Abstract
Objective
Vascular remodeling diseases (VRD) are mainly characterized by inflammation and a vascular smooth muscle cells (VSMCs) proproliferative and anti-apoptotic phenotype. Recently, the activation of the advanced glycation endproducts receptor (RAGE) has been shown to promote VSMC proliferation and resistance to apoptosis in VRD in a signal transducer and activator of transcription (STAT)3-dependant manner. Interestingly, we previously described in both cancer and VRD that the sustainability of this proproliferative and antiapoptotic phenotype requires activation of the transcription factor NFAT (nuclear factor of activated T-cells). In cancer, NFAT activation is dependent of the oncoprotein provirus integration site for Moloney murine leukemia virus (Pim1), which is regulated by STAT3 and activated in VRD. Therefore, we hypothesized that RAGE/STAT3 activation in VSMC activates Pim1, promoting NFAT and thus VSMC proliferation and resistance to apoptosis.
Methods/Results
In vitro, freshly isolated human carotid VSMCs exposed to RAGE activator Nε-(carboxymethyl)lysine (CML) for 48 hours had (1) activated STAT3 (increased P-STAT3/STAT3 ratio and P-STAT3 nuclear translocation); (2) increased STAT3-dependent Pim1 expression resulting in NFATc1 activation; and (3) increased Pim1/NFAT-dependent VSMC proliferation (PCNA, Ki67) and resistance to mitochondrial-dependent apoptosis (TMRM, Annexin V, TUNEL). Similarly to RAGE inhibition (small interfering RNA [siRNA]), Pim1, STAT3 and NFATc1 inhibition (siRNA) reversed these abnormalities in human carotid VSMC. Moreover, carotid artery VSMCs isolated from Pim1 knockout mice were resistant to CML-induced VSMC proliferation and resistance to apoptosis. In vivo, RAGE inhibition decreases STAT3/Pim1/NFAT activation, reversing vascular remodeling in the rat carotid artery-injured model.
Conclusion
RAGE activation accounts for many features of VRD including VSMC proliferation and resistance to apoptosis by the activation of STAT3/Pim1/NFAT axis. Molecules aimed to inhibit RAGE could be of a great therapeutic interest for the treatment of VRD.
Keywords: apoptosis, oncogenes, vascular biology, vascular muscle
Vascular remodeling disease (VRD) such as hypertension, atherosclerosis and postangioplasty restenosis are characterized by increased media wall thickness and neointimal formation. This histological remodeling has been attributed in part to an increase in vascular smooth muscle cell (VSMC) proliferation and resistance to apoptosis.1–3 This proproliferative and antiapoptotic phenotype is mediated by a variety of growth factors (platelet derived growth factor), cytokines, and chemokines4 – 6 stimulating prosurvival factors like survivin, which by inhibiting apoptosis7 and promoting mitotic progression enhances neointimal formation.8 We recently described that the sustainability of this phenotype is due to the activation of the transcription factor NFAT (nuclear factor of activated T-cell).1,9 Once activated, NFAT promotes inflammation by stimulating cytokine production10 and VSMC proliferation by decreasing K+ channel,1 such as Kv1.5, which promotes [Ca2+]i and apoptosis resistance, in part by increasing Bcl2 expression. This results in subsequent mitochondrial membrane potential (ΔΨm) hyperpolarization, and trapping of proapoptotic factors, such as cytochrome c, in the mitochondria.11 The mechanisms accounting for NFAT activation in VRD remain undetermined.
Advanced glycation end products (AGEs) are now recognized as bioactive circulating molecules able to enhance proliferative pathways through their receptor RAGE.12 Circulating AGEs are particularly elevated in diabetic13 and hypertensive subjects14,15 and contribute to vascular remodeling processes associated with inflammation.16 –18 Although AGEs are part of a wide family of molecules, only a few have been characterized and identified in human tissues. These include Nε-(carboxymethyl)lysine (CML), pentosidine, pyrraline, and immidazole,19 with only CML being able to increase RAGE signaling.17 Finally, because CML is the most commonly encountered AGE in vivo,20 is present in atherosclerotic lesions,21,22 and its binding to RAGE correlates with carotid artery diameter in normoglycemic patients,23 we decided to focus our study on the role of CML/RAGE interaction in VRD.
Although RAGE activation in neointimal formation has been established,24 the mechanism by which RAGE promotes VRD remains unknown. Recently, RAGE activation has been associated with an increase in signal transducer and activator of transcription (STAT)3 pathway.25 STAT3 has been shown to be implicated in VSMC proliferation.26 – 28 In cancer, STAT3 promotes the expression of the Provirus integration site for Moloney murine leukemia virus (Pim1),29 a protooncogene encoding a serine/threonine protein kinase.30 Over-expression of Pim1 is linked to the development and progression of several cancers by increasing cell proliferation/survival and resistance to apoptosis.31–33 Furthermore, it has been demonstrated that Pim1 could also be implicated in VRD.34 Interestingly, Pim1 activation enhanced NFATc1– 4 activity in rat PC12 cells and lymphoid cells.35 Our team recently showed that the STAT3/Pim1/NFATc2 axis is responsible for abnormal SMC proliferation in pulmonary arteries.27 Thus, the RAGE-dependent activation of STAT3 in VSMCs could trigger Pim1/NFAT axis activation and promote VRD.
The present study demonstrated, indeed, that RAGE activation triggers STAT3 accounting for a Pim1-dependent NFAT activation promoting VSMC proliferation and resistance to apoptosis. Similarly to RAGE inhibition, Pim1 silencing prevented and reversed RAGE-dependent proproliferative and antiapoptotic phenotype seen in cultured VSMCs stimulated by CML. In vivo, RAGE inhibition decreases STAT3/Pim1/NFAT activation preventing vascular remodeling in carotid artery postangioplasty.
Methods
An expanded Methods section is available at http://atvb.ahajournals.org.
Cell Culture
Primary cultured human carotid artery smooth muscle cells (CASMCs) from 2 healthy donors were used (less passage 6), see the expanded supplemental Methods section. Mouse carotid artery smooth muscle cells were freshly isolated from Pim1 knockout (KO) mice and proper control littermates. Final concentration of 1 μg/mL of CML-bovine serum albumin (BSA) was used as RAGE agonist, and NFAT competitor peptide, VIVIT, was used at 4 μmol/L. CASMCs were transfected by CaPO4 precipitation with 20 nmol/L small interfering RNA (siRNA) oligonucleotides.
ELISA Assay
CML auto-antibody and direct CML ELISA assays (Circulex) were performed following manufacturer’s instructions. Serum antibodies anti-CML levels were studied in human subjects with history of cardiovascular disease (n=15) and controls (n=44). Cardiovascular disease was defined as history of coronary artery revascularization, myocardial infarction with abnormal wall motion by ultrasound, ischemic stroke, and symptomatic peripheral vascular disease. The protocol was approved by the institutional review board and patients provided informed consent. No sex-based or racial/ethnic-based differences were present.
Whole Cell Patch Clamping
Whole cell patch clamping was performed on cells voltage-clamped at a holding potential of −70 mV. Currents were evoked by 200 ms test pulses from −70 to +70 mV with 20mV steps as previously described.1 To quantify the effects of CML-BSA on voltage-gated K+ current (Kv), 4-aminopyridine 1 mmol/L (4-AP), a Kv channel blocker, was used.1
Confocal Microscopy/Immunofluorescence, Immunoblotting
Sections of carotids and human CASMCs were used for immunfluorescence staining (see the expanded supplemental Methods section).
Nuclear Translocation Assays
STAT3 and NFATc1 activity were estimated by the percentage of cells presenting a P-STAT3 or NFATc1 nuclear pattern.
Luciferase Assay
Cells were lysed, and luminescence was detected using the Luciferase Assay System (Promega) according to the manufacturer’s instructions. Luminescence counts were standardized to protein content.
Carotid Artery (Balloon) Injury Model
Three groups of rats were studied. Sham operated and rats with angioplasty received either negative control siRNA (siSCRM) or siRAGE (both 10 μmol/L).
