Abstract
Introduction:
Nondaily smoking represents a substantial and growing fraction of smokers, many of whom do not consider themselves smokers or at risk of tobacco-related diseases and, so, may be less responsive to counseling content contained in traditional cessation interventions. This study compares the effects brief counseling interventions (<20 min) focused on the harm smoking does to themselves (harm to self, HTS) versus the harm their secondhand smoke (SHS) does to others (harm to others, HTO) among nondaily smokers.
Methods:
Randomized trial of 52 nondaily smokers (smoked in the past week, but not daily) recruited between September 2009 and June 2010; 40 completed the study. We measured changes in motivation and smoking status at 3 months postintervention.
Results:
There was a difference in quitting between the two groups, with 9.5% (2 out of 21) for HTS and 36.8% (7 out of 19) for HTO subjects reporting not smoking any cigarettes in the prior week (p = .06 by Fisher exact test and .035 by likelihood-ratio chi-square). Motivation and self-efficacy increased from baseline to 3-month follow-up, but not differentially by intervention group.
Conclusions:
Consistent with findings from research conducted by the tobacco industry as early as the 1970s that concluded that social smokers feel immune from the personal health effects of tobacco but are concerned about the consequences of their SHS on others, educating nondaily smokers about the dangers of SHS to others appears to be a more powerful cessation message than traditional smoking cessation counseling that emphasizes the harmful consequences to the smoker.
Introduction
Nondaily smoking increased through the 1990s, and in 2010 represented 21.8% of U.S. smokers (Centers for Disease Control and Prevention, 2003, 2011) In California, between 1992 and 2008, nondaily smokers doubled from 14.8% to 28.1% of smokers (Al-Delaimy et al., 2010). Previously considered a behavior associated with initiation or cessation, nondaily smoking is now recognized as a growing stable consumption pattern (Gilpin, Cavin, & Pierce, 1997; Hassmiller, Warner, Mendez, Levy, & Romano, 2003; Oksuz, Mutlu, & Malhan, 2007; Schane, Glantz, & Ling, 2009a; Wortley, Husten, Trosclair, Chrismon, & Pederson, 2003).
Compared with daily smokers, nondaily smokers tend to be younger, better educated, Black and Hispanic, and wealthier (Gilpin et al., 1997; Hassmiller et al., 2003; Wortley et al., 2003). Nondaily smokers often do not consider themselves smokers and, consequently, are under-recognized by clinicians (Schane et al., 2009a); up to 42% classify themselves as nonsmokers when questioned about tobacco product use (Fergusson & Horwood, 1995). Even though nondaily smoking increases risk for many diseases (Schane, Ling, & Glantz, 2010; Surgeon General, 2004), this propensity to self-identify as a nonsmoker reinforces nondaily smokers’ belief that nondaily smoking does not carry health risks (Tong, Ong, Vittinghoff, & Perez-Stable, 2006).
Nondaily smokers report greater intention to quit and are more likely to succeed than everyday smokers (Hennrikus, Jeffery, & Lando, 1996; Sargent, Mott, & Stevens, 1998). Among 12–18 year olds, occasional smoking is associated with a sevenfold increase in cessation compared with everyday smoking (Sargent et al., 1998). While prime targets for intervention (Hassmiller et al., 2003; Wortley et al., 2003), nondaily smokers may require a new treatment paradigm. Standard cessation counseling that focuses on personal health risks may not motivate them to quit, because they tend to minimize health risks due to their tobacco use (Hyland, Rezaishiraz, Bauer, Giovino, & Cummings, 2005; Tong et al., 2006), In contrast, observational data support counseling nondaily smokers on the dangers that their secondhand smoke (SHS) poses to others as a promising cessation message (Schane & Glantz, 2008; Tong et al., 2006).
Public education campaigns about the dangers of SHS (California Environmental Protection Agency, 2005; Surgeon General, 2006) have been a staple of state tobacco control programs since California first started focusing on them in 1989 (Goldman & Glantz, 1998). Having a smokefree home is associated with increased smoking cessation among adult smokers (Mills, Messer, Gilpin, & Pierce, 2009).
The pediatric literature reports that counseling parents about the dangers their SHS poses to their children (Caponnetto, Polosa, & Best, 2008) leads to changes in parental smoking. Of 19 trials with parental SHS reduction as their primary target, those that focused on parental education and counseling were most effective at reducing children’s SHS exposure measured by reduced self-reported parental cigarette consumption, home air nicotine levels, or children’s urinary cotinine (Baheiraei et al., 2011; Tyc, Hovell, & Winickoff, 2008). Mothers who received counseling that focused on ways to reduce childhood exposure to parental tobacco smoke significantly reduced their cigarette consumption more than mothers in the control group (Hovell et al., 2000). Studies of children with asthma showed decreases in exposure to cigarette smoke and reductions in illness and health care utilization when parents participated in SHS counseling interventions (Tyc et al., 2008; Wahlgren, Hovell, Meltzer, Hofstetter, & Zakarian, 1997). While the SHS counseling studies were not powered to study cessation as a primary outcome, many showed increased quit rates among parents (Hovell et al., 1994, 2002; McIntosh, Clark, & Howatt, 1994; Tyc et al., 2008).
We conducted a pilot randomized trial of adult nondaily smokers to compare the efficacy of counseling on the dangers of SHS exposure to others versus counseling on personal health risks.
Methods
Procedures
From September 2009 to June 2010, we recruited nondaily smokers in the San Francisco Bay Area with fliers, newspaper advertisements, and website postings. Respondents were telephone screened to ensure that they smoked at least 100 cigarettes in their lifetime, smoked at least once in the past seven days but not on every day, were 18 years or older, and spoke English. Intention to quit smoking was not required for study participation. Subjects were excluded if they had an exhaled carbon monoxide (CO) exceeding 10 ppm.
