Abstract
Based on phenomenological similarities between anhedonia (reward deficits) associated with drug withdrawal and the negative symptoms of schizophrenia, we showed previously that the atypical antipsychotic clozapine attenuated reward deficits associated with psychostimulant withdrawal. Antagonism of α2 adrenergic and 5-HT2A receptors may contribute to these effects of clozapine. We investigated here whether blockade of α2 or 5-HT2A receptors by idazoxan and M100907, respectively, would reverse anhedonic aspects of psychostimulant withdrawal. Idazoxan treatment facilitated recovery from spontaneous nicotine, but not amphetamine, withdrawal by attenuating reward deficits and increased number of somatic signs. Thus, α2 adrenoceptor blockade may have beneficial effects against nicotine withdrawal and may be involved in the effects of clozapine previously observed. M100907 worsened the anhedonia associated with nicotine and amphetamine withdrawal suggesting that monotherapy with M100907 may exacerbate the expression of the negative symptoms of schizophrenia or nicotine withdrawal symptoms in people, including schizophrenia patients, attempting to quit smoking.
Keywords: DHβE, brain reward thresholds, somatic signs of withdrawal, anhedonia
1. Introduction
Cessation of chronic drug use leads to a withdrawal syndrome characterized by affective symptoms including inability to experience pleasure, that is, anhedonia (American Psychiatric Association, 1994). Anhedonia is also one of the negative symptoms of schizophrenia and a core symptom of major depression (American Psychiatric Association, 1994). This phenomenological similarity suggests that similar neurobiological substrates may underlie these phenomena (Markou and Kenny, 2002; Paterson and Markou, 2007). We hypothesize here that the study of anhedonia associated with withdrawal states experienced after termination of chronic administration of various drugs of abuse, such as amphetamine or nicotine, may have heuristic value in identifying targets for therapeutic intervention for anhedonia characterizing a variety of psychiatric disorders. In support of this hypothesis, we previously showed that treatment with the atypical antipsychotic clozapine partially attenuated the magnitude and duration of the anhedonic aspects of nicotine or amphetamine withdrawal, especially in a subset of rats that showed resistance to the initial aversive effects of clozapine (Semenova and Markou, 2003). This partial efficacy of clozapine in attenuating anhedonia may be attributable to actions of clozapine on multiple neurotransmitter receptors (Baldessarini and Frankenburg, 1991; Meltzer, 1994; Brunello et al., 1995; Goudie et al., 1998). Thus, we sought to elucidate the receptor actions of clozapine that may mediate the previously shown partial effectiveness of clozapine treatment in attenuating the anhedonic aspects of psychostimulant withdrawal in rats.
The present study investigated whether blockade of α2 adrenergic or serotonin-2A (5-HT2A) receptors ameliorates the anhedonic aspects of drug withdrawal. The rationale for this work was based on evidence that clozapine has antagonist actions at α2A and 5-HT2A receptors (Baldessarini and Frankenburg, 1991; Meltzer, 1994; Brunello et al., 1995; Goudie et al., 1998). These actions of clozapine have been suggested to contribute to its limited therapeutic effects against the negative symptoms of schizophrenia (Meltzer et al., 1989; Carlsson et al., 1999; Weiner et al., 2001; Davis et al., 2003; Svensson, 2003; Wadenberg et al., 2007; Jones and McCreary, 2008; Meltzer and Huang, 2008; Leucht et al., 2009). Specifically, we investigated whether administration of idazoxan, an antagonist at presynaptic inhibitory α2 adrenergic receptors (Doxey et al., 1983; Tao and Hjorth, 1992; de Boer et al., 1996) and M100907, a highly selective 5-HT2A receptor antagonist (Palfreyman et al., 1993; de Paulis, 2001), would reverse the reward deficits and/or somatic signs associated with spontaneous nicotine and amphetamine withdrawal in rats. Additionally, we investigated the effects of idazoxan and M100907 on brain reward function during nicotine withdrawal precipitated by the administration of the nicotinic acetylcholine receptor antagonist dihydro-β-erythroidine. Differential effects of drug treatment in reversing brain reward deficits during precipitated and spontaneous nicotine withdrawal have been observed previously (Bruijnzeel et al., 2007).
Clinical studies indicated that drugs affecting noradrenergic (NA) transmission improved smoking cessation outcomes (Schnoll and Lerman, 2006; Lerman et al., 2007; Buchhalter et al., 2008). Relatively few studies have examined changes in NA neurotransmission, specifically α2 adrenergic receptor function, during psychostimulant withdrawal. The α2 adrenergic receptor agonist clonidine reduced nicotine withdrawal in humans (Glassman et al., 1988; Prochazka et al., 1992; Gourlay et al., 1994). However, other studies in humans reported no or very limited effectiveness of clonidine against nicotine withdrawal (Nana and Praditsuwan, 1998; Gourlay et al., 2004) suggesting that the blockade of α2 NA receptor may be a better strategy to ameliorate nicotine withdrawal than α2 NA agonist actions. In animals, a plethora of findings indicate that the stimulation of α2 adrenergic receptors reduced different aspects of opiate withdrawal (for reviews, Maldonado, 1997; Smith and Aston-Jones, 2008). Evidence for an ameliorative effect of NA manipulations on the anhedonic aspects of nicotine and/or amphetamine withdrawal in animal models is lacking. Further, decreased 5-HT function is associated with nicotine or cocaine withdrawal (Parsons et al., 1995; 1996; Harrison et al., 2001; Kenny and Markou, 2001). The effects of M100907 on animal models of anhedonia have not been investigated. Most of the animal models investigated the effects of M100907 have relevance to the positive symptoms of schizophrenia (Sorensen et al., 1993; Carlsson, 1995; Sipes and Geyer, 1995; Varty et al., 1999; Barr et al., 2004). Clinical trials with M100907 in neuroleptic-responsive schizophrenia patients have yielded mixed results (de Paulis, 2001), suggesting that more research is needed.
In the present study, the discrete trial intracranial self-stimulation (ICSS) procedure was used to measure anhedonia associated with psychostimulant withdrawal. ICSS directly activates the same neuronal circuits that are activated by natural reinforcers, and thus, provides a direct measure of brain reward function (Phillips et al., 1989; Wise et al., 1992). Brain reward thresholds are assessed by measuring the minimal electrical current intensity for which the animal is willing to respond to receive the stimulation. Thresholds are derived by recording the subject's responses as the current intensity of the electrical stimulus is varied systemically in alternating descending and ascending series. Elevations in thresholds reflect a decrease in the rewarding and motivational value of the stimulation and are an operational measure of anhedonia associated with drug withdrawal (Markou and Kenny, 2002; Semenova and Markou, 2003; Paterson and Markou, 2007). Because of direct activation of the brain reward pathways, there is no satiation, tolerance or sensitization to the rewarding effects of ICSS. Importantly, the discrete trial ICSS procedure used in this study allows the assessment of reward thresholds that are independent of response rates and are not affected by nonspecific motor effects of manipulation (Markou and Koob, 1992). Thus, a major advantage of this procedure is that it provides a quantitative measure of reward (brain reward thresholds measured in μA) that is extremely stable over periods of months under baseline conditions, allows repeated testing of subjects, and shows the predictable and reliable effects of pharmacological manipulations on brain reward function (Kornetsky et al., 1979; Markou and Koob, 1992).
2. Experimental procedures
2.1. Subjects
Male Wistar rats (Charles River; 320-360 g at the beginning of the experiments) were housed in groups of two in a humidity- and temperature-controlled vivarium on a 12 h light/dark cycle. Rats had ad libitum access to food and water throughout the course of the studies except during testing. Training and testing occurred during the dark cycle. All experiments were in accordance with the guidelines of the American Association for the Accreditation of Laboratory Animal Care and the National Research Council's Guide for Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee.
2.2. Drugs
Nicotine bitartrate (Sigma, St. Louis, MO) was subcutaneously (s.c.) delivered through 7, 14, or 28 day osmotic minipumps (9 mg/kg/day nicotine salt, 3.16 mg/kg/day base). D-amphetamine sulfate (National Institute on Drug Abuse, Bethesda, MD) was injected intraperitoneally (i.p.) three times per day according to an escalating dose regimen or delivered through osmotic minipumps (5 mg/kg/day for 7 days). Idazoxan hydrochloride (Sigma, St. Louis, MO) was administered i.p. in the dose range of 0.5-5.0 mg/kg. The nicotinic acetylcholine receptor antagonist dihydro-β-erythroidine hydrobromide (DHβE, Sigma, St. Louis, MO) was administered s.c. at a dose of 3 mg/kg. Corresponding control rats were treated (s.c., i.p., or through minipumps) with saline. All compounds, with the exception of M100907, were dissolved in saline and administered in a volume of 1 ml/kg. M100907 (commissioned for custom synthesis by ANAWA Trading SA, Zurich, Switzerland) for systemic administration was dissolved in sterile 0.9% saline with 1/3 or 1/4 equivalent HCl 0.1N and injected i.p. in a volume of 2 ml/kg. The M100907 solution, delivered by 28 day osmotic minipumps at a dose of 0.5 mg/kg/day salt, was dissolved in 0.1 M HCl and diluted with 0.9% saline (approximately 1/2 of the volume) according to the rats' body weights. Control rats were treated (i.p. or through osmotic minipumps) with the vehicle solution, which contained the same amount of HCl 0.1N.
2.3. Intracranial self-stimulation apparatus, surgery, and procedure
The ICSS apparatus, surgery, and procedure were identical to those described previously (Harrison et al., 2001; Semenova and Markou, 2003). Briefly, training and testing occurred in 16 sound-attenuated plexiglas chambers (San Diego Instruments, San Diego, CA) that contained a metal wheel manipulandum. Brain stimulation was delivered by constant current stimulators (San Diego Instruments, San Diego, CA). Subjects were prepared with bipolar stainless steel electrodes (Plastics One, Roanoke, VA) in the posterior lateral hypothalamus (anterior/posterior, −0.5 mm from bregma; lateral, ±1.7 mm; dorsal/ventral, −8.3 mm from dura) (Pellegrino et al., 1986) with the incisor bar elevated 5.0 mm above the interaural line.
The ICSS procedure was a modification of a procedure originally developed by Kornetsky and colleagues (Kornetsky et al., 1979). This discrete-trial current-threshold procedure has been described in detail by Markou and Koob (Markou and Koob, 1991; 1992). At the start of each trial, rats received a noncontingent electrical stimulus (100 Hz rectangular cathodal pulses). During the following 7.5 s limited hold (if the rats responded by turning the wheel manipulandum; positive response) they received a second contingent stimulus identical to the previous noncontingent stimulus. During a 2 s period immediately after a positive response, further responses had no consequences. If no response occurred during the 7.5 s limited hold, a negative response was recorded. The intertrial interval (ITI), which followed the limited hold period, had an average duration of 10 s (7.5-12.5 s). Responses occurring during the ITI resulted in a further 12.5 s delay of the onset of the next trial. Stimulation intensities varied according to the psychophysical method of limits. Thus, rats received four alternating series of ascending and descending current intensities starting with a descending series. Within each series, the stimulus intensity was altered by 5 μA steps between each set of trials (3 trials/set). The initial stimulus intensity was set at 30-40 μA above the baseline current threshold for each rat. A test session typically lasted 30 min and provided brain reward thresholds as the dependent variable. The threshold for each descending series was defined as the stimulus intensity between a successful completion of a set of trials (two consecutive sets), during which the rat failed to respond positively on two or more of the three trials for two consecutive steps. For the ascending series, the reverse situation defined the threshold. Thus, current thresholds were recorded for each of the four series, and the mean of these values was taken as the threshold for that session. Baseline reward thresholds were considered stable when less than 10% variation occurred over 5 consecutive days. The latency between the onset of the noncontingent stimulus at the start of each trial and a positive response was recorded as the response latency. The response latency for each test session was defined as the mean response latency of all trials during which a positive response occurred.
2.4. Ratings of somatic signs of nicotine withdrawal
Somatic signs of nicotine withdrawal were counted under white light conditions in cylindrical Plexiglas chambers (diameter 15 cm) with sawdust bedding on the floor. Each subject was observed for 10 min by an observer blind to the subjects' treatments. The standard checklist used was adapted from an opiate withdrawal signs checklist (Malin et al., 1992; Epping-Jordan et al., 1998). The following signs were recorded: blinks, body and head shakes, chews, cheek tremors, teeth chattering, escape attempts, foot licks, genital licks, gasps, writhes, scratches, ptosis, and piloerection. Multiple successive counts of any sign required a distinct pause between episodes. Continuous signs (e.g., tremor, teeth chatter) that lasted longer than 5 sec, were scored as the total number of 5 sec episodes. Ptosis was counted as 1 for appearance or 0 for non-appearance during the 10 min period of observation.
