Abstract
Reports that maternal diet influences coat color in mouse offspring carrying the agouti Avy allele have received considerable attention because the range, from pseudoagouti (brown) to yellow, predicts adult health outcomes, especially disposition toward obesity and diabetes, in yellower mice. Bisphenol A (BPA), an endocrine-disrupting compound with estrogenic properties, fed to a/a dams harboring Avy/a conceptuses has been reported to induce a significant shift toward yellower mice, whereas consumption of either genistein (G) alone or in combination with BPA led to greater numbers of healthy, brown offspring. Groups of C57/B6 a/a females, which are nonagouti, were fed either a phytoestrogen-free control diet or one of six experimental diets: diets 1–3 contained BPA (50 mg, 5 mg, and 50 μg BPA/kg food, respectively); diet 4 contained G (250 mg/kg food); diet 5 contained G plus BPA (250 and 50 mg/kg food, respectively); and diet 6 contained 0.1 μg of ethinyl estradiol (EE)/kg food. Mice were bred to Avy/a males over multiple parities. In all, 2,824 pups from 426 litters were born. None of the diets provided any significant differences in relative numbers of brown, yellow, or intermediate coat color Avy/a offspring. However, BPA plus G (P < 0.0001) and EE diets (P = 0.005), but not the four others, decreased the percentage of black (a/a) to Avy/a offspring from the expected Mendelian ratio of 1:1. Data suggest that Avy/a conceptuses, which may possess a so-called “thrifty genotype,” are at a competitive advantage over a/a conceptuses in certain uterine environments.
Keywords: endocrine disruption, pregnancy, viable yellow mouse, epigenetics, metabolic disease
The viable yellow (Avy/a) mouse strain (1, 2) provided the earliest model for studying epigenetic inheritance in mammals (3, 4) and also affords insight into the so-called “metabolic syndrome,” as animals with the more extreme yellow coat color (Fig. 1) exhibit maturity-onset obesity, accompanied by diabetes (1, 2, 5). By contrast, their brown-coated (pseudoagouti) siblings remain healthy, despite being genetically identical to the yellow mice (1, 2). The observation that the coat color of these mice and their associated degree of metabolic disease can be modulated by diet provided to the dam during early development of her offspring (3, 4, 6) has also raised considerable medical interest, as it is becoming clear that both the quantity and quality of the food a pregnant woman consumes during her pregnancy can either enhance or reduce the risks of her infant developing adult disease, presumably by modulating epigenetic modifications on genes encoding key metabolic enzymes and hormones (7–9).
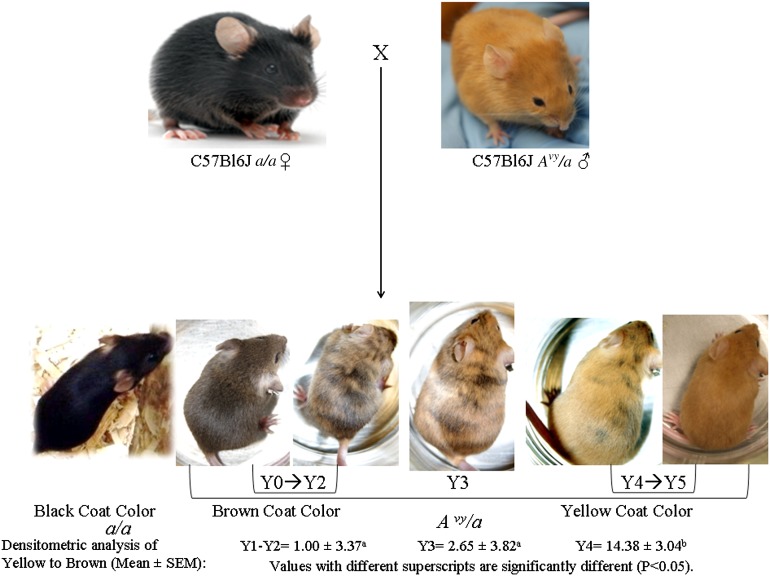
Fig. 1.
Breeding scheme design for the maternal diet experiments. Black (a/a) females were placed on one of the seven diets described in Materials and Methods 2 wk before breeding to Avy/a males, who were maintained on the AIN93G CTL diet, when not paired with breeder females. The dams were continued on their respective diets throughout gestation and lactation. All offspring were placed on the CTL diet at weaning. At weaning, offspring were classified into one of four coat color groups [black (a/a), brown Y0–Y2 (Avy/a), intermediate mottled Y3 (Avy/a), and yellow Y4–Y5 (Avy/a)]. The numbers below these coat colors represent the average ± SEM densitometric ratio of yellow to brown associated with the Y1–Y2, Y3, and Y4 assignments. Survival of pups born to weaning was >90%.