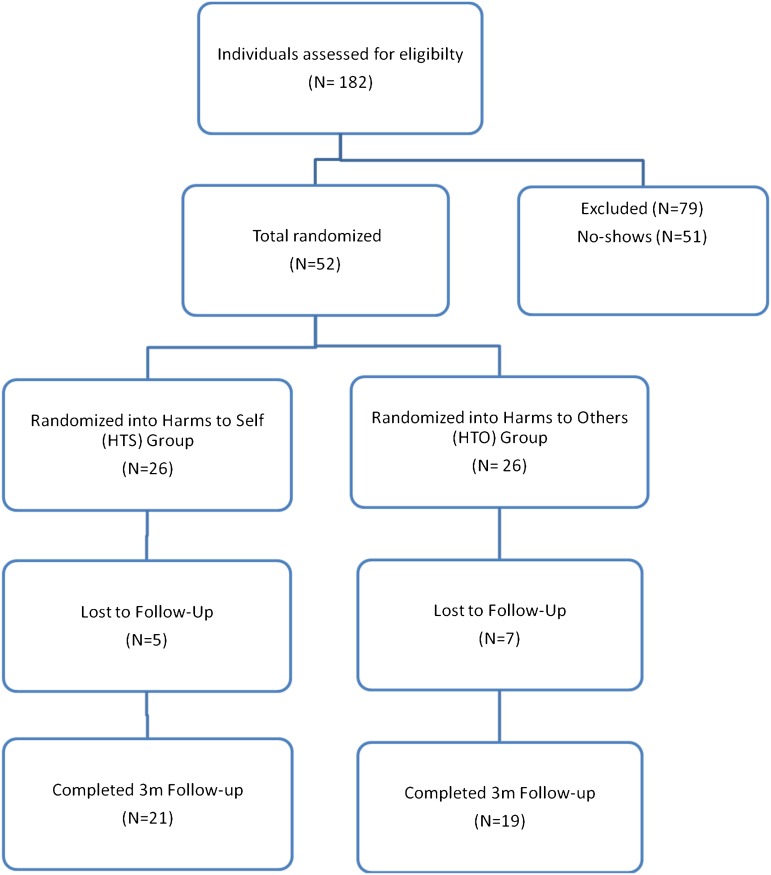
Participants were randomized (Figure 1) at enrollment using a random sequence created by SAG using the random number generator in Minitab 14 to receive one individual 15-min counseling session on either: (a) the personal health risks of smoking and personal benefits of quitting (Harms to Self, HTS group) or (b) the dangers of exposing nonsmokers to SHS and benefits to others of quitting (Harms to Others, HTO group). Following the counseling, participants viewed a 4-min montage of antitobacco California Department of Public Heath advertisements. We kept the interventions under 20 min to make it possible to incorporate into clinical practice and because counseling patients longer than 10 min generates better cessation outcomes than shorter interventions (Stevens, Severson, Lichtenstein, Little, & Leben, 1995; Stewart & Rosser, 1982; Surgeon General, 2008).
Figure 1.
Consort diagram. Of the 79 ineligible respondents, 46 were ineligible because they answered “yes” to the question do they smoke everyday on the telephone screen, 27 because they answered “yes” to smoking products other than tobacco (e.g., hookah, cigars), four were ineligible because the answered “no” to smoking more than 100 cigarettes in their lifetime, and two were ineligible because they answered “yes” to currently receiving treatment for their smoking elsewhere.
Participants were paid $25 for their time at enrollment and again at the 3-month follow-up.
Procedures were approved by the University of California San Francisco (UCSF) Committee on Human Research and participants provided informed consent.
Interventions
In the Harms to Self (HTS) group, subjects were informed by the study nurse that their smoking places them at increased risk for medical conditions, including heart disease, obstructive lung disease (emphysema/bronchitis), cancer, infection, impaired fertility/impotence, periodontal disease, osteoporosis, poor wound healing, and accelerated aging (facial wrinkling; Surgeon General, 2004). The nurse also provided information on some of the common chemicals in cigarette smoke and how these substances have other applications, including ammonia (floor/toilet cleaner), butane (lighter fluid), cadmium (rechargeable batteries), carbon monoxide (car exhaust fumes), and formaldehyde (body tissue preservative). Subjects were told that: tobacco is the leading cause of preventable disease, disability, and death in the United States; nicotine is at least as addictive as cocaine or heroin and may even be more addictive than these drugs; the portion of U.S. deaths attributable to smoking: lung cancer (90%), emphysema/bronchitis (85%), mouth cancer (70%), throat cancer (50%), bladder cancer (50%), esophageal cancer (40%), and pancreatic cancer (35%); and the risk of coronary artery disease is 70% higher among smokers (Surgeon General, 2004). They were then shown eight 30-s antismoking advertisements that stressed the risks to smokers posed by smoking (Nicotine Soundbites, Poisons, Other Ways to Use a Cigarette, Icons, Debi, Gala Event, Left Behind, Echo).