2.5. Osmotic minipump implantation and removal
Rats were anesthetized with a isoflurane/oxygen vapor mixture (1-2%), and an osmotic minipump [models: 7-day 2ML1 (10 μl/hr), 14-day 2ML2 (5 μl/hr), or 28-day 2ML4 (2.5 μl/hr), Alzet Osmotic Pumps, Cupertino, CA] was inserted subcutaneously (back of the animal parallel to the spine) with the flow-moderator directed posteriorly. The wound was closed with 9 mm stainless steel wound clips (Becton Dickinson Primary Care Diagnostics, Sparks, MD), and antibacterial Bacitracin ointment was applied to the incision area. On day 7, 14, or 28 the minipumps were surgically removed using the aforementioned procedure. The concentration of all drugs was adjusted to compensate for differences in the rats' body weights at the time of implantation.
In Experiment 5, two subsequent minipump implantation procedures were involved (the first minipump contained M100907/vehicle, and the second minipump contained either nicotine/saline or amphetamine/saline; Fig. 1). Minipumps delivered (i) 0.5 mg/kg/day M100907 for 28 days or vehicle (control groups) or (ii) 9 mg/kg/day (3.16 mg/kg/day base) nicotine for 14 days or 5 mg/kg/day amphetamine for 7 days. If an animal was required to have two minipumps, then the minipumps were placed in different parts of the body (left or right side of the back of the animal).
Figure 1.
Diagram of Experiment 5 showing the sequence of chronic concurrent treatment with M100907 and nicotine (A) or amphetamine (B). Arrows indicate osmotic minipump implantation/removal time-points.
2.6. Experimental designs
Experiment 1: Effects of acute idazoxan and acute M100907 treatment on brain reward thresholds under baseline conditions
This experiment was designed to determine the idazoxan and M100907 doses that minimally affected brain reward thresholds under baseline conditions that would be used in the subsequent experiments. Naive rats were injected with idazoxan (0, 0.5, 1.0, and 5 mg/kg, salt, i.p., n = 10) or M100907 (0, 0.25, 1.0, 2.0, 4.0, 0.01, and 0.1 mg/kg, salt, i.p., n = 8) 30 min before the ICSS session using a within-subjects Latin square design with at least 3 days between each drug administration, during which time rats were tested in the ICSS procedure without treatment.
Experiment 2: Effects of acute idazoxan (Experiment 2A) and acute M100907 (Experiment 2B) treatment on brain reward thresholds and somatic signs during spontaneous nicotine withdrawal
Rats were prepared with 7-day osmotic minipumps delivering 9 mg/kg/day nicotine salt (two groups of n = 12 per group for idazoxan; two groups of n = 3-4 per group for M100907) or saline (two groups of n = 12 per group for idazoxan; two groups of n = 4 per group for M100907). On day 7, the minipumps were removed, and ICSS thresholds were assessed 6, 24, 48, 72, 96, 120, and 144 h after minipump removal and at 24 h intervals thereafter until thresholds returned to baseline levels. Based on thresholds obtained at the 6 h time-point of nicotine withdrawal, rats were assigned to experimental groups such that the mean thresholds and standard deviations of the groups were approximately equal, thus equating the magnitude of the drug withdrawal effect before the idazoxan/vehicle or M100907/vehicle treatment. Somatic signs were assessed on day 1 and 2 of nicotine withdrawal. Idazoxan (1 mg/kg, i.p.), M100907 (0.1 mg/kg, i.p.), or vehicle (control groups) was administered 30 min before the ICSS session on day 1 (24 h time-point) of nicotine withdrawal. Immediately after each ICSS session (approximately 30 min duration), all animals were observed for somatic signs of withdrawal for 10 min.
Experiment 3: Effects of acute idazoxan and acute M100907 treatment on brain reward thresholds during DHβE-precipitated nicotine withdrawal
Rats were prepared with 28-day osmotic minipumps delivering 9 mg/kg/day nicotine salt (n = 12 for idazoxan, n = 7 for M100907) or vehicle (n = 12 for idazoxan, n = 7 for M100907). Starting on day 6 of chronic nicotine delivery, a period of time that was shown previously to be sufficient for the induction of nicotine withdrawal (Watkins et al., 2000; Kenny et al., 2003), rats were injected with DHβE (3 mg/kg, s.c., 10 min before the ICSS session) and idazoxan (0, 0.5, 1.0, and 5 mg/kg, i.p.) or M100907 (0, 0.01, 0.1, and 1.0 mg/kg, i.p.) 30 min before the ICSS session using a within-subjects Latin square design with 2 days between each drug administration, during which time rats were tested in the ICSS procedure without treatment.
Experiment 4: Effects of acute idazoxan (Experiment 4A) and acute M100907 (Experiment 4B) treatment on brain reward thresholds during spontaneous amphetamine withdrawal
The amphetamine administration regimen used here was a modification of that used originally by Leith and Barrett (Leith and Barrett, 1976) and identical to that used previously in our laboratory (Lin et al., 1999; Harrison et al., 2001; Markou et al., 2005). Specifically, amphetamine was injected three times per day (8 am, 2 pm, and 8 pm) for 4 days in a rising-dose regimen starting at 1 mg/kg and maintained at 5 mg/kg (i.e., 1, 2, 3, 4, 5, 5, 5, 5, 5, 5, 5, 5 mg/kg, total dose 50 mg/kg over 4 days; i.p.). Control rats were injected with saline at the same time-points. ICSS reward thresholds were assessed 12, 18, 36, 42, 60, 84, 108, 132, and 156 h after the last amphetamine or saline injection. Idazoxan (1 mg/kg, i.p.) or saline was administered acutely prior to the 36 h ICSS session. M100907 (0.1 mg/kg, i.p.) or vehicle was administered acutely prior to the 18 h test session. This testing time-point was selected based on the time-course of threshold elevations previously observed during amphetamine withdrawal (Lin et al., 1999; Harrison et al., 2001). The experimental groups were the following for Experiment 4A: saline-saline (n = 9), saline-idazoxan (n = 9), amphetamine-saline (n = 10), amphetamine-idazoxan (n = 10). The experimental groups were the following for Experiment 4B: saline-saline (n = 6), vehicle-M100907 (n = 9), amphetamine-saline (n = 8), amphetamine-M100907 (n = 9).
Experiment 5: Effects of chronic M100907 treatment on brain reward thresholds during spontaneous nicotine or amphetamine withdrawal
The experimental design of this experiment is presented in Fig. 1. Rats were prepared with 28-day osmotic minipumps delivering 0.5 mg/kg/day M100907 (n = 14) or vehicle (n = 16). These rats were then divided into six experimental groups. For Experiment 5A, rats that received minipumps delivering M100907 or vehicle were prepared with a second 14-day minipump delivering either 9 mg/kg/day nicotine salt (vehicle-nicotine, n = 6; M100907-nicotine, n = 5) or vehicle (vehicle-vehicle, n = 5; M100907-vehicle, n = 5) on day 10 of the experiment, resulting in four experimental groups (Fig. 1A). For Experiment 5B, the remaining two groups of rats that received minipumps delivering M100907 or vehicle were prepared with a second 7-day minipump delivering 5 mg/kg/day amphetamine (vehicle-amphetamine, n = 5; M100907-amphetamine, n = 4) on day 17 of the experiment (Fig. 1B). In this experiment, we chose to use amphetamine delivery via minipumps, instead of repeated injections, because the effects of chronic M100907 treatment on both nicotine and amphetamine withdrawal were investigated concurrently with all treatment groups subjected to the same experimental conditions (e.g., minipump implantation/removal surgeries). Our previous studies showed that the magnitude and duration of amphetamine withdrawal was similar after the termination of amphetamine delivery via minipumps (Paterson et al., 2000) or repeated injections (Lin et al., 1999; 2000; Harrison et al., 2001; Markou et al., 2005). Control groups vehicle-vehicle and M100907-vehicle were the same for Experiments 5A and 5B because the two experiments were conducted concurrently. On day 23 of the experiment, minipumps containing nicotine, amphetamine, or the corresponding control solutions were removed, while the first implanted minipump that contained M100907/vehicle remained implanted. ICSS reward thresholds were assessed for the next 5 days of nicotine/amphetamine withdrawal (days 23-27) after minipump removal. Somatic signs were assessed during 3 consecutive days of nicotine withdrawal on days 23, 24, and 25 of the experiment. On day 27 of the experiment, minipumps containing M100907 or vehicle were removed, and ICSS reward thresholds continued to be assessed for the next 5 days of M100907/vehicle withdrawal. No ICSS test sessions were conducted on the days of minipump implantation (days 1, 10, and 17 of the experiment).
2.7. Statistical analyses
All analyses were performed using the Biomedical Computer Programs for Personal Computers Statistical Package (BMDP, Los Angeles, CA). All data were analyzed with the appropriate mixed-design ANOVAs, with within- or between-subjects factors based on the experimental design. Statistically significant interactions were followed by Newman-Keuls post hoc tests. Nonparametric tests (exact Fisher's test and χ2 test) were used to compare the percentage of rats that showed withdrawal defined as threshold elevations more than 10% from baseline threshold levels. The criterion for significance was set at the 0.05 level.
Thresholds were expressed as a percentage of the mean of the last five baseline values before the drug treatments. Somatic sign data were expressed as the total number of somatic signs observed during the 10 min observation period.
3. Results
No statistically significant differences were observed among mean absolute values of baseline thresholds of the various groups in the different experiments (average group mean thresholds ± SEM: Experiment 1 [102.23 ± 13.22 μA, 120.01 ± 27.67 μA], Experiment 2 [118.8 ± 6.51 μA, 136 ± 11.07 μA], Experiment 3A and B [104.53 ± 10.72 μA, 120.92 ± 7.39 μA], Experimental 4A and B [121.91 ± 5.64 μA, 105.77 ± 5.21 μA], Experiment 5 [118.18 ± 5.4 μA]).
Data on response latencies were collected and analyzed, but are not presented here. The effects of chronic nicotine/amphetamine exposure and nicotine/amphetamine withdrawal on response latencies were similar to those reported in previously published work (Epping-Jordan et al., 1998; Lin et al., 1999; 2000; Harrison and Markou, 2001; Semenova and Markou, 2003; Markou et al., 2005). There were no significant effects of idazoxan treatment on response latencies during either chronic nicotine/amphetamine exposure or during nicotine/amphetamine withdrawal. Similarly, there were no significant effects of M100907 treatment on response latencies, except in Experiment 5. In Experiment 5, M100907 increased response latencies (F1,28 = 9.54, p < 0.01), but post-hoc test did not reveal significance for specific data points (data not shown).
3.1. Experiment 1: Effects of acute idazoxan and acute M100907 treatment on brain reward thresholds under baseline conditions
Idazoxan treatment had no effect on brain reward thresholds under baseline conditions (Table 1). Idazoxan at the dose of 1.5 mg/kg was found to generate approximately 75% α2 adrenergic receptor occupancy with ED50 value of 0.43 mg/kg that was similar to that of clozapine (Marcus et al., 2005). Thus, the dose of 1 mg/kg was chosen for the subsequent experiments assessing the acute effects of idazoxan on nicotine or amphetamine withdrawal.
Table 1.
Brain reward thresholds after acute administration of idazoxan or M100907 (i.p., 30 min before the ICSS session). Data are expressed as a percentage of baseline thresholds (mean ± SEM). No statistically significant differences were observed between idazoxan- and vehicle-treated rats. M100907 significantly elevated thresholds compared with the vehicle-treated group (*p < 0.05, **p < 0.01, Newman-Keuls post hoc test).
| Dose (mg/kg) | Threshold (% of baseline) |
|---|---|
| Idazoxan (n = 10) | |
| vehicle | 100.34 ± 4.54 |
| 0.5 | 100.56 ± 4.05 |
| 1.0 | 103.93 ± 3.67 |
| 5.0 | 104.5 ± 2.76 |
| M100907 (n = 8) | |
| vehicle | 95.84 ± 4.01 |
| 0.25 | 113.71 ± 2.8** |
| 1.0 | 110.95 ± 2.2** |
| 2.0 | 112.49 ± 4.52** |
| 4.0 | 105.77 ± 2.84* |
| 0.01 | 101.02 ± 2.23 |
| 0.1 | 103.74 ± 1.84 |
M100907 treatment significantly elevated brain reward thresholds at the dose range of 0.25-4 mg/kg (F4,28 = 5.12, p < 0.05; Table 1). Lower doses of M100907 (0.01 and 0.1 mg/kg), administered after completion of the Latin square, did not change thresholds. Thus, the 0.1 mg/kg dose was chosen for the subsequent experiments assessing the acute effects of M100907 on nicotine or amphetamine withdrawal.