The heterogeneity in color of Avy/a mice stems from differences in the control of expression of the agouti (A) gene, and specifically the Avy allele (3, 4, 6, 10). Mice (11, 12) and a wide range of animals, including humans (13, 14), possess a single agouti gene locus that, if transcribed, is responsible for encoding the agouti signaling protein (ASIP). Mouse ASIP is a 131-aa, paracrine signaling molecule, whose binding to the melanocortin-1 receptor prevents α-melanocyte-stimulating hormone signaling, thereby downregulating synthesis of brown/black pigments and increasing synthesis of yellow/red (pheomelanin) pigments during the midstage of the hair growth cycle (15). The broad spectrum of coat colors among siblings, ranging from full yellow to pseudoagouti (brown), and the mottled patterning observed in the mice intermediate between these extremes, arises from the labile state of the Avy allele and its variable expression. The increased appetite and associated fat mass of the yellower mice, in turn, is due to ectopic expression of ASIP outside the hair follicle, most critically in the hypothalamus (11), where it antagonizes the melanocortin 4 receptor (MC4R) (15), and the pancreas (16).
Analysis of the Avy and the related Aiapy allele revealed an inserted retrotransposon (an intracisternal A particle or IAP) positioned upstream (in pseudoexon 1a) of the customary (exon 2) transcriptional start site of the wild type A gene (10, 17). This insertion contains a cryptic promoter in its 5′-long-term repeat (LTR) region capable of driving agouti gene expression and overriding the control mechanisms that normally limit the production of ASIP to certain stages of the hair follicle cycle (3, 4, 17–19). In such mice, the fur is completely brown (pseudoagouti) when the cryptic promoter within the IAP is silent, but yellow when the promoter is fully active. This range of phenotypes is correlated with the degree of cytosine methylation of the 5′-LTR of the IAP, with CpG island hypermethylation associated with the pseudoagouti phenotype and hypomethylation with yellow fur and broad ectopic expression. When this epigenetic modification is passed through the male germ line, however, it is believed to be completely erased and then variably reconstituted during development of the F1 embryos, resulting in litters in which the pups can range from completely yellow to fully brown (10). Subsequent studies from several groups of investigators showed that a diet enriched in methyl supplements provided to a/a pregnant dams carrying Avy/a conceptuses could influence coat color and associated adult health of the resulting pups, presumably by driving increased CpG methylation within the IAP sequence during conceptus development when the methylation marks are restored, albeit inconsistently (3, 4, 6, 20).
Other supplemented diets may also alter the spectrum of coat colors within litters born to a/a dams after they had been bred to Avy/a males (21–23). For example, the phytoestrogen genistein (G), fed at 250 mg/kg feed weight (fw), has been reported to shift the coat color balance toward brown and more healthy offspring (23), whereas relatively high (50 mg/kg fw) feed concentration of the endocrine-disrupting compound (EDC) bisphenol A (BPA), favored the birth of greater numbers of yellow, presumably more unhealthy mice with a hypomethylated IAP (22). In a more recent paper, extremely low doses of BPA (50 µg and 50 ng/kg fw) were reported to influence coat color redistribution compared with controls (21) and presumably the tendencies of the mice to develop age-onset obesity and diabetes. This observation is important, as BPA is a chemical ubiquitous in the environment and may disrupt a range of processes controlled through steroid receptors, particularly ESR1 (24–26). In view of the importance of the effects of diet on the expression of the Avy allele and considerable media and scientific attention paid to the papers describing the phenomenon (22, 23), we have examined the coat color pattern distribution of Avy/a offspring born to a/a dams exposed to three doses of BPA, ranging from relatively high (50 mg/kg fw) to low (50 µg/kg fw), but all within the “no observable adverse effect level” (27). We have also tested whether the upper 50-mg dose of BPA in combination with G, G alone, and ethinyl estradiol (EE), which provides a positive estrogen control, had any effects on coat color relative to controls where the dam had been fed a phytoestrogen-free refined diet. Additionally, we examined the ratio of a/a to Avy/a pups to determine whether developmental exposure to BPA or any of the other compounds acted as a selective barrier to one genotype over the other, i.e., a/a-nonagouti versus Avy/a-agouti. Finally, we determined whether exposure to BPA, G, and EE had effects relative to parity (and hence age of the dams), because earlier studies have indicated that diet-induced biases in offspring sex ratio become more extreme in late parities than at first parity when the mice are young adults (28, 29).
Results
Effects of BPA and G on Litter Size and Sex Ratio.
The mean litter size for all of the maternal diet groups was 6.6 pups. Although the litter size for the EE group was slightly greater than for the controls (CTL) (P < 0.03), there was no overall difference when all of the groups were compared together (P = 0.4) (Table S1). Although more male than female pups were born to dams in the CTL and EE groups relative to the expected 1:1 ratio (fraction males, 0.57 and 0.55, respectively; P < 0.05), none of the other groups showed a significant deviation in sex ratio of total pups born from 0.5, and none, including the CTL and EE groups, differed significantly in the number of male- versus female-biased litters that were born.