In the Harms to Others (HTO) group, subjects were informed that their tobacco use places their nonsmoking friends and family members at an increased risk of heart disease, peripheral vascular disease, cancer, respiratory infections (bronchitis and pneumonia), and compromised lung function and that the chemicals in SHS have other applications, as described to subjects in the HTS group. They were educated about further key health risks associated with passive smoking: approximately 3,000 nonsmokers die each year from lung cancer due to SHS; more than 35,000 nonsmokers die each year from heart disease due to SHS; smoking around nonsmokers will increase their female family members’ and friends’ risk of breast cancer and cervical cancer; parents who smoke around their children can increase their child’s risk of sudden infant death syndrome, asthma induction and exacerbation, eye/ear/nose irritation and infection, and can impair childhood brain development; and smoking around friends/family/significant others will compromise fertility in both nonsmoking men and women (California Environmental Protection Agency, 2005; Surgeon General, 2006). They were then shown eight 30-s antismoking advertisements that stressed the risks to nonsmokers posed by SHS (Victim Wife, Baby Blocks, Clinical, Inhaler, Baby Smokers, Cereal, Apartment, Take it Outside).
Participants who expressed interest in quitting over the course of the study were referred to the UCSF Tobacco Education Center, which provides individual and group counseling and pharmacotherapy (Surgeon General, 2008).
Measures
At baseline, subjects reported age, sex, race/ethnicity, education level, current income, employment status, occupation, and marital status. Personal smoking history was assessed including the number and duration of prior quit attempts, the number of friends/family members who smoked, and home smoking rules.
The primary outcome measure was 7-day point prevalence abstinence at the 3-month follow-up. Self-report is a reliable measure of smoking status in the absence of perceived response demand between the interviewer and the respondent (Glasgow et al., 1993; Patrick et al., 1994; Velicer, Prochaska, Rossi, & Snow, 1992). To eliminate this potential bias, we included biochemical validation with CO≤5 ppm and urinary cotinine (SRNT Subcommittee on Biochemical Verification, 2002). (Cotinine was only measured at the 3-month follow-up). Cotinine was determined by the UCSF Tobacco Biomarker Core Laboratory using high sensitivity liquid chromatography tandem mass spectrometry with a lower limit of detection of 0.02–1.0 ng/ml (Jacob et al., 2011) with a 16 ng/ml cutpoint to maximize sensitivity and specificity for distinguishing smokers from nonsmokers (Benowitz, Bernert, Caraballo, Holiday, & Wang, 2009; Benowitz, Dains, et al., 2009).
Secondary outcomes were increases in motivation and beliefs in one’s ability to quit and stay quit (self-efficacy) from baseline to 3-month follow-up assessed with the Contemplation Ladder (Biener & Abrams, 1991) and Thoughts about Abstinence scale (TAA; Hall, Havassy, & Wasserman, 1990); reduction in cigarettes smoked in the past week at baseline and 3-month follow-up; making a quit attempt; and reports of seeking intensive cessation treatment between the intervention and 3-month follow-up. Corresponding to the stages of change construct, the Contemplation Ladder visually resembles a 10-rung ladder to assess a smoker’s position on a continuum ranging from having no thoughts of quitting to engaging in action to change their smoking behavior (Biener & Abrams, 1991; Prochaska & DiClemente, 1983). The TAA scale uses three Likert scales from 0 to 10 to assess desire to quit smoking, perceived success with quitting, and anticipated difficulty with staying quit. A fourth item assesses abstinence goal coded as no goal (0), complete sustained abstinence (2), or an intermediate goal, such as smoking reduction (1). Engagement in more formal cessation treatments such as those available at the UCSF Tobacco Education Center (e.g., cessation groups and pharmacotherapy) and alternative treatments (e.g., hypnosis, acupuncture) were coded for seeking formal assistance with quitting versus not and coded as quitting cold turkey (i.e., without additional counseling support or cessation pharmacotherapy).
At the end of the baseline visit, after the intervention, participants were asked to rate their acceptability of the antismoking advertisements they viewed on 10 point scales (how much they liked the videos, how familiar they were with the information contained in the videos, and how much additional information they learned). Lastly, they indicated “yes” or “no” whether the videos made them feel uncomfortable.
Statistical Methods
Analyses were conducted using SPSS version 17.0 and Stata version 10.1. Because of the small sample size, the primary outcome variable (smoking at 3-month follow-up) was assessed using a two-tailed Fisher exact test. Due to the Fisher test's conservatism (Berkson, 1978; D’Agostino, Chase, & Belanger, 1988; Liddell, 1976), we also report the likelihood-ratio chi-square test. Comparisons between baseline conditions and comparisons between subjects who completed the study and those who were lost to follow-up was done using t tests, Kruskal–Wallis tests, or Fisher exact tests, depending on the type of data. Repeated measures analyses of variance (ANOVA) was used to test the main effect of changes over time in motivation and self-efficacy following the interventions and the interaction of changes over time and the intervention.
Results
Sample Characteristics
Fifty two nondaily smokers, 26 in each experimental group, entered the study (Table 1). They were similar on all but one tobacco use or demographic variable, contemplation ladder score (p = .0504). People in the sample smoked a median of 6.5 cigarettes/week (Interquartile range 2.8–15.0) and few participants (6 out of 52 = 11.5%) identified quitting for good as their goal.
Table 1.