3.2. Experiment 2A: Effects of acute idazoxan treatment on brain reward thresholds and somatic signs during spontaneous nicotine withdrawal
A significant main effect of chronic nicotine administration (7-day treatment) was observed on thresholds (F5,230 = 3.43, p < 0.05; Fig. 2A). Post hoc analyses revealed significantly decreased thresholds in nicotine-exposed rats compared with saline-exposed rats on day 1 of nicotine delivery.
Figure 2.
The effects of idazoxan treatment on brain reward thresholds. (A) Data on thresholds are presented as a percentage of baseline thresholds (mean ± SEM) before nicotine/saline exposure. **p < 0.01, *p < 0.05, statistically significant differences between thresholds of nicotine- and saline-exposed rats treated with saline. ##p < 0.01, statistically significant differences between thresholds of nicotine- and saline-exposed rats treated with idazoxan. @p < 0.05, statistically significant differences between thresholds of nicotine-exposed rats treated with idazoxan and saline. (B) Probit analysis of the number of subjects that show an effect (i.e., more than 10% elevation in thresholds). *p < 0.05, significant percentage of rats that showed withdrawal. Abbreviation p1-p6 corresponds to pump days 1-6.
After removal of the minipumps, elevations in thresholds were evident in rats undergoing spontaneous nicotine withdrawal, reflected in a significant effect of nicotine exposure (F1,44 = 18.81, p < 0.0001; Fig. 2A). An ANOVA revealed a significant Nicotine exposure × Idazoxan treatment interaction (F1,44 = 6.84, p < 0.01), although the three-way Day × Nicotine/Saline exposure × Idazoxan/Vehicle treatment interaction was not significant. Post hoc analyses revealed significantly higher thresholds in nicotine-exposed rats treated with vehicle compared with nicotine-exposed rats treated with idazoxan (1 mg/kg) at 48 and 72 hr post-nicotine, indicating that idazoxan significantly attenuated the threshold elevations associated with nicotine withdrawal. To further investigate the significant interaction between Nicotine exposure and Idazoxan treatment, non-parametric probit analysis of the number of subjects that showed an effect (i.e., more than 10% elevation in thresholds) demonstrated that idazoxan pretreatment reduced the duration of nicotine withdrawal (effective time, ET50 = 43 h) compared with vehicle-pretreated rats (ET50 = 59.7 h) (Fig. 2B). However, the factor Day of withdrawal was not significant because nicotine withdrawal was short. Finally, the percentage of nicotine-withdrawing rats showing threshold elevations (i.e., more than 10% above baseline thresholds) was significantly smaller in the group treated with idazoxan compared with the group treated with vehicle during days 1 and 2 of nicotine withdrawal (Fisher's exact test, p < 0.05; Fig. 2C). Idazoxan treatment did not induce changes in thresholds of saline-treated rats.
Somatic signs of nicotine withdrawal were increased in nicotine- compared with saline-withdrawing rats (Nicotine exposure, F1,44 = 15.95, p < 0.001) without any effect of idazoxan treatment (Fig. 3). Post-hoc analyses revealed that nicotine withdrawing rats treated with either saline or idazoxan exhibited significantly more somatic signs of withdrawal compared to the corresponding control group (Fig. 3). Nevertheless, a strong tendency toward idazoxan reducing the number of somatic signs in nicotine-withdrawing rats was observed (Nicotine exposure × Idazoxan treatment interaction, F1,44 = 3.85, p < 0.056). Further analyses were performed on somatic signs data on day 1 of nicotine withdrawal to evaluate the percentage of rats showing withdrawal somatic signs more than the upper confidence limit of the corresponding control group. Results showed that only 50% of nicotine-withdrawing rats treated with idazoxan exhibited increased number of somatic signs compared to 100% of nicotine-withdrawing rats treated with saline, indicating attenuation of somatic aspects of nicotine withdrawal with idazoxan treatment (Fisher's exact test, p < 0.05). In both control groups, 25% of saline-“withdrawing” rats treated with either saline or idazoxan exhibited somatic signs. This pattern of results reflects partial efficacy of idazoxan treatment on the expression of somatic signs during nicotine withdrawal. No effect of idazoxan was observed on somatic signs in saline-“withdrawing” rats.
Figure 3.
The effects of idazoxan treatment on somatic signs during spontaneous nicotine withdrawal (mean ± SEM). *p < 0.05, **p < 0.01, statistically significant differences between somatic signs of nicotine-exposed rats treated with idazoxan or saline compared with those of saline-exposed rats treated with idazoxan or saline (Newman-Keuls post-hoc test).
Experiment 2B: Effects of acute M100907 treatment on brain reward thresholds and somatic signs during spontaneous nicotine withdrawal
During 7 days of nicotine exposure, we observed no significant main effect of chronic Nicotine exposure or Day or a Day × Nicotine exposure interaction (Fig. 4A). Nicotine withdrawal induced threshold elevations in nicotine-exposed rats (Nicotine exposure, F1,13 = 13.40, p < 0.01; Day × Nicotine exposure interaction, F4,52 = 6.27, p < 0.0003; Fig. 4A). We found no effect of acute M100907 treatment on thresholds during spontaneous nicotine withdrawal.
Figure 4.
The effects of M100907 treatment on brain reward thresholds (A) and somatic signs (B) during spontaneous nicotine withdrawal (mean ± SEM). Data on thresholds are presented as a percentage of baseline thresholds before nicotine/saline exposure. *p < 0.05, **p < 0.01, statistically significant differences between somatic signs of nicotine-exposed rats treated with M100907 or vehicle compared with saline-exposed rats treated with M100907 or vehicle. Abbreviation p1-p6 corresponds to pump days 1-6. @, statistically significant main effect of nicotine withdrawal on thresholds (p < 0.01) independent of M100907 treatment.
Somatic signs of nicotine withdrawal were increased in nicotine-withdrawing rats compared with saline-withdrawing rats (Nicotine exposure, F1,11 = 8.89, p < 0.01), and this effect was dependent on treatment with M100907 (main effect of M100907, F1,11 = 7.55, p < 0.05; Nicotine exposure × Day of withdrawal × M100907 treatment interaction, F1,11 = 7.03, p < 0.05; Fig. 4B). Post hoc analyses revealed that nicotine-withdrawing rats treated with either saline or M100907 exhibited significantly more somatic signs of withdrawal compared to corresponding control groups (Fig. 4B). Further, M100907 treatment increased the number of somatic signs observed during nicotine withdrawal (Fig. 4B).
3.3. Experiment 3: Effects of acute idazoxan (Experiment 3A) and acute M100907 (Experiment3B) treatment on brain reward thresholds during DHβE-precipitated nicotine withdrawal
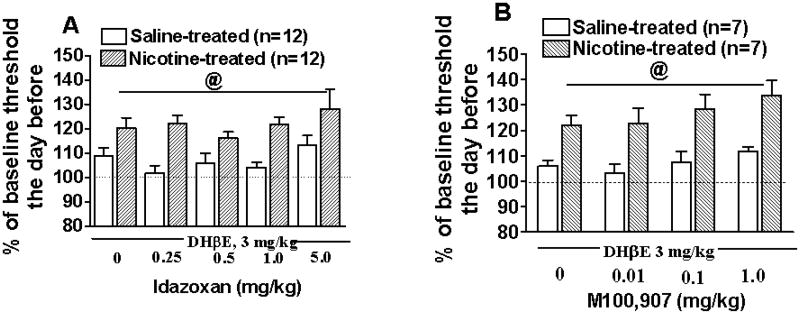
In both Experiments 3A and 3B, DHβE (3 mg/kg) administration precipitated nicotine withdrawal, reflected in threshold elevations (main effect of Nicotine exposure, F1,22 = 45.21 and F1,12 = 42.51, respectively, p < 0.0001) in nicotine-exposed rats compared with saline-treated rats (Fig. 5). Treatment with either idazoxan or M100907 at all doses did not reverse threshold elevations associated with DHβE-precipitated nicotine withdrawal (Fig. 5).
Figure 5.
Effects of idazoxan (A) and M100907 (B) treatment on brain reward thresholds during DHβE-precipitated nicotine withdrawal (mean ± SEM). Data on thresholds are presented as percent of the baseline thresholds obtained the day before DHβE-precipitated withdrawal. @, statistically significant main effect of precipitated nicotine withdrawal on thresholds (p < 0.0001) independent of idazoxan or M100907 treatment.
3.4. Experiment 4A: Effects of acute idazoxan treatment on brain reward thresholds during spontaneous amphetamine withdrawal
Amphetamine-exposed rats exhibited elevated brain reward thresholds compared with saline-exposed rats after termination of amphetamine administration (Amphetamine exposure, F1,34 = 95.73, p < 0.0001; Day of withdrawal × Amphetamine exposure interaction, F5,170 = 24.20, p < 0.0001; Fig. 6A). Acute idazoxan treatment had no effect on thresholds in either saline- or amphetamine-exposed rats during amphetamine/saline withdrawal (Fig. 6A).
Figure 6.
The effects of idazoxan (A) and M100907 (B) treatment on brain reward thresholds during amphetamine withdrawal (mean ± SEM). No effect of idazoxan or M100907 treatment was observed on brain reward thresholds during amphetamine withdrawal. @, p < 0.0001, significant main effect of amphetamine withdrawal.
Experiment 4B: Effects of acute M100907 treatment on brain reward thresholds during spontaneous amphetamine withdrawal
Amphetamine-exposed rats exhibited elevated brain reward thresholds compared with saline-exposed rats after termination of amphetamine administration (main effect of Amphetamine exposure, F1,28 = 49.7, p < 0.0001; Day of withdrawal × Amphetamine exposure interaction, F4,112 = 11.59, p < 0.0001; Fig. 6B). M100907 treatment had no effect on thresholds in either saline- or amphetamine-exposed rats during amphetamine/saline withdrawal (Fig. 6B).
3.5. Experiment 5: Effects of chronic M100907 treatment on brain reward thresholds during spontaneous nicotine and amphetamine withdrawal
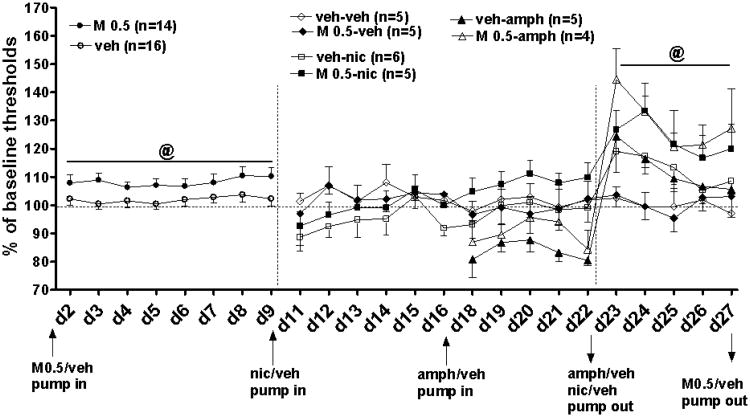
Chronic M100907 administration for 9 days significantly elevated brain reward thresholds (F1,28 = 8.11, p < 0.01; Fig. 7). Concurrent administration of M100907 and nicotine during days 11-22 had no significant effect on thresholds reflected by a nonsignificant three-way M100907 treatment × Nicotine exposure × Day of drug exposure interaction (Fig. 7). We found, however, a significant effect of Day of drug exposure (nicotine and M100907) on thresholds (F10,170 = 2.5, p < 0.01) and a significant Day × M100907 treatment interaction (F10,170 = 3.5, p < 0.001), attributable to a trend toward threshold elevations in the groups treated with M100907 and exposed to nicotine on days 18-22 of the experiment.
Figure 7.
The effects of chronic M100907 treatment on brain reward thresholds under baseline conditions, during concurrent administration with nicotine/amphetamine, and during nicotine/amphetamine withdrawal (mean ± SEM). Arrows indicate osmotic minipump implantation/removal. Abbreviation d2-d27 corresponds to experimental days 2-27. @, p < 0.01 significant main effect of M100907 treatment under baseline conditions and during nicotine/amphetamine withdrawal revealed in ANOVA analyses.