Effects of BPA and G on Inheritance of Coat Color Patterns.
Previous reports have indicated that diet supplementation with either BPA and/or G can shift coat color patterns in Avy/a mice in a predictable manner when analyzed by χ2 analysis (21–23). Therefore, we first analyzed coat color differences between brown (Y0–Y2) and yellow (Y4–Y5) with this type of analysis. No significant shifts in coat color patterning were observed (P < 0.07) (Table S2). The tendency of the low-dose BPA and the G diets, both of which caused an apparent shift toward yellow, are the main contributors to the borderline P value. However, because the χ2 analysis fails to consider unequal sample size and dam/litter effects, more appropriate analyses were performed to take into account these factors.
We then used PROC GLIMMIX with a multinomial analysis and first considered all Avy/a offspring, including those with brown (Y0–Y2), intermediate (Y3), and yellow (Y4–Y5) coat color patterns, and again did not demonstrate any significant shifts toward either yellow or brown by any of the maternal diets (Table 1). The distribution of brown (Y0–Y2) relative to yellow (Y4–Y5) coat color mice for the seven different diets was also compared (Table 1). Analyses of these data by PROC GLIMMIX with a binomial comparison (brown, Y0–Y2, versus yellow, Y4–Y5) again failed to show any significant change in the probability (range of P values: P = 0.94, EE versus BPA plus G; P = 0.11, EE versus low dose BPA and EE versus G) of brown or yellow coat color pups in comparison with either the CTL or other diet groups (Table 1 and Table S3). For all groups combined, the mean fraction of brown and yellow coat color pups compared with the total number of Avy/a offspring was 36.38% and 43.73%, respectively. As the original classifications, which were performed over a 3-y period, were made by visual inspection using the same two independent observers (D.A.W. and P.T.S.) for each litter, an independent classification was performed on digital comparisons of variably mottled (n = 27), control mice from the later breeding experiments (Fig. 1 and Fig. S1). An advantage of this technique is that it provided numerical ratios for the relative areas of yellow and brown fur for each of the Y1–Y4 mice diet comparisons (Fig. 1 and Fig. S1). These more objective numerical measurements supported the earlier visual assessments.
Table 1.
Total pup information and percentage of Avy/a coat color offspring compared with total number of Avy/a offspring born
| Maternal diet | Total dams, n | Total litters, n | Pups born, n | Brown (Y0–Y2) (Avy/a) pups, n | Intermediate (Y3) (Avy/a) pups, n | Yellow (Y4–Y5) (Avy/a) pups, n | Brown (Avy/a) pups, % | Intermediate (Y3) (Avy/a) pups, % | Yellow (Avy/a) pups, % |
| Control | 39 | 84 | 530 | 104 | 65 | 118 | 36.2 | 22.7 | 41.1 |
| Low-dose BPA | 21 | 67 | 426 | 75 | 36 | 121 | 32.3 | 15.5 | 52.2 |
| Middle-dose BPA | 15 | 27 | 149 | 30 | 4 | 44 | 38.5 | 5.1 | 56.4 |
| Upper-dose BPA | 34 | 76 | 520 | 94 | 68 | 101 | 35.7 | 25.9 | 38.4 |
| Upper-dose BPA + G | 26 | 61 | 407 | 108 | 60 | 110 | 38.9 | 21.6 | 39.6 |
| G | 14 | 46 | 299 | 47 | 24 | 77 | 31.8 | 16.2 | 52.0 |
| EE | 34 | 65 | 493 | 116 | 57 | 119 | 39.7 | 19.5 | 40.8 |
The data were then analyzed to determine whether there was any change in Avy/a offspring coat color in relation to the parity of the dams. Because there were insufficient brown and yellow coat color Avy/a offspring born across the later two parities for the middle-dose (5 mg/kg fw) BPA dams, this dietary group could not be analyzed. When all of the remaining treatment groups were considered, there was a marginal change in offspring coat color from parity 1 to parity 3 (P = 0.046). However, when analyses were performed within treatment group, some differences were observed with parity (Table S4). In particular, for the upper-dose BPA group, there were more brown (Y0–Y2) offspring born in parity 3 relative to parity 2 (69.3% versus 34.4%, respectively, P < 0.004). There were, however, no differences in offspring coat color between parity 1 compared with parity 3 in this group (Table S4). Likewise, for the BPA-plus-G group, there were no differences between parity 1 and parities 2 and 3, but the percentage of brown offspring decreased from parity 2 to parity 3 [brown coat color (Y0–Y2) offspring: parity 1, 50.1%; parity 2, 58.4%; parity 3, 37.6%; P = 0.38, 0.15, 0.02, respectively] (Table S4). The significance of these changes relative to parity is unclear and again point to inconsistencies in the relationship between diet and coat color.
Effects of Diet on Ratio of Nonagouti (a/a) to Agouti (Avy/a) Offspring.