Statistics
Data are presented as mean±SD with Mann-Whitney U test for human serum CML levels and mean±SEM for all other studies. Normality of our data were assessed by the Shapiro-Wilk normality test. All our data were normally distributed (P>0.05). For comparison between 2 means, we used unpaired Student t-test and for comparison between more than 2 means, we used 1-way ANOVA followed by Tukey-Kramer tests. Probability values less than 0.001 (***), 0.01 (**), and 0.05 (*) were considered as statistically significant.
Results
CML-Circulating Levels are Increased in Patient With Vascular Diseases
It was recently described that plasma CML levels are significantly higher in patients presenting an increased carotid diameter or an acute myocardial infarction.23,36 In order to extend these observations, we studied CML plasma levels in a cohort of patients with cardiovascular disease as defined by a history of coronary artery revascularization (60%), ischemic stroke (20%), and symptomatic peripheral vascular disease (33%). Plasma levels of antibodies anti-CML were measured by ELISA assay.37 There is a 52% increase (P=0.012) in CML plasma levels in patients with cardiovascular diseases compared with control patients (Table). This result suggests that CML is implicated in many vascular dysfunctions; thus, a better understanding of its role may be of great therapeutic interest.
Table.
Nε-(Carboxymethyl)lysine Levels in Controls and in Patients with Cardiovascular Disease
| Control (n=44) | CVD (n=15) | P Value | |
|---|---|---|---|
| Age (years) | 57±8 | 56±11 | ns |
| Male (%) | 68% | 73% | ns |
| Albumin (g/L) | 40.0±3.8 | 38.7±2.4 | ns |
| Ab anti-CML (ng/L) | 25.7±26.5 | 54.4±51.4 | 0.012 |
| CML-Albumin ratio (μg/g) | 0.64±0.64 | 1.43±1.43 | 0.007 |
Values are means±SD; Bold values indicate P values showing differences between patients with and without cardiovascular diseases. CML indicates Nε-(carboxymethyl)lysine; CVD, cardiovascular disease; ns, not significant.
CML Triggers STAT3 Activation Through a RAGE-Dependent Mechanism in CASMCs
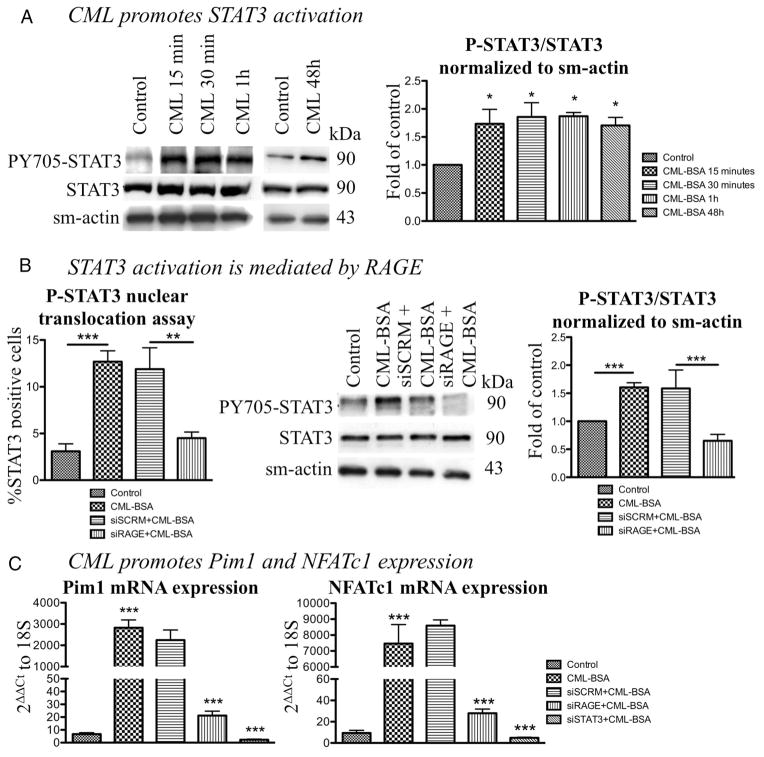
To determine whether CML triggers STAT3 activation, CASMCs were exposed to CML-BSA (1 μg.mL−1) (dose previously used and shown to have biological effects38) for 15, 30, 60 minutes and 48 hours. After a 15-minute exposition, CML-BSA–treated cells showed a sustained 1.5-fold increase in STAT3 activation measured by immunoblots (ie, Y705-phosphorylated STAT3/total STAT3 ratio normalized to smooth muscle actin) compared with control CASMCs (n=3, P<0.05; Figure 1A). This finding was confirmed by a STAT3 nuclear translocation assay on CASMCs (Figure 1B). CML-BSA increases the percentage of PY705-STAT3 (green) colocalizing with the nucleus (blue) producing a yellow pattern (n=50 CASMC/experiment for 4 experiments, P<0.01; Supplemental Figure IA). To determine whether RAGE is implicated in the CML-BSA– dependent activation of STAT3, CASMCs were exposed to CML-BSA in presence of either RAGE siRNA or its control (siSCRM). RAGE inhibition reverses CML-BSA– dependent STAT3 activation (decreased PY705-STAT3/STAT3 ratio in immunoblots, n=4, P<0.05 and yellow staining in nuclear translocation assay, n=50 CASMCs/experiment for 4 experiments, P<0.05) compared with siSCRM (Figure 1B and Supplemental Figure IA).
Figure 1.
Nε-(carboxymethyl)lysine-bovine serum albumin (CML-BSA) enhances advanced glycation endproducts receptor (RAGE) expression and promotes STAT3/Pim1/NFATc1 axis. A, CML-BSA promotes STAT3 activation after only a 15-minutes exposition and its activation is maintained for 48 hours (1.5-fold activation; immunoblot, n=3, P<0.05). B, STAT3 activation is measured by nuclear translocation assay (n=50 CASMC/experiment for 4 experiments, P<0.01). CML-BSA induces a 80% increase in STAT3 activation and RAGE blockade (by siRNA 20 nmol/L) decreases the activation by 60%. We also confirmed that STAT3 activation was mediated by RAGE by immunoblot, showing that CML-BSA induces a 1.6-fold increase and RAGE blockade decreases STAT3 activation (n=4, P<0.01). C, CML-BSA triggers Pim1 and NFATc1 expression (800-fold increase; qRT-PCR, n=4, P<0.001). RAGE and STAT3 inhibition by siRNA induce a 150- and 300-fold decrease in Pim1 and NFATc1 expression, respectively (qRT-PCR).
