Abstract
Agrobacterium tumefaciens is a broad host range plant pathogen that combinatorially recognizes diverse host molecules including phenolics, low pH, and aldose monosaccharides to activate its pathogenic pathways. Chromosomal virulence gene E (chvE) encodes a periplasmic-binding protein that binds several neutral sugars and sugar acids, and subsequently interacts with the VirA/VirG regulatory system to stimulate virulence (vir) gene expression. Here, a combination of genetics, X-ray crystallography, and isothermal calorimetry reveals how ChvE binds the different monosaccharides and also shows that binding of sugar acids is pH dependent. Moreover, the potency of a sugar for vir gene expression is modulated by a transport system that also relies on ChvE. These two circuits tune the overall system to respond to sugar concentrations encountered in vivo. Finally, using chvE mutants with restricted sugar specificities, we show that there is host variation in regard to the types of sugars that are limiting for vir induction.
Keywords: protein crystallization, sugar binding protein, sugar binding specificity, host recognition, ABC transporter
Broad host range bacterial pathogens confront a variety of problems in the context of regulating their virulence mechanisms. Because these mechanisms are usually energy intensive, the controlling system is expected to exhibit very tight regulation—activating virulence pathways under conditions that are not suitable for pathogenic activity would be wasteful (1). However, the pathogen needs to recognize diverse hosts, suggesting that the control system must be very flexible in terms of signal perception. Agrobacterium tumefaciens is a broad host range plant pathogen that has the intriguing capacity to recognize several chemically distinct types of host molecules and to activate virulence (vir) gene expression only when the appropriate combination of signals is perceived. Moreover, within each class of signals, there is unusual chemical diversity, providing an important means of broadening host range. The focus of this paper is on the periplasmic sugar binding protein encoded by the chromosomal virulence gene E (chvE) of A. tumefaciens, which recognizes diverse aldose monosaccharides including arabinose, glucose, galactose, fucose, and xylose as well as sugar acids such as galacturonic and glucuronic acids (2–4).
ChvE works together with the VirA/VirG two-component system of A. tumefaciens to integrate information from several different host-derived signals and activate virulence gene expression. ChvE binds aldose monosaccharides, whereas VirA recognizes plant phenolic derivatives; low pH impinges on the system at multiple levels. Previously, we found that there was little correlation between the in vitro dissociation constants for binding of various sugars to ChvE and the corresponding efficacy of these sugars to activate vir gene expression (2). Here, we resolve this apparent paradox by considering ChvE’s activity in the context of the complex interplay between pathogen and host. Specifically, we show (i) how the host’s low physiological pH modulates the affinity of a subgroup of sugars for ChvE, and (ii) that the pathogen’s sugar transport systems markedly decrease the effective steady-state concentration of a second group of inducing sugars. Finally, we develop tools that have now allowed us to determine which sugars are used for signaling in a given host environment providing insight into the ability of A. tumefaciens to thrive and adapt to diverse environments.
ChvE is a periplasmic sugar binding protein (5) that interacts with a bacterial two-component signaling system; binding of sugars to ChvE activates the histidine kinase VirA to phosphorylate its cognate transcriptional factor, VirG. This activation leads to the insertion of the T-DNA from A. tumefaciens Ti plasmid into host plant cells, ultimately leading to uncontrolled cell division and tumor formation (6–8). Besides its activities affecting the VirA/VirG system, ChvE also functions as a sugar binding protein in association with the MmsAB transporter (9). In this regard, ChvE is similar to the Escherichia coli maltose-binding protein (MBP), which interacts with both a chemoreceptor and the maltose transporter (10). The activity of the maltose transporter modulates the apparent steady-state concentration of maltose in the periplasm, thereby modulating the dose-dependent response of the chemoreceptor activity to this sugar. Here, we explore the situation that ensues with ChvE, which has a broad specificity for multiple monosaccharides (11), not all of which are necessarily substrates for its MmsAB transporter system. In comparison with most periplasmic sugar binding proteins, ChvE is particularly interesting in that it recognizes a broad set of sugars. On a molar basis, galacturonic acid and glucuronic acid are more potent vir-inducing sugars than galactose or glucose when tested in vitro (11). To date, however, the biochemical basis for this difference has not been elucidated nor has it been possible to determine whether the sugar acids or neutral sugars are more critical (or even involved) in vir induction during the interaction of Agrobacterium with its hosts.
The current paper addresses the question of how neutral sugars and sugar acids are bound by purified ChvE, as well as how this binding is related to signaling and virulence. In a previous paper (2), we used a fluorescence assay to measure binding of neutral sugars to ChvE and found that they were bound at concentrations far below those required for signaling. By contrast, we found that the strongly activating sugar acids failed to bind to ChvE at neutral pH, even at concentrations far higher than required for signaling. Here, we show that ChvE’s affinity for sugar acids is pH dependent, increasing at the low pH that is encountered at an infection site on the plant (8). Genetic studies demonstrate that the activity of the MmsAB ABC transporter (9) vastly decreases the sensitivity of A. tumefaciens to sugars that are transported via the ChvE/MmsAB system. The molecular basis for the recognition of sugars and sugar acids was elucidated by solving the crystal structures of ChvE bound to either neutral sugar or sugar acid. Using this information, we have generated ChvE mutant proteins that are capable of recognizing sugars but not sugar acids. These ChvE variants were used to define significant differences in the sugars that serve as vir-inducing agents in Kalanchoe daigremontiana and Nicotiana tabacum leaves.