Because the breeding scheme involved Avy/a males bred to a/a females, a 1:1 ratio of agouti (Avy/a) to black (a/a) offspring born was predicted on the basis of Mendelian inheritance. A binomial analysis was, therefore, used to determine whether any of the diets caused the fraction of Avy/a pups born to deviate from 0.5. Only the BPA-plus-G and EE groups demonstrated a significant distortion in pup genotype (BPA plus G: Avy/a = 68.3%, P < 0.0001; EE: Avy/a = 59.2%, P = 0.005) (Table 2). The data were also compared across dietary groups by binomial PROC GLIMMIX procedures. There was a reduced fraction of a/a pups in the BPA plus G group relative to all other dietary groups, except the middle-dose BPA group (P < 0.07) (Table 2). Overall, 61 of 426 litters completely lacked black pups, but the relative number of completely Avy/a litters showed no significant differences across varying maternal diet groups, although the statistical power of the analysis was weak because of low litter numbers.
Table 2.
Percentage of a/a versus Avy/a offspring
| Maternal diet | Total no. of a/a pups born | Total no. of Avy/a pups born | a/a offspring, % | Avy/a offspring, % |
| Control | 243 | 287 | 45.9 | 54.2 |
| Low-dose BPA | 194 | 232 | 45.5 | 54.5 |
| Middle-dose BPA | 71 | 78 | 47.7 | 52.3 |
| Upper-dose BPA | 257 | 263 | 49.4 | 50.5 |
| Upper-dose BPA + G | 129 | 278 | 31.7* | 68.3 |
| G | 151 | 148 | 50.5 | 49.5 |
| EE | 201 | 292 | 40.8** | 59.2 |
Bolded values differed significantly from expected 1:1 Mendelian ratio by *P < 0.0001 and **P = 0.005.
Because the mice were first received in 2004 and later rederived through embryo transfer to establish a second colony at a different location, a single breeding scheme has been used, namely mating a/a (nonagouti) females to Avy/a males. Nonetheless, the observation that a significant number of litters contained no black a/a pups raised concerns that unintended matings by Avy/Avy males had occurred. To rule out this possibility, PCR analysis was used to assess copy number of the Avy allele by using DNA from any male that had failed to produce a black a/a offspring in any pairing with an a/a female. Controls included proven heterozygous males, who had generated at least one a/a pup (Fig. S2). All of the questionable males proved to be heterozygous (Avy/a) based on densitometry analysis comparing known heterozygous males to uncertain heterozygous males. Black (a/a, nonagouti) males were also analyzed as a negative control. Therefore, the skewing of agouti to nonagouti offspring could not be attributed to the mistaken genotype of the male partners.
We also used a binomial distribution to determine whether the ratio of a/a to Avy/a offspring changed across parities (Table S5). There were too few pups born in later parities for the low- and middle-dose BPA group to provide analyzable data, but when all groups were combined the fraction of a/a mice changed significantly over parity (parity 1, 47.6%; parity 2, 41.9%; parity 3, 38.4%; P < 0.005). The overall change from parity 1 to 3 originated primarily from a shift in the percentage of a/a offspring in the BPA-plus-G group (Table S5). By parity 3, this dietary group only birthed 18.6% black mice, a value significantly different from that of parity 1 (P = 0.01) (Table S5). No other differences in the fraction of a/a compared with Avy/a offspring born relative to parity was evident in any of the other treatment groups.
Discussion
Many chronic, adult disorders have their origins during early development (7–9). For example, food restriction during the first trimester of human pregnancy frequently leads to adult onset of obesity, diabetes, hypertension, and other aspects of ill health (30–34), a phenomenon that has been reproduced in several animal models (35–37). In addition, in utero exposure of test animals to industrial chemicals that mimic the action of steroid hormones can also have serious, although sometimes subtle, consequences with regard to development of the genitalia, the brain, and other organ systems, and may also increase the frequency of adult diseases, including cancer (26, 38–43). Hence, the claim that the viable yellow (Avy/a) mouse could serve as a biosensor (44) for exposure to EDC, and might even be used to study the ability of nutritional supplements to counteract such environmental insults, provided an exciting prospect for risk assessment and even dietary recommendations for pregnant women. The experiments described herein were not designed because there were doubts about the reproducibility of the earlier studies (21–23), but so that neurological effects of developmental exposure to BPA, G, and combinations of the two, on adult mouse behaviors could be examined in C57/B6 Avy/a mice over the full spectrum of yellow to brown coat colors. The expectation was that abnormalities in behavior caused by BPA exposure in utero (41, 45–51) might be partially or even totally offset by maternal consumption of G, a natural product found in plants, especially soybeans, and widely used as a nutritional supplement by humans (52, 53). We also predicted that any positive and negative effects on mouse behaviors would be correlated with changes in the coat color of the individual mice. Unexpectedly, the anticipated changes in coat color, such as a shift toward brown with G and toward yellow with BPA, did not materialize in the F1 pups with any of the seven experimental diets. Accordingly, we continued to breed the mothers while they were still exposed to the diets to increase the power of the analyses. Over the course of the full study, we ultimately obtained data on 426 litters and 2,824 pups, with litter numbers/diet that far exceeded those analyzed in any comparable study (21–23) (Table 1 and Table S6). Even with such numbers, we failed to observe any shift among Avy/a coat morphs in any of the treatment groups. In this sense, our results are consistent with another study that reported that a soy protein isolate (SPI) diet failed to produce a shift toward brown in Avy/a offspring compared with a casein-based control diet (54). Both our experiment and those performed by Badger et al. (54) contrast with the original report that gestational exposure of a/a dams to G increased the percentage of brown (pseudoagouti) Avy/a offspring born after breeding Avy/a males to a/a females (23).
