Abstract
Introduction:
Smokers with a faster rate of nicotine metabolism, estimated using the ratio of 3′-hydroxycotinine (3-HC) to cotinine, have lower plasma nicotine levels and are more likely to relapse with 21 mg nicotine patch therapy, than smokers with slower rates of nicotine metabolism. Thus, faster metabolizers of nicotine may require a higher nicotine patch dose to achieve cessation.
Methods:
This proof of concept randomized placebo-controlled trial evaluated the efficacy and safety of 8 weeks of 42 mg transdermal nicotine versus 21 mg, among 87 fast metabolizers of nicotine (3-HC/cotinine ≥ 0.18).
Results:
After 1 week of treatment, an intent-to-treat (ITT) analysis showed that participants treated with 42 mg nicotine had significantly higher expired-air carbon monoxide (CO)-confirmed 24-hr abstinence (75% vs. 58.1%; OR = 3.21; 95% CI: 1.12–9.24, p = .03) but not 7-day abstinence (50% vs. 34.9%; OR = 2.02; 95% CI: 0.82–4.94, p = .13). After 8 weeks of treatment, ITT analysis showed that participants treated with 42 mg nicotine had marginally higher rates of CO-confirmed 24-hr abstinence (45.5% vs. 30.2%; OR = 2.32; 95% CI: 0.92–5.92, p = .08) but not 7-day abstinence (29.6% vs. 23.3%; OR = 1.52, 95% CI: 0.57–4.07, p = .41). Percent nicotine and cotinine replacement were significantly greater for 42 mg nicotine versus 21 mg (p < .005). There were no significant differences between treatment arms in the frequency of severe side effects and serious adverse events or blood pressure during treatment (p > .10).
Conclusions:
Further examination of the efficacy of 42 mg nicotine patch therapy for fast metabolizers of nicotine is warranted.
Introduction
Transdermal nicotine is a popular and effective treatment for smoking cessation (Stead et al., 2008; West et al., 2001). However, two clinical trials have indicated that smokers who are fast metabolizers of nicotine, estimated by the ratio of 3′-hydroxycotinine (3-HC) to its precursor cotinine, show significantly lower rates of cessation when treated with the standard, 21 mg dose of transdermal nicotine, compared with slower metabolizers (Lerman et al., 2006; Schnoll et al., 2009). Bupropion and varenicline may be plausible treatment alternatives for these smokers; however, faster metabolizers of nicotine (i.e., those with 3-HC/cotinine values which fall in the top three quartiles of 3-HC/cotinine distribution) also show significantly lower levels of plasma nicotine during 21 mg nicotine patch treatment versus slow metabolizers (i.e., those with 3-HC/cotinine values which fall within the first quartile of the 3-HC/cotinine distribution; Lerman et al., 2006; Schnoll et al., 2009). These findings suggest that fast metabolizers of nicotine may require a higher dose of nicotine patch in order to achieve successful abstinence when using transdermal nicotine.
Previous clinical trials have examined doses of transdermal nicotine substantially higher than the standard 21-mg dose. Although these trials have demonstrated that higher doses of transdermal nicotine are safe, compared with the standard dose of transdermal nicotine (21 mg), higher doses (42 mg) do not generally result in higher smoking cessation rates (Fiore et al., 2008; Stead et al., 2008). However, no clinical trial of high dose transdermal nicotine has targeted smokers who are fast metabolizers of nicotine. As a next step toward improving response rates to transdermal nicotine by considering smoker’s interindividual variability in nicotine metabolism, we conducted this proof of concept study to evaluate the differential efficacy of standard (21 mg) versus high dose (42 mg) transdermal nicotine in smokers who were selected as fast metabolizers of nicotine. Specifically, we hypothesized that quit rates would be significantly higher for participants treated with 42 mg of transdermal nicotine, versus 21 mg of transdermal nicotine, and that there would be no significant differences between treatment groups in terms of severe side effects and serious adverse events.
Methods
Participants
Media ads asked treatment-seeking smokers to call for information about a smoking cessation program and to have their initial eligibility reviewed. Participants interested in the clinical trial and initially eligible attended an in-person session to determine their final eligibility. This session included a medical and psychiatric evaluation, including an electrocardiogram (ECG), and the collection of saliva to determine the 3-HC/cotinine ratio. To be eligible, participants had to be aged between 18 and 55, smoke 10 cigarettes/day or more, and able to communicate in English. In addition, based on prior studies of the effect of rate of nicotine metabolism on response to nicotine patch (Lerman et al., 2006; Schnoll et al., 2009), participants had to have a 3-HC/cotinine ratio that placed them in the top 3 quartiles of the 3-HC/cotinine ratio distribution to be consider fast nicotine metabolizers (i.e., top 75% of the 3-HC/cotinine ratio distribution), which was ≥ .18 based on Dempsey et al. (2004).