Results
Affinity of ChvE for Neutral Sugars and Sugar Acids.
In our previous studies using fluorescence spectroscopy of ChvE at neutral pH, we found that neutral sugars were bound much more tightly and sugar acids were bound much more weakly than would be inferred from the dose–response curves for vir gene induction. We therefore suspected that the binding of sugar acids alone would be pH dependent, becoming far more favorable as the pH was lowered to the value seen at the site of the injured plant leaf. Here, we confirm this possibility by using isothermal calorimetry (ITC), which also allows one to monitor proton uptake/release associated upon binding (12). Galactose binds tightly at both pH 5.5 and 7.5 (Table S1). In contrast, glucuronic acid binds very weakly at pH 7.5, but shows a favorable association at pH 5.5 for glucuronic acid (Table S1), which is close to the pH in an infected leaf (6). We also observed submicromolar dissociation constants for the neutral inducing sugars galactose, arabinose, and glucose at pH 5.5 (Fig. S1 and Table S2).
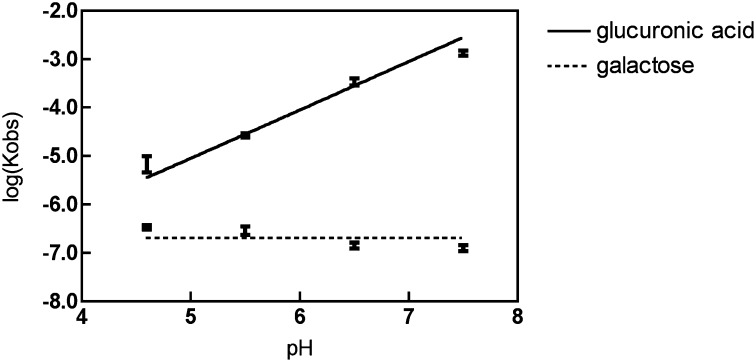
As the pH decreased from 7.5 to 4.6, the affinity of glucuronic acid gradually increased by three orders of magnitude (Table S1). A plot of the log of the disassociation constant for binding of glucuronic acid vs. pH showed that a single proton is taken up when the ChvE binds the galacuronic acid (Fig. 1 and Scheme S1). The uptake of a single proton was confirmed by measuring the buffer dependence of the binding enthalpy (12) (Fig. S2). To determine whether the carboxylate group of glucuronic acid was required for this pH-dependent association, we examined the binding of glucuronamide, which has a neutral carboxamide group in place of its carboxylate. Indeed, this compound binds very tightly in a pH-independent manner (Kd of ∼50 nM), and it also is a very potent activator of vir gene induction (Fig. S3). These data show that the carboxylate of glucuronic acid is the molecular feature that defines its pH-dependent binding.
Fig. 1.
The dependence of disassociation constant to pH: logKobs vs. pH for both glucuronic acid and galactose.
Relationship Between Kd and EC50.
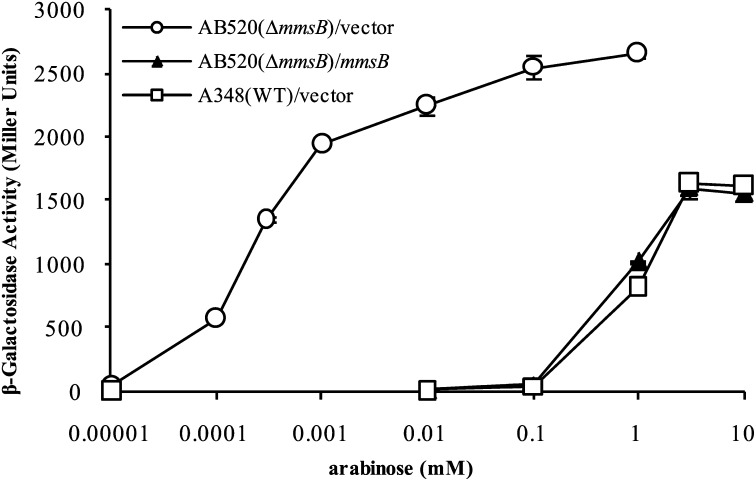
The concentrations of the neutral sugars, such as glucose, galactose, and arabinose, that yield half-maximal induction of vir gene expression (their EC50 values) are several orders of magnitude higher than expected based on their dissociation constants for binding to ChvE (Table 1), whereas EC50 of the sugar acids is near their Kd. Besides its involvement in vir induction, ChvE also plays a critical role in sugar utilization as the sugar binding protein that delivers sugars to the ABC transporter MmsAB (9). To determine whether ChvE’s interplay with the MmsAB system affects its capacity to function with the vir-inducing system of Agrobacterium, we compared the response of our wild-type strain (A348) with a similar strain (AB520) that carries an in-frame deletion of mmsB (∆mmsB). Remarkably, compared with wild type, the EC50 for the response to arabinose for vir induction in the ∆mmsB strain decreased more than three orders of magnitude (Fig. 2) to ∼1 µM, close to its Kd. The effect was abolished when the ∆mmsB strain carried a plasmid (pJZ19) capable of expressing mmsB (Fig. 2). Similar results were observed when galactose was tested (Table 1 and Fig. S4A). To determine whether transporter activity, as opposed to the simple presence of MmsB, is responsible for the relative sugar insensitivity described above, strains carrying a deletion of mmsA or a point mutation in the mmsA ATP binding site were tested and found to be significantly more sensitive to the vir-inducing sugar, arabinose (Fig. S4B). These results demonstrate that a functional MmsAB transporter has profound effects on the sensitivity of Agrobacterium’s vir-inducing machinery to sugars. We next tested the responses of these strains to sugar acids. The mmsB deletion strain grows poorly in medium containing neutral sugars as a carbon source (9), but grows as well as wild type when glucuronic acid is used as the sole carbon source (Fig. S5A), suggesting the sugar acids are not substrates for the MmsAB transport system. Interestingly, the dose–response to glucuronic acid for induction of vir gene expression is not affected by deletion of mmsB (Fig. S5B). These results suggest that the transporter needs to be functioning in the transport process to exert an effect on the EC50 of the sugars.