CML-BSA–Dependent STAT3 Activation Increases Pim1 and NFATc1
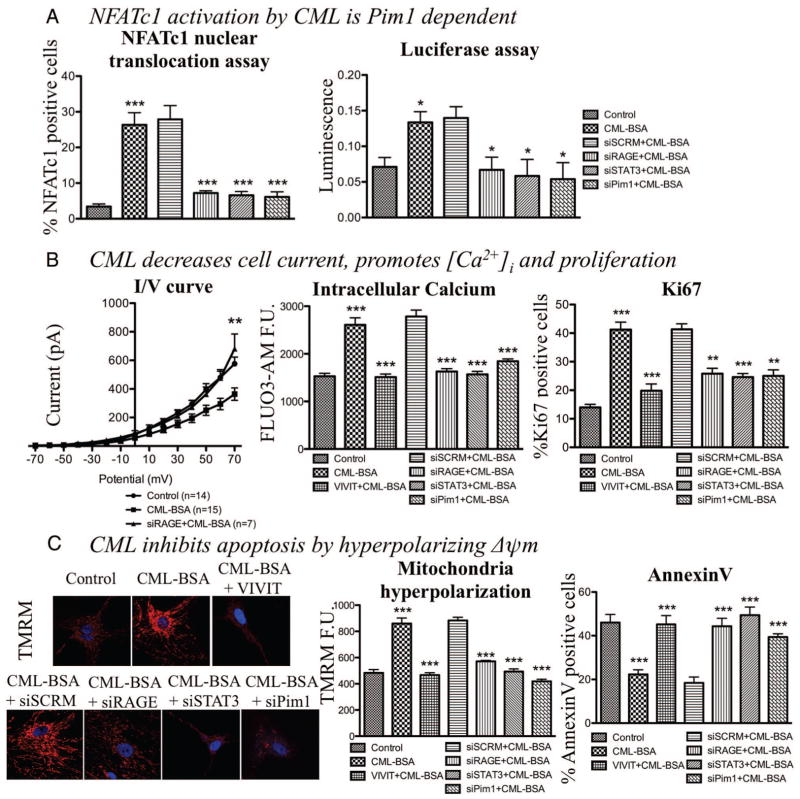
Because STAT3 has been demonstrated as a putative regulator of the NFAT activator Pim1 in cancer cells,39,40 and as Pim1/NFAT is implicated in VRD,41 we measured whether the CML-BSA– dependent STAT3 activation in CASMCs increases Pim1-dependent NFATc1 (the NFAT isoform expressed in CASMC42) expression and activation. Using both qRT-PCR and immunoblot, we demonstrated that CML-BSA significantly increases Pim1 and NFATc1 mRNA (300- and 800-fold increase, n=4, P<0.001, respectively; Figure 1C) and protein levels (both 2.4-fold increase; n=4, P<0.05; Supplemental Figure IB). Interestingly, the timing of Pim1/NFAT protein activation occurs 30 minutes post-CML-BSA exposure, while STAT3 activation occurred within 15 minutes, suggesting, as in cancer, a possible implication of STAT3 in Pim1/NFAT regulation. This was confirmed by qRT-PCR, measuring Pim1 and NFATc1 mRNA levels in with RAGE and STAT3 siRNAs. Both siRAGE and siSTAT3 decreased Pim1 and NFATc1 expression compared with siSCRM (140- and 300-fold decrease, n=4, P<0.001, respectively; Figure 1C). (Note that RAGE, STAT3, and Pim1 siRNAs efficiencies were confirmed; see Supplemental Figure IIA, IIB, and IIC). NFATc1 activation, measured by nuclear translocation and luciferase assays, confirmed this finding (n=50 CASMC/experiment for 4 experiments, P<0.001 and n=5, P<0.05, respectively; Figure 2A).
Figure 2.
Nε-(carboxymethyl)lysine-bovine serum albumin (CML-BSA) enhances Pim1 and nuclear factor of activated T-cells (NFAT)c1 expression and activation, which promotes cell proliferation and inhibits apoptosis in human carotid artery smooth muscle cells. A, CML-BSA also promotes NFATc1 activation, ie, translocation to the nucleus, through a RAGE/STAT3/Pim1-dependent mechanism (over 30% increase; immunofluorescence, n=50 CASMC/experiment for 4 experiments, P<0.001), also measured by luciferase assay (n=5, P<0.05). B, CML-BSA decreases K+ current (n=7 per group, P<0.01), which promotes intracellular calcium entry measured by FLUO3-AM by immunofluorescence (1.7-fold increase, n=50 CASMC/experiment for 5 experiments, P<0.001), which enhances cell proliferation. Cell proliferation was measured by activation of proliferation factor Ki67 (25% increase, n=50 CASMC/experiment for 5 experiments, P<0.001). To calculate the percentage of proliferation: cells positive for proliferation factors activated divided by total number of cell [visualized by 4,6-diamidino-2-phenylindole (DAPI)]. C, CML-BSA hyperpolarizes mitochondrial membrane potential measured by tetramethylrhodamine methyl ester (1.9-fold increase, n=50 CASMC/experiment for 5 experiments, P<0.001) which blocks cell death. CML-BSA inhibits apoptosis in CASMCs put in 0.1% of fetal bovine serum to stimulate apoptosis in normal cells (starvation) (n=50 CASMC/experiment for 5 experiments, P<0.05). Apoptosis was measured by calculating the percentage of positive AnnexinV cells, divided by total number of cell (visualized by DAPI). CML-BSA induced apoptosis resistance is mediated by RAGE/STAT3/Pim1/NFAT pathway, showed by an increase of apoptosis when either one of these effectors is blocked (by VIVIT 4 μmol/L or siRNA 20 nmol/L).
Because the Akt/GSK3β pathway can also activate Pim1/NFAT in CASMC, we studied whether CML-BSA increases the Akt/GSK3β pathway. CML-BSA does not increase Akt expression or activation as measured by immunoblot (ie, PS473-Akt/Akt ratio, n=3; Supplemental Figure IIIA).
Finally, in addition to the NFAT axis, STAT3 is recognized as an activator of the prosurvival protein survivin, which is critical in the remodeling process of VRD.8,43,44 To determine whether CML-BSA– dependent activation of STAT3 triggers survivin in VRD, survivin expression was measured in CML-BSA–treated CASMCs in presence of either RAGE or STAT3 siRNA or their proper control. Both RAGE and STAT3 inhibition decreased survivin expression (n=4, P<0.01; Supplemental Figure IIIB).
CML-BSA Enhances CASMC Proliferation and Decreases Apoptosis Through a RAGE/Pim1/NFATc1-Dependent Mechanism
We previously showed that NFATc1 activation in VRD accounts for the sustainability of the proproliferative and antiapoptotic phenotype of CASMCs by decreasing whole cell K+ current, depolarizing CASMC membrane potential, and increasing [Ca2+]i and mitochondrial membrane potential (ΔΨm) hyperpolarization.1 To determine whether these effects were mediated by the CML-BSA– dependent activation of Pim1/NFAT through RAGE, we measured K+ current (patch clamp) [Ca2+]i (FLUO3); proliferation (Ki67 and PCNA); ΔΨm (TMRM); and apoptosis (TUNEL and annexinV) in CASMCs treated with CML-BSA in presence of either Pim1 siRNA, NFAT inhibitor (VIVIT) (VIVIT efficiency is shown in Supplemental Figure IID, and the control peptide is not shown in graph because it has no effect as previously described,41 Supplemental Figure IIE), siRAGE, or siSTAT3.
Using whole cell patch clamping, we demonstrated that CML-BSA decreases voltage-gated K+ current (n=at least 7 per group, P<0.05; Figure 2B cell capacity were not different between CASMCs and average around 30pF), which is restored when RAGE is inhibited. As shown in Supplemental Figure IVA, CML-BSA has less 4-AP-sensitive current than control or siRAGE-treated CASMCs (n=5 per group, P<0.05). Because 4-AP is a voltage-dependant potassium channel blocker, the current diminution is a consequence of a decrease of cell membrane potassium channels (Kv1.5, for example) which confirms our hypothesis because NFAT is responsible of diminution of K channels transcription.11 Representative currents of each conditions are shown in Supplemental Figure IVB.
Decrease of K+ current causes an increase of CASMCs [Ca2+]i (1.7-fold increase, n=50 CASMC/experiment for 5 experiments, P<0.001; Figure 2B and Supplemental Figure IVA) which stimulates cell proliferation (25% increase, n=50 CASMC/experiment for 5 experiments, P<0.001) (Ki67, Figure 2B and PCNA, Supplemental Figure IIIC). Pim1, NFATc1, RAGE, or STAT3 inhibition reversed these effects (at least 30% decrease) (n=50 CASMC/experiment for 5 experiments, P<0.005) (Figure 2B and Supplemental Figure IIIC). The fact that either siPim1, VIVIT, siRAGE, or siSTAT3 normalized [Ca2+]i and proliferation in CML-BSA–treated CASMCs with the same efficiency, suggests that their effects are not additive and that indeed the calcium-dependent proliferation is mediated by the RAGE/STAT3/Pim1/NFAT axis.