Individuals were excluded if they had a health condition that may increase risk of adverse reactions to the nicotine patch (e.g., uncontrolled hypertension); if they reported current use of a smoking cessation medication (e.g., varenicline), antipsychotics, antidepressants, anxiolytics, or stimulants; if they had a recent history (<12 months) of substance abuse or dependence; or if they reported a history or a current diagnosis of psychosis, major depression, an anxiety disorder, or bipolar disorder assessed by the MINI International Neuropsychiatric Interview (Sheehan et al., 1998). Female participants who were pregnant or nursing were excluded.
The accrual and retention data are shown in Figure 1. Of the 1,074 individuals screened by telephone, 432 were considered eligible for, and interested in, the in-person eligibility session; 206 individuals attended the in-person screening. Of these, 165 individuals provided saliva for 3-HC/cotinine analysis (2 refused and 39 were ineligible) and 125 samples yielded 3-HC/cotinine values ≥ .18, which was 75.8% of the samples tested, consistent with the cutoff selected to represent the top three quartiles of the 3-HC/cotinine ratio distribution. The intent-to-treat (ITT) sample was comprised of 87 individuals (eight participants refused and 30 individuals were considered ineligible), 44 randomized to high dose patch and 43 randomized to standard dose patch.
Figure 1.
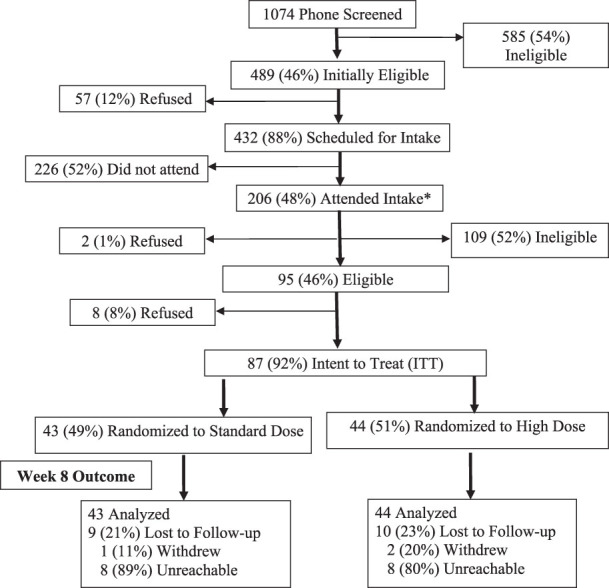
CONSORT Diagram. Note. There were no significant differences between treatment arms with respect to the number of participants who were lost to follow-up, withdrew from the trial, or were not reachable for biochemical confirmation of smoking status; * indicates where sample was collected to determine 3-HC/cotinine ratio.
Procedures
The study procedures were approved by the University of Pennsylvania Institutional Review Committee and by the U.S. Food and Drug Administration under IND#78,265. During the in-person eligibility assessment, medical and psychiatric screening occurred, including an ECG that was reviewed by the study physician, and a saliva sample was collected and sent to the Clinical Pharmacology laboratory at San Francisco General Hospital/UCSF for determination of 3-HC/cotinine ratio. Eligible participants were called by telephone to schedule their Week −1 session, where a baseline assessment was completed and they were randomized to 42 mg nicotine (two 21 mg Nicoderm CQ patches; GlaxoSmithKline, Research Triangle Park, NC) or 21 mg nicotine (one 21 mg Nicoderm CQ patch and one placebo Nicoderm CQ patch). Medication was taken for 8 weeks, from Week 0 to Week 8. All participants received five behavioral counseling sessions, two in-person and three by phone, to prepare for a target quit date, manage cravings to smoke, and avoid relapse (Weeks −1, 0, 1, 3, and 5). The counseling model was based on established guidelines (Fiore et al., 2008) and past studies that assessed the 3-HC/cotinine ratio (Schnoll et al., 2009). Participants began their assigned dose (21 mg or 42 mg) at Week 0 as in past trials of high dose transdermal nicotine (e.g., Kalman et al., 2004) and the step-down procedure, whereby the patch dose is reduced during the treatment course, was not used in order to standardize the nicotine patch dosing across treatment arms and since previous studies have shown little variation in cessation outcomes with tapered versus full dose therapy (Stapleton et al., 1995). Side effects and patch adherence were assessed at Weeks −1, 0, 1, 3, 5, and 8, an ECG was administered at Weeks 1 and 8, and blood pressure was assessed at Weeks 1 and 8. Saliva was collected at Week 1 to assess nicotine and cotinine levels. Self-reported abstinence, verified with breath CO, was assessed at Weeks 1 and 8.