Baseline Descriptive Characteristics of the Sample by Group
| Variables | HTS condition | HTO condition | p Valuesa | |
| Mean (SD)a median (IQR)b or % (n)c | Mean (SD), median (IQR), or % (n) | HTS vs. HTO at baseline | Completed vs. lost to follow-up | |
| Enrolled in study | 26 | 26 | ||
| Completed study | 21 | 19 | ||
| Smoking characteristics | ||||
| CO level (ppm) | 5.81 (4.09) | 6.27 (5.19) | .723 | .859 |
| Age first tried cigarettes | 14.92 (3.44) | 14.58 (3.42) | .718 | .178 |
| Age smoked regularly | 19.96 (5.67) | 19.12 (3.29) | .514 | .216 |
| Years of smoking regularly | 14.00 (11.30) | 12.24 (10.30) | .575 | .435 |
| Usual cigarettes/week | 4.75 (2.75, 15) | 8.50 (2.75, 15) | .451 | .549 |
| Contemplation ladder | 5.23 (1.86) | 6.16 (1.4) | .050 | .615 |
| Desire to quit smoking | 5.62 (2.86) | 6.19 (2.26) | .423 | .261 |
| Expected success in quitting smoking | 5.88 (2.53) | 6.56 (2.04) | .301 | .412 |
| Perceived difficulty with staying smokefree once abstinent | 6.04 (3.07) | 5.52 (2.68) | .524 | .135 |
| Goal of complete abstinence | 7.7% (2) | 15.4% (4) | .754 | .465 |
| Number of prior quit attempts | 2 (0, 5) | 3 (0, 6) | .599 | .576 |
| Duration prior quit attempts (weeks) | 10 (2, 9) | 3 (14, 90) | .862 | .844 |
| Number of family/friends who smoke | 2.38 (.85) | 2.65 (1.50) | .429 | .550 |
| Smoking allowed in the home | 42.9% (9) | 57.1% (12) | .400 | .739 |
| Age | 33.83 (11.51) | 31.48 (10.71) | .468 | .179 |
| Gender | .404 | .026 | ||
| Female | 38.7% (10) | 53.8% (14) | ||
| Male | 57.7% (15) | 46.2% (12) | ||
| Transgender | 3.8% (1) | 0% (0) | ||
| Ethnicity | .775 | .139 | ||
| Caucasian | 57.7% (15) | 57.7% (15) | ||
| Black | 3.8% (1) | 11.5% (3) | ||
| Asian | 23.1% (6) | 19.2% (5) | ||
| Other | 15.4% (4) | 11.5% (3) | ||
| Education | .552 | .126 | ||
| High school degree | 30.8% (8) | 23.1% (6) | ||
| 2-year college degree | 26.9% (7) | 23.1% (6) | ||
| 4-year college degree | 26.9% (7) | 38.5% (10) | ||
| Postgraduate degree | 15.4% (4) | 15.4% (4) | ||
| Household income | .564 | .524 | ||
| <$20,000 | 46.2% (12) | 34.6% (9) | ||
| $20,000–$50,000 | 26.9% (7) | 46.2% (12) | ||
| $50,000–$100,000 | 19.2% (5) | 11.5% (3) | ||
| >$100,000 | 7.7% (2) | 7.7% (2) | ||
| Employment status | .879 | .999 | ||
| Unemployed | 38.5% (10) | 30.8% (8) | ||
| Outside employment | 34.6% (9) | 46.2% (12) | ||
| Self-employed | 23.1% (6) | 19.2% (5) | ||
| Retired | 3.8% (1) | 3.8% (1) | ||
| Working full time | .618 | .301 | ||
| No | 69.2% (18) | 69.2% (18) | ||
| Yes | 30.8% (8) | 30.8% (8) | ||
| Marital status | .506 | .874 | ||
| Single/never married | 76.9% (20) | 80.8% (21) | ||
| Married/cohabitating | 15.4% (4) | 3.8% (1) | ||
| Separated/divorced/widowed | 7.6% (2) | 15.4% (4) | ||
Note. HTS = harms to self; HTO = harm to others
Normally distributed, compared with t tests.
Compared with Kruskal–Wallis tests.
Compared with Fisher exact tests.
Over the 3-month study, sample retention was 77%: 21 HTS participants and 19 HTO participants completed the 3-month follow-up (Figure 1). Study completers (n = 40) and those lost to follow-up (n = 12) did not differ on any of the measured baseline demographic or tobacco use characteristics except gender distribution (p = .026; Table 1).
Abstinence Status
The study outcome was bioconfirmed tobacco abstinence at the 3-month follow-up. Of the 40 participants who completed the 3-month follow-up, one in the HTO intervention self-reported not smoking any cigarettes in the past seven days, yet had a urinary cotinine of 22 ng/ml, so was classified as a smoker. Eight had urinary cotinine levels below 16 ng/ml but reported smoking in the prior 7 days and so were classified as smokers (six in the HTS group and two in the HTO group). There was a difference in abstinence between the two groups, with 9.5% (2 out of 21) of the HTS and 36.8% (7 out of 19) of the HTO subjects reporting not smoking any cigarettes in the prior week (p = .06 by Fisher exact test and .035 by likelihood-ratio chi-square; Figure 2).
Figure 2.
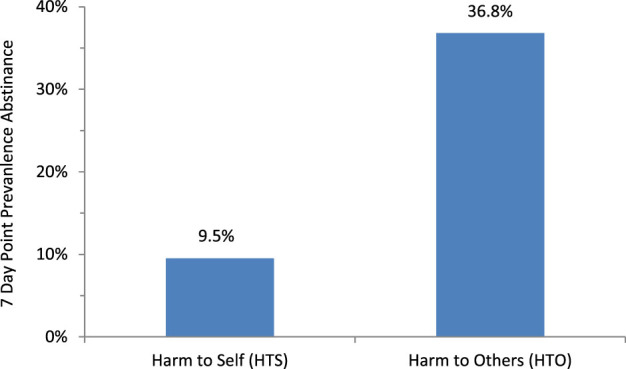
Abstinence rates by condition. Harm to Others was associated with higher abstinence rates than Harm to Self (HTS; p = .06 by Fisher exact test and .035 by likelihood-ratio chi-square).