Concurrent administration of M100907 and amphetamine during days 18-22 had no significant effect on thresholds reflected by a nonsignificant three-way M100907 treatment × Amphetamine exposure × Day of drug exposure interaction (Fig. 7). We found, however, a significant effect of M100907 treatment on thresholds (F1,15 = 7.83, p < 0.01), attributable to a trend toward a reversal of decreased thresholds induced by amphetamine on days 20 and 21 of the experiment.
Chronic M100907 delivery during nicotine withdrawal did not produce significant main or interaction effects on thresholds (Fig. 7). Visual inspection of the data revealed that nicotine-withdrawing rats treated with vehicle exhibited threshold elevations, but this effect was not significant because of the small size of the experimental groups. Treatment with M100907 induced larger threshold elevations (M100907 treatment effect, F1,17 = 14.10, p < 0.01; Day × M100907 treatment interaction, F4,68 = 4.85, p < 0.01) in nicotine-withdrawing rats compared with saline-treated rats, indicating a worsening of nicotine withdrawal by M100907. Somatic signs tended to be increased in nicotine-withdrawing rats treated with vehicle. Continuous administration of M100907 induced a larger increase of somatic signs in nicotine-withdrawing rats compared with nicotine-withdrawing vehicle-treated rats, and this effect was seen during the entire period of testing (3 days; Table 2). ANOVA of the number of somatic signs revealed a significant main effect of M100907 treatment (F1,17 = 9.47, p < 0.01) but no other main or interaction effects. M100907 had no effect on somatic signs in vehicle-treated control rats. Because of the lack of any indication of a beneficial effect of M100907 on any aspect of nicotine withdrawal, replication of this experiment to increase statistical power was not warranted. The effects of nicotine withdrawal without any treatment on threshold elevations and somatic signs have been repeatedly shown to be statistically reliable (Epping-Jordan et al., 1998; Harrison and Markou, 2001; Semenova and Markou, 2003; Markou et al., 2005).
Table 2.
Total number of somatic signs (± SEM) during nicotine withdrawal and chronic treatment with M100907 (0.5 mg/kg/day, days 23-25). M100907 tended to exacerbate the increases in the number of somatic signs seen in nicotine-withdrawing rats and had no effect on somatic signs of vehicle-withdrawing rats.
| Day of withdrawal | veh-veh (n = 5) | M 0.5-veh (n = 5) | M 0.5-nic (n = 5) | veh-nic (n = 6) |
|---|---|---|---|---|
| 10 h | 7.8 ± 1.39 | 6.0 ± 0.61 | 17.2 ± 4.29 | 14.7 ± 3.09 |
| Day 2 | 10.6 ± 4.51 | 8.0 ± 1.27 | 17.6 ± 5.35 | 13.0 ± 1.74 |
| Day 3 | 8.4 ± 1.86 | 8.2 ± 1.08 | 13.2 ± 2.58 | 9.0 ± 1.92 |
Chronic M100907 delivery during amphetamine withdrawal did not produce significant main or interaction effects on thresholds (Fig. 7). Visual inspection of the data revealed that amphetamine-withdrawing rats treated with vehicle showed threshold elevations, but this effect only showed a trend because of the small size of the experimental groups (Amphetamine exposure, F1,15 = 3.5, p < 0.08). Treatment with M100907 induced larger threshold elevations (main effect of M100907 treatment, F1,15 = 18.87, p < 0.001; Day × M100907 treatment interaction, F4,60 = 4.73, p < 0.01) in amphetamine-withdrawing rats, indicating a worsening of amphetamine withdrawal by M100907. Because of the lack of any indication of a beneficial effect of M100907 on any aspect of amphetamine withdrawal, replication of this experiment to increase statistical power was not warranted. Thresholds elevations during amphetamine withdrawal without any treatment have been repeatedly shown to be statistically reliable (Epping-Jordan et al., 1998; Lin et al., 1999; Harrison and Markou, 2001; Semenova and Markou, 2003; Markou et al., 2005).
After minipump removal, M100907 treatment had no withdrawal effect on reward thresholds and no Day × Treatment interaction (Table 3).
Table 3.
Brain reward thresholds during withdrawal from chronic treatment with M100907 (M, 0.5 mg/kg/day). Data are presented as a percentage of baseline thresholds (mean ± SEM). There were no significant differences between M100907 and vehicle treated rats.
| Day of withdrawal | M 0.5 - vehicle (n=5) | vehicle-vehicle (n=5) |
|---|---|---|
| 8hrs | 92.52±2.42 | 99.54±4.7 |
| day 2 | 92.26±5.78 | 99.73±5.17 |
| day 3 | 93.31±4.41 | 102.67±6.14 |
| day 4 | 94.82±5.28 | 98.13±3.57 |
| day 5 | 92.73±6.7 | 100.22±4.62 |
4. Discussion
The results of the present study indicate that acute idazoxan (1 mg/kg) administration attenuated the magnitude and shortened the duration of the threshold elevations associated with spontaneous, but not DHβE-precipitated, nicotine withdrawal and tended to decrease the number of somatic signs of nicotine withdrawal. Idazoxan treatment had no effect on either the magnitude or duration of amphetamine withdrawal. Acute administration of M100907 (0.1 mg/kg) did not affect threshold elevations associated with either nicotine or amphetamine withdrawal and tended to worsen the somatic aspects of nicotine withdrawal. Chronic administration of M100907 (0.5 mg/kg/day) worsened the affective signs of both spontaneous nicotine and amphetamine withdrawal and worsened the somatic signs of nicotine withdrawal.
Effects of idazoxan
In the present study, idazoxan administration at the dose range of 1-5 mg/kg had no effect on brain reward thresholds under baseline conditions. Similarly, in the rate-frequency ICSS procedure, idazoxan treatment at the dose of 1 mg/kg had no effect on reward thresholds; while a higher idazoxan dose (3 mg/kg) induced a depression in self-stimulation response rates (Montgomery et al., 2003). During spontaneous withdrawal, post-hoc analyses showed that acute idazoxan treatment had no significant effect on threshold elevations 30 min after its administration. However, probit non-parametric statistical analyses that took into consideration individual rats' responses to idazoxan indicated reversal of the threshold elevations associated with nicotine withdrawal in 50% of the rats treated with idazoxan at this time-point (Figure 2B). Additionally, acute idazoxan treatment shortened the duration of nicotine withdrawal (ET50 = 43 h) compared with vehicle-treated nicotine-withdrawing rats (ET50 = 59.7 h). Furthermore, similar to ICSS threshold elevations, idazoxan treatment also attenuated the number of somatic signs of nicotine withdrawal in 50% of rats treated with idazoxan on day 1 post-nicotine. Thus, idazoxan administration during nicotine withdrawal triggered a cascade of neurochemical processes in different neurotransmitter systems resulting in facilitation of recovery from acute withdrawal. These findings cannot be attributed to the delayed effects of idazoxan, because the half-life of idazoxan ranged from 26 to 37 min after intravenous administration independent of idazoxan dose used (1-10 mg/kg) (Valles et al., 1989). The neurobiological mechanisms underlying the partial effectiveness of idazoxan to alleviate the affective aspects of nicotine withdrawal remain to be elucidated. Taken together, the idazoxan-induced facilitation of recovery from nicotine withdrawal indicated that blockade of α2 receptors may have beneficial effects in the treatment of nicotine withdrawal in human smokers during quit attempts.
In contrast to the acute effects of idazoxan, acute clozapine treatment reversed somatic, but not anhedonic, aspects of nicotine withdrawal in rats (Semenova and Markou, 2003). Moreover, acute clozapine administration had small anhedonic effects in control rats. Similarly, in the rate-frequency ICSS procedure, acute administration of the typical antipsychotic haloperidol or the atypical antipsychotics clozapine or olanzapine induced depression in ICSS responding (Montgomery et al., 1999; 2003). Interestingly, concurrent administration of clozapine and clonidine, an α2 adrenergic receptor agonist, facilitated recovery from clozapine-induced response depression in the ICSS procedure (Montgomery et al., 1999), indicating involvement of α2 adrenergic receptors in the effects of clozapine. Conversely, concurrent administration of idazoxan or clonidine failed to significantly alter the self-stimulation response depression induced by haloperidol or olanzapine (Montgomery et al., 2003). Unlike clozapine, haloperidol and olanzapine have no or low, respectively, affinity for α2 receptors (Bymaster et al., 1996), an observation that likely explains the pattern of results reported by Montgomery and co-workers. Thus, taken together these data suggest that α2 receptor blockade may contribute to the effects of clozapine.
Although data on the specific role of α2 receptors in nicotine dependence is limited (Buchhalter et al., 2008), antidepressant medications have been proposed (nortriptyline: (Prochazka et al., 1998; Hughes et al., 2004; Wagena et al., 2005) or established (bupropion: (Hurt et al., 1997; Jorenby et al., 1999; Shiffman et al., 2000; Lerman et al., 2002; Piasecki et al., 2003; Dwoskin et al., 2006) as effective anti-smoking medications. Among pharmacological effects that may mediate nortriptyline and bupropion anti-smoking properties is modulation of the adrenergic system via norepinephrine reuptake inhibition. Specifically, nortriptyline preferentially blocks NA reuptake (Frazer, 2001), and bupropion inhibits the dopamine and the NA transporters (Ferris and Beaman, 1983; Ferris et al., 1983; Damaj et al., 2004). In animals, acute (Cryan et al., 2003) or chronic (Paterson et al., 2008a) bupropion administration reversed both the somatic and affective aspects of nicotine withdrawal in rats. Further, chronic, but not acute, treatment with the NA reuptake inhibitor desipramine reversed reward deficits of nicotine withdrawal in rats (Paterson et al., 2008b). In the present study, the demonstrated partial effectiveness of idazoxan in reversing both the affective and somatic aspects of nicotine withdrawal may be related to the actions of idazoxan at presynaptic α2 adrenergic receptors that lead to increased NA neurotransmission (Nutt et al., 1997; Fernandez-Pastor and Meana, 2002). Consistent with this notion, limited antidepressant effects of idazoxan treatment have been reported in depressed patients (Pinder and Sitsen, 1987; Osman et al., 1989). However, in the forced swim test that assesses antidepressant activity in rodents, idazoxan treatment alone was ineffective (Taksande et al., 2009; Zhang et al., 2009). Further, in the forced swim test, idazoxan blocked, rather than enhanced, the antidepressant-like effects of the tricyclic antidepressant desipramine (Zhang et al., 2009) and the selective 5-HT reuptake inhibitors paroxetine and fluoxetine (Taksande et al., 2009). These findings cannot be explained by presynaptic actions of idazoxan resulting in increases of NA release. Idazoxan is an imidazoline 2 receptor antagonist (Parini, 1995; Garcia-Sevilla et al., 1996). It has been reported that the distribution pattern of α2 adrenergic receptors and imidazoline receptors are independent from each other (De Vos et al., 1991; De Vos et al., 1994). Recent findings indicated the involvement of imidazoline 2 receptors that also involved actions at monoamine oxidase inhibition mechanism in the neuropathology of depression (Nutt et al., 1995; Garcia-Sevilla et al., 1996; Lalies et al., 1999; Halaris and Piletz, 2003). Further, there is a limited evidence that agmatine, an endogenous ligand for the imidazoline receptors, attenuated naloxone-induced somatic aspects of morphine (Aricioglu-Kartal and Uzbay, 1997) and alcohol withdrawal signs (Uzbay et al., 2000) in morphine-dependent and alcohol dependent rats, respectively. Thus, it is possible that the antagonist actions of idazoxan at postsynaptic α2 adrenergic receptors and/or imidazoline 2 receptors are involved in idazoxan-induced blockade of antidepressant-like activity of monoamine reuptake inhibitors in the forced swim test (Taksande et al., 2009; Zhang et al., 2009). Altogether, these findings indicate that presynaptic α2 adrenergic receptor blockade may be involved in the antidepressant properties of idazoxan, as reflected in reversal of the anhedonic and somatic aspects of spontaneous nicotine withdrawal, but partial beneficial effect of idazoxan treatment may also be attributable to antagonism of imidazoline 2 receptors.