CML-BSA significantly increased ΔΨm hyperpolarization (greater red staining) (Figure 2D). Once again, RAGE, STAT3, Pim1, and NFATc1 inhibition (siRNA and VIVIT peptide) normalized ΔΨm compared, respectively, to siSCRM (for RAGE, STAT3, and Pim1) and to CML-BSA–treated cells (for NFAT) (1.9-fold increase, n=50 CASMC/experiment for 5 experiments, P<0.001) (Figure 2C). ΔΨm normalization by RAGE/STAT3/NFAT inhibition reverses the resistance to serum starvation (0.1% FBS for 24 hours) induced apoptosis measured by AnnexinV and TUNEL (n=50 CASMC/experiment for 5 experiments, P<0.05) (Figure 2C and Supplemental Figure IIID, respectively). This finding demonstrates that, as for proliferation, apoptosis resistance in CML-BSA–treated CASMCs is due to the activation of the RAGE/STAT3/NFAT axis.
Pim1 KO Mice Are Resistant to RAGE-Induced VSMC Proliferation and Resistance to Apoptosis
To demonstrate that RAGE-dependent VSMC proliferation and resistance to apoptosis rely on Pim1/NFATc1 activation, we freshly isolated CASMCs from Pim1 KO mice and littermate control mice. VSMCs were then exposed to CML-BSA for 48 hours. As in humans, in control VSMCs, CML-BSA triggers STAT3 activation, ie, P-STAT3 nuclear translocation (n=30 cells per condition per experiment for 3 experiments, P<0.001; Supplemental Figure VA) in both wild type (WT) and Pim1 KO mice. CML-BSA also triggers a RAGE-dependent NFATc1 activation in Pim1 WT-CASMCs (nuclear translocation assay) (n=30 cells per condition per experiment for 3 experiments, P<0.05) (Supplemental Figure VB) promoting calcium release and proliferation (n=30 CASMC/experiment for 3 experiments, P<0.05; Supplemental Figure VC), as well as mitochondrial membrane hyperpolarization and resistance to apoptosis (n=30 CASMC/experiment for 3 experiments, P<0.05; Supplemental Figure VD). In Pim1 KO-CASMC, CML-BSA failed to activate NFATc1 and to promote proliferation and resistance to apoptosis (Supplemental Figure V).
Taken together, these findings demonstrated that CML-BSA triggers NFATc1 expression through STAT3 and NFATc1 activation through Pim1. Lack of NFATc1 activation in Pim1 KO-CASMCs demonstrated that the RAGE-dependent proliferation and resistance to apoptosis in CML-BSA–stimulated CASMCs rely mainly on the Pim1/NFATc1 axis.
CML-BSA Promotes the RAGE/STAT3/NFAT-Dependent Proproliferative and Antiapoptotic Phenotype Through the Activation of a Positive Feedback Loop Increasing RAGE Expression
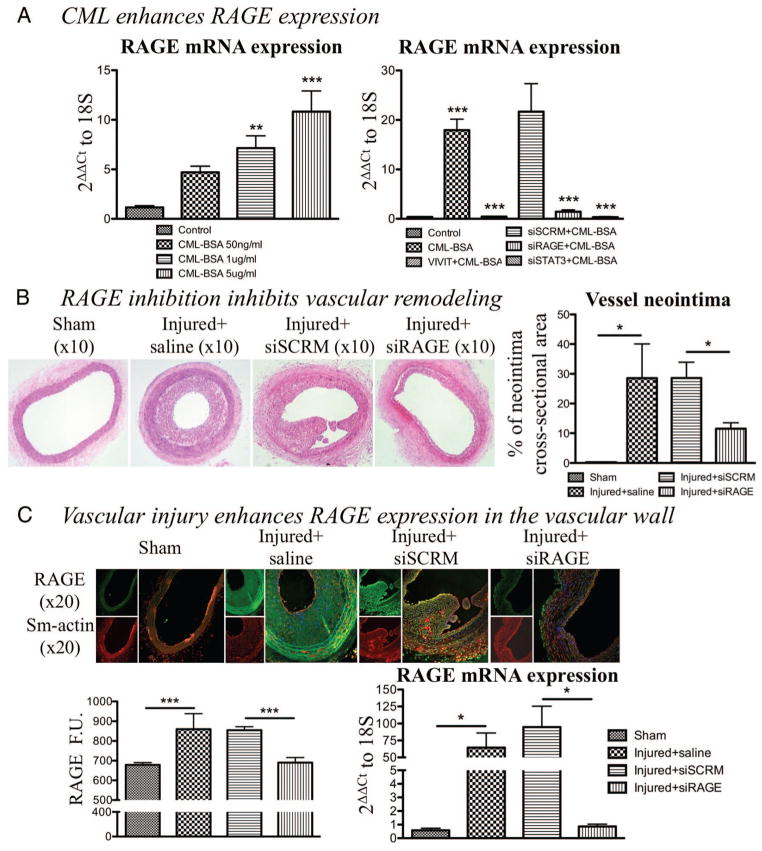
RAGE expression is described in VRD as dependant of the amount of circulating CML.15,45 We confirmed this topic by studying RAGE mRNA level (qRT-PCR) in CASMCs exposed to increasing doses of CML-BSA. As shown in Figure 3A, CML-BSA promotes RAGE expression in a dose-dependent manner (n=4, P<0.01). To elucidate the mechanism explaining how CML enhances RAGE expression, we measured RAGE protein expression in CASMCs exposed to CML-BSA for 15, 30, and 60 minutes. RAGE expression increases after 60 minutes of CML-BSA exposure (n=4, P<0.01; Supplemental Figure IC). Considering that STAT3 is activated at 15 minutes (Figure 1A), NFAT at 60 minutes (Supplemental Figure IB), and that both STAT3 and NFAT are transcription factors, we speculated that either STAT3 or NFAT could be implicated in the regulation of RAGE at the transcriptional level. RAGE expression was measured in presence of siSTAT3 or NFAT inhibitor (VIVIT). STAT3 and NFATc1 inhibition decreased by 15-fold CML-BSA–dependent RAGE mRNA level. This suggests the effective NFATc1 implication in the transcriptional regulation of RAGE (n=4, P<0.001; Figure 3A). Further experiments are required to elucidate the exact mechanisms and will be the purpose of a future study.
Figure 3.
Enhanced advanced glycation endproducts receptor (RAGE) expression stimulates vascular remodeling. A, Nε-(carboxymethyl)lysine-bovine serum albumin (CML-BSA) enhances RAGE expression in a dose-dependent manner (measured by qRT-PCR normalized with 18S; n=4, P<0.01). Furthermore, RAGE expression in enhanced through a positive feedback loop including STAT3 and nuclear factor of activated T-cells (NFAT) because their blockade (siSTAT3 20 nmol/L and VIVIT 4 μmol/L, respectively) reduces RAGE expression (qRT-PCR, n=4, P<0.001). B, After H&E staining, neointima cross-sectional areas were measured. RAGE blockade showed decreased neointima area compared with injured carotids (55% decrease, n=5 per group, P<0.05). C, RAGE expression is enhanced in the injured vascular wall and siRAGE efficiency are shown by immunofuorescence, which corresponds to RAGE protein expression (n=5 per group, P<0.001) and qRT-PCR (n=5 per group, P<0.05).