Given that we tested for differences in 22 baseline variables, it was not surprising that we found one significant change between the baseline characteristics of the two treatment groups, contemplation ladder score, and one significant difference between those who completed the study and those lost to follow-up, gender. To test whether either of these differences could substantially confound the results, we computed separate logistic regressions to predict smoking status at follow-up as a function of intervention group and baseline contemplation ladder score or gender. These variables did not even approach significance (p = .825 for contemplation ladder score and .989 for gender) nor had much effect on the significance of the effect of the intervention (p = .065 for intervention when controlling for contemplation ladder and .055 when controlling for gender).
Change in Motivation
The repeated measures ANOVAs (Table 2) revealed increases over time in motivation (Contemplation Ladder, p < .001; TAA Desire, p < .001) and self-efficacy (TAA Success, p = .008), but not perceived difficulty of staying abstinent (TAA Difficulty, p = .942). These changes were similar for both intervention groups (p ≥ .247 for the interactions of intervention group by time for all three variables). At the 3-month follow-up, 17.9% of the sample identified quitting for good as their goal, with no difference by intervention group (p = .999). Among those abstinent at the 3-month follow-up, three of nine identified sustained abstinence as their goal.
Table 2.
Repeated Measures Analysis of Variances Testing the Main Effect of Changes Over Time in Motivation and Self-efficacy and the Interaction of Changes Over Time by Intervention Condition (p values)
| Variable | Contemplation ladder | Desire to quit | Perceived success in quitting smoking | Anticipated difficulty in staying smokefree once abstinent |
| Intervention (main effect) | 0.343 | 0.441 | 0.449 | 0.505 |
| Time (main effect) | <0.001 | <0.001 | 0.008 | 0.942 |
| Intervention × time interaction | 0.247 | 0.998 | 0.485 | 0.535 |
Quit Attempts and Engagement in More Intensive Cessation Treatment
Most participants reported trying to reduce or quit smoking between the counseling intervention and 3-month follow-up with no significant difference by group: 94.7% HTO versus 85.7% HTS (p = .607). The data suggested that more HTS (28.6%) than HTO (5.3%) participants sought intensive cessation treatment (p = .095). For both interventions all of those who were abstinent at the 3-month follow-up reported quitting cold turkey.
Smoking Reduction
At the 3-month follow-up, the change in cigarettes smoked in the past seven days among those who were still smoking was comparable for the two groups: HTS median change = 0 cigarettes/week (IQR = −2 to +6) and HTO median change 0 (IQR = −3 to +10; p = .634). The treatment effect was on quitting, not on reduction of cigarettes per week among continuing smokers.
Intervention Acceptability Ratings
Participant ratings of acceptability of the intervention videos did not vary significantly by group (p ≥ .360). On 10-point scales where 10 was the strongest positive valence and 0 the lowest, the subjects independent of intervention had a mean of 6.48 ± 2.80 (SD) for liking the videos, 7.44 ± 1.96 for familiarity with the content, and 4.65 ± 3.01 for degree of new information learned from the videos. In both groups, 31% of participants (8 per group) reported the videos made them feel uncomfortable. Participants who were abstinent at the 3-month follow-up reported liking the videos at baseline more than participants who were still smoking (8.22 ± 1.86 vs. 6.16 ± 2.77, p = .043). Familiarity, new information, and discomfort ratings did not predict abstinence status.
Discussion
This paper reports the first randomized trial of using counseling about SHS (HTO) as a cessation method for adult nondaily smokers. While we hypothesized that a focus on HTO would lead to greater motivation to quit than an intervention focused on HTS, our findings indicated comparable increases in motivation for both interventions. Ratings of liking, familiarity, and learning also were comparable for the two interventions. Yet there was more than a threefold greater abstinence in the HTO group that the HTS group (Figure 2).
Both interventions impacted motivation to quit, but only the HTO led to actual behavior change at the 3-month follow-up. Most participants attempted to quit smoking but the HTO group was more successful at being abstinent at 3-month follow-up. It may be that the HTO message has equal impact on motivation but greater impact on actual abstinence; motivation to quit and ability to stay abstinent may be different.
The finding that concern over the effects of SHS is a more potent instigator for behavior change than concern about HTS is consistent with the tobacco industry’s understanding of intermittent “social smokers” (Schane, Glantz, & Ling, 2009b). In particular, tobacco industry research as early as the 1970s identified characteristics of social smokers that included a denial of personal nicotine addiction, self-identification as a nonsmoker, and perceived immunity to personal health effects of tobacco but concern about the consequences of their SHS on others.
Education about SHS also may be relevant for prevention efforts. A prospective study of 295 adolescents found that youth who perceived greater harms from SHS were significantly less likely to initiate tobacco use (Song, Glantz, & Halpern-Felsher, 2009). As more and more people become intermittent smokers, our results suggest that media campaigns about SHS will not only encourage protection of nonsmokers from SHS but may also be a cessation intervention for nondaily smokers.
An unanticipated finding in this study was that traditional biomarkers (SRNT Subcommittee on Biochemical Verification, 2002) were not sufficient to identify intermittent smokers. In contrast to the situation in which heavier smokers tend to under-report their smoking when compared with biomarkers, 8 of the 17 (47%) subjects who had levels of cotinine below the cutpoint (16 ng/ml) considered consistent with nonsmoking nevertheless reported having smoked in the last week and four of the eight subjects smoked so infrequently that they had cotinine values below the limit of detection. (Use of cotinine as a biomarker may have been made more accurate by considering different urinary cotinine cutpoints for different racial/ethnic groups cutpoints [Benowitz, Bernert, et al., 2009]; our study was too small to investigate this possibility.) This finding points to the need to use biomarkers with a longer half-life such as metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (Hecht et al., 1999) and the utility of combining self-report with biomarkers in studies of intermittent smokers.