Assessments
Covariates
At the baseline session (Week −1), sociodemographic (e.g., age, race, sex) and smoking (e.g., cigarettes per day, Fagerström Test for Nicotine Dependence [FTND]; Fagerström et al., 1996; Heatherton et al., 1991) data were collected.
3-HC/Cotinine and Nicotine Levels
Saliva samples collected during eligibility assessment were examined to determine 3-HC/cotinine using liquid chromatography with tandem mass spectrometry (Dempsey et al., 2004). The 3-HC/cotinine ratio has been shown to be independent from time since last cigarette and stable over repeated measures (Lea et al., 2006; Mooney et al., 2008). While plasma was used previously to ascertain the rate of nicotine metabolism, the reliability and validity of using saliva samples for 3-HC/cotinine has also been demonstrated (Dempsey et al., 2004) and was selected for this study given its ease of collection, storage, and shipping. A 3-HC/cotinine value of ≥ .18 was used, a priori, to select participants with 3-HC/cotinine values that were within the top three quartiles of the 3-HC/cotinine saliva distribution (Dempsey et al., 2004). It is worth noting that this value is lower than the value used to identify the top three quartiles of the 3-HC/cotinine distribution when plasma is used (i.e., 0.23–0.26; Lerman et al., 2006; Schnoll et al., 2009). Nicotine and cotinine levels in saliva at baseline and Week 1 were also determined using liquid chromatography with tandem mass spectrometry (Dempsey et al., 2004) in order to determine nicotine and cotinine percent replacement, versus baseline levels. To ensure that the Week 1 measures of nicotine and cotinine levels were not confounded by smoking, Week 1 saliva samples were collected only among participants who were confirmed abstinent using CO breath samples (≤ 10 ppm).
Patch Adherence
Patch adherence was measured by self-report. At each assessment from Week 0–8, participants indicated if they used the patches on each day. Participants were classified as compliant for a week if they used patches on 6 days or more each week (Schnoll et al., 2010).
Side Effects
Side effects were assessed using a symptom checklist from past trials (Schnoll et al., 2010). The checklist was administered at Weeks −1, 0, 1, 3, 5, and 8. Each symptom (e.g., nausea, skin reaction) was rated from 1 (none) to 4 (severe). Participants were instructed to contact study personnel if they experienced any serious medical problems between assessments. Adverse events were considered serious if the participant considered them debilitating or if they required hospitalization. Serious adverse events were reported to the University of Pennsylvania IRB and were classified as related or unrelated to treatment arm allocation. At Weeks 1 and 8, an ECG was administered and blood pressure was assessed.
Smoking Cessation
At Week 1 and Week 8 (end of treatment), participant quit rates were assessed. A time-line follow-back measure was used to assess daily smoking from Week 0 to Week 8. A breath sample was collected at Week 1 and Week 8 to verify self-reported abstinence using a CO monitor. Two primary outcome measures were used for this study. First, smoking cessation was defined as self-reported abstinence (not even a puff) for the 7 days prior to the Week 1 and Week 8 assessment and a CO of 10 ppm or less. This is a common measure in the smoking cessation clinical trial literature and was used as the outcome measure in past trials assessing 3-HC/cotinine and nicotine patch treatment response (Lerman et al., 2006; Schnoll et al., 2009). Second, smoking cessation was defined as self-reported abstinence (not even a puff) for the 24 hr prior to the Week 1 and Week 8 assessment and a CO of 10 ppm or less. This is the most appropriate point prevalence measure to use with CO as the form of biochemical verification, given the half-life of CO (Hughes et al., 2003). Cotinine could not be used to verify self-reported abstinence since participant use of transdermal nicotine could confound the measurement. As a secondary outcome measure at Week 8 only, we examined continuous abstinence (no self-reported smoking from Week 0 to Week 8; Hughes et al., 2003). This secondary outcome was not verified with CO.
Statistical Analysis
The characteristics of the sample were examined using descriptive statistics and the treatment arms were compared with respect to potential covariates (e.g., sex, FTND) using chi-square and t tests. The difference in the rate of completion of the Week 8 assessment across treatment arm was assessed and potential covariates associated with Week 8 completion rates were evaluated using chi-square and t tests (e.g., sex, FTND).