RAGE Inhibition In Vivo Prevents Vascular Remodeling Processes Through the Inhibition of the Pim1/NFAT Axis
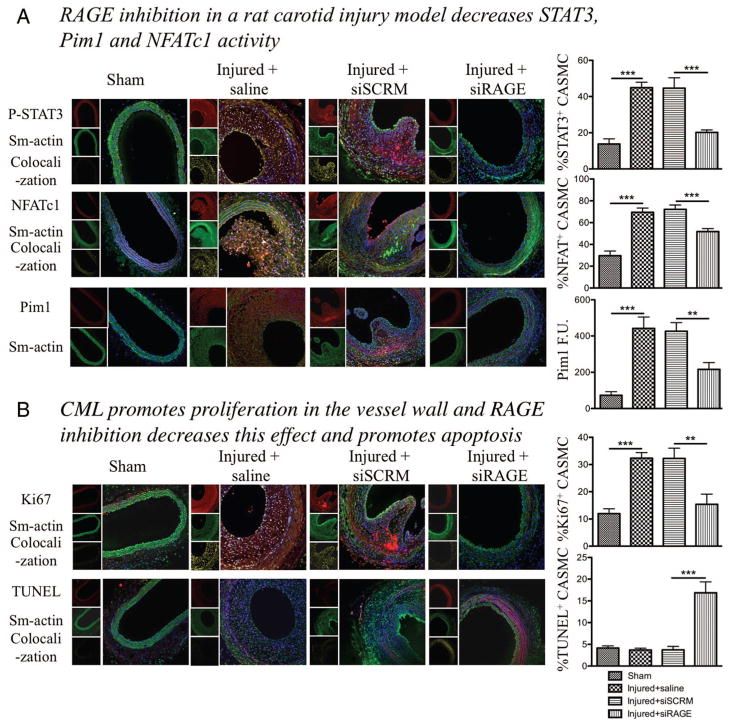
Vascular remodeling was observed before sacrifice using echography Doppler by measuring carotid wall thickness and internal diameter of the vessel. Injured rats treated with siRNA directed against RAGE showed less remodeling, ie, a thinner artery wall and a larger internal diameter of the vessel (n=5 per group, P<0.05; Supplemental Figure VIA) compared with rats treated with a control siRNA. Animals were euthanized 14 days after injury. We confirmed that the injury increased the amount of CML-BSA in the carotid by Western blot (n=4, P<0.01; Supplemental Figure VIB). Using H&E staining on harvested carotid, paraffin embedded, we measured neointimal cross-sectional area in control injured carotid arteries and injured carotids transfected with siSCRM (Figure 3B). As expected, injured carotids transfected with a siRNA directed against RAGE showed 55% less neointimal thickening (P<0.05) as compared with siSCRM (n=5 rats per group; Figure 3B). In vivo, RAGE inhibition was confirmed at the RNA level by qRT-PCR and at the protein level by immunofluorescence (n=5 rats per group, P<0.05) (Figure 3C). Immunofluorescent staining of smooth muscle actin (in green) indicates that neointimal formation is mainly constituted of smooth muscle cells, confirming the impact of the RAGE/STAT3/Pim1/NFATc1 axis on SMC, which enhance their proliferation and progressive obstruction of the lumen. Triple staining with either NFATc1 or P-STAT3 (in red), α-smooth muscle actin (green) and 4,6-diamidino-2-phenylindole (DAPI) showed that STAT3 and NFATc1 activation are increased in CASMCs of siSCRM injured carotids (showed by the colocalization in yellow of P-STAT3/DAPI or NFATc1/DAPI) compared with sham. siRAGE injured carotids at the opposite showed a decreased NFATc1 and STAT3 activation compared with siSCRM treated ones (n=50 CASMC/rat, 5 rats per group, P<0.001; Figure 4A), and the two proteins are mainly present in the cytosol. To confirm our hypothesis that NFATc1 activation is due to the presence of Pim1, we studied Pim1 expression by immunostaining and qRT-PCR. As expected, Pim1 was significantly increased in injured carotid arteries (saline or siSCRM) compare to sham, while Pim1 expression is decreased in siRAGE-treated rats compared with siSCRM (n=50 CASMC/rat, 5 rats per group, P<0.01; Figure 4A and Supplemental Figure VIC). Immunostaining for Ki67 (in red), α-smooth muscle actin (green), and DAPI showed that carotids injury is associated in saline or control siRNA with a increase of CASMC proliferation compared with sham (measured by the the nuclear localization of Ki67) (n=50 CASMC/rat, 5 rats per group, P<0.01; Figure 4B). In siRAGE injured carotids the proliferation rate of CASMCs is decreased compared with siSCRM-treated injured carotids, showing that interruption of proliferative signal progression by RAGE inhibition have a powerful nonproiferative effect on CASMCs in vivo, mainly by inhibiting the activation of the STAT3/Pim1/NFATc1 axis. Furthermore, triple staining with TUNEL (in red), α-smooth muscle actin (green), and DAPI showed that siRAGE carotids presented significantly more apoptotic CASMCs than any other group (n=50 CASMC/rat, 5 rats per group, P<0.001). These results suggest that localized RAGE inhibition decreases postinjury carotid artery remodeling in rats, mainly by inhibiting the STAT3/Pim1/NFATc1 axis.
Figure 4.
Nε-(carboxymethyl)lysine-bovine serum albumin (CML-BSA) injected in rats stimulates the STAT3/Pim1/NFATc1 pathway and enhances vascular smooth muscle cells proliferation. A, CML-BSA promotes STAT3 activation through advanced glycation endproducts receptor (RAGE) in the vascular wall (immunofluorescence, n=5 rats per group, P<0.001). STAT3 activation correlates with nuclear factor of activated T-cells (NFAT) c1 activation (immunofluorescence, n=5 rats per group, P<0.001). Both STAT3 and NFAT are transcription factors and are present in the nucleus when activated. Thus, colocalization between either P-STAT3 or NFATc1 (red) and the nucleus blue, is showed by a yellow pattern, which is decreased when carotids were treated with RAGE siRNA. Pim1 expression is also enhanced in injured carotid and is inhibited when RAGE is blocked. Pim1 quantification was measured by fluorescence quantification (immunofluorescence, n=5 rats per group, P<0.01). B, RAGE/STAT3/Pim1/NFATc1 axis is responsible of cell proliferation, measured by Ki67 (immunofluorescence, n=5 rats per group, P<0.01). RAGE inhibition increases apoptosis in the vessel wall, measured by TUNEL (immunofluorescence, n=5 rats per group, P<0.01). For all staining, to calculate percentage of activation or proliferation, cells that had positive staining in nucleus were considered positive, which was divided by the total amount of cell (counted with DAPI). Again, yellow pattern shows colocalization between proliferation and apotosis factors (red) and the nucleus (blue).
Discussion
We showed for the first time that RAGE activation in both humans’ and rodents’ CASMCs triggers the activation of the STAT3/Pim1/NFAT axis accounting for CASMC proliferation and resistance to apoptosis. Moreover, using Pim1 KO mice we showed that the RAGE-dependent CASMC proliferation and resistance to apoptosis rely on the activation of NFATc1 by Pim1, a mechanism also found in neoplasic processes.
As in cancer cells, Pim1/NFATc1 activation hyperpolarized mitochondrial membrane potential (suppressing CASMC apoptosis) and increased [Ca2+]i (promoting CASMC proliferation). These finding are in accordance with previous published studies (including ours) showing that Pim1 (through Bcl-2 associated death promoter phosphorylation46) and NFAT (through pyruvate kinase regulation41) hyperpolarized ΔΨm. Moreover, we extensively published in the past, in both cancer and VSMCs, that NFAT activation decreases K+ channels, including Kv1.5, depolarizing VSMCs and opening the voltage-gated calcium channels thus increasing [Ca2+]i. Thus, by blocking Pim1/NFAT axis, RAGE inhibition restores K+ current, [Ca2+]i and depolarizes ΔΨm. In vivo, we demonstrated that localized RAGE inhibition after 30 minutes of siRNA instillation prevented carotid artery postinjury remodeling in rats by decreasing the activation of Pim1/NFATc1 axis and thus decreasing CASMC proliferation and resistance to apoptosis. Although this finding could be seen as methodologically surprising, such approach has shown similar results in the past as shown in Lipskaia et al47 confirming the translational potential of this intervention.
We are the first linking Pim1/NFATc1 activation to RAGE in CASMCs and vascular diseases. Previous studies have described the activation of the calcineurin/NFAT axis in cultured pig CASMCs in response to AGE.48 Nonetheless, the mechanism accounting for NFAT activation (Pim1) and its implication in pathological processes including human CASMCs was not demonstrated. The role of RAGE in NFAT activation has been also observed in Alzheimer’s disease,49 further validating our findings.