In contrast to the partial reversal of spontaneous nicotine withdrawal by idazoxan, idazoxan administration did not prevent threshold elevations associated with DHβE-precipitated nicotine withdrawal. This dissociation in the effects of idazoxan cannot be explained by the differences in the severity of withdrawal because both methods of withdrawal induction induced a similar 25% elevation in brain reward thresholds. Differential effects of idazoxan treatment on spontaneous and antagonist-precipitated nicotine withdrawal may be attributed to the duration of the two types of withdrawal and/or different neurochemical mechanisms underlying these two types of withdrawal. Specifically, DHβE-induced nicotine withdrawal lasts only few hours and idazoxan effects may be counteracted with the chronic nicotine exposure via minipumps that occurs in these animals. Further, DHβE-precipitated withdrawal involves direct blockade of nicotinic acetylcholine receptors. By contrast, spontaneous nicotine withdrawal is longer (2-3 days) than precipitated withdrawal and associated with changes in multiple receptor/neurotransmitter systems. Our previous findings demonstrated that spontaneous nicotine or amphetamine withdrawal can be reversed with a single acute drug treatment targeting different neurotransmitter systems/receptors (Harrison et al., 2001; Cryan et al., 2003; Kenny et al., 2003; Markou et al., 2005). These findings provide some support to our hypothesis that acute drug treatment triggers a cascade of neurochemical changes resulting in long-lasting reversal of spontaneous withdrawal after a single drug dose (see above).
Another possible explanation may be related to the fact that in the precipitated withdrawal experiment, idazoxan was administered before the onset of withdrawal, while in the spontaneous withdrawal experiment, idazoxan was administered after the onset of withdrawal. Our results are consistent with findings showing differential effects of drug treatment on spontaneous and antagonist-precipitated nicotine withdrawal, although the direction of the effect was opposite to results obtained in the present study. Specifically, administration of the corticotropin-releasing factor receptor antagonist D-Phe CRF12-41 prevented nicotine withdrawal precipitated by the nicotinic acetylcholine receptor antagonist mecamylamine, but had no effect on spontaneous nicotine withdrawal (Bruijnzeel et al., 2007). These findings suggest that antagonist-precipitated withdrawal differentially affects stress-systems compared to spontaneous withdrawal, including changes in NA transmission. During spontaneous nicotine withdrawal, nicotine-stimulated NA release in hippocampal slices was decreased in mice injected chronically with nicotine twice daily for 10 days and withdrawn for 14 h after the last injection (Jacobs et al., 2002). By contrast, antagonist-precipitated morphine withdrawal elicited increases in forebrain NA transmission including the bed nucleus stria terminalis and the amygdala (Van Bockstaele et al., 2008). Consistent with the opposite direction of NA transmission during spontaneous and antagonist-precipitated withdrawal, acute treatment with idazoxan attenuated both affective and somatic aspects of spontaneous nicotine withdrawal in the present study; while both idazoxan and yohimbine worsened antagonist-precipitated morphine withdrawal (Coupar, 1992; Sharif and El-Kadi, 1996; El-Kadi and Sharif, 1997). Furthermore, chronic co-administration of idazoxan or yohimbine with morphine attenuated the withdrawal signs precipitated with naloxone in morphine-dependent mice (El-Kadi and Sharif, 1997). Thus, based on these findings, we predict that chronic treatment with idazoxan may worsen somatic and affective aspects of nicotine withdrawal. Taken together, our results suggest that the changes in monoaminergic neurotransmission, induced by α2 adrenergic antagonism, are not sufficient to prevent the effects of acute nicotinic acetylcholine receptor blockade after a period of chronic nicotine exposure.
Contrary to our prediction, idazoxan treatment had no effect on amphetamine withdrawal. These results may be attributed to the fact that idazoxan-induced changes in monoaminergic neurotransmission were not sufficient to reverse the larger magnitude of amphetamine withdrawal (approximately 40% above baseline level) compared with the magnitude of nicotine withdrawal (approximately 25% above baseline level). Prevention of nicotine withdrawal when chronic desipramine treatment (Paterson et al., 2008b) was co-administered with nicotine suggests that chronic concurrent administration of idazoxan and amphetamine may prevent, or at least reduce, the affective signs of amphetamine withdrawal. We previously reported attenuation of cocaine withdrawal in rats treated with desipramine, and this effect was correlated with β-adrenoceptor downregulation (Markou et al., 1992). Thus, actions at β-adrenoceptors, but not α2 adrenoceptors, may be involved in the expression of amphetamine withdrawal.
Psychostimulant, including nicotine, withdrawal has been associated with decreases in dopaminergic and serotonergic neurotransmission in the nucleus accumbens (Parsons et al., 1991b; a; Parsons et al., 1995; Fung et al., 1996; Parsons et al., 1996; Hildebrand et al., 1998; Hildebrand et al., 1999; Nomikos et al., 1999; Carboni et al., 2000; Hildebrand and Svensson, 2000; Rada et al., 2001; Rahman et al., 2004; Vacca et al., 2007). The partial effectiveness of idazoxan in attenuating nicotine withdrawal may be attributed to idazoxan-induced increase in dopamine (Hertel et al., 1999b) and serotonin (Garratt et al., 1991; Matsumoto et al., 1998) release in the medial prefrontal cortex, but not in the nucleus accumbens (Hertel et al., 1999b). The negative symptoms of schizophrenia have been hypothesized to result from frontal hypodopaminergia (Howes and Kapur, 2009). The superiority of clozapine over typical antipsychotics in the treatment of the negative symptoms of schizophrenia has been suggested to be attributable to combined blockade of α2 and dopamine D2 receptors resulting in a marked increase in dopamine transmission in the rat prefrontal cortex (Nomikos et al., 1994; Ichikawa et al., 2002). Supporting this hypothesis, treatment with idazoxan potentiated the effects of raclopride, a dopamine D2/3 antagonist, on dopamine release in the prefrontal cortex (Hertel et al., 1999a; Hertel et al., 1999b; Wadenberg et al., 2007). Similarly, combined idazoxan treatment with typical or atypical antipsychotics potentiated glutamate release in the prefrontal cortex, but not in the nucleus accumbens (Marcus et al., 2005). Consistent with the above, concurrent treatment with idazoxan and fluphenazine, a typical neuroleptic, resulted in significant reductions of global psychosis and both positive and negative symptoms in schizophrenia patients (Litman et al., 1996). Altogether these findings suggest, that idazoxan treatment in combination with clozapine or D2 receptors antagonist may reverse anhedonia associated with psychostimulant withdrawal more effectively than idazoxan alone.
Effects of M100907
In contrast to idazoxan treatment, acute treatment with M100907 had no effect on either the magnitude or duration of nicotine withdrawal and non-significantly worsened the somatic signs of nicotine withdrawal. Furthermore, M100907 exacerbated the threshold elevations induced in nicotine-exposed rats by administration of the nicotinic acetylcholine receptor antagonist DHbE. Chronic administration of M100907 elevated reward thresholds in vehicle-exposed rats, indicating mild aversive effects of M100907. Similar effects were seen after chronic clozapine administration (Semenova and Markou, 2003) or co-administration of fluoxetine or paroxetine, serotonin reuptake inhibitors, with p-MPPI, 5-HT1A receptor antagonist, a drug combination that acutely increases serotonin neurotransmission (Harrison et al., 2001; Markou et al., 2005). Moreover, chronic M100907 did not have significant effects on the reward-enhancing effects associated with chronic administration of nicotine or amphetamine.
Both spontaneous and mecamylamine- or DHβE-precipitated nicotine withdrawal result in increased susceptibility to DOI-induced (5-HT2 receptor agonist, [±]-1-[2,5-dimethoxy-4-iodophenyl]-2-aminopropane hydrochloride) wet-dog shakes in rats (Suemaru et al., 2001) and head-twitches in mice (Yasuda et al., 2002), suggesting increased sensitivity of 5-HT2 receptors during nicotine withdrawal. Increases in serotonin neurotransmission induced by co-administration of serotonin reuptake inhibitors with a 5-HT1A receptor antagonist reversed elevations in brain reward thresholds in nicotine- and amphetamine-withdrawing rats (Harrison et al., 2001; Markou et al., 2005).
In contrast to earlier work (Schmidt and Fadayel, 1995), recent studies showed that M100907 administration did not increase either dopamine or serotonin release in either the medial prefrontal cortex or the nucleus accumbens (Ichikawa et al., 1998; Gobert and Millan, 1999; Rollema et al., 2000; Pehek et al., 2001; Lopez-Gil et al., 2009) and did not affect the spontaneous firing activity of locus coeruleus neurons (Szabo and Blier, 2001). Therefore, it is not surprising that M100907 did not reverse or prevent anhedonia during psychostimulant withdrawal. Interestingly, however, M100907 potentiated haloperidol-induced dopamine release in the medial prefrontal cortex, while inhibiting it in the nucleus accumbens (Gobert and Millan, 1999; Liegeois et al., 2002; Li et al., 2005). 5-HT2 receptor blockade in combination with selective dopamine D2 receptor antagonists has been suggested to increase dopamine output in the prefrontal cortex (Andersson et al., 1995) by enhancing the effect of 5-HT1A receptor stimulation by endogenous serotonin (Ichikawa et al., 2001). These findings indicate that 5-HT2A receptor blockade in combination with D2 receptor blockade may be involved in the beneficial effects of clozapine against the negative symptoms of schizophrenia and, possibly, anhedonic aspects of nicotine withdrawal.
In conclusion, idazoxan attenuated the anhedonic aspects of spontaneous nicotine, but not amphetamine, withdrawal, suggesting that antagonist actions at α2 adrenoceptors may be involved in the attenuation of nicotine withdrawal with clozapine. By contrast, blockade of 5-HT2A receptors with M100907 worsened the “anhedonia” associated with nicotine and amphetamine withdrawal. These results suggest that monotherapy with M100907 may exacerbate the expression of the negative symptoms of schizophrenia in humans or exacerbate nicotine withdrawal symptoms in schizophrenia patients during attempts to quit smoking. Combined blockade of 5-HT2 and dopamine D2 or other receptors may be related to clozapine's therapeutic efficacy against the negative symptoms of schizophrenia (Weinberger, 1987; Horacek et al., 2006; Meltzer and Huang, 2008), and the anhedonia associated with psychostimulant withdrawal (Semenova and Markou, 2003).
Acknowledgments
The authors would like to thank Mrs. Jessica Benedict and Ms. Chelsea Onifer for technical assistance, and Mr. Michael Arends for editorial assistance. The authors would also like to thank Dr. John Cryan for conducting preliminary studies investigating the effects of idazoxan on amphetamine withdrawal that facilitated the design of the reported studies with idazoxan.
Role of the funding source: This work was supported by National Institute of Mental Health grant MH62527 to AM. The NIMH had no further role in the study design, collection, analysis and interpretation of data, writing of the report or the decision to submit the article for publication.
Footnotes
Contributors: S.S. was involved in conception and design of the study, performed the experiments and the statistical analyses, and wrote the manuscript. A.M. was involved in the conception and design of the study, supervised all phases of the experiments and data analyses, and contributed to the writing of the manuscript.