Second, we examined differences in abstinence rates across the treatment arms using logistic regression. Models controlled for variables related to smoking (sex, FTND) and nicotine metabolism (baseline 3-HC/cotinine, race) as done previously (Schnoll et al., 2009). We conducted an ITT analysis, with missing outcome data considered smokers, and a completers-only analysis (including only participants who completed the Week 8 assessment; n = 69). Separate models were conducted for the measures of smoking cessation.
Third, we compared the treatment arms in terms of percent nicotine and cotinine replacement, comparing pretreatment nicotine and cotinine levels to the Week 1 levels. These analyses were conducted only among participants confirmed abstinent with CO at Week 1. t tests were used to compare percent replacement between standard and high dose participants.
Fourth, the frequency of severe side effects, serious adverse events, and abnormal ECG results, and the mean systolic and diastolic blood pressure, were compared across treatment arms. For severe side effects, chi-square was used to evaluate differences in frequencies across treatment arms at Weeks 1, 3, 5, and 8. Chi-square was also used to evaluate differences in the frequency of serious adverse events reported at any time during the treatment period. For ECG results, chi-square was used to evaluate differences in the frequency of abnormal ECG findings at Weeks 1 and 8. We used t tests to evaluate differences in baseline versus Week 1 and baseline versus Week 8 systolic and diastolic blood pressure by treatment arm. Lastly, chi-square was used to evaluate differences in patch adherence at each week of treatment across the two treatment arms.
Results
Sample Characteristics and Covariates
On average, participants were 38 years old (SD = 9 years). Fifty-two percent of the sample was female, 70% of the sample had a high school diploma or higher, and 69% of the sample was of European ancestry. The average FTND score was 4.7 (SD = 2.1), the average number of cigarettes per day at baseline was 16.9 (SD = 7.3 cigarettes), the average cotinine level was 218.3 (SD = 115.0, range = 8.5–473.4), and the average 3-HC/cotinine rate at baseline was 0.46 (SD = 0.29, range = 0.19–2.34). There were no significant differences in these characteristics across the two treatment arms (p > 0.10; see Table 1).
Table 1.
Participant Characteristics by Treatment Arm Assignment
| Characteristic | Standard dose (n = 43) | High dose (n = 44) | Overall (n = 87) |
| Sex (% female) | 51.2 | 52.2 | 51.7 |
| Age, M (SD) | 37.1 (9.0) | 38.8 (9.1) | 38.0 (9.0) |
| Education (% beyond high school) | 62.8 | 77.3 | 70.1 |
| Race (% European ancestry) | 72.1 | 65.9 | 69.0 |
| FTND, M (SD) | 4.5 (2.0) | 4.9 (2.1) | 4.7 (2.1) |
| Cigarettes per day, M (SD) | 16.3 (6.7) | 17.5 (7.9) | 16.9 (7.3) |
| Cotinine, M (SD) | 227.6 (121.4) | 209.7 (109.7) | 218.3 (115.0) |
| 3′-hydroxycotinine/cotinine, M (SD) | .43 (.22) | .49 (.35) | .46 (.29) |
Note. FTND = Fagerström Test for Nicotine Dependence; n = number of participants.
There were no significant differences across treatment arms in the rate of completion of the Week 1 or 8 assessments (p > .10). Sex, age, level of education, and race were not related to the likelihood of completion of the assessments (p > .10). However, participants who did not complete the Week 8 assessment tended to have higher FTND scores (p = .06) and smoke more cigarettes per day at baseline (p = .04). FTND score, which includes cigarettes per day, was included as a covariate in subsequent models of treatment arm effect on quit rates.
Treatment Arm Effect on Abstinence
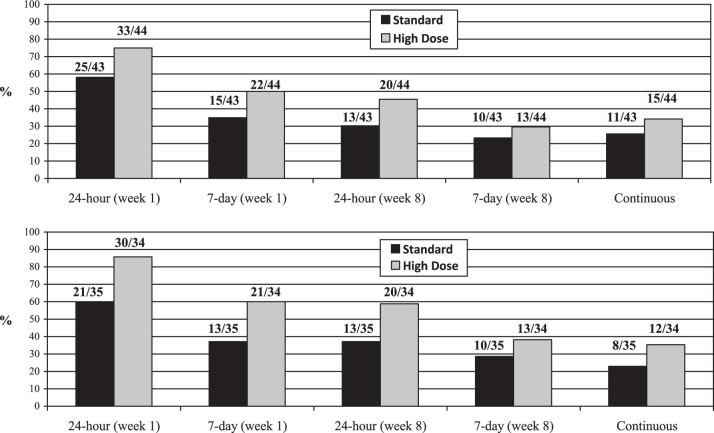
The top of Figure 2 presents the quit rates across treatment arm for the ITT analyses. At Week 1, a significantly greater proportion of participants treated with high dose nicotine reported 24-hr abstinence, CO-confirmed, versus standard dose participants (75% vs. 58.1%; OR = 3.22, 95% CI: 1.12–9.24, p = .03). The comparison between treatment groups at Week 1 for 7-day point prevalence abstinence, CO-confirmed, was not significantly different (50% vs. 34.8%; OR = 2.02, 95% CI: 0.83–4.94, p = .13). At Week 8, 45.5% of high dose participants reported 24-hr abstinence, CO-confirmed, compared with 30.2% of standard dose participants, which was marginally significant (OR = 2.32, 95% CI: 0.92–5.92, p = .08). The comparison between treatment groups at Week 8 for 7-day abstinence, CO-confirmed, was not significantly different (29.6% vs. 23.3%; OR = 1.52, 95% CI: 0.57–4.07, p = .41). Lastly, for continuous abstinence, 34.1% of high dose participants were abstinent, versus 25.6% of standard dose participants (OR = 1.49, 95% CI: 0.58–3.82, p = .40).