A contributing factor to the contrasting outcomes could be the statistical methods used to analyze data. The previous reports were analyzed by χ2 methodology (21–23). As coat colors ranged from all brown (pseudoagouti) to all yellow, we decided that such categorical data were more appropriately analyzed by using PROC GLIMMIX with multinomial and binomial comparisons. By collapsing those animals within a treatment group into two categories, one with a greater percentage of brown coat color (Y0–Y2), the other with the greater percentage of yellow coats (Y4–Y5), and ignoring the more “difficult” Y3 category, we were able to perform a binomial analysis to determine that none of the maternal diets shifted the probability of brown or yellow coat color relative to each other (Table 1). This method also treats the dam ID as the experimental unit and thus controls for any potential litter effect, whereas previous studies treated the individual offspring as the experimental unit (21–23). However, for thoroughness, a similar χ2 analysis to that used in the other studies was also used, and this statistical method also failed to reveal any differences in coat color correlating with diet.
It should be emphasized that some inconsistencies with the observation that BPA and G, together or in combination, can influence coat color have emerged earlier. For example, as noted above, one report suggested that a soy-based diet had no ability to modulate coat color in Avy/a offspring (54), whereas another (21) observed that exposures of BPA at 50 mg, 50 µg, and 50 ng/kg fw each significantly altered coat colors compared with controls, but did so in an inconsistent manner, with only the higher dose generating a shift toward yellow. In another study, a combination of BPA with G produced a higher proportion of brown mice, whereas a high dose of BPA shifted the balance of coat colors toward yellow (22). Even in our datasets, it is evident that the distribution of coat colors showed shifts in some nutritional groups according to the parity of the dam, especially at parities 2 and 3, but again these changes were inconsistent (Table S4). Although such variability might conceivably be due to breeding the same dams to different Avy/a males, i.e., paternal effects, such a phenomenon must be considered unlikely, as the epigenetic modifications controlling Avy/a expression are believed to be erased completely when the allele is passed through the male germ line (10), and the inbred nature of the mice most likely precludes any variation between conceptuses in expression of DNA methyl transferases and other epigenetic modifiers. However, if the female is the Avy/a parent, epigenetic inheritance through the oocyte is observed, such that a yellow dam will produce proportionately more yellow pups than a brown dam (4, 55), due presumably to incomplete loss of epigenetic “marks” (10). Unfortunately, our records do not describe the degree of brown mottling for all breeder males used, as no inheritance of the epigenetic modification linked to male coat color was anticipated. Finally, a further possible cause for inconsistent patterns of coat color could be the changing gut flora of the pregnant dams, which might be expected to show fluctuations in composition over parity, animal age, and environmental background, and influence the nature of the compounds to which the developing offspring are exposed (56–60).
Other than the fact that coat color was not consistently influenced by either G or BPA in the diet, our most notable finding was that a/a dams on the EE and especially the G–plus–high-BPA diet gave birth to fewer nonagouti (a/a) than agouti (Avy/a) offspring, and that this effect became more pronounced with increased parity (or age) of the dams (Table 2 and Table S5). One previous study (Table S6) indicated no significant deficit of black pups in diets supplemented with BPA plus G (22), although all of the data were most likely obtained from parity 1 when effects are least obvious (Table S5). Possibly an estrogen-enriched reproductive tract environment caused by exposure to EE or BPA plus G favored Avy/a over a/a conceptuses, but it is unclear why the shift became more pronounced with parity. Also, it remains to be determined when the bias to Avy/a offspring occurred during pregnancy. Mice are known to ovulate more oocytes and produce more blastocysts than pups born (61). Thus, a litter size of around six to seven pups, as noted here for dams on a C57/B6 background, is an outcome of competition for space and resources in the uterus. Possibly the uterine environment found in the dams adapted to the EE or BPA-plus-G diets provided some advantage to Avy/a conceptuses.