The activation of Pim1/NFAT by RAGE that we described for the first time may account for many of the abnormalities seen in VRD reinforcing the importance of our findings. In fact by their effects on mitochondrial membrane potential, Pim1/NFAT may contribute to the activation of the transcription factor HIF-1 (hypoxic-inducible factor 1) which we previously showed to be implicated in VRD.2 The sustained mitochondrial hyperpolarization induced by Pim1/NFATc1 triggers the metabolic unbalanced known as Warburg paradox in both cancer50,51 and VRD,52 contributing to the activation of the transcription factor HIF-12 further enhancing VSMC proliferation. Moreover, the activation of NFAT by RAGE could explain how AGE promotes cytokines and proinflammatory molecules, such as interleukin-1 and TNF-α53,54 which are implicated in VRD.55 Indeed NFAT regulates the expression of many cytokines including the ones implicated in VRD.56 Therefore the inhibition of RAGE could not only inhibit the proproliferative and antiapoptotic phenotype but also block inflammation, another major player in VRD.
Our findings postulate that NFAT activation could maintain RAGE activation efficiency by the activation of a positive feedback loop enhancing RAGE expression in proliferative CASMCs (Figure 5). Although the exact mechanism by which NFAT increases RAGE remains to be established several possibilities exist: (1) As NFAT is a transcription factors, it could directly bind the RAGE promoter and increase RAGE expression. Nonetheless, based on in silico analysis (UCSC gene browser database), no NFAT binding sites are present within the RAGE promoter region. (2) NFAT could indirectly activate RAGE by promoting other factors, eg, NFκB and HIF-1, which are increased in VRD and known to enhance RAGE expression by a direct interaction with RAGE promoter region.24,25,45 Interestingly, recent studies in cancer have suggested the existence of positive cooperation (enhancing NFκB effects) between NFκB and NFATc1.57 Nonetheless, more experiments are required to demonstrate such cooperation in VRD.
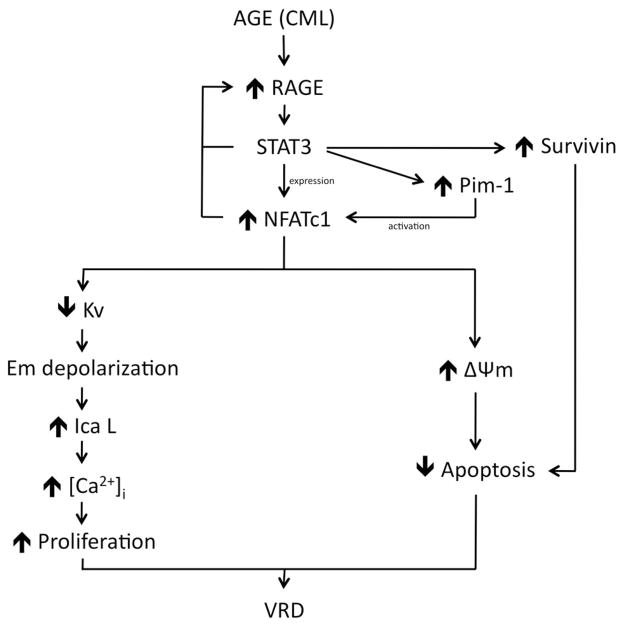
Figure 5.
Suggested pathway. Advanced glycation end product (AGE) molecules such as Nε-(carboxymethyl)lysine-bovine serum albumin (CML-BSA) activate STAT3 through their receptor advanced glycation endproducts receptor (RAGE). STAT3 is responsible for enhancing Survivin, Pim1, and nuclear factor of activated T-cells (NFAT) expressions. Pim1 is responsible of NFAT activation and NFAT stimulates proliferation through a decrease in Kv channel, which causes cell membrane (Em) depolarization. This depolarization causes a calcium intake through opening voltage-gated calcium channel known as long-lasting voltage-gated Ca channel ICaL which stimulates proliferation. On the other end, NFAT inhibits apoptosis by mitochondria membrane hyperpolarization. Survivin helps maintain resistance to apoptosis. Enhanced proliferation and resistance to apoptosis are responsible of vascular remodeling disease phenotype.
In addition to our studies, the role of RAGE in VRD has been previously described24,25,45 along with the putative therapeutic benefit that RAGE inhibition might have. Nonetheless, we are the first group providing a complete mechanism (common to many other diseases like cancer and Alzheimer disease) explaining how a sustained activation of RAGE triggers vascular remodeling processes. We demonstrated that RAGE effects in VRD rely on Pim1 dependent activation of NFAT, which could explain many features seen in VRD including HIF-1 associated metabolic disorders and inflammation. Thus, we offer new therapeutic perspectives, not only for vascular diseases, but also possibly for other diseases sharing common features with VRD including cancer.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by grants from the Canadian Institutes of Health Research (CIHR; entitled “Experimental Therapies for Vascular Remodeling Diseases” to S.B.) and the Heart and Stroke Foundation of Canada (S.B.). Sébastien Bonnet holds a Canada Research Chair. Mohsen Agharazii holds a clinician-scientist scholarship from Fonds de la Recherche en Santé du Québec (FRSQ). Jolyane Meloche is a recipient of a Summer Studentship Research Project Awards from The Canadian Hypertension Society and a Graduate Scholarship from La Société Québécoise d’Hypertension Artérielle (SQHA). Roxane Paulin also has a Graduate Scholarship from La Société Québécoise d’Hypertension Artérielle (SQHA).
Footnotes
Disclosures
None.
References
- 1.Bonnet S, Paulin R, Sutendra G, Dromparis P, Roy M, Watson KO, Nagendran J, Haromy A, Dyck JR, Michelakis ED. Dehydroepiandrosterone reverses systemic vascular remodeling through the inhibition of the Akt/GSK3-{beta}/NFAT axis. Circulation. 2009;120:1231–1240. doi: 10.1161/CIRCULATIONAHA.109.848911. [DOI] [PubMed] [Google Scholar]
- 2.Lambert CM, Roy M, Robitaille GA, Richard DE, Bonnet S. HIF-1 inhibition decreases systemic vascular remodelling diseases by promoting apoptosis through a hexokinase 2-dependent mechanism. Cardiovasc Res. 88:196–204. doi: 10.1093/cvr/cvq152. [DOI] [PubMed] [Google Scholar]
- 3.Doran AC, Meller N, McNamara CA. Role of smooth muscle cells in the initiation and early progression of atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28:812– 819. doi: 10.1161/ATVBAHA.107.159327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Libby P, Clinton SK. Cytokines as mediators of vascular pathology. Nouv Rev Fr Hematol. 1992;34 (Suppl):S47–S53. [PubMed] [Google Scholar]
- 5.Schwartz LB, Radic ZS, O’Donohoe MK, Mikat EM, McCann RL, Hagen PO. Saphenous vein endothelium-dependent relaxation in patients with peripheral vascular disease. Ann Vasc Surg. 1992;6:425– 432. doi: 10.1007/BF02006997. [DOI] [PubMed] [Google Scholar]
- 6.Ross JG, Hussey DH, Mayr NA, Davis CS. Acute and late reactions to radiation therapy in patients with collagen vascular diseases. Cancer. 1993;71:3744–3752. doi: 10.1002/1097-0142(19930601)71:11<3744::aid-cncr2820711144>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 7.Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3:917–921. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- 8.Blanc-Brude OP, Yu J, Simosa H, Conte MS, Sessa WC, Altieri DC. Inhibitor of apoptosis protein survivin regulates vascular injury. Nat Med. 2002;8:987–994. doi: 10.1038/nm750. [DOI] [PubMed] [Google Scholar]