Figure 2.
Abstinence rates across treatment arms, ITT (top) and completers-only (bottom). Note. ITT indicates intent-to-treat analysis; bars show proportion quit, and numbers atop columns show number of participants.
The bottom of Figure 2 presents the quit rates across treatment arm for the completers-only analyses. At Week 1, a significantly greater proportion of participants treated with high dose nicotine reported 24-hr abstinence, CO-confirmed, versus standard dose participants (88.2% vs. 60%; OR = 4.84, 95% CI: 1.32–17.80, p = .02). The comparison between treatment groups at Week 1 for 7-day point prevalence abstinence, CO-confirmed, approached significance (61.8% vs. 37.1%; OR = 2.39, 95% CI: 0.88–6.50, p = .09). At Week 8, 58.8% of high dose participants reported 24-hr abstinence, CO-confirmed, compared with 37.1% of standard dose participants, which was marginally significant (OR = 2.68, 95% CI: 0.98–7.31, p = .08). The comparison between treatment groups at Week 8 for 7-day abstinence, CO-confirmed, was not significantly different (38.2% vs. 28.6%; OR = 1.64, 95% CI: 0.58–4.59, p = .35). Lastly, for continuous abstinence, 35.3% of high dose participants were abstinent, vs. 22.9% of standard dose participants (OR = 1.93, 95% CI: 0.66–5.69, p = .23).
Percent Nicotine and Cotinine Replacement
Nicotine and cotinine replacement were significantly greater for those who received high dose transdermal nicotine versus participants who received the standard dose. Participants on high dose nicotine patch showed, on average, a 164% (SD = 112.5%, range = 24.5%–435.7%) replacement of their baseline nicotine versus 85% (SD = 63.3%, range = 8.6%–221.1%) for standard dose nicotine patch participants (t(40) = 2.68, p = .005). Participants on high dose nicotine patch showed, on average, a 194% (SD = 137.2%, range = 49.4%–541.5%) replacement of their baseline cotinine compared with 88% (SD = 87.8%, range = 6.3%–215.9%) for standard dose nicotine patch participants (t(48) = 3.33, p = .0008).
Side Effects and Adherence
There were no significant differences in the frequency of self-reported severe side effects from the checklist administered at Weeks 1, 3, 5, and 8 between the treatment arms (see Supplementary Table 1; all p > .10). There was one serious adverse event (hospitalization for severe influenza) reported among high dose participants compared with zero among standard dose participants, but this event was not considered related to treatment. There were no abnormal results from ECG recordings at Week 1 and Week 8. Further, there were no significant differences in the change in systolic or diastolic blood pressure over the course of treatment between the treatment arms. Lastly, there were no significant differences across treatment arms in the rate of nicotine patch adherence at each week (p > .10). Adherence rates for standard dose participants across the treatment weeks were 88.4%, 83.7%, 74.4%, 79.1%, 69.8%, 65.1%, 53.5%, and 48.8%. Adherence rates for the high dose participants across the treatment phase were 88.1%, 84.1%, 79.6%, 75.0%, 77.3%, 72.7%, 59.1%, and 56.8%.
Discussion
This proof of concept clinical trial was designed to determine if there was any indication that high dose transdermal nicotine is more effective than standard dose transdermal nicotine for fast metabolizers of nicotine and to assess the safety of high dose transdermal nicotine in this population. Previous clinical trials have shown that the standard 21 mg dose of transdermal nicotine is less efficacious for fast metabolizers of nicotine, compared with smokers with slower nicotine metabolism and yields significantly lower rates of nicotine and cotinine during treatment (Lerman et al., 2006; Schnoll et al., 2009). As such, we hypothesized that 42 mg of nicotine may increase quit rates, versus the standard dose, among fast metabolizers of nicotine, without increasing side effects.