One theory that might account for these findings is that of the “thrifty genotype” (62, 63), which proposes that environmental inputs, particularly lack of access to food, might select against members of the population with less “thrifty” genes by providing a competitive advantage to individuals possessing such a cadre of genes. The downside of this benefit is that it probably predisposes infants for risk of developing metabolic syndrome if food is plentiful (62). A few genes that are candidates for thriftiness have been proposed (64), but none positively identified. ASIP might be a potential contender for such a role through its action in metabolic signaling. By antagonizing melanocortin receptors (2, 65), including MC4R in the hypothalamus, ASIP increases appetite and possibly also aspects of energy metabolism (66). Additionally, in humans, a species conspicuously lacking agouti hair coloring, ASIP is expressed in adipose tissue and the pancreas, influences levels of STAT1, STAT3, peroxisome proliferator-activated receptor-γ), and fatty acid synthase, and promotes pancreatic insulin release (67, 68). Moreover, A mRNA levels in adipose tissue are significantly elevated in subjects with type 2 diabetes (69). As A expression occurs in both the fetus and placenta of the mouse in midgestation (70), conceptuses expressing it might, under certain circumstances, have some subtle advantage in utero over black (a/a) siblings that are incapable of producing ASIP at all.
It is clear that the agouti (A) allele has been under strong positive selection in wild populations of rodents (71–74), presumably because coat color provides camouflage. Is it also possible that ASIP expression has adaptive value as a metabolic signaling hormone under other circumstances, for example in the placenta? In the case of mice, it is conceivable that developmental exposure of conceptuses to estrogenic compounds leads to increased expression of ASIP, thereby providing a metabolic advantage to Avy genotypes. Alternatively, dam exposure to such chemicals may influence the uterine environment in such a way that Avy/a conceptuses thrive better than their a/a siblings. Rodent models that express ASIP to varying degrees under normal promoter control may be useful animal models for testing these hypotheses (75).
In conclusion, our data contrast with results of others (21–23) and indicate that exposure of Avy/a conceptuses to genistein and BPA through maternal diet does not cause any consistent shift in offspring coat color relative to controls. However, two diets likely to promote an enriched estrogenic environment distorted the anticipated 1:1 ratio of agouti Avy/a to nonagouti (a/a) offspring in a/a × Avy/a crosses in favor of the latter. This effect became more pronounced with parity, possibly because the expression of ASIP provides a short-term, competitive advantage.
Materials and Methods
Animal Husbandry.
All experiments were approved by University of Missouri Animal Care and Use Committee and performed in accordance with National Institutes of Health Animal Care and Use Guidelines. The original founder VY/WffC3Hf/NCTR-Avy/a animals were donated in 2004 from Dr. G. Wolff (National Center for Toxicological Research/Food and Drug Administration, Jefferson, AR) and maintained in the small animal unit in the Animal Sciences Research Center. In 2009, a second colony was established at the Bond Life Sciences Center vivarium after rederiving the line by embryo transfer. The mice at the second location were also bred according to the same scheme to maintain the heterozygosity of the Avy allele on a C57BL/6J background.
Virgin a/a (C57BL/6J) females, 6–8 wk of age, purchased from The Jackson Laboratory were placed on the control AIN93G diet with 7% corn oil by weight (CTL) for 2 wk after arrival at the animal housing facility to eliminate any background BPA exposure. After 2 wk, females were randomly assigned to receive one of seven diets: (i) CTL (n = 37); (ii) AIN93G supplemented with 50 µg of BPA/kg fw [low dose (L)] (n = 21); (iii) AIN93G supplemented with 5 mg of BPA/kg fw [middle dose (M)] (n = 14); (iv) AIN93G supplemented with 50 mg of BPA/kg fw [upper dose (U)] (n = 33); (v) 50 mg BPA/kg fw plus 250 mg G/kg fw (BPA plus G) (n = 26); (vi) 250 mg G/kg fw (n = 14); or (vii) AIN93G diet supplemented with 0.1 parts per billion of EE (n = 34), as a positive control for studies on compounds with an anticipated estrogenic action (76). Based on the average daily food consumption of a/a mice (77), the intake of BPA on the U, M, and L diets approximated 6.5 µg, 0.65 µg, and 6.5 ng/g body weight, respectively. The females were housed two per cage and were randomly bred to Avy/a males that had previously been maintained on the CTL diet (Fig. 1). Hence, males were only exposed to the experimental diets while they were occupying the same cage as a female. The coat colors of the males ranged from Y0 to Y5 (fully brown, to fully yellow with intermediate patterns).
The doses of BPA, G, and EE were chosen on the basis of previous published studies (21–23, 41, 78). We have previously performed a comprehensive pharmokinetic analysis with C57BL/6J a/a adult females on a diet supplemented with 100 mg deuterated [dimethyl-d6]-BPA (BPA-d6)/kg fw. A maximum active BPA serum concentration of approximately of 18.8 ± 4.4 ng/mL was achieved within 6 h of the mice being placed on this diet (77), a value similar to that observed in adult humans (79–81). Additional details are provided in SI Text.
Offspring Coat Color Analyses.