- 9.Bonnet S, Dumas-de-La-Roque E, Begueret H, Marthan R, Fayon M, Dos Santos P, Savineau JP, Baulieu EE. Dehydroepiandrosterone (DHEA) prevents and reverses chronic hypoxic pulmonary hypertension. Proc Natl Acad Sci U S A. 2003;100:9488–9493. doi: 10.1073/pnas.1633724100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- 11.Bonnet S, Archer SL, Allalunis-Turner J, Haromy A, Beaulieu C, Thompson R, Lee CT, Lopaschuk GD, Puttagunta L, Harry G, Hashimoto K, Porter CJ, Andrade MA, Thebaud B, Michelakis ED. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell. 2007;11:37–51. doi: 10.1016/j.ccr.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt AM, Yan SD, Wautier JL, Stern D. Activation of receptor for advanced glycation end products: a mechanism for chronic vascular dysfunction in diabetic vasculopathy and atherosclerosis. Circ Res. 1999;84:489– 497. doi: 10.1161/01.res.84.5.489. [DOI] [PubMed] [Google Scholar]
- 13.Ahmed KA, Muniandy S, Ismail IS. Role of N-(carboxymethyl)lysine in the development of ischemic heart disease in type 2 diabetes mellitus. J Clin Biochem Nutr. 2007;41:97–105. doi: 10.3164/jcbn.2007014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McNulty M, Mahmud A, Feely J. Advanced glycation end-products and arterial stiffness in hypertension. Am J Hypertens. 2007;20:242–247. doi: 10.1016/j.amjhyper.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 15.Wautier MP, Massin P, Guillausseau PJ, Huijberts M, Levy B, Boulanger E, Laloi-Michelin M, Wautier JL. N(carboxymethyl)lysine as a biomarker for microvascular complications in type 2 diabetic patients. Diabetes Metab. 2003;29:44–52. doi: 10.1016/s1262-3636(07)70006-x. [DOI] [PubMed] [Google Scholar]
- 16.Zhang H, Park Y, Wu J, Chen X, Lee S, Yang J, Dellsperger KC, Zhang C. Role of TNF-alpha in vascular dysfunction. Clin Sci (Lond) 2009;116:219–230. doi: 10.1042/CS20080196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sparvero LJ, Asafu-Adjei D, Kang R, Tang D, Amin N, Im J, Rutledge R, Lin B, Amoscato AA, Zeh HJ, Lotze MT. RAGE (Receptor for Advanced Glycation Endproducts), RAGE ligands, and their role in cancer and inflammation. J Transl Med. 2009;7:17. doi: 10.1186/1479-5876-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hudson BI, Bucciarelli LG, Wendt T, Sakaguchi T, Lalla E, Qu W, Lu Y, Lee L, Stern DM, Naka Y, Ramasamy R, Yan SD, Yan SF, D’Agati V, Schmidt AM. Blockade of receptor for advanced glycation endproducts: a new target for therapeutic intervention in diabetic complications and inflammatory disorders. Arch Biochem Biophys. 2003;419:80– 88. doi: 10.1016/j.abb.2003.08.030. [DOI] [PubMed] [Google Scholar]
- 19.Rashid G, Benchetrit S, Fishman D, Bernheim J. Effect of advanced glycation end-products on gene expression and synthesis of TNF-alpha and endothelial nitric oxide synthase by endothelial cells. Kidney Int. 2004;66:1099–1106. doi: 10.1111/j.1523-1755.2004.00860.x. [DOI] [PubMed] [Google Scholar]
- 20.Ikeda K, Higashi T, Sano H, Jinnouchi Y, Yoshida M, Araki T, Ueda S, Horiuchi S. N (epsilon)-(carboxymethyl)lysine protein adduct is a major immunological epitope in proteins modified with advanced glycation end products of the Maillard reaction. Biochemistry. 1996;35:8075– 8083. doi: 10.1021/bi9530550. [DOI] [PubMed] [Google Scholar]
- 21.Schleicher ED, Wagner E, Nerlich AG. Increased accumulation of the glycoxidation product N(epsilon)-(carboxymethyl)lysine in human tissues in diabetes and aging. J Clin Invest. 1997;99:457– 468. doi: 10.1172/JCI119180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakata N, Imanaga Y, Meng J, Tachikawa Y, Takebayashi S, Nagai R, Horiuchi S, Itabe H, Takano T. Immunohistochemical localization of different epitopes of advanced glycation end products in human atherosclerotic lesions. Atherosclerosis. 1998;141:61–75. doi: 10.1016/s0021-9150(98)00149-x. [DOI] [PubMed] [Google Scholar]
- 23.Baumann M, Richart T, Sollinger D, Pelisek J, Roos M, Kouznetsova T, Eckstein HH, Heemann U, Staessen JA. Association between carotid diameter and the advanced glycation end product N-epsilon-carboxymethyllysine (CML) Cardiovasc Diabetol. 2009;8:45. doi: 10.1186/1475-2840-8-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou Z, Wang K, Penn MS, Marso SP, Lauer MA, Forudi F, Zhou X, Qu W, Lu Y, Stern DM, Schmidt AM, Lincoff AM, Topol EJ. Receptor for AGE (RAGE) mediates neointimal formation in response to arterial injury. Circulation. 2003;107:2238–2243. doi: 10.1161/01.CIR.0000063577.32819.23. [DOI] [PubMed] [Google Scholar]
- 25.Sakaguchi T, Yan SF, Yan SD, Belov D, Rong LL, Sousa M, Andrassy M, Marso SP, Duda S, Arnold B, Liliensiek B, Nawroth PP, Stern DM, Schmidt AM, Naka Y. Central role of RAGE-dependent neointimal expansion in arterial restenosis. J Clin Invest. 2003;111:959–972. doi: 10.1172/JCI17115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grote K, Luchtefeld M, Schieffer B. JANUS under stress–role of JAK/STAT signaling pathway in vascular diseases. Vascul Pharmacol. 2005;43:357–363. doi: 10.1016/j.vph.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 27.Paulin R, Courboulin A, Meloche J, Mainguy V, Dumas de la Roque E, Saksouk N, Cote J, Provencher S, Sussman MA, Bonnet S. Signal transducers and activators of transcription-3/pim1 axis plays a critical role in the pathogenesis of human pulmonary arterial hypertension. Circulation. 123:1205–1215. doi: 10.1161/CIRCULATIONAHA.110.963314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Courboulin A, Paulin R, Giguere NJ, Saksouk N, Perreault T, Meloche J, Paquet ER, Biardel S, Provencher S, Cote J, Simard MJ, Bonnet S. Role for miR-204 in human pulmonary arterial hypertension. J Exp Med. 208:535–548. doi: 10.1084/jem.20101812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zemskova M, Sahakian E, Bashkirova S, Lilly M. The PIM1 kinase is a critical component of a survival pathway activated by docetaxel and promotes survival of docetaxel-treated prostate cancer cells. J Biol Chem. 2008;283:20635–20644. doi: 10.1074/jbc.M709479200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Padma R, Nagarajan L. The human PIM-1 gene product is a protein serine kinase. Cancer Res. 1991;51:2486–2489. [PubMed] [Google Scholar]
- 31.Beier UH, Weise JB, Laudien M, Sauerwein H, Gorogh T. Overexpression of Pim-1 in head and neck squamous cell carcinomas. Int J Oncol. 2007;30:1381–1387. [PubMed] [Google Scholar]
- 32.Chiang WF, Yen CY, Lin CN, Liaw GA, Chiu CT, Hsia YJ, Liu SY. Up-regulation of a serine-threonine kinase protooncogene Pim-1 in oral squamous cell carcinoma. Int J Oral Maxillofac Surg. 2006;35:740–745. doi: 10.1016/j.ijom.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 33.Cibull TL, Jones TD, Li L, Eble JN, Ann Baldridge L, Malott SR, Luo Y, Cheng L. Overexpression of Pim-1 during progression of prostatic adenocarcinoma. J Clin Pathol. 2006;59:285–288. doi: 10.1136/jcp.2005.027672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Katakami N, Kaneto H, Hao H, Umayahara Y, Fujitani Y, Sakamoto K, Gorogawa S, Yasuda T, Kawamori D, Kajimoto Y, Matsuhisa M, Yutani C, Hori M, Yamasaki Y. Role of pim-1 in smooth muscle cell proliferation. J Biol Chem. 2004;279:54742–54749. doi: 10.1074/jbc.M409140200. [DOI] [PubMed] [Google Scholar]