Table 1.
Dissociation constants of various sugars and their EC50 for vir gene expression in A. tumefaciens wild-type strain A348 and mmsB deletion strain AB520
Fig. 2.
Effects of mmsB deletion on sugar dose–response for vir induction. mmsB deletion strain AB520 carrying an mmsB complementation plasmid pJZ19 or empty vector pBBR1MCS-5. Wild-type strain A348 carrying vector pBBR1MCS-5 was used as a control. All samples were assayed in triplicate, and results are plotted as means with SD.
Crystallographic Structure of ChvE Bound to Sugars and Sugar Acids.
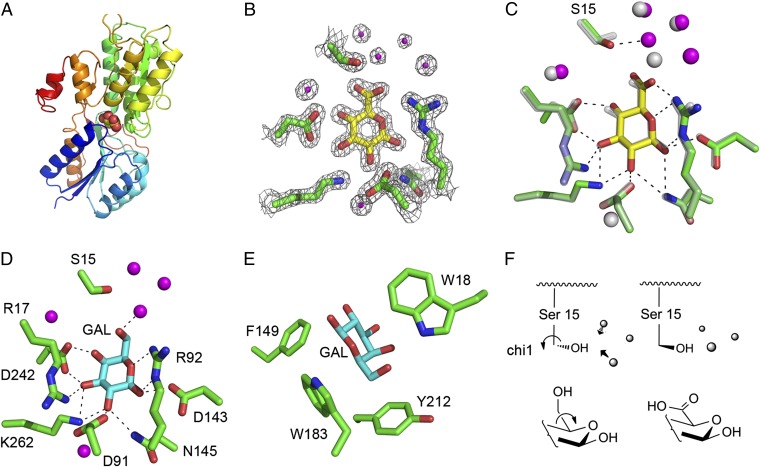
To help understand the binding specificity of ChvE to protonated sugar acids and neutral sugars, we used a crystallographic approach to get structural information. Galactose-bound ChvE and glucuronic acid-bound ChvE were crystallized at approximately pH 5, yielding crystals that diffract to 1.8 and 1.75 Å, respectively (Table S3). The structures were solved by molecular replacement using a homologous ribose-binding protein (PDB ID code 1DRK) as the initial search model (13). The overall fold of the protein is typical of the closed form of periplasmic-binding proteins (13–15) (Fig. 3A) and binds the ligand as the pyranose β anomer (Fig. 3B). The overall structure of the protein is in very good agreement with that predicted from genetic studies and homology modeling (2).
Fig. 3.
Crystal structures of ChvE with galactose and glucuronic acid. (A) Structure of ChvE bound with glucuronic acid (sphere representation). (B) Electron density map surrounding the glucuronic acid binding site (σ level 2). (C) Structure alignment between the wild-type ChvE with two different sugars, galactose (gray) and glucuronic acid (yellow). The bound waters are shown as spheres for galactose (gray) and glucuronic acid (magenta). (D and E) Hydrogen bond network and aromatic residues around the galactose binding site. (F) Change in the rotamer conformation of Ser-15 and rearrangement of the water molecules.
The backbones of ChvE with either galactose or glucuronic acid bound are identical within experimental error (rmsd of 0.127 Å for all of the backbone atoms), and the pyranose rings overlap closely (Fig. 3C). Asp-242 interacts bivalently with the 3- and 4-hydroxyl groups of the sugar, whereas the essential residue Arg-92 (2) forms a three-centered interaction with the ring oxygen, the 1-hydroxyl and 6-hydroxyl (or carboxylate in glucuronic acid). Arg-17, Asp-91, Asp-143, Asn-145, and Lys-262 are also involved in the hydrogen bond network with the sugar (Fig. 3D). Also, aromatic side chains of Phe-149, Tyr-212, and the essential residues Trp-18 and Trp-183 (2) surround the ligand (Fig. 3E). The ring of Trp-183 is roughly coplanar to the pyranose rings.
The crystal structures of ChvE nicely explain its known specificity for pyranose sugars with equatorial hydroxyls at C-1, C-2, and C-3 (11). Consistent with this specificity, the C-1 through C3 hydroxyls are clamped into place by two to three direct hydrogen bonds with protein ligands. By comparison, the anomeric C4 hydroxyl forms only a single hydrogen bond to Asp-242 in either the axial (galactose) or equatorial (glucuronic acid) configurations. Finally, the variable C5 substituent (carboxyl or CH2OH) projects toward a narrow water-filled cavity leading toward the protein surface. The carboxyl group of glucuronic acid is accommodated by a simple change in the χ1 rotamer of Ser-15 and rearrangement of the water molecules within the cavity (Fig. 3F). The sugar-contacting residues include three acidic (Asp-91, Asp-143, Asp-242) and three basic (Arg-17, Arg-92, Lys-262) residues, suggesting the site is net neutral in the pH range of 4.5–7.5. Thus, if glucuronic acid were to bind in its ionized form, it would become buried in the protein interior without a compensating cationic residue. Protonation of the glucuronic acid upon binding would greatly decrease the unfavorable free energy of dehydrating an uncompensated charge, explaining the marked pH dependence of binding of sugar acids.