At the time the offspring were weaned and culled as adults, they were photographed and an assignment of genotype (a/a versus Avy/a) and coat color pattern (black, brown: Y0–Y2; mottled with equivalent amount of brown to yellow banding: Y3; or yellow: Y4–Y5) was determined based on previous studies (Fig. 1) (3, 4, 82). Additional details are provided in SI Text.
PCR Analysis to Confirm Genotype Status of Presumptive Avy/a Breeder Males.
PCR analysis was performed on the initial breeder males, as illustrated on the Mutant Mouse Regional Resource Center website (www.mmrrc.org/strains/375/ctr_protocol.pdf). Additional details are provided in SI Text.
Statistical Analyses.
No differences were observed in any parameter between animals reared in the two colonies. Therefore, results were combined. Rather than simply using χ2 procedures for analyzing offspring coat color data (21–23), we chose to use the more appropriate generalized linear mixed model procedures, which models categorical data with PROC GLIMMIX (SAS, version 9.2, software; SAS Institute). The basic linear statistical model included the fixed effects of diet, sex, and the interaction of diet by sex. The random effect of dam ID within diet mean square was used as the denominator of F for testing diet, and the random residual means square was used as the denominator of F for sex and the interaction of diet by sex. The PROC GLIMMIX procedure adjusted for unequal dams per diet and unequal pups per dam per diet. Dam ID was considered the experimental unit rather than each individual offspring in contrast to the approach used by others (21–23). Additional details are provided in SI Text.
Supplementary Material
Acknowledgments
We thank Kelcie Declue, Leslie Miller, Roxanne E. Gelven, and Henry A. Kelley for assistance in taking care of the mice; Dr. Jessica Flowers at Harlan-Teklad for her assistance in preparing the diets used herein; Dr. Craig A. Cooney for advice on the densitometric analysis; and Howard Wilson for assisting in the digital assessments for the Avy mice images. We also thank Dr. Fred vom Saal for comments on the manuscript. This work was supported by National Institutes of Health (NIH) Challenge Grant RC1 ES018195 (to C.S.R.) and Food for the 21st Century Program from the University of Missouri (R.M.R.). The University of Missouri Office of Animal Resources supported the rederivation embryo transfers to create additional Avy/a breeder males from the Mutant Mouse Regional Resource Center (University of Missouri, Columbia), which is supported by NIH Grant U42RR14821.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1220230110/-/DCSupplemental.
References
- 1.Wolff GL, Roberts DW, Galbraith DB. Prenatal determination of obesity, tumor susceptibility, and coat color pattern in viable yellow (Avy/a) mice. The yellow mouse syndrome. J Hered. 1986;77(3):151–158. doi: 10.1093/oxfordjournals.jhered.a110206. [DOI] [PubMed] [Google Scholar]
- 2.Wolff GL, Roberts DW, Mountjoy KG. Physiological consequences of ectopic agouti gene expression: The yellow obese mouse syndrome. Physiol Genomics. 1999;1(3):151–163. doi: 10.1152/physiolgenomics.1999.1.3.151. [DOI] [PubMed] [Google Scholar]
- 3.Cooney CA, Dave AA, Wolff GL. Maternal methyl supplements in mice affect epigenetic variation and DNA methylation of offspring. J Nutr. 2002;132(8, Suppl):2393S–2400S. doi: 10.1093/jn/132.8.2393S. [DOI] [PubMed] [Google Scholar]
- 4.Wolff GL, Kodell RL, Moore SR, Cooney CA. Maternal epigenetics and methyl supplements affect agouti gene expression in Avy/a mice. FASEB J. 1998;12(11):949–957. [PubMed] [Google Scholar]
- 5.Klebig ML, Wilkinson JE, Geisler JG, Woychik RP. Ectopic expression of the agouti gene in transgenic mice causes obesity, features of type II diabetes, and yellow fur. Proc Natl Acad Sci USA. 1995;92(11):4728–4732. doi: 10.1073/pnas.92.11.4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Waterland RA, Jirtle RL. Transposable elements: Targets for early nutritional effects on epigenetic gene regulation. Mol Cell Biol. 2003;23(15):5293–5300. doi: 10.1128/MCB.23.15.5293-5300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Godfrey KM, et al. Epigenetic gene promoter methylation at birth is associated with child’s later adiposity. Diabetes. 2011;60(5):1528–1534. doi: 10.2337/db10-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lillycrop KA. Effect of maternal diet on the epigenome: Implications for human metabolic disease. Proc Nutr Soc. 2011;70(1):64–72. doi: 10.1017/S0029665110004027. [DOI] [PubMed] [Google Scholar]
- 9.Weaver IC. Shaping adult phenotypes through early life environments. Birth Defects Res C Embryo Today. 2009;87(4):314–326. doi: 10.1002/bdrc.20164. [DOI] [PubMed] [Google Scholar]