There was some indication that high dose nicotine increased quit rates initially, 1 week after treatment began. Participants who received high dose nicotine reported significantly greater 24-hr abstienence, CO-confirmed, at the Week 1 assessment in both the ITT and the completers-only analysis. The absolute differences in abstinence rates were 16.9% in the ITT analysis and a 28.2% in the completers-only analysis and the ORs were greater than 3.0. In addition, participants treated with high dose nicotine reported marginally statistically greater 7-day point prevalence abstinence, CO-confirmed, at the Week 1 assessment in the completers-only analysis. The difference in quit rate was 24.7% and the OR was greater than 2.3.
While there were no statistically significant differences in measures of abstinence at the end of treatment between the treatment groups, there was a trend toward statistical significance in the ITT and completers-only analyses for 24-hr point prevalence abstinence, CO confirmed. The differences in quit rates between the treatment arms for this measure of abstinence were 15.3% for the ITT analysis and a 21.7% for the completers-only analysis with ORs greater than 2.0. In contrast, for 7-day point prevalence abstinence, confirmed with CO, and for continuous abstinence, there were no differences in quit rates across the treatment arms. However, for proof of concept clinical trials like the present trial, Hughes et al. (2003) advises the use of 24-hr point prevalence as an outcome measure, not 7-day point prevalence, if CO is used for biochemical verification, given the half-life of CO. Thus, overall, there is some indication that a higher dose of nicotine may increase quit rates for fast metabolizers of nicotine, compared with the standard dose, but this hypothesis should be tested in an adequately powered randomized clinical trial.
One potential explanation for the increased quit rates among participants who received high dose transdermal nicotine is that 42 mg of nicotine provides faster metabolizers of nicotine with greater replacement of nicotine during abstinence. The present results provide some support for this possible mechanism. Study participants had substantially greater replacement of their nicotine during abstinence if they received high dose transdermal nicotine compared with standard nicotine dose. Nicotine levels after 1 week of nicotine patch treatment have been related to subsequent quit rates, albeit weakly (Lerman et al., 2006); thus, it is plausible that this enhanced nicotine replacement from high dose transdermal nicotine can translate into higher quit rates. However, a substantially larger sample is needed to formally assess this mediational relationship.
Finally, the present results indicate that using 42 mg of transdermal nicotine is safe among fast nicotine metabolizers. This study conducted a fairly rigorous assessment of side effects and possible adverse events, administering a side effects checklist at four time points, administering an ECG and assessing blood pressure at two time points, and providing participants with the opportunity to report any serious health concern during the 8-week treatment phase. Across all of these measures, there was no indication of safety concerns for the 42 mg dose, even with substantial percent replacement of nicotine. There was no significant increase in the frequency of patch-related side effects (e.g., skin reaction, nausea, dizziness) and no indication of adverse cardiovascular effects from the 42-mg dose. Further, as another indication that the 42-mg dose of transdermal nicotine was well-tolerated, there was no difference in the rate of participant withdrawal from the study and no difference in the rate of participant adherence with patch use recommendations across the treatment arms. These results converge with the general literature on the use of higher doses of transdermal nicotine (see Dale et al., 1995; Fredrickson et al., 1995; Hatsukami et al., 2007; Hughes et al., 1999).
These results should be considered in light of study limitations. First, this was a proof of concept trial and, as such, was inadequately powered to detect statistically significant treatment arm effects and did not include a long-term follow-up assessment. As such, the present results should be interpreted to suggest that future trials to explore the long-term efficacy of high dose transdermal nicotine for fast metabolizers of nicotine are warranted and to offer estimates of effect size for such a trial. Second, the present study included treatment-seeking smokers, comprised of participants who reported a lower rate of pretreatment cigarettes per day, versus other trials with fast metabolizers of nicotine (Strasser et al., 2011), and included a sample rigorously screened for medical conditions and under age 55. While treatment-seeking smokers are likely to be the most valid group to evaluate the efficacy of new medications for nicotine dependence (Perkins et al., 2010), the present sample may not represent the U.S. population of smokers. Third, saliva was used to determine 3-HC/cotinine ratio, whereas previous trials have used plasma. This resulted in the use of a 3-HC/cotinine ratio cutoff for study inclusion that was lower than in previous trials which may have affected the study results. Nevertheless, the use of saliva to determine 3-HC/cotinine ratio has been validated (Dempsey et al., 2004) and, as expected, 75.8% of the samples had 3-HC/cotinine values above the cutoff of 0.18. Lastly, we decided against a factorial design that would have included slow metabolizers of nicotine in this trial and thus, cannot discern that the present effects are unique to fast metabolizers of nicotine.