Structure/Function Analysis of Sugar Binding.
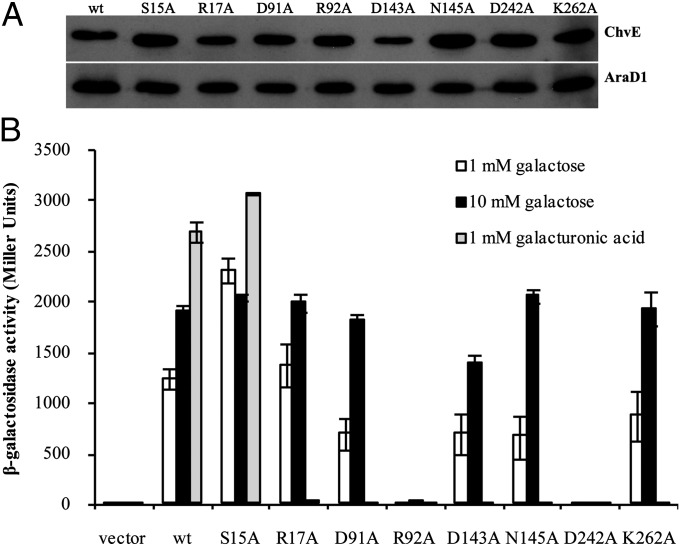
To identify mutants with altered specificity for monosaccharides, each residue involved in hydrogen bonding to the sugars was individually mutated to Ala (Materials and Methods). Each mutant strain except D143A accumulated wild-type levels of ChvE (Fig. 4A). Examination of vir induction revealed that R92 and D242 are absolutely required for induction by galactose (Fig. 4B). These two side chains clasp the ligand in multivalent interactions (Fig. 3D), and R92 was previously identified as essential in a genetic screen (2). Other mutations (D91A, N145A, K262A) had reduced activity at a concentration of galactose (1 mM) near the EC50 for wild type; at higher galactose concentration (e.g., 10 mM), they yielded vir induction at wild-type levels (Fig. 4B). ChvES15A and ChvER17A appeared to yield a near wild-type response at both 1 and 10 mM galactose. In contrast to this array of responses to galactose, each substitution tested except S15A prevented response to galacturonic acid, even at 1 mM, a 10-fold higher concentration than the EC50 of wild-type ChvE for this ligand. Cells expressing ChvES15A responded as well as cells expressing ChvEwt to galacturonic acid, suggesting that replacing this Ser with the smaller residue Ala may allow extra solvent to access the binding pocket to interact with the carboxylate group. Finally, we also examined ChvE mutants carrying a replacement of Lys-262 with each amino acid; all but K262I were insensitive to glucuronic acid (Fig. S6). Taken together, the genetic studies presented here indicate that the capacity to recognize the sugar acids is much more sensitive to alterations at the binding site than in this case for the neutral sugars.
Fig. 4.
β-Galactosidase assays of ChvE binding site mutants in response to different sugars. (A) Expression levels of mutant ChvE proteins. A constitutively expressed His-tagged AraD1 was used as loading control. (B) Effects of mutation on induction of PvirB-lacZ in the presence of different sugars.
What Are the Critical vir Inducers at the in planta Infection Site?
The chvE mutants described above allows us to determine the relative role of sugar acids and neutral sugars in the tumor initiation process. In the vir induction assays described above (Fig. 4B and Fig. S6), the strain expressing ChvER92A did not respond to any of the tested sugars, whereas the strain expressing ChvEK262L responded like wild type to neutral sugars but did not respond to sugar acids. Strains expressing ChvEN145A and ChvEK262A showed a reduced response to neutral sugars but no induction in response to sugar acids. If the neutral sugars are the predominantly available inducer in the plant, then a ChvEK262L-containing strain should initiate tumors at levels similar to wild type. If the sugar acids, as opposed to neutral sugars, are critical signals in the leaf explant system, then tumor initiation would be greatly reduced in response to this strain. Various versions of chvE were moved into strain AB300 (∆chvE) and tested for their capacity to induce tumors on Kalanchoe daigremontiana leaves or in the tobacco leaf explant system, both of which are routinely used to study Agrobacterium virulence (16, 17). Interestingly, although the strain expressing ChvEK262L yielded a wild-type response in the tobacco leaf explant system, it was vastly less virulent than wild type when tested on Kalanchoe (Fig. 5). Strains expressing ChvEN145A, ChvEK262A, and ChvEK262S were reduced in virulence compared with wild type in the tobacco system but were avirulent on Kalanchoe leaves, whereas, as expected, a strain expressing ChvER92A was avirulent in both assays (Fig. 5 and Table S4). These results indicate that the sugar acids are an important component of vir gene induction in the Kalanchoe leaves, whereas the neutral sugars appear most critical in the tobacco leaf explant system.
Fig. 5.