Conflict of interest: A.M. declares that she has received contract research support from Intracellular Therapeutics, Inc., Lundbeck Research USA, Inc., Bristol Myers Squibb, La Roche Inc, Astra-Zeneca and Pfizer, and honorarium/consulting fee from Abbott GmbH and Company and Pfizer during the past 3 years. There are no actual or potential financial conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Press; Washington, DC: 1994. [Google Scholar]
- Andersson JL, Nomikos GG, Marcus M, Hertel P, Mathe JM, Svensson TH. Ritanserin potentiates the stimulatory effects of raclopride on neuronal activity and dopamine release selectivity in the mesolimbic dopaminergic system. Naunyn Schmiedebergs Arch Pharmacol. 1995;352:374–385. doi: 10.1007/BF00172774. [DOI] [PubMed] [Google Scholar]
- Aricioglu-Kartal F, Uzbay IT. Inhibitory effect of agmatine on naloxone-precipitated abstinence syndrome in morphine dependent rats. Life Sci. 1997;61:1775–1781. doi: 10.1016/s0024-3205(97)00801-1. [DOI] [PubMed] [Google Scholar]
- Baldessarini RJ, Frankenburg FR. Clozapine. A novel antipsychotic agent. N Engl J Med. 1991;324:746–754. doi: 10.1056/NEJM199103143241107. [DOI] [PubMed] [Google Scholar]
- Barr AM, Lehmann-Masten V, Paulus M, Gainetdinov RR, Caron MG, Geyer MA. The selective serotonin-2A receptor antagonist M100907 reverses behavioral deficits in dopamine transporter knockout mice. Neuropsychopharmacology. 2004;29:221–228. doi: 10.1038/sj.npp.1300343. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, Zislis G, Wilson C, Gold MS. Antagonism of CRF receptors prevents the deficit in brain reward function associated with precipitated nicotine withdrawal in rats. Neuropsychopharmacology. 2007;32:955–963. doi: 10.1038/sj.npp.1301192. [DOI] [PubMed] [Google Scholar]
- Brunello N, Masotto C, Steardo L, Markstein R, Racagni G. New insights into the biology of schizophrenia through the mechanism of action of clozapine. Neuropsychopharmacology. 1995;13:177–213. doi: 10.1016/0893-133X(95)00068-O. [DOI] [PubMed] [Google Scholar]
- Buchhalter AR, Fant RV, Henningfield JE. Novel pharmacological approaches for treating tobacco dependence and withdrawal: current status. Drugs. 2008;68:1067–1088. doi: 10.2165/00003495-200868080-00005. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, Calligaro DO, Falcone JF, Marsh RD, Moore NA, Tye NC, Seeman P, Wong DT. Radioreceptor binding profile of the atypical antipsychotic olanzapine. Neuropsychopharmacology. 1996;14:87–96. doi: 10.1016/0893-133X(94)00129-N. [DOI] [PubMed] [Google Scholar]
- Carboni E, Bortone L, Giua C, Di Chiara G. Dissociation of physical abstinence signs from changes in extracellular dopamine in the nucleus accumbens and in the prefrontal cortex of nicotine dependent rats. Drug Alcohol Depend. 2000;58:93–102. doi: 10.1016/s0376-8716(99)00064-2. [DOI] [PubMed] [Google Scholar]
- Carlsson A, Waters N, Carlsson ML. Neurotransmitter interactions in schizophrenia-therapeutic implications. Eur Arch Psychiatry Clin Neurosci. 1999;249(4):37–43. doi: 10.1007/pl00014183. [DOI] [PubMed] [Google Scholar]
- Carlsson ML. The selective 5-HT2A receptor antagonist MDL 100,907 counteracts the psychomotor stimulation ensuing manipulations with monoaminergic, glutamatergic or muscarinic neurotransmission in the mouse--implications for psychosis. J Neural Transm Gen Sect. 1995;100:225–237. doi: 10.1007/BF01276460. [DOI] [PubMed] [Google Scholar]
- Coupar IM. Effect of alpha 2-adrenoceptor agonists on the expression of morphine-withdrawal in rats. Naunyn Schmiedebergs Arch Pharmacol. 1992;345:553–557. doi: 10.1007/BF00168948. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Bruijnzeel AW, Skjei KL, Markou A. Bupropion enhances brain reward function and reverses the affective and somatic aspects of nicotine withdrawal in the rat. Psychopharmacology (Berl) 2003;168:347–358. doi: 10.1007/s00213-003-1445-7. [DOI] [PubMed] [Google Scholar]
- Damaj MI, Carroll FI, Eaton JB, Navarro HA, Blough BE, Mirza S, Lukas RJ, Martin BR. Enantioselective effects of hydroxy metabolites of bupropion on behavior and on function of monoamine transporters and nicotinic receptors. Mol Pharmacol. 2004;66:675–682. doi: 10.1124/mol.104.001313. [DOI] [PubMed] [Google Scholar]
- Davis JM, Chen N, Glick ID. A meta-analysis of the efficacy of second-generation antipsychotics. Arch Gen Psychiatry. 2003;60:553–564. doi: 10.1001/archpsyc.60.6.553. [DOI] [PubMed] [Google Scholar]
- de Boer TH, Nefkens F, van Helvoirt A, van Delft AM. Differences in modulation of noradrenergic and serotonergic transmission by the alpha-2 adrenoceptor antagonists, mirtazapine, mianserin and idazoxan. J Pharmacol Exp Ther. 1996;277:852–860. [PubMed] [Google Scholar]
- de Paulis T. M-100907 (Aventis) Curr Opin Investig Drugs. 2001;2:123–132. [PubMed] [Google Scholar]
- De Vos H, Bricca G, De Keyser J, De Backer JP, Bousquet P, Vauquelin G. Imidazoline receptors, non-adrenergic idazoxan binding sites and alpha 2-adrenoceptors in the human central nervous system. Neuroscience. 1994;59:589–598. doi: 10.1016/0306-4522(94)90179-1. [DOI] [PubMed] [Google Scholar]
- De Vos H, Convents A, De Keyser J, De Backer JP, Van Megen IJ, Ebinger G, Vauquelin G. Autoradiographic distribution of alpha 2 adrenoceptors, NAIBS, and 5-HT1A receptors in human brain using [3H]idazoxan and [3H]rauwolscine. Brain Res. 1991;566:13–20. doi: 10.1016/0006-8993(91)91675-q. [DOI] [PubMed] [Google Scholar]
- Doxey JC, Roach AG, Smith CF. Studies on RX 781094: a selective, potent and specific antagonist of alpha 2-adrenoceptors. Br J Pharmacol. 1983;78:489–505. doi: 10.1111/j.1476-5381.1983.tb08809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwoskin LP, Rauhut AS, King-Pospisil KA, Bardo MT. Review of the pharmacology and clinical profile of bupropion, an antidepressant and tobacco use cessation agent. CNS Drug Rev. 2006;12:178–207. doi: 10.1111/j.1527-3458.2006.00178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Kadi AO, Sharif SI. The influence of chronic treatment with clonidine, yohimbine and idazoxan on morphine withdrawal. Psychopharmacology (Berl) 1997;132:67–73. doi: 10.1007/s002130050321. [DOI] [PubMed] [Google Scholar]
- Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393:76–79. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- Fernandez-Pastor B, Meana JJ. In vivo tonic modulation of the noradrenaline release in the rat cortex by locus coeruleus somatodendritic alpha(2)-adrenoceptors. Eur J Pharmacol. 2002;442:225–229. doi: 10.1016/s0014-2999(02)01543-1. [DOI] [PubMed] [Google Scholar]
- Ferris RM, Beaman OJ. Bupropion: a new antidepressant drug, the mechanism of action of which is not associated with down-regulation of postsynaptic beta-adrenergic, serotonergic (5-HT2), alpha 2-adrenergic, imipramine and dopaminergic receptors in brain. Neuropharmacology. 1983;22:1257–1267. doi: 10.1016/0028-3908(83)90198-3. [DOI] [PubMed] [Google Scholar]
- Ferris RM, Cooper BR, Maxwell RA. Studies of bupropion's mechanism of antidepressant activity. J Clin Psychiatry. 1983;44:74–78. [PubMed] [Google Scholar]
- Frazer A. Serotonergic and noradrenergic reuptake inhibitors: prediction of clinical effects from in vitro potencies. J Clin Psychiatry. 2001;62(12):16–23. [PubMed] [Google Scholar]
- Fung YK, Schmid MJ, Anderson TM, Lau YS. Effects of nicotine withdrawal on central dopaminergic systems. Pharmacol Biochem Behav. 1996;53:635–640. doi: 10.1016/0091-3057(95)02063-2. [DOI] [PubMed] [Google Scholar]
- Garcia-Sevilla JA, Escriba PV, Sastre M, Walzer C, Busquets X, Jaquet G, Reis DJ, Guimon J. Immunodetection and quantitation of imidazoline receptor proteins in platelets of patients with major depression and in brains of suicide victims. Arch Gen Psychiatry. 1996;53:803–810. doi: 10.1001/archpsyc.1996.01830090049008. [DOI] [PubMed] [Google Scholar]
- Garratt JC, Crespi F, Mason R, Marsden CA. Effects of idazoxan on dorsal raphe 5-hydroxytryptamine neuronal function. Eur J Pharmacol. 1991;193:87–93. doi: 10.1016/0014-2999(91)90204-4. [DOI] [PubMed] [Google Scholar]
- Glassman AH, Stetner F, Walsh BT, Raizman PS, Fleiss JL, Cooper TB, Covey LS. Heavy smokers, smoking cessation, and clonidine. Results of a double-blind, randomized trial. Jama. 1988;259:2863–2866. [PubMed] [Google Scholar]
- Gobert A, Millan MJ. Modulation of dialysate levels of dopamine, noradrenaline, and serotonin (5-HT) in the frontal cortex of freely-moving rats by (-)-pindolol alone and in association with 5-HT reuptake inhibitors: comparative roles of beta-adrenergic, 5-HT1A, and 5-HT1B receptors. Neuropsychopharmacology. 1999;21:268–284. doi: 10.1016/S0893-133X(99)00035-4. [DOI] [PubMed] [Google Scholar]
- Goudie AJ, Smith JA, Taylor A, Taylor MA, Tricklebank MD. Discriminative stimulus properties of the atypical neuroleptic clozapine in rats: tests with subtype selective receptor ligands. Behav Pharmacol. 1998;9:699–710. doi: 10.1097/00008877-199812000-00006. [DOI] [PubMed] [Google Scholar]
- Gourlay S, Forbes A, Marriner T, Kutin J, McNeil J. A placebo-controlled study of three clonidine doses for smoking cessation. Clin Pharmacol Ther. 1994;55:64–69. doi: 10.1038/clpt.1994.11. [DOI] [PubMed] [Google Scholar]
- Gourlay SG, Stead LF, Benowitz NL. Clonidine for smoking cessation. Cochrane Database Syst Rev. 2004:CD000058. doi: 10.1002/14651858.CD000058.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halaris A, Piletz JE. Relevance of imidazoline receptors and agmatine to psychiatry: a decade of progress. Ann N Y Acad Sci. 2003;1009:1–20. doi: 10.1196/annals.1304.001. [DOI] [PubMed] [Google Scholar]
- Harrison AA, Liem YT, Markou A. Fluoxetine combined with a serotonin-1A receptor antagonist reversed reward deficits observed during nicotine and amphetamine withdrawal in rats. Neuropsychopharmacology. 2001;25:55–71. doi: 10.1016/S0893-133X(00)00237-2. [DOI] [PubMed] [Google Scholar]
- Harrison AA, Markou A. Serotonergic manipulations both potentiate and reduce brain stimulation reward in rats: involvement of serotonin-1A receptors. J Pharmacol Exp Ther. 2001;297:316–325. [PubMed] [Google Scholar]
- Hertel P, Fagerquist MV, Svensson TH. Enhanced cortical dopamine output and antipsychotic-like effects of raclopride by alpha2 adrenoceptor blockade. Science. 1999a;286:105–107. doi: 10.1126/science.286.5437.105. [DOI] [PubMed] [Google Scholar]
- Hertel P, Nomikos GG, Svensson TH. Idazoxan preferentially increases dopamine output in the rat medial prefrontal cortex at the nerve terminal level. Eur J Pharmacol. 1999b;371:153–158. doi: 10.1016/s0014-2999(99)00175-2. [DOI] [PubMed] [Google Scholar]
- Hildebrand BE, Nomikos GG, Hertel P, Schilstrom B, Svensson TH. Reduced dopamine output in the nucleus accumbens but not in the medial prefrontal cortex in rats displaying a mecamylamine-precipitated nicotine withdrawal syndrome. Brain Res. 1998;779:214–225. doi: 10.1016/s0006-8993(97)01135-9. [DOI] [PubMed] [Google Scholar]
- Hildebrand BE, Panagis G, Svensson TH, Nomikos GG. Behavioral and biochemical manifestations of mecamylamine-precipitated nicotine withdrawal in the rat: role of nicotinic receptors in the ventral tegmental area. Neuropsychopharmacology. 1999;21:560–574. doi: 10.1016/S0893-133X(99)00055-X. [DOI] [PubMed] [Google Scholar]