The study has several limitations. Abstinence from cigarette smoking in a 7-day period could also be related to illness or other environmental or social variables not just the experimental intervention. Designed as a pilot study with the goal of examining initial evidence of efficacy, the sample size was small. Nevertheless, the sample size was adequate to detect an effect of HTO counseling on cessation. Few participants endorsed a goal of complete and sustained abstinence and assessment of intervention effects beyond 3 months follow-up would be desirable. There are data, however, indicating that self-reported cessation among smokers who quit at 3 months is not detectably different from those who quit at 6 months (Gilpin et al., 1997). The study was conducted in California, which has a longstanding tobacco control program with an emphasis on SHS harms and many state and local laws restricting smoking, which may have particularly sensitized respondents to the HTO message. All the advertisements used in the study (for both interventions) were from California. Lastly, five of the eight HTO antitobacco advertisements were focused on the harmful SHS effects on children. The study surveys did not include assessment of children under the care of participants and, as noted in the Introduction, concern about the effects of SHS on their children, has been linked to changes in parents’ smoking behavior. This construct would be of interest as a potential moderator of treatment effects in future investigations. Nondaily smokers are a rapidly growing group, many of whom do not consider themselves smokers. Current clinical practice guidelines do not recommend pharmacotherapy for nondaily smokers and viable alternative treatment paradigms are needed. This is the first study to focus intervention messaging on the harms of SHS exposure for encouraging abstinence among nondaily smoking adults. The finding of a more than threefold increase in abstinence relative to an active intervention comparison group is encouraging. Consistent with findings from research conducted by the tobacco industry since as early as the 1970s (Schane et al., 2009b), educating nondaily smokers about the dangers of SHS for others may be a more powerful cessation message than traditional smoking cessation counseling about the harm smoking does to the smoker.
Funding
This work was supported by a National Cancer Institute training grant R25 CA-113710 and NRSA fellowship F32 CA-141930 to Dr. Schane, the William Cahan Endowment provided to Dr. Glantz by the Flight Attendant Medical Research Institute, and research awards to Dr. Prochaska from the National Institute of Mental Health R01 MH083684, and the UCSF Helen Diller Family Comprehensive Cancer Center and the California Tobacco-Related Disease Research Program 13-KT-0152. The funding agencies played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Declaration of Interests
Dr. Prochaska holds an Investigator Initiated Research Award from Pfizer, Inc., WS981308, titled “A Two-Part Pilot Study of Dosing, Safety and Efficacy of Varenicline Initiated during an Acute Smokefree Hospitalization and Continued Post-Hospitalization.” The other authors have nothing to declare. All authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Acknowledgments
We thank Torsten Neilands and Kevin Delucchi for advice on statistical issues.
References
- Al-Delaimy WK, White MM, Mills AL, Pierce JP, Emory K, Boman M, Smith J, et al. Final summary report of: Two decades of the California Tobacco Control Program: California Tobacco Survey, 1990–2008. 2010. Retrieved from http://www.cdph.ca.gov/programs/tobacco/Documents/CDPH_CTS2008%20summary%20report_final.pdf. [Google Scholar]
- Baheiraei A, Kharaghani R, Mohsenifar A, Kazemnejad A, Alikhani S, Milani HS, et al. Reduction of secondhand smoke exposure among healthy infants in Iran: Randomized Controlled Trial. Nicotine & Tobacco Research. 2011;13:840–847. doi: 10.1093/ntr/ntr085. Epub 2011 Apr 2018. doi:ntr085 [pii] 10.1093/ntr/ntr085. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Bernert JT, Caraballo RS, Holiday DB, Wang J. Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. American Journal of Epidemiology. 2009;169:236–248. doi: 10.1093/aje/kwn301. doi:kwn301 [pii] 10.1093/aje/kwn301. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Dains KM, Dempsey D, Herrera B, Yu L, Jacob P., 3rd Urine nicotine metabolite concentrations in relation to plasma cotinine during low-level nicotine exposure. Nicotine & Tobacco Research. 2009;11:954–960. doi: 10.1093/ntr/ntp092. doi:ntp092 [pii] 10.1093/ntr/ntp092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkson J. In dispraise of the exact test. Journal of Statistical Planning and Inference. 1978;2:27–42. doi:10.1016/0378-3758(78)90019-8. [Google Scholar]
- Biener L, Abrams DB. The Contemplation Ladder: Validation of a measure of readiness to consider smoking cessation. Health Psychology. 1991;10:360–365. doi: 10.1037//0278-6133.10.5.360. doi:10.1037/0278-6133.10.5.360. [DOI] [PubMed] [Google Scholar]