Response of tobacco leaf explants and Kalanchoe leaves to strains carrying mutant versions of ChvE. Strain AB300 (ΔchvE) transformed with vector pBBR1MCS-5 containing various chvE mutants and tested in the tobacco and Kalanchoe tumorigenesis assays as described in Materials and Methods.
Discussion
These studies address three major issues concerning ChvE function as it pertains to the pathogenic processes of Agrobacterium. In particular, we demonstrate the following: (i) the physical basis for binding of sugar acids to ChvE revolves around protonation of the ligand; (ii) the discrepancy between the Kd for neutral sugars and their EC50 for vir induction results from the presence and apparent activity of the ChvE-dependent ABC transport system; and (iii) the ability of mutant strains to respond to neutral sugars but not sugar acids indicates host variation in the role played by these two vir-inducing agents.
An intriguing feature of ChvE compared with a canonical sugar binding protein such as the glucose-galactose binding protein (GGBP) or arabinose binding protein (ABP) is that it responds to a wide variety of sugars including sugar acids (11). This makes biological sense because Agrobacterium is likely to find itself in a variety of plant environments in which any one of these sugars may be present. The capacity to recognize the various sugars may thereby broaden the host range of this pathogen. Although they are structurally diverse, all activating sugars have equatorial hydroxyls at C1, C2, and C3, and this forms a dense network of hydrogen bonds to polar residues in ChvE. Both axial and equatorial hydroxyls are recognized by Asp-242 at C4 position and the C5 substituent and projects to a water-filled cavity capable of accommodating both CH2OH and COOH groups. This contrasts with ABP, which binds C5 substituent in an apolar pocket (18). The charge-balanced region that interacts with the C5 substituent favors binding of neutral sugars or sugar acids in the noncharged form at low pH. This contrasts with periplasmic sugar binding proteins such as TogB, which binds anionic sugar derivatives in a cationic pocket (19). Interestingly, the binding of sugar acids was highly sensitive to mutation at all sites in the binding pocket, except Ser-15. Additional structural and biochemical analysis would be required to explain this sensitivity to mutation. However, its sensitivity to both mutation and pH suggest fine-tuning might contribute to known acid-dependent signaling of Agrobacterium in situations in which sugar acids are the activating sugars.
The EC50 of ChvE-binding ligands for induction of vir gene expression by Agrobacterium strains was not always reflective of their affinity to ChvE (Table 1 and Fig. 2). Because our earlier work (2, 9) strongly suggested that ChvE interacts with the MmsAB transporter, we tested a strain carrying a deletion of mmsB for its capacity to respond to sugar in the vir gene expression assay. These results revealed an EC50 for neutral sugars (Table 1 and Fig. 2) near the Kd of ChvE for that sugar, representing an approximate change in EC50 of three log orders and indicating that the transporter (MmsAB) actively counteracts the effects of the ligand detector (ChvE) in the vir induction process. Previous studies most closely related to this involve the MBP and its role in bacterial chemotaxis (20, 21). In this case, ligand (maltose)-occupied MBP can bind to either the TAR chemoreceptor or to MalF of the maltose transporter (the equivalent transport protein to MmsB). Maltose sensitivity for chemotaxis using mutants lacking MalF (22) was 10-fold greater (0.1 vs. 1 mM) than that of strains carrying wild-type MalF (10), although still significantly higher than the Kd of MBP for maltose (1–3 μM) (23). Similarly, deletion of the LrsC transporter of AI-2 increased the sensitivity of LrsB (AI-2 binding protein)-mediated chemotaxis by approximately two log orders (24). Other recent work has also found interactions between ABC transport systems and histidine sensor kinases (25, 26). As in the case of ChvE, these transporters are necessary for the detection of the signaling ligand by the kinase. Additionally, a recent report (27) indicates that deletion of the Mtr indole/tryptophan transporter in E. coli affects the indole-mediated tolerance of antibiotic treatment, presumably by increasing the concentration of indole in the periplasmic space, although the signaling mechanism controlling this has not been elucidated.
Our results suggest that transporter activity is required for the suppression of sugar sensitivity for vir gene induction by MmsAB: not only does a deletion of mmsB exhibit this phenotype (Fig. 2) but so too does a point mutation in MmsA that is predicted to eliminate its capacity to bind ATP and has been shown to affect sugar utilization (Fig. S4B) (9). In contrast, based on utilization studies the sugar acids appear not to be transported by the MmsAB system, and their EC50 is close to the Kd even in the wild-type strain. The biochemical mechanism(s) underlying the effect of MmsAB activity on the EC50 of neutral sugars for vir induction has not been established. Three alternatives that are not mutually exclusive include the following: (i) MmsAB activity lowers the concentration of the sugar in the periplasmic space; (ii) functioning MmsAB transporters kinetically outcompete VirA for ligand-bound ChvE; and/or (iii) functioning MmsAB interacts with VirA in a fashion that represses its activity. Although we consider explanation i to be the most likely, we cannot rule out the other possibilities.
The biological significance of the “suppression” of sugar sensitivity by the MmsAB transporter becomes clearer when the relative concentrations of inducing sugars in the soil or in the plant are considered. Depending on the soil’s particular nature, the concentration of inducing sugars in the soil is in the 10–100 µM range (28), whereas their concentrations in the tobacco leaf explants—at least at an infection site—appear to be significantly higher, in the millimolar and higher range (see below). The suppression of sugar sensitivity, then, can be viewed as a remarkable strategy to maintain tight, high-affinity, and highly specific binding of the sugar, while lowering its EC50 to the physiological range encountered at the infection site. This situation is reminiscent of potassium or proton channels, in which the first ions are bound very tightly relative to the physiological concentrations (thereby providing ion selectivity), while negative cooperativity in the binding of additional ions allows the system to also respond in the physiological ionic concentration range (29). Thus, although the molecular mechanisms are vastly different, one sees in both cases modulation of a tight, specific high-affinity interaction to allow response to a ligand present at much greater concentrations.