- Hildebrand BE, Svensson TH. Intraaccumbal mecamylamine infusion does not affect dopamine output in the nucleus accumbens of chronically nicotine-treated rats. J Neural Transm. 2000;107:861–872. doi: 10.1007/s007020070038. [DOI] [PubMed] [Google Scholar]
- Horacek J, Bubenikova-Valesova V, Kopecek M, Palenicek T, Dockery C, Mohr P, Hoschl C. Mechanism of action of atypical antipsychotic drugs and the neurobiology of schizophrenia. CNS Drugs. 2006;20:389–409. doi: 10.2165/00023210-200620050-00004. [DOI] [PubMed] [Google Scholar]
- Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophrenia bulletin. 2009;35:549–562. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J, Stead L, Lancaster T. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2004:CD000031. doi: 10.1002/14651858.CD000031.pub2. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Sachs DP, Glover ED, Offord KP, Johnston JA, Dale LC, Khayrallah MA, Schroeder DR, Glover PN, Sullivan CR, Croghan IT, Sullivan PM. A comparison of sustained-release bupropion and placebo for smoking cessation. N Engl J Med. 1997;337:1195–1202. doi: 10.1056/NEJM199710233371703. [DOI] [PubMed] [Google Scholar]
- Ichikawa J, Ishii H, Bonaccorso S, Fowler WL, O'Laughlin IA, Meltzer HY. 5-HT(2A) and D(2) receptor blockade increases cortical DA release via 5-HT(1A) receptor activation: a possible mechanism of atypical antipsychotic-induced cortical dopamine release. J Neurochem. 2001;76:1521–1531. doi: 10.1046/j.1471-4159.2001.00154.x. [DOI] [PubMed] [Google Scholar]
- Ichikawa J, Kuroki T, Dai J, Meltzer HY. Effect of antipsychotic drugs on extracellular serotonin levels in rat medial prefrontal cortex and nucleus accumbens. Eur J Pharmacol. 1998;351:163–171. doi: 10.1016/s0014-2999(98)00308-2. [DOI] [PubMed] [Google Scholar]
- Ichikawa J, Li Z, Dai J, Meltzer HY. Atypical antipsychotic drugs, quetiapine, iloperidone, and melperone, preferentially increase dopamine and acetylcholine release in rat medial prefrontal cortex: role of 5-HT1A receptor agonism. Brain Res. 2002;956:349–357. doi: 10.1016/s0006-8993(02)03570-9. [DOI] [PubMed] [Google Scholar]
- Jacobs I, Anderson DJ, Surowy CS, Puttfarcken PS. Differential regulation of nicotinic receptor-mediated neurotransmitter release following chronic (-)-nicotine administration. Neuropharmacology. 2002;43:847–856. doi: 10.1016/s0028-3908(02)00166-1. [DOI] [PubMed] [Google Scholar]
- Jones CA, McCreary AC. Serotonergic approaches in the development of novel antipsychotics. Neuropharmacology. 2008 doi: 10.1016/j.neuropharm.2008.05.025. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Leischow SJ, Nides MA, Rennard SI, Johnston JA, Hughes AR, Smith SS, Muramoto ML, Daughton DM, Doan K, Fiore MC, Baker TB. A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. N Engl J Med. 1999;340:685–691. doi: 10.1056/NEJM199903043400903. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Gasparini F, Markou A. Group II metabotropic and alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA)/kainate glutamate receptors regulate the deficit in brain reward function associated with nicotine withdrawal in rats. J Pharmacol Exp Ther. 2003;306:1068–1076. doi: 10.1124/jpet.103.052027. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. Neurobiology of the nicotine withdrawal syndrome. Pharmacol Biochem Behav. 2001;70:531–549. doi: 10.1016/s0091-3057(01)00651-7. [DOI] [PubMed] [Google Scholar]
- Kornetsky C, Esposito RU, McLean S, Jacobson JO. Intracranial self-stimulation thresholds: a model for the hedonic effects of drugs of abuse. Arch Gen Psychiatry. 1979;36:289–292. doi: 10.1001/archpsyc.1979.01780030055004. [DOI] [PubMed] [Google Scholar]
- Lalies MD, Hibell A, Hudson AL, Nutt DJ. Inhibition of central monoamine oxidase by imidazoline2 site-selective ligands. Ann N Y Acad Sci. 1999;881:114–117. doi: 10.1111/j.1749-6632.1999.tb09350.x. [DOI] [PubMed] [Google Scholar]
- Leith NJ, Barrett RJ. Amphetamine and the reward system: evidence for tolerance and post-drug depression. Psychopharmacologia. 1976;46:19–25. doi: 10.1007/BF00421544. [DOI] [PubMed] [Google Scholar]
- Lerman C, LeSage MG, Perkins KA, O'Malley SS, Siegel SJ, Benowitz NL, Corrigall WA. Translational research in medication development for nicotine dependence. Nat Rev Drug Discov. 2007;6:746–762. doi: 10.1038/nrd2361. [DOI] [PubMed] [Google Scholar]
- Lerman C, Roth D, Kaufmann V, Audrain J, Hawk L, Liu A, Niaura R, Epstein L. Mediating mechanisms for the impact of bupropion in smoking cessation treatment. Drug Alcohol Depend. 2002;67:219–223. doi: 10.1016/s0376-8716(02)00067-4. [DOI] [PubMed] [Google Scholar]
- Leucht S, Kissling W, Davis JM. Second-generation antipsychotics for schizophrenia: can we resolve the conflict? Psychol Med. 2009:1–12. doi: 10.1017/S0033291709005455. [DOI] [PubMed] [Google Scholar]
- Li Z, Ichikawa J, Huang M, Prus AJ, Dai J, Meltzer HY. ACP-103, a 5-HT2A/2C inverse agonist, potentiates haloperidol-induced dopamine release in rat medial prefrontal cortex and nucleus accumbens. Psychopharmacology (Berl) 2005;183:144–153. doi: 10.1007/s00213-005-0170-9. [DOI] [PubMed] [Google Scholar]
- Liegeois JF, Ichikawa J, Meltzer HY. 5-HT(2A) receptor antagonism potentiates haloperidol-induced dopamine release in rat medial prefrontal cortex and inhibits that in the nucleus accumbens in a dose-dependent manner. Brain Res. 2002;947:157–165. doi: 10.1016/s0006-8993(02)02620-3. [DOI] [PubMed] [Google Scholar]
- Lin D, Koob GF, Markou A. Differential effects of withdrawal from chronic amphetamine or fluoxetine administration on brain stimulation reward in the rat--interactions between the two drugs. Psychopharmacology (Berl) 1999;145:283–294. doi: 10.1007/s002130051060. [DOI] [PubMed] [Google Scholar]
- Lin D, Koob GF, Markou A. Time-dependent alterations in ICSS thresholds associated with repeated amphetamine administrations. Pharmacol Biochem Behav. 2000;65:407–417. doi: 10.1016/s0091-3057(99)00213-0. [DOI] [PubMed] [Google Scholar]
- Litman RE, Su TP, Potter WZ, Hong WW, Pickar D. Idazoxan and response to typical neuroleptics in treatment-resistant schizophrenia. Comparison with the atypical neuroleptic, clozapine. Br J Psychiatry. 1996;168:571–579. doi: 10.1192/bjp.168.5.571. [DOI] [PubMed] [Google Scholar]
- Lopez-Gil X, Artigas F, Adell A. Role of different monoamine receptors controlling MK-801-induced release of serotonin and glutamate in the medial prefrontal cortex: relevance for antipsychotic action. Int J Neuropsychopharmacol. 2009;12:487–499. doi: 10.1017/S1461145708009267. [DOI] [PubMed] [Google Scholar]
- Maldonado R. Participation of noradrenergic pathways in the expression of opiate withdrawal: biochemical and pharmacological evidence. Neurosci Biobehav Rev. 1997;21:91–104. doi: 10.1016/0149-7634(95)00061-5. [DOI] [PubMed] [Google Scholar]
- Malin DH, Lake JR, Newlin-Maultsby P, Roberts LK, Lanier JG, Carter VA, Cunningham JS, Wilson OB. Rodent model of nicotine abstinence syndrome. Pharmacol Biochem Behav. 1992;43:779–784. doi: 10.1016/0091-3057(92)90408-8. [DOI] [PubMed] [Google Scholar]
- Marcus MM, Jardemark KE, Wadenberg ML, Langlois X, Hertel P, Svensson TH. Combined alpha2 and D2/3 receptor blockade enhances cortical glutamatergic transmission and reverses cognitive impairment in the rat. Int J Neuropsychopharmacol. 2005;8:315–327. doi: 10.1017/S1461145705005328. [DOI] [PubMed] [Google Scholar]
- Markou A, Harrison AA, Chevrette J, Hoyer D. Paroxetine combined with a 5-HT(1A) receptor antagonist reversed reward deficits observed during amphetamine withdrawal in rats. Psychopharmacology (Berl) 2005;178:133–142. doi: 10.1007/s00213-004-2008-2. [DOI] [PubMed] [Google Scholar]
- Markou A, Hauger RL, Koob GF. Desmethylimipramine attenuates cocaine withdrawal in rats. Psychopharmacology (Berl) 1992;109:305–314. doi: 10.1007/BF02245878. [DOI] [PubMed] [Google Scholar]
- Markou A, Kenny PJ. Neuroadaptations to chronic exposure to drugs of abuse: relevance to depressive symptomatology seen across psychiatric diagnostic categories. Neurotox Res. 2002;4:297–313. doi: 10.1080/10298420290023963. [DOI] [PubMed] [Google Scholar]
- Markou A, Koob GF. Postcocaine anhedonia. An animal model of cocaine withdrawal. Neuropsychopharmacology. 1991;4:17–26. [PubMed] [Google Scholar]
- Markou A, Koob GF. Construct validity of a self-stimulation threshold paradigm: effects of reward and performance manipulations. Physiol Behav. 1992;51:111–119. doi: 10.1016/0031-9384(92)90211-j. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Yoshioka M, Togashi H, Mori K, Ueno K, Saito H. Effects of idazoxan on dopamine release in the prefrontal cortex of freely moving rats. Eur J Pharmacol. 1998;343:165–170. doi: 10.1016/s0014-2999(97)01544-6. [DOI] [PubMed] [Google Scholar]
- Meltzer HY. An overview of the mechanism of action of clozapine. J Clin Psychiatry. 1994;55(B):47–52. [PubMed] [Google Scholar]
- Meltzer HY, Huang M. In vivo actions of atypical antipsychotic drug on serotonergic and dopaminergic systems. Progress in brain research. 2008;172:177–197. doi: 10.1016/S0079-6123(08)00909-6. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Matsubara S, Lee JC. The ratios of serotonin2 and dopamine2 affinities differentiate atypical and typical antipsychotic drugs. Psychopharmacol Bull. 1989;25:390–392. [PubMed] [Google Scholar]
- Montgomery AM, Grottick AJ, Herberg LJ. Rapid recovery of self-stimulation responding from depression by clozapine is prevented by the alpha 2-adrenoceptor agonist, clonidine. Behav Pharmacol. 1999;10:475–482. doi: 10.1097/00008877-199909000-00006. [DOI] [PubMed] [Google Scholar]
- Montgomery AM, Grottick AJ, Herberg LJ. Alpha2-adrenoceptor antagonism is neither sufficient nor necessary for the distinctive action of atypical neuroleptics on intracranial self-stimulation in the rat. Behav Pharmacol. 2003;14:307–314. doi: 10.1097/01.fbp.0000080415.40539.b9. [DOI] [PubMed] [Google Scholar]
- Nana A, Praditsuwan R. Clonidine for smoking cessation. J Med Assoc Thai. 1998;81:87–93. [PubMed] [Google Scholar]
- Nomikos GG, Hildebrand BE, Panagis G, Svensson TH. Nicotine withdrawal in the rat: role of alpha7 nicotinic receptors in the ventral tegmental area. Neuroreport. 1999;10:697–702. doi: 10.1097/00001756-199903170-00007. [DOI] [PubMed] [Google Scholar]
- Nomikos GG, Iurlo M, Andersson JL, Kimura K, Svensson TH. Systemic administration of amperozide, a new atypical antipsychotic drug, preferentially increases dopamine release in the rat medial prefrontal cortex. Psychopharmacology (Berl) 1994;115:147–156. doi: 10.1007/BF02244765. [DOI] [PubMed] [Google Scholar]
- Nutt DJ, French N, Handley S, Hudson A, Husbands S, Jackson H, Jordan S, Lalies MD, Lewis J, Lione L, et al. Functional studies of specific imidazoline-2 receptor ligands. Ann N Y Acad Sci. 1995;763:125–139. doi: 10.1111/j.1749-6632.1995.tb32397.x. [DOI] [PubMed] [Google Scholar]
- Nutt DJ, Lalies MD, Lione LA, Hudson AL. Noradrenergic mechanisms in the prefrontal cortex. J Psychopharmacol. 1997;11:163–168. doi: 10.1177/026988119701100209. [DOI] [PubMed] [Google Scholar]
- Osman OT, Rudorfer MV, Potter WZ. Idazoxan: a selective alpha 2-antagonist and effective sustained antidepressant in two bipolar depressed patients. Arch Gen Psychiatry. 1989;46:958–959. doi: 10.1001/archpsyc.1989.01810100100021. [DOI] [PubMed] [Google Scholar]