Nevertheless, the present study provides an indication that high dose transdermal nicotine may increase abstinence rates among fast metabolizers of nicotine and that this treatment approach is likely to be safe. It is worth noting how the present results compare to a previous study which compared 21 mg and 42 mg nicotine patches but did not restrict eligibility to fast metabolizers of nicotine (Kalman et al., 2004). In contrast to the present results and contrary to expectation, Kalman et al. (2004) found higher quit rates (p < .08) among the 21 mg nicotine patch group, compared with 42 mg, at the end-of-treatment. An increase in adverse events among participants in the 42 mg dose condition was postulated as a possible explanation for the finding, a result that may have occurred from the inclusion of slow metabolizers of nicotine who received 42 mg nicotine patches. The current sample size, however, was small and the results concerning treatment arm effects on abstinence rates were significant only at Week 1 and then approached statistical significance at Week 8. Thus, the results should only be interpreted to provide support for the need to conduct an adequately powered clinical trial of high dose transdermal nicotine for fast metabolizers of nicotine. Such a trial is recommended, given the paucity of efficacious treatments for these smokers, who may represent 75% of the population distribution of smokers, and the possibility that such a trial may yield information essential for guiding a personalized treatment for nicotine dependence.
Supplementary Material
Supplementary Table 1 can be found online at http://www.ntr.oxfordjournals.org.
Funding
This research was supported by grants from the National Institute on Drug Abuse (NIDA; R21 DA026889, R01 DA025078, P30 DA12393), by a grant from the NIDA and the National Cancer Institute (NCI; P50 CA143187), and by a grant from the NIDA, the NCI, the National Institute of General Medical Sciences, and the National Human Genome Research Institute (U01 DA020830).
Declaration of Interests
Dr. RAS has served as a consultant to GlaxoSmithKline, the company that manufactures the nicotine patch used in this study. However, GSK did not provide medication or financial support for this study. Dr. RFT has shares in Nicogen Research Inc., a company focused on novel smoking cessation treatment approaches. Dr. RFT has also consulted for Novartis and McNeil pharmaceuticals on smoking cessation approaches. Dr. NLB serves as a consultant to pharmaceutical companies that market smoking cessation medications and has served as a paid expert witness in litigation against tobacco companies.
Acknowledgments
The authors would like to thank the following individuals who participated or assisted in the implementation of this research project: Caryn Lerman, Elisa Martinez, Angela Pinto, Freda Patterson, Ainsley Backman, Carolina Miranda, Lynne Kohler, Ben Spears, and Sophie Feller. We also thank Lisa Yu and Trisha Mao for performing the analytical chemistry for this study.
References
- Dale LC, Hurt RD, Offord KP, Croghan IT, Hays JT, Gomez-Dahl L, et al. High-dose nicotine patch therapy. Percentage of replacement and smoking cessation. Journal of the American Medical Association. 1995;274:1353–1358. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8528039. [PubMed] [Google Scholar]
- Dempsey D, Tutka P, Jacob P, 3rd, Allen F, Schoedel K, et al. Nicotine metabolite ratio as an index of cytochrome P450 2A6 metabolic activity. Clinical Pharmacology & Therapeutics. 2004;76:64–72. doi: 10.1016/j.clpt.2004.02.011. doi:10.1016/j.clpt.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Fagerström KO, Kunze M, Schoberberger R, Breslau N, Hughes JR, Hurt RD, et al. Nicotine dependence versus smoking prevalence: Comparisons among countries and categories of smokers. Tobacco Control. 1996;5:52–56. doi: 10.1136/tc.5.1.52. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8795860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, et al. Treating tobacco use and dependence: 2008 Update. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service; 2008. Retrieved from http://www.surgeongeneral.gov/tobacco/treating_tobacco_use08.pdf. [Google Scholar]