A key objective of our studies has been to develop tools to help delineate which particular vir-inducing sugars are limiting for vir induction at the plant wound site. Mutagenesis at the sugar binding site of ChvE yielded three general classes of mutant proteins: those that did not support vir gene expression in response to either neutral sugars or sugar acids (e.g., ChvER92A), those that supported a decreased but readily observable response to neutral sugars, and did not respond to sugar acids (e.g., ChvEN145A, ChvEK262A, ChvEK262S), and those that supported a near wild-type response to neutral sugars, but had no response to sugar acids (e.g., ChvEK262L). Interestingly, Kalanchoe daigremontiana leaves and Nicotiana tabacum leaf explants responded quite differently to the mutant strains. When ChvEK262L was tested in the tobacco leaf explant tumorigenesis assay, tumor initiation was similar to wild type (Fig. 5), indicating that the sugar acids must not be a limiting vir-inducing signal in the case of the wounded leaf explant. In contrast, this same strain had significantly reduced virulence on Kalanchoe leaves, indicating that the sugar acids are quite important as vir inducers in that system. When ChvEK262A, ChvEN145A, and ChvEK262S were tested, tumor initiation on tobacco was reduced compared with wild type, whereas there was no tumor formation observed on the Kalanchoe leaves. Because vir gene expression assays of strains carrying these versions of ChvE indicated a ∼10-fold reduction in the sensitivity of these strains for the neutral sugars (e.g., EC50 approached 10 mM rather than 1–3 mM normally observed for galactose, arabinose, or glucose), we can also conclude that the neutral sugars are the limiting vir-inducing sugars in tobacco and that the available (to the bacterium) sugar concentration at the leaf wound site must near 1–3 mM range. The sugar levels at the Kalanchoe leaf infection site appear to be lower, because the ChvEK262A and ChvEN145A versions, which respond to these neutral sugar concentrations with some vir induction (Fig. 4 and Fig. S6), do not support tumor formation. Moreover, the critical nature of the sugar acids as vir inducers in the Kalanchoe system provides an example of selection pressure that would maintain the capacity of ChvE to recognize that class of sugars.
In summary, the results demonstrate that, by manipulating the ligand binding properties of ChvE, it will be possible to define the host sugar “signal landscape” (6), i.e., the spatial and temporal distribution of host-derived signals that activate virulence gene expression. Moreover, this will allow for characterization of that landscape in a variety of hosts as well as the role it may play in the identification by the pathogen of host cells competent for transformation and tumor formation.
Materials and Methods
Protein Expression and Purification.
The wild-type ChvE expression and purification is described in SI Text.
To remove residual sugar, purified protein was first exhaustively dialyzed against 2 M guanidine⋅HCl, 25 mM Tris⋅HCl, pH 8.0, and then refolded slowly by further dialysis in 25 mM Mes, 50 mM NaCl, pH 5.5, buffer and concentrated.
Isothermal Titration Calorimetry.
Binding of the various inducing sugars to ChvE was determined by ITC with an ITC200 microcalorimeter (MicroCal) as described in SI Text.
Crystallization, X-Ray Diffraction, and Structure Determination of Galactose and Glucuronic Acid-Bound ChvE.
Both galactose-bound and glucuronic acid-bound ChvE were crystallized using the hanging-drop vapor diffusion method as described in SI Text. Diffraction data were collected at the Beamline ×25 at Brookhaven National Laboratory. The data collection and structure determination are described in SI Text.
vir Gene Induction Assays.
pSW209Ω (30) is a reporter plasmid that carries a PvirB::lacZ fusion. AB300 is an A. tumefaciens A348 derivative strain with chvE deletion. DC1 is AB300 transformed with plasmid pSW209Ω (2). Wild-type chvE and the site-directed mutants driven by a constitutive expressed promoter PN25 in vector pBBR1MCS-5 (31) were transformed into DC1 by electroporation. Strains were first grown in MG/L (32) medium plus appropriate antibiotics overnight at 25 °C and used to inoculate into AB induction (ABI) medium (32) containing 0.25% glycerol plus galactose or glucuronic acid at 10 µM AS. After a 24-h induction, strains were assayed for β-galactosidase by the method of Miller (33).
Alanine Scanning Mutagenesis of Sugar Binding Pocket and Random Mutagenesis of ChvEK262.
The alanine scanning mutagenesis for the eight residues in ChvE sugar binding pocket was carried out using a QuikChange site-directed mutagenesis kit (Stratagene) with plasmid pGN102 (carries wild-type chvE) (2) as template. Each mutant version of ChvE, under the control of the PN25 promoter, to eliminate possible issues related to the sugar-inducible wild type promoter (9), was cloned into vector pBBR1MCS-5 and moved into AB300 (ΔchvE) that also carried the PvirB-lacZ reporter plasmid pSW209Ω. Random mutagenesis of ChvEK262 was carried out using degenerate primers (SI Text).
Plant Tumor Assays.