- California Environmental Protection Agency. Health effects of exposure to environmental tobacco smoke. 2005. Retrieved from http://www.oehha.ca.gov/air/environmental_tobacco/2005etsfinal.html. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caponnetto P, Polosa R, Best D. Tobacco use cessation counseling of parents. Current Opinion in Pediatrics. 2008;20:729–733. doi: 10.1097/mop.0b013e328317f1d2. doi:10.1097/MOP.0b013e328317f1d2. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Prevalence of current cigarette smoking among adults and changes in prevalence of current and some day smoking-United States, 1996–2001. Morbidity & Mortality Weekly Report. 2003;52:303–307. Retrieved from http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5214a5212.htm. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Vital signs: Current cigarette smoking among adults aged ≥18 years—United States, 2005–2010. Morbidity & Mortality Weekly Report. 2011;60:1207–1212. Retrieved from http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6035a1205.htm?s_cid=mm6035a1205_w. [PubMed] [Google Scholar]
- D’Agostino RB, Chase W, Belanger A. The appropriateness of some common procedures for testing equality of two independent binomial proportions. American Statistician. 1988;42:198–202. doi:10.2307/2685002. [Google Scholar]
- Fergusson DM, Horwood LJ. Transitions to cigarette smoking during adolescence. Addictive Behaviors. 1995;20:627–642. doi: 10.1016/0306-4603(95)00023-6. doi:10.1016/0306-4603(95)00023-6. [DOI] [PubMed] [Google Scholar]
- Gilpin E, Cavin SW, Pierce JP. Adult smokers who do not smoke daily. Addiction. 1997;92:473–480. doi:10.1111/j.1360-0443.1997.tb03379.x. [PubMed] [Google Scholar]
- Glasgow RE, Mullooly JP, Vogt TM, Stevens VJ, Lichtenstein E, Hollis JF, et al. Biochemical validation of smoking status: Pros, cons, and data from four low-intensity intervention trials. Addictive Behaviors. 1993;18:511–527. doi: 10.1016/0306-4603(93)90068-k. doi:10.1016/0306-4603(93)90068-K. [DOI] [PubMed] [Google Scholar]
- Goldman LK, Glantz SA. Evaluation of antismoking advertising campaigns. Journal of the American Medical Association. 1998;279:772–777. doi: 10.1001/jama.279.10.772. doi:10.1001/jama.279.10.772. [DOI] [PubMed] [Google Scholar]
- Hall SM, Havassy BE, Wasserman DA. Commitment to abstinence and acute stress in relapse to alcohol, opiates, and nicotine. Journal of Consulting and Clinical Psychology. 1990;58:175–181. doi: 10.1037//0022-006x.58.2.175. doi:apa.org/psycinfo/1990-20863-001. [DOI] [PubMed] [Google Scholar]
- Hassmiller KM, Warner KE, Mendez D, Levy DT, Romano E. Nondaily smokers: Who are they? American Journal of Public Health. 2003;93:1321–1327. doi: 10.2105/ajph.93.8.1321. doi:10.2105/AJPH.93.8.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht SS, Carmella SG, Chen M, Dor Koch JF, Miller AT, Murphy SE, et al. Quantitation of urinary metabolites of a tobacco-specific lung carcinogen after smoking cessation. Cancer Research. 1999;59:590–596. Retrieved from http://cancerres.aacrjournals.org/content/559/593/590.full. [PubMed] [Google Scholar]
- Hennrikus DJ, Jeffery RW, Lando HA. Occasional smoking in a Minnesota working population. American Journal of Public Health. 1996;86:1260–1266. doi: 10.2105/ajph.86.9.1260. doi:10.1136/tc.11.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovell MF, Meltzer SB, Wahlgren DR, Matt GE, Hofstetter CR, Jones JA, et al. Asthma management and environmental tobacco smoke exposure reduction in Latino children: A controlled trial. Pediatrics. 2002;110:946–956. doi: 10.1542/peds.110.5.946. doi:10.1542/peds.110.5.946. [DOI] [PubMed] [Google Scholar]
- Hovell MF, Meltzer SB, Zakarian JM, Wahlgren DR, Emerson JA, Hofstetter CR, et al. Reduction of environmental tobacco smoke exposure among asthmatic children: A controlled trial. Chest. 1994;106:440–446. doi: 10.1378/chest.106.2.440. doi:10.1378/chest.106.2.440. [DOI] [PubMed] [Google Scholar]
- Hovell MF, Zakarian JM, Matt GE, Hofstetter CR, Bernert JT, Pirkle J. Effect of counselling mothers on their children's exposure to environmental tobacco smoke: Randomised controlled trial. British Medical Journal. 2000;321:337–342. doi: 10.1136/bmj.321.7257.337. doi:10.1136/bmj.321.7257.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyland A, Rezaishiraz H, Bauer J, Giovino GA, Cummings KM. Characteristics of low-level smokers. Nicotine & Tobacco Research. 2005;7:461–468. doi: 10.1080/14622200500125369. doi:10.1080/14622200500125369. [DOI] [PubMed] [Google Scholar]
- Jacob P, 3rd, Yu L, Duan M, Ramos L, Yturralde O, Benowitz NL. Determination of the nicotine metabolites cotinine and trans-3’-hydroxycotinine in biologic fluids of smokers and non-smokers using liquid chromatography-tandem mass spectrometry: Biomarkers for tobacco smoke exposure and for phenotyping cytochrome P450 2A6 activity. Journal of Chromatography B Analytical Technology in Biomedical & Life Sciences. 2011;879:267–276. doi: 10.1016/j.jchromb.2010.12.012. doi:S1570-0232(10)00767-1 [pii] 10.1016/j.jchromb.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddell D. Practical tests of 2x2 contingency tables. Statistican. 1976;25:295–304. doi:10.2307/2988087. [Google Scholar]
- McIntosh NA, Clark NM, Howatt WF. Reducing tobacco smoke in the environment of the child with asthma: A cotinine-assisted, minimal-contact intervention. Journal of Asthma. 1994;31:453–462. doi: 10.3109/02770909409089487. doi:10.3109/02770909409089487. [DOI] [PubMed] [Google Scholar]
- Mills AL, Messer K, Gilpin EA, Pierce JP. The effect of smoke-free homes on adult smoking behavior: A review. Nicotine & Tobacco Research. 2009;11:1131–1141. doi: 10.1093/ntr/ntp122. doi:10.1093/ntr/ntp122. [DOI] [PubMed] [Google Scholar]