- 35.Glazova M, Aho TL, Palmetshofer A, Murashov A, Scheinin M, Koskinen PJ. Pim-1 kinase enhances NFATc activity and neuroendocrine functions in PC12 cells. Brain Res Mol Brain Res. 2005;138:116–123. doi: 10.1016/j.molbrainres.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 36.Kralev S, Zimmerer E, Brueckmann M, Lang S, Kalsch T, Rippert A, Lin J, Borggrefe M, Hammes HP, Suselbeck T. Elevation of the glycoxidation product N(epsilon)-(carboxymethyl)lysine in patients presenting with acute myocardial infarction. Clin Chem Lab Med. 2009;47:446– 451. doi: 10.1515/CCLM.2009.100. [DOI] [PubMed] [Google Scholar]
- 37.Mitsuhashi T, Vlassara H, Founds HW, Li YM. Standardizing the immunological measurement of advanced glycation endproducts using normal human serum. J Immunol Methods. 1997;207:79– 88. doi: 10.1016/s0022-1759(97)00110-5. [DOI] [PubMed] [Google Scholar]
- 38.Kobayashi S, Nomura M, Nishioka T, Kikuchi M, Ishihara A, Nagai R, Hagino N. Overproduction of N(epsilon)-(carboxymethyl)lysine-induced neovascularization in cultured choroidal explant of aged rat. Biol Pharm Bull. 2007;30:133–138. doi: 10.1248/bpb.30.133. [DOI] [PubMed] [Google Scholar]
- 39.Manukyan I, Galatioto J, Mascareno E, Bhaduri S, Siddiqui MA. Cross-talk between calcineurin/NFAT and Jak/STAT signalling induces cardioprotective alphaB-crystallin gene expression in response to hypertrophic stimuli. J Cell Mol Med. 14:1707–1716. doi: 10.1111/j.1582-4934.2009.00804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen X, Xu H, Yuan P, Fang F, Huss M, Vega VB, Wong E, Orlov YL, Zhang W, Jiang J, Loh YH, Yeo HC, Yeo ZX, Narang V, Govindarajan KR, Leong B, Shahab A, Ruan Y, Bourque G, Sung WK, Clarke ND, Wei CL, Ng HH. Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell. 2008;133:1106–1117. doi: 10.1016/j.cell.2008.04.043. [DOI] [PubMed] [Google Scholar]
- 41.Bonnet S, Rochefort G, Sutendra G, Archer SL, Haromy A, Webster L, Hashimoto K, Bonnet SN, Michelakis ED. The nuclear factor of activated T cells in pulmonary arterial hypertension can be therapeutically targeted. Proc Natl Acad Sci U S A. 2007;104:11418–11423. doi: 10.1073/pnas.0610467104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chow W, Hou G, Bendeck MP. Glycogen synthase kinase 3beta regulation of nuclear factor of activated T-cells isoform c1 in the vascular smooth muscle cell response to injury. Exp Cell Res. 2008;314:2919–2929. doi: 10.1016/j.yexcr.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 43.Chen F, Xu Y, Luo Y, Zheng D, Song Y, Yu K, Li H, Zhang L, Zhong W, Ji Y. Down-regulation of Stat3 decreases invasion activity and induces apoptosis of human glioma cells. J Mol Neurosci. 40:353–359. doi: 10.1007/s12031-009-9323-3. [DOI] [PubMed] [Google Scholar]
- 44.Wang GJ, Sui XX, Simosa HF, Jain MK, Altieri DC, Conte MS. Regulation of vein graft hyperplasia by survivin, an inhibitor of apoptosis protein. Arterioscler Thromb Vasc Biol. 2005;25:2081–2087. doi: 10.1161/01.ATV.0000183885.66153.8a. [DOI] [PubMed] [Google Scholar]
- 45.Naka Y, Bucciarelli LG, Wendt T, Lee LK, Rong LL, Ramasamy R, Yan SF, Schmidt AM. RAGE axis: Animal models and novel insights into the vascular complications of diabetes. Arterioscler Thromb Vasc Biol. 2004;24:1342–1349. doi: 10.1161/01.ATV.0000133191.71196.90. [DOI] [PubMed] [Google Scholar]
- 46.Hu XF, Li J, Vandervalk S, Wang Z, Magnuson NS, Xing PX. PIM-1-specific mAb suppresses human and mouse tumor growth by decreasing PIM-1 levels, reducing Akt phosphorylation, and activating apoptosis. J Clin Invest. 2009;119:362–375. doi: 10.1172/JCI33216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lipskaia L, del Monte F, Capiod T, Yacoubi S, Hadri L, Hours M, Hajjar RJ, Lompre AM. Sarco/endoplasmic reticulum Ca2+-ATPase gene transfer reduces vascular smooth muscle cell proliferation and neointima formation in the rat. Circ Res. 2005;97:488– 495. doi: 10.1161/01.RES.0000180663.42594.aa. [DOI] [PubMed] [Google Scholar]
- 48.David KC, Scott RH, Nixon GF. Advanced glycation endproducts induce a proliferative response in vascular smooth muscle cells via altered calcium signaling. Biochem Pharmacol. 2008;76:1110–1120. doi: 10.1016/j.bcp.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 49.Cho HJ, Son SM, Jin SM, Hong HS, Shin DH, Kim SJ, Huh K, Mook-Jung I. RAGE regulates BACE1 and Abeta generation via NFAT1 activation in Alzheimer’s disease animal model. FASEB J. 2009;23:2639–2649. doi: 10.1096/fj.08-126383. [DOI] [PubMed] [Google Scholar]
- 50.Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–270. [PubMed] [Google Scholar]
- 51.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 52.McMurtry MS, Archer SL, Altieri DC, Bonnet S, Haromy A, Harry G, Puttagunta L, Michelakis ED. Gene therapy targeting survivin selectively induces pulmonary vascular apoptosis and reverses pulmonary arterial hypertension. J Clin Invest. 2005;115:1479–1491. doi: 10.1172/JCI23203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813– 820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 54.Miyata T, Hori O, Zhang J, Yan SD, Ferran L, Iida Y, Schmidt AM. The receptor for advanced glycation end products (RAGE) is a central mediator of the interaction of AGE-beta2microglobulin with human mononuclear phagocytes via an oxidant-sensitive pathway. Implications for the pathogenesis of dialysis-related amyloidosis. J Clin Invest. 1996;98:1088–1094. doi: 10.1172/JCI118889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lambert CM, Roy M, Meloche J, Robitaille GA, Agharazii M, Richard DE, Bonnet S. Tumor necrosis factor inhibitors as novel therapeutic tools for vascular remodeling diseases. Am J Physiol Heart Circ Physiol. 299:H995–H1001. doi: 10.1152/ajpheart.00562.2010. [DOI] [PubMed] [Google Scholar]
- 56.Nilsson LM, Sun ZW, Nilsson J, Nordstrom I, Chen YW, Molkentin JD, Wide-Swensson D, Hellstrand P, Lydrup ML, Gomez MF. Novel blocker of NFAT activation inhibits IL-6 production in human myometrial arteries and reduces vascular smooth muscle cell proliferation. Am J Physiol Cell Physiol. 2007;292:C1167–C1178. doi: 10.1152/ajpcell.00590.2005. [DOI] [PubMed] [Google Scholar]
- 57.Xue J, Thippegowda PB, Hu G, Bachmaier K, Christman JW, Malik AB, Tiruppathi C. NF-kappaB regulates thrombin-induced ICAM-1 gene expression in cooperation with NFAT by binding to the intronic NF-kappaB site in the ICAM-1 gene. Physiol Genomics. 2009;38:42–53. doi: 10.1152/physiolgenomics.00012.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.