Nicotiana tabacum cvH425 leaf explant assay.
A. tumefaciens strains were grown overnight in MG/L medium plus appropriate antibiotic, spun down, and then resuspended to an OD600 of 0.2 in liquid MS medium lacking sucrose. Mature leaves (∼20 cm) from vegetative greenhouse-grown plants were surface sterilized followed by excision of ∼4-mm2 explants lacking major vessels, as described (17). Leaf pieces were briefly (∼1 min) immersed in the bacterial suspension, then blotted on a sterile paper towel to remove excess liquid and placed onto MS (17) medium. After 2 d, the leaf pieces were washed in liquid MS medium and transferred to MS medium containing 200 µg/mL timentin and 200 µg/mL vancomycin to eliminate the bacteria. These were cultured at 25 °C in the dark for 12 d and then photographed.
Kalanchoe daigremontiana leaf assays.
Expanded leaves of greenhouse grown plants were scratched with a sterile 18-gauge needle and inoculated with 2 µL of bacteria grown above except resuspended to an OD600 of 0.1 in liquid MS medium lacking sucrose. Leaves were photographed 21 d after inoculation.
Supplementary Material
Acknowledgments
We acknowledge Dr. Fanglian He, who first noted that glucuronic acid did not bind ChvE at pH 7, and Mr. Cush El, who assisted in the plant transformation assays. We also thank Dr. Rudresh Acharya, Dr. Hyunil Jo in the laboratory, and Dr. Hengming Ke at University of North Carolina for discussions, and beamline X25 at National Synchrotron Light Source for diffraction data collection. W.F.D. acknowledges support from National Institutes of Health Grants GM54616 and AI74866, as well as the Materials Research Science and Engineering Centers program of the National Science Foundation. A.N.B. acknowledges support from National Science Foundation Grants IOS-0818613 and IOS11211019.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org [PDB ID codes 3URM (wild-type ChvE with galactose) and 3UUG (wild-type ChvE with glucuronic acid)].
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1215033110/-/DCSupplemental.
References
- 1.Platt TG, Bever JD, Fuqua C. A cooperative virulence plasmid imposes a high fitness cost under conditions that induce pathogenesis. Proc Biol Sci. 2012;279(1734):1691–1699. doi: 10.1098/rspb.2011.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.He F, et al. Molecular basis of ChvE function in sugar binding, sugar utilization, and virulence in Agrobacterium tumefaciens. J Bacteriol. 2009;191(18):5802–5813. doi: 10.1128/JB.00451-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cangelosi GA, Ankenbauer RG, Nester EW. Sugars induce the Agrobacterium virulence genes through a periplasmic binding protein and a transmembrane signal protein. Proc Natl Acad Sci USA. 1990;87(17):6708–6712. doi: 10.1073/pnas.87.17.6708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shimoda N, Toyoda-Yamamoto A, Aoki S, Machida Y. Genetic evidence for an interaction between the VirA sensor protein and the ChvE sugar-binding protein of Agrobacterium. J Biol Chem. 1993;268(35):26552–26558. [PubMed] [Google Scholar]
- 5.Fukami-Kobayashi K, Tateno Y, Nishikawa K. Domain dislocation: A change of core structure in periplasmic binding proteins in their evolutionary history. J Mol Biol. 1999;286(1):279–290. doi: 10.1006/jmbi.1998.2454. [DOI] [PubMed] [Google Scholar]
- 6.McCullen CA, Binns AN. Agrobacterium tumefaciens and plant cell interactions and activities required for interkingdom macromolecular transfer. Annu Rev Cell Dev Biol. 2006;22:101–127. doi: 10.1146/annurev.cellbio.22.011105.102022. [DOI] [PubMed] [Google Scholar]
- 7.Gelvin SB. Agrobacterium and plant genes involved in T-DNA transfer and integration. Annu Rev Plant Physiol Plant Mol Biol. 2000;51:223–256. doi: 10.1146/annurev.arplant.51.1.223. [DOI] [PubMed] [Google Scholar]
- 8.Brencic A, Winans SC. Detection of and response to signals involved in host-microbe interactions by plant-associated bacteria. Microbiol Mol Biol Rev. 2005;69(1):155–194. doi: 10.1128/MMBR.69.1.155-194.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao J, Binns AN. Characterization of the mmsAB-araD1 (gguABC) genes of Agrobacterium tumefaciens. J Bacteriol. 2011;193(23):6586–6596. doi: 10.1128/JB.05790-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Conway C, Rosato M, Suh Y, Manson MD. Maltose chemotaxis involves residues in the N-terminal and C-terminal domains on the same face of maltose-binding protein. J Biol Chem. 1992;267(32):22813–22820. [PubMed] [Google Scholar]
- 11.Ankenbauer RG, Nester EW. Sugar-mediated induction of Agrobacterium tumefaciens virulence genes: Structural specificity and activities of monosaccharides. J Bacteriol. 1990;172(11):6442–6446. doi: 10.1128/jb.172.11.6442-6446.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Velázquez-Campoy A, Ohtaka H, Nezami A, Muzammil S, Freire E (2004) Isothermal titration calorimetry. Current Protocols in Cell Biology (Wiley, New York), pp 17.8.1–17.8.24. [DOI] [PubMed]