- Oksuz E, Mutlu ET, Malhan S. Characteristics of daily and occasional smoking among youths. Public Health. 2007;121:349–356. doi: 10.1016/j.puhe.2006.12.007. doi:10.1016/j.puhe.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Patrick D, Cheadle A, Thompson D, Diehr P, Koepsell T, Kinne S. The validity of self-reported smoking: A review and meta-analysis. American Journal of Public Health. 1994;84:1086–1093. doi: 10.2105/ajph.84.7.1086. Retrieved from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1614767/pdf/amjph1600458-1610032.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: Toward an integrative model of change. Journal of Consulting and Clinical Psychology. 1983;51:390–395. doi: 10.1037//0022-006x.51.3.390. doi:apa.org/psycinfo/1983-26480-001. [DOI] [PubMed] [Google Scholar]
- Sargent JD, Mott LA, Stevens M. Predictors of smoking cessation in adolescents. Archives of Pediatrics and Adolescent Medicine. 1998;152:388–393. doi: 10.1001/archpedi.152.4.388. Retrieved from http://archpedi.ama-assn.org/cgi/content/abstract/152/384/388. [DOI] [PubMed] [Google Scholar]
- Schane R, Glantz S. Education on the dangers of passive smoking: A cessation strategy past due. Circulation. 2008;118:1521–1523. doi: 10.1161/CIRCULATIONAHA.108.805259. doi:118/15/1521 [pii] 10.1161/CIRCULATIONAHA.108.805259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schane R, Glantz S, Ling P. Nondaily Smoking: An Increasingly Prevalent Pattern. Archives of Internal Medicine. 2009a;26:1742–1744. doi: 10.1001/archinternmed.2009.315. Retrieved from http://www.ajpmonline.org/article/S0749-3797%2809%2900301-2900308/abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schane R, Glantz S, Ling P. Social smoking: Implications for public health, clinical practice, and intervention research. American Journal of Preventive Medicine. 2009b;37:124–131. doi: 10.1016/j.amepre.2009.03.020. doi:10.1016/j.amepre.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schane R, Ling P, Glantz S. Health effects of light and intermittent smoking: A review. Circulation. 2010;121:1518–1522. doi: 10.1161/CIRCULATIONAHA.109.904235. doi:121/13/1518 [pii] 10.1161/CIRCULATIONAHA.109.904235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song AV, Glantz SA, Halpern-Felsher BL. Perceptions of second-hand smoke risks predict future adolescent smoking initiation. Journal of Adolescent Health. 2009;45:618–625. doi: 10.1016/j.jadohealth.2009.04.022. doi:S1054-139X(09)00182-7 [pii] 10.1016/j.jadohealth.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine & Tobacco Research. 2002;4:149–159. doi: 10.1080/14622200210123581. doi:10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- Stevens VJ, Severson H, Lichtenstein E, Little SJ, Leben J. Making the most of a teachable moment: A smokeless-tobacco cessation intervention in the dental office. American Journal of Public Health. 1995;85:231–235. doi: 10.2105/ajph.85.2.231. doi:10.2105/AJPH.85.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart PJ, Rosser WW. The impact of routine advice on smoking cessation from family physicians. Canadian Medical Association Journal. 1982;126:1051–1054. Retrieved from http://www.cmaj.ca/content/1126/1059/1051.abstract. [PMC free article] [PubMed] [Google Scholar]
- Surgeon General; P. H. Service. The Health consequences of smoking. Rockville, MD: US Department of Health and Human Services; 2004. Retrieved from http://www.surgeongeneral.gov/library/smokingconsequences/ [Google Scholar]
- Surgeon General. The health consequences of involuntary exposure to tobacco smoke: United States. Public Health Service. Office of the Surgeon General; 2006. Retrieved from http://www.surgeongeneral.gov/library/secondhandsmoke/report/ [Google Scholar]
- Surgeon General; P. H. Service. Treating tobacco use and dependence: 2008 update-clinical practice guidelines. US Department of Health and Human Services; 2008. Retrieved from http://www.surgeongeneral.gov/tobacco/treating_tobacco_use08.pdf. [Google Scholar]
- Tong EK, Ong MK, Vittinghoff E, Perez-Stable EJ. Nondaily smokers should be asked and advised to quit. American Journal of Preventive Medicine. 2006;30:23–30. doi: 10.1016/j.amepre.2005.08.048. doi:10.1016/j.amepre.2005.08.048. [DOI] [PubMed] [Google Scholar]
- Tyc VL, Hovell MF, Winickoff J. Reducing secondhand smoke exposure among children and adolescents: Emerging issues for intervening with medically at-risk youth. Journal of Pediatric Psychology. 2008;33:145–155. doi: 10.1093/jpepsy/jsm135. doi:10.1093/jpepsy/jsm135. [DOI] [PubMed] [Google Scholar]
- Velicer WF, Prochaska JO, Rossi JS, Snow MG. Assessing outcome in smoking cessation studies. Psychology Bulletin. 1992;111:23–41. doi: 10.1037/0033-2909.111.1.23. Retrieved from http://psycnet.apa.org/index.cfm?fa=buy.optionToBuy&id=1992-17575-17001. [DOI] [PubMed] [Google Scholar]
- Wahlgren DR, Hovell MF, Meltzer SB, Hofstetter CR, Zakarian JM. Reduction of environmental tobacco smoke exposure in asthmatic children. A 2-year follow-up. Chest. 1997;111:81–88. doi: 10.1378/chest.111.1.81. doi:10.1378/chest.111.1.81. [DOI] [PubMed] [Google Scholar]
- Wortley PM, Husten CG, Trosclair A, Chrismon J, Pederson LL. Nondaily smokers: A descriptive analysis. Nicotine & Tobacco Research. 2003;5:755–759. doi: 10.1080/1462220031000158753. doi:10.1080/1462220031000158753. [DOI] [PubMed] [Google Scholar]