- Palfreyman MG, Schmidt CJ, Sorensen SM, Dudley MW, Kehne JH, Moser P, Gittos MW, Carr AA. Electrophysiological, biochemical and behavioral evidence for 5-HT2 and 5-HT3 mediated control of dopaminergic function. Psychopharmacology (Berl) 1993;112:S60–67. doi: 10.1007/BF02245008. [DOI] [PubMed] [Google Scholar]
- Parini A. Imidazoline binding sites. Pharmacological and molecular characteristics. Ann N Y Acad Sci. 1995;763:100–105. doi: 10.1111/j.1749-6632.1995.tb32394.x. [DOI] [PubMed] [Google Scholar]
- Parsons LH, Koob GF, Weiss F. Serotonin dysfunction in the nucleus accumbens of rats during withdrawal after unlimited access to intravenous cocaine. J Pharmacol Exp Ther. 1995;274:1182–1191. [PubMed] [Google Scholar]
- Parsons LH, Koob GF, Weiss F. Extracellular serotonin is decreased in the nucleus accumbens during withdrawal from cocaine self-administration. Behav Brain Res. 1996;73:225–228. doi: 10.1016/0166-4328(96)00101-5. [DOI] [PubMed] [Google Scholar]
- Parsons LH, Smith AD, Justice JB., Jr Basal extracellular dopamine is decreased in the rat nucleus accumbens during abstinence from chronic cocaine. Synapse. 1991a;9:60–65. doi: 10.1002/syn.890090109. [DOI] [PubMed] [Google Scholar]
- Parsons LH, Smith AD, Justice JB., Jr The in vivo microdialysis recovery of dopamine is altered independently of basal level by 6-hydroxydopamine lesions to the nucleus accumbens. J Neurosci Methods. 1991b;40:139–147. doi: 10.1016/0165-0270(91)90063-6. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Balfour DJ, Markou A. Chronic bupropion differentially alters the reinforcing, reward-enhancing and conditioned motivational properties of nicotine in rats. Nicotine Tob Res. 2008a;10:995–1008. doi: 10.1080/14622200802097571. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Markou A. Animal models and treatments for addiction and depression co-morbidity. Neurotox Res. 2007;11:1–32. doi: 10.1007/BF03033479. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Myers C, Markou A. Effects of repeated withdrawal from continuous amphetamine administration on brain reward function in rats. Psychopharmacology (Berl) 2000;152:440–446. doi: 10.1007/s002130000559. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Semenova S, Markou A. The effects of chronic versus acute desipramine on nicotine withdrawal and nicotine self-administration in the rat. Psychopharmacology (Berl) 2008b doi: 10.1007/s00213-008-1144-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pehek EA, McFarlane HG, Maguschak K, Price B, Pluto CP. M100,907, a selective 5-HT(2A) antagonist, attenuates dopamine release in the rat medial prefrontal cortex. Brain Res. 2001;888:51–59. doi: 10.1016/s0006-8993(00)03004-3. [DOI] [PubMed] [Google Scholar]
- Pellegrino L, Pellegrino A, Cushman A. A Stereotaxic Atlas of the Rat Brain. Plenum Press; New York: 1986. [Google Scholar]
- Phillips AG, Blaha CD, Fibiger HC. Neurochemical correlates of brain-stimulation reward measured by ex vivo and in vivo analyses. Neurosci Biobehav Rev. 1989;13:99–104. doi: 10.1016/s0149-7634(89)80017-x. [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Jorenby DE, Smith SS, Fiore MC, Baker TB. Smoking withdrawal dynamics: I. Abstinence distress in lapsers and abstainers. J Abnorm Psychol. 2003;112:3–13. [PubMed] [Google Scholar]
- Pinder RM, Sitsen JM. Alpha 2-adrenoceptor antagonists as antidepressants: the search for selectivity. Psychopharmacol Ser. 1987;3:107–112. doi: 10.1007/978-3-642-71288-3_13. [DOI] [PubMed] [Google Scholar]
- Prochazka AV, Petty TL, Nett L, Silvers GW, Sachs DP, Rennard SI, Daughton DM, Grimm RH, Jr, Heim C. Transdermal clonidine reduced some withdrawal symptoms but did not increase smoking cessation. Arch Intern Med. 1992;152:2065–2069. [PubMed] [Google Scholar]
- Prochazka AV, Weaver MJ, Keller RT, Fryer GE, Licari PA, Lofaso D. A randomized trial of nortriptyline for smoking cessation. Arch Intern Med. 1998;158:2035–2039. doi: 10.1001/archinte.158.18.2035. [DOI] [PubMed] [Google Scholar]
- Rada P, Jensen K, Hoebel BG. Effects of nicotine and mecamylamine-induced withdrawal on extracellular dopamine and acetylcholine in the rat nucleus accumbens. Psychopharmacology (Berl) 2001;157:105–110. doi: 10.1007/s002130100781. [DOI] [PubMed] [Google Scholar]
- Rahman S, Zhang J, Corrigall WA. Effects of nicotine preexposure on sulpiride-induced dopamine release in the nucleus accumbens. Eur J Pharmacol. 2004;494:31–34. doi: 10.1016/j.ejphar.2004.04.044. [DOI] [PubMed] [Google Scholar]
- Rollema H, Lu Y, Schmidt AW, Sprouse JS, Zorn SH. 5-HT(1A) receptor activation contributes to ziprasidone-induced dopamine release in the rat prefrontal cortex. Biol Psychiatry. 2000;48:229–237. doi: 10.1016/s0006-3223(00)00850-7. [DOI] [PubMed] [Google Scholar]
- Schmidt CJ, Fadayel GM. The selective 5-HT2A receptor antagonist, MDL 100,907, increases dopamine efflux in the prefrontal cortex of the rat. Eur J Pharmacol. 1995;273:273–279. doi: 10.1016/0014-2999(94)00698-7. [DOI] [PubMed] [Google Scholar]
- Schnoll RA, Lerman C. Current and emerging pharmacotherapies for treating tobacco dependence. Expert Opin Emerg Drugs. 2006;11:429–444. doi: 10.1517/14728214.11.3.429. [DOI] [PubMed] [Google Scholar]
- Semenova S, Markou A. Clozapine treatment attenuated somatic and affective signs of nicotine and amphetamine withdrawal in subsets of rats exhibiting hyposensitivity to the initial effects of clozapine. Biol Psychiatry. 2003;54:1249–1264. doi: 10.1016/s0006-3223(03)00240-3. [DOI] [PubMed] [Google Scholar]
- Sharif SI, El-Kadi AO. Modification of naloxone-precipitated withdrawal symptoms in mice by drugs acting on alpha(2)-adrenoceptors. Behav Pharmacol. 1996;7:334–340. doi: 10.1097/00008877-199608000-00004. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Johnston JA, Khayrallah M, Elash CA, Gwaltney CJ, Paty JA, Gnys M, Evoniuk G, DeVeaugh-Geiss J. The effect of bupropion on nicotine craving and withdrawal. Psychopharmacology (Berl) 2000;148:33–40. doi: 10.1007/s002130050022. [DOI] [PubMed] [Google Scholar]
- Sipes TE, Geyer MA. DOI disruption of prepulse inhibition of startle in the rat is mediated by 5-HT(2A) and not by 5-HT(2C) receptors. Behav Pharmacol. 1995;6:839–842. [PubMed] [Google Scholar]
- Smith RJ, Aston-Jones G. Noradrenergic transmission in the extended amygdala: role in increased drug-seeking and relapse during protracted drug abstinence. Brain structure & function. 2008;213:43–61. doi: 10.1007/s00429-008-0191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen SM, Kehne JH, Fadayel GM, Humphreys TM, Ketteler HJ, Sullivan CK, Taylor VL, Schmidt CJ. Characterization of the 5-HT2 receptor antagonist MDL 100907 as a putative atypical antipsychotic: behavioral, electrophysiological and neurochemical studies. J Pharmacol Exp Ther. 1993;266:684–691. [PubMed] [Google Scholar]
- Suemaru K, Araki H, Kitamura Y, Yasuda K, Gomita Y. Cessation of chronic nicotine administration enhances wet-dog shake responses to 5-HT2 receptor stimulation in rats. Psychopharmacology (Berl) 2001;159:38–41. doi: 10.1007/s002130100866. [DOI] [PubMed] [Google Scholar]
- Svensson TH. Alpha-adrenoceptor modulation hypothesis of antipsychotic atypicality. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1145–1158. doi: 10.1016/j.pnpbp.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Szabo ST, Blier P. Functional and pharmacological characterization of the modulatory role of serotonin on the firing activity of locus coeruleus norepinephrine neurons. Brain Res. 2001;922:9–20. doi: 10.1016/s0006-8993(01)03121-3. [DOI] [PubMed] [Google Scholar]
- Taksande BG, Kotagale NR, Tripathi SJ, Ugale RR, Chopde CT. Antidepressant like effect of selective serotonin reuptake inhibitors involve modulation of imidazoline receptors by agmatine. Neuropharmacology. 2009;57:415–424. doi: 10.1016/j.neuropharm.2009.06.035. [DOI] [PubMed] [Google Scholar]
- Tao R, Hjorth S. Alpha 2-adrenoceptor modulation of rat ventral hippocampal 5-hydroxytryptamine release in vivo. Naunyn Schmiedebergs Arch Pharmacol. 1992;345:137–143. doi: 10.1007/BF00165728. [DOI] [PubMed] [Google Scholar]
- Uzbay IT, Yesilyurt O, Celik T, Ergun H, Isimer A. Effects of agmatine on ethanol withdrawal syndrome in rats. Behav Brain Res. 2000;107:153–159. doi: 10.1016/s0166-4328(99)00127-8. [DOI] [PubMed] [Google Scholar]
- Vacca G, Ahn S, Phillips AG. Effects of short-term abstinence from escalating doses of D-amphetamine on drug and sucrose-evoked dopamine efflux in the rat nucleus accumbens. Neuropsychopharmacology. 2007;32:932–939. doi: 10.1038/sj.npp.1301173. [DOI] [PubMed] [Google Scholar]
- Valles J, Prunonosa J, Menargues A, Nomen M, Obach R. Oral idazoxan bioavailability in rat. Relevance of intestinal and hepatic first-pass effect. Drug Metab Dispos. 1989;17:673–676. [PubMed] [Google Scholar]
- Van Bockstaele EJ, Qian Y, Sterling RC, Page ME. Low dose naltrexone administration in morphine dependent rats attenuates withdrawal-induced norepinephrine efflux in forebrain. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1048–1056. doi: 10.1016/j.pnpbp.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varty GB, Bakshi VP, Geyer MA. M100907, a serotonin 5-HT2A receptor antagonist and putative antipsychotic, blocks dizocilpine-induced prepulse inhibition deficits in Sprague-Dawley and Wistar rats. Neuropsychopharmacology. 1999;20:311–321. doi: 10.1016/S0893-133X(98)00072-4. [DOI] [PubMed] [Google Scholar]
- Wadenberg ML, Wiker C, Svensson TH. Enhanced efficacy of both typical and atypical antipsychotic drugs by adjunctive alpha2 adrenoceptor blockade: experimental evidence. Int J Neuropsychopharmacol. 2007;10:191–202. doi: 10.1017/S1461145706006638. [DOI] [PubMed] [Google Scholar]
- Wagena EJ, Knipschild P, Zeegers MP. Should nortriptyline be used as a first-line aid to help smokers quit? Results from a systematic review and meta-analysis. Addiction. 2005;100:317–326. doi: 10.1111/j.1360-0443.2005.00998.x. [DOI] [PubMed] [Google Scholar]
- Watkins SS, Stinus L, Koob GF, Markou A. Reward and somatic changes during precipitated nicotine withdrawal in rats: centrally and peripherally mediated effects. J Pharmacol Exp Ther. 2000;292:1053–1064. [PubMed] [Google Scholar]
- Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- Weiner DM, Burstein ES, Nash N, Croston GE, Currier EA, Vanover KE, Harvey SC, Donohue E, Hansen HC, Andersson CM, Spalding TA, Gibson DF, Krebs-Thomson K, Powell SB, Geyer MA, Hacksell U, Brann MR. 5-hydroxytryptamine2A receptor inverse agonists as antipsychotics. J Pharmacol Exp Ther. 2001;299:268–276. [PubMed] [Google Scholar]
- Wise RA, Bauco P, Carlezon WA, Jr, Trojniar W. Self-stimulation and drug reward mechanisms. Ann N Y Acad Sci. 1992;654:192–198. doi: 10.1111/j.1749-6632.1992.tb25967.x. [DOI] [PubMed] [Google Scholar]
- Yasuda K, Suemaru K, Araki H, Gomita Y. Effect of nicotine cessation on the central serotonergic systems in mice: involvement of 5-HT(2) receptors. Naunyn Schmiedebergs Arch Pharmacol. 2002;366:276–281. doi: 10.1007/s00210-002-0592-4. [DOI] [PubMed] [Google Scholar]
- Zhang HT, Whisler LR, Huang Y, Xiang Y, O'Donnell JM. Postsynaptic alpha-2 adrenergic receptors are critical for the antidepressant-like effects of desipramine on behavior. Neuropsychopharmacology. 2009;34:1067–1077. doi: 10.1038/npp.2008.184. [DOI] [PMC free article] [PubMed] [Google Scholar]