- 13.Björkman AJ, et al. Probing protein-protein interactions. The ribose-binding protein in bacterial transport and chemotaxis. J Biol Chem. 1994;269(48):30206–30211. [PubMed] [Google Scholar]
- 14.Magnusson U, et al. Hinge-bending motion of D-allose-binding protein from Escherichia coli: Three open conformations. J Biol Chem. 2002;277(16):14077–14084. doi: 10.1074/jbc.M200514200. [DOI] [PubMed] [Google Scholar]
- 15.Vyas MN, Vyas NK, Quiocho FA. Crystallographic analysis of the epimeric and anomeric specificity of the periplasmic transport/chemosensory protein receptor for D-glucose and D-galactose. Biochemistry. 1994;33(16):4762–4768. doi: 10.1021/bi00182a003. [DOI] [PubMed] [Google Scholar]
- 16.Ward JE, Jr, Dale EM, Binns AN. Activity of the Agrobacterium T-DNA transfer machinery is affected by virB gene products. Proc Natl Acad Sci USA. 1991;88(20):9350–9354. doi: 10.1073/pnas.88.20.9350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Banta LM, Joerger RD, Howitz VR, Campbell AM, Binns AN. Glu-255 outside the predicted ChvE binding site in VirA is crucial for sugar enhancement of acetosyringone perception by Agrobacterium tumefaciens. J Bacteriol. 1994;176(11):3242–3249. doi: 10.1128/jb.176.11.3242-3249.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quiocho FA, Vyas NK. Novel stereospecificity of the L-arabinose-binding protein. Nature. 1984;310(5976):381–386. doi: 10.1038/310381a0. [DOI] [PubMed] [Google Scholar]
- 19.Abbott DW, Boraston AB. Specific recognition of saturated and 4,5-unsaturated hexuronate sugars by a periplasmic binding protein involved in pectin catabolism. J Mol Biol. 2007;369(3):759–770. doi: 10.1016/j.jmb.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 20.Bordignon E, Grote M, Schneider E. The maltose ATP-binding cassette transporter in the 21st century—towards a structural dynamic perspective on its mode of action. Mol Microbiol. 2010;77(6):1354–1366. doi: 10.1111/j.1365-2958.2010.07319.x. [DOI] [PubMed] [Google Scholar]
- 21.Hazelbauer GL, Falke JJ, Parkinson JS. Bacterial chemoreceptors: High-performance signaling in networked arrays. Trends Biochem Sci. 2008;33(1):9–19. doi: 10.1016/j.tibs.2007.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manson MD, Boos W, Bassford PJ, Jr, Rasmussen BA. Dependence of maltose transport and chemotaxis on the amount of maltose-binding protein. J Biol Chem. 1985;260(17):9727–9733. [PubMed] [Google Scholar]
- 23.Wandersman C, Schwartz M, Ferenci T. Escherichia coli mutants impaired in maltodextrin transport. J Bacteriol. 1979;140(1):1–13. doi: 10.1128/jb.140.1.1-13.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hegde M, et al. Chemotaxis to the quorum-sensing signal AI-2 requires the Tsr chemoreceptor and the periplasmic LsrB AI-2-binding protein. J Bacteriol. 2011;193(3):768–773. doi: 10.1128/JB.01196-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hiron A, Falord M, Valle J, Débarbouillé M, Msadek T. Bacitracin and nisin resistance in Staphylococcus aureus: A novel pathway involving the BraS/BraR two-component system (SA2417/SA2418) and both the BraD/BraE and VraD/VraE ABC transporters. Mol Microbiol. 2011;81(3):602–622. doi: 10.1111/j.1365-2958.2011.07735.x. [DOI] [PubMed] [Google Scholar]
- 26.Ouyang J, Tian XL, Versey J, Wishart A, Li YH. The BceABRS four-component system regulates the bacitracin-induced cell envelope stress response in Streptococcus mutans. Antimicrob Agents Chemother. 2010;54(9):3895–3906. doi: 10.1128/AAC.01802-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vega NM, Allison KR, Khalil AS, Collins JJ. Signaling-mediated bacterial persister formation. Nat Chem Biol. 2012;8(5):431–433. doi: 10.1038/nchembio.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nguyen CGA. Short-term utilisation of 14C-[U]glucose by soil microorganisms in relation to carbon availability. Soil Biol Biochem. 2011;33(1):53–60. [Google Scholar]
- 29.Grigoryan G, Moore DT, DeGrado WF. Transmembrane communication: General principles and lessons from the structure and function of the M2 proton channel, K+ channels, and integrin receptors. Annu Rev Biochem. 2011;80:211–237. doi: 10.1146/annurev-biochem-091008-152423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y, Mukhopadhyay A, Howitz VR, Binns AN, Lynn DG. Construction of an efficient expression system for Agrobacterium tumefaciens based on the coliphage T5 promoter. Gene. 2000;242(1–2):105–114. doi: 10.1016/s0378-1119(99)00541-7. [DOI] [PubMed] [Google Scholar]
- 31.Kovach ME, et al. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene. 1995;166(1):175–176. doi: 10.1016/0378-1119(95)00584-1. [DOI] [PubMed] [Google Scholar]
- 32.Wise AA, Liu Z, Binns AN. Culture and maintenance of Agrobacterium strains. Methods Mol Biol. 2006;343:3–13. doi: 10.1385/1-59745-130-4:3. [DOI] [PubMed] [Google Scholar]
- 33.Miller J. Experiments in Molecular Genetics. 1972. (Cold Spring Harbor Laboratory, Cold Spring Harbor, NY) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.