- 10.Morgan HD, Sutherland HG, Martin DI, Whitelaw E. Epigenetic inheritance at the agouti locus in the mouse. Nat Genet. 1999;23(3):314–318. doi: 10.1038/15490. [DOI] [PubMed] [Google Scholar]
- 11.Bultman SJ, Michaud EJ, Woychik RP. Molecular characterization of the mouse agouti locus. Cell. 1992;71(7):1195–1204. doi: 10.1016/s0092-8674(05)80067-4. [DOI] [PubMed] [Google Scholar]
- 12.Miller MW, et al. Cloning of the mouse agouti gene predicts a secreted protein ubiquitously expressed in mice carrying the lethal yellow mutation. Genes Dev. 1993;7(3):454–467. doi: 10.1101/gad.7.3.454. [DOI] [PubMed] [Google Scholar]
- 13.Kwon HY, et al. Molecular structure and chromosomal mapping of the human homolog of the agouti gene. Proc Natl Acad Sci USA. 1994;91(21):9760–9764. doi: 10.1073/pnas.91.21.9760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson BD, et al. Structure and function of ASP, the human homolog of the mouse agouti gene. Hum Mol Genet. 1995;4(2):223–230. doi: 10.1093/hmg/4.2.223. [DOI] [PubMed] [Google Scholar]
- 15.Cone RD, et al. The melanocortin receptors: Agonists, antagonists, and the hormonal control of pigmentation. Recent Prog Horm Res. 1996;51:287–317, discussion 318. [PubMed] [Google Scholar]
- 16.Mansour M, et al. Pancreatic neuronal melanocortin-4 receptor modulates serum insulin levels independent of leptin receptor. Endocrine. 2010;37(1):220–230. doi: 10.1007/s12020-009-9289-5. [DOI] [PubMed] [Google Scholar]
- 17.Michaud EJ, et al. Differential expression of a new dominant agouti allele (Aiapy) is correlated with methylation state and is influenced by parental lineage. Genes Dev. 1994;8(12):1463–1472. doi: 10.1101/gad.8.12.1463. [DOI] [PubMed] [Google Scholar]
- 18.Duhl DM, Vrieling H, Miller KA, Wolff GL, Barsh GS. Neomorphic agouti mutations in obese yellow mice. Nat Genet. 1994;8(1):59–65. doi: 10.1038/ng0994-59. [DOI] [PubMed] [Google Scholar]
- 19.Chen Y, Duhl DM, Barsh GS. Opposite orientations of an inverted duplication and allelic variation at the mouse agouti locus. Genetics. 1996;144(1):265–277. doi: 10.1093/genetics/144.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cropley JE, Suter CM, Beckman KB, Martin DI. CpG methylation of a silent controlling element in the murine Avy allele is incomplete and unresponsive to methyl donor supplementation. PLoS One. 2010;5(2):e9055. doi: 10.1371/journal.pone.0009055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson OS, et al. Epigenetic responses following maternal dietary exposure to physiologically relevant levels of bisphenol A. Environ Mol Mutagen. 2012;53(5):334–342. doi: 10.1002/em.21692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dolinoy DC, Huang D, Jirtle RL. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc Natl Acad Sci USA. 2007;104(32):13056–13061. doi: 10.1073/pnas.0703739104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dolinoy DC, Weidman JR, Waterland RA, Jirtle RL. Maternal genistein alters coat color and protects Avy mouse offspring from obesity by modifying the fetal epigenome. Environ Health Perspect. 2006;114(4):567–572. doi: 10.1289/ehp.8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubin BS. Bisphenol A: An endocrine disruptor with widespread exposure and multiple effects. J Steroid Biochem Mol Biol. 2011;127(1–2):27–34. doi: 10.1016/j.jsbmb.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Schug TT, Janesick A, Blumberg B, Heindel JJ. Endocrine disrupting chemicals and disease susceptibility. J Steroid Biochem Mol Biol. 2011;127(3–5):204–215. doi: 10.1016/j.jsbmb.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM. Bisphenol-A and the great divide: A review of controversies in the field of endocrine disruption. Endocr Rev. 2009;30(1):75–95. doi: 10.1210/er.2008-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cox KH, Gatewood JD, Howeth C, Rissman EF. Gestational exposure to bisphenol A and cross-fostering affect behaviors in juvenile mice. Horm Behav. 2010;58(5):754–761. doi: 10.1016/j.yhbeh.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenfeld CS, et al. Striking variation in the sex ratio of pups born to mice according to whether maternal diet is high in fat or carbohydrate. Proc Natl Acad Sci USA. 2003;100(8):4628–4632. doi: 10.1073/pnas.0330808100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alexenko AP, et al. The contrasting effects of ad libitum and restricted feeding of a diet very high in saturated fats on sex ratio and metabolic hormones in mice. Biol Reprod. 2007;77(4):599–604. doi: 10.1095/biolreprod.107.062174. [DOI] [PubMed] [Google Scholar]
- 30.Carroll D, et al. Systolic blood pressure reactions to acute stress are associated with future hypertension status in the Dutch Famine Birth Cohort Study. