cortex: a review of evidence from a dual-process perspective. Neuropsychologia. 2008;46:1787–1799. doi: 10.1016/j.neuropsychologia.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villian N., Desgranges B., Viader F., de la Sayette V., Mezenge F., Landeau B., Chetelat G. Relationships between hippocampal atrophy, white matter disruption, and gray matter hypometabolism in Alzheimer's disease. Journal of Neuroscience. 2008;28:6174–6181. doi: 10.1523/JNEUROSCI.1392-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waber D.P., De Moor C., Forbes P.W., Almli C.R., Botteron K.N., Leonard G., Rumsey J. The NIH MRI study of normal brain development: performance of a population based sample of healthy children aged 6 to 18 years on a neuropsychological battery. Journal of the International Neuropsychological Society. 2007;13:729–746. doi: 10.1017/S1355617707070841. [DOI] [PubMed] [Google Scholar]
- Wagner A.D., Shannon B.J., Kahn I., Buckner R.L. Parietal lobe contributions to episodic memory retrieval. Trends in Cognitive Sciences. 2005;9:445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Wendelken C., Baym C.L., Gazzaley A., Bunge S.A. Neural evidence of weaker attentional modulation in children relative to young adults. Developmental Cognitive Neuroscience. 2011;1:175–186. doi: 10.1016/j.dcn.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalley M.G., Rugg M.D., Smith A.P., Dolan R.J., Brewin C.R. Incidental retrieval of emotional contexts in post-traumatic stress disorder and depression: an fMRI study. Brain and Cognition. 2009;69:98–107. doi: 10.1016/j.bandc.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler M.E., Buckner R.L. Functional dissociation among components of remembering: control, perceived oldness, and content. Journal of Neuroscience. 2003;23:3869–3880. doi: 10.1523/JNEUROSCI.23-09-03869.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler M.E., Buckner R.L. Functional–anatomic correlates of remembering and knowing. NeuroImage. 2004;21:1337–1349. doi: 10.1016/j.neuroimage.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Woodcock R.W., Johnson M.B. DLM Teaching Resources; Allen, TX: 1989. Manual for Woodcock-Johnson Psychoeducational Battery-Revised. [Google Scholar]
- Wu T.C., Wilde E.A., Bigler E.D., Yallampalli R., McCauley S.R., Troyanskaya M., Levin H.S. Evaluating the relationship between memory functioning and cingulum bundles in acute mild traumatic brain injury using diffusion tensor imaging. Journal of Neurotrauma. 2010;27:303–307. doi: 10.1089/neu.2009.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas A.P. The nature of recollection and familiarity: a review of 30 years of research. Journal of Memory and Language. 2002;46:441–517. [Google Scholar]
- Yurgelun-Todd D.A., Killgore W.D., Cintron C.B. Cognitive correlates of medial temporal lobe development across adolescence: a magnetic resonance imaging study. Perceptual and Motor Skills. 2003;96:3–17. doi: 10.2466/pms.2003.96.1.3. [DOI] [PubMed] [Google Scholar]