- Fredrickson PA, Hurt RD, Lee GM, Wingender L, Croghan IT, Lauger G, et al. High dose transdermal nicotine therapy for heavy smokers: Safety, tolerability and measurement of nicotine and cotinine levels. Psychopharmacology (Berlin) 1995;122:215–222. doi: 10.1007/BF02246542. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8748390. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Mooney M, Murphy S, LeSage M, Babb D, Hecht S. Effects of high dose transdermal nicotine replacement in cigarette smokers. Pharmacology, Biochemistry & Behavior. 2007;86:132–139. doi: 10.1016/j.pbb.2006.12.017. doi:10.1016/j.pbb.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström K.-O. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1932883. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: Issues and recommendations. Nicotine & Tobacco Research. 2003;5:13–25. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12745503. [PubMed] [Google Scholar]
- Hughes JR, Lesmes GR, Hatsukami DK, Richmond RL, Lichtenstein E, Jorenby DE, et al. Are higher doses of nicotine replacement more effective for smoking cessation? Nicotine & Tobacco Research. 1999;1:169–174. doi: 10.1080/14622299050011281. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/21652735. [DOI] [PubMed] [Google Scholar]
- Kalman D, Kahler CW, Tirch D, Kaschub C, Penk W, Monti PM. Twelve-week outcomes from an investigation of high-dose nicotine patch therapy for heavy smokers with a past history of alcohol dependence. Psychology of Addictive Behaviors. 2004;18:78–82. doi: 10.1037/0893-164X.18.1.78. doi:10.1037/0893-164X.18.1.78. [DOI] [PubMed] [Google Scholar]
- Lea RA, Dickson S, Benowitz NL. Within-subject variation of the salivary 3HC/COT ratio in regular daily smokers: Prospects for estimating CYP2A6 enzyme activity in large-scale surveys of nicotine metabolic rate. Journal of Analytical Toxicology. 2006;30:386–389. doi: 10.1093/jat/30.6.386. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/16872570. [DOI] [PubMed] [Google Scholar]
- Mooney ME, Li ZZ, Murphy SE, Pentel PR, Le C, Hatsukami DK. Stability of the nicotine metabolite ratio in ad libitum and reducing smokers. Cancer, Epidemiology, Biomarkers, and Prevention. 2008;17:1396–1400. doi: 10.1158/1055-9965.EPI-08-0242. doi:10.1158/1055-9965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman C, Tyndale R, Patterson F, Wileyto EP, Shields PG, Pinto A, et al. Nicotine metabolite ratio predicts efficacy of transdermal nicotine for smoking cessation. Clinical Pharmacology & Therapeutics. 2006;79:600–608. doi: 10.1016/j.clpt.2006.02.006. doi:10.1016/j.clpt.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Lerman C, Fonte CA, Mercincavage M, Stitzer ML, Chengappa KN, et al. Cross-validation of a new procedure for early screening of smoking cessation medications in humans. Clinical Pharmacology & Therapeutics. 2010;88:109–114. doi: 10.1038/clpt.2010.65. doi:10.1038/clpt.2010.65. [DOI] [PubMed] [Google Scholar]
- Schnoll RA, Patterson F, Wileyto EP, Tyndale RF, Benowitz N, Lerman C. Nicotine metabolic rate predicts successful smoking cessation with transdermal nicotine: A validation study. Clinical Pharmacology & Therapeutics. 2009;92:6–11. doi: 10.1016/j.pbb.2008.10.016. doi:10.1016/j.pbb.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnoll RA, Patterson F, Wileyto EP, Heitjan DF, Shields AE, Asch DA, et al. Efficacy of extended duration transdermal nicotine therapy: A randomized trial. Annals of Internal Medicine. 2010;152:144–151. doi: 10.7326/0003-4819-152-3-201002020-00005. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/20124230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998;59(Suppl 20):22–33. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9881538. [PubMed] [Google Scholar]
- Stapleton J, Russell M, Feyerabend C, Wiseman SM, Gustavsson G, Sawe U, et al. Dose effects and predictors of outcome in a randomized trial of transdermal nicotine patches in general practice. Addiction. 1995;90:31–42. doi: 10.1046/j.1360-0443.1995.901316.x. doi:10.1046/j.1360-0443. [DOI] [PubMed] [Google Scholar]
- Stead LR, Bullen P, Mant C, Lancaster T. Nicotine replacement therapy for smoking cessation. Cochrane Database of Systematic Reviews. 2008;(21):CD000146. doi: 10.1002/14651858.CD000146.pub3. doi:10.1002/14651858. [DOI] [PubMed] [Google Scholar]
- Strasser AA, Benowitz NL, Pinto AG, Tang KZ, Hecht SS, Carmella SG, et al. Nicotine metabolite ratio predicts smoking topography and carcinogen biomarker level. Cancer Epidemiology Biomarkers & Prevention. 2011;20:234–238. doi: 10.1158/1055-9965.EPI-10-0674. doi:10.1158/1055-9965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West R, Hajek P, Nilsson F, Foulds J, May S, Meadows A. Individual differences in preferences for and responses to four nicotine replacement products. Psychopharmacology (Berlin) 2001;153:225–230. doi: 10.1007/s002130000577. doi:10.1007/s002130000577. [DOI] [PubMed] [Google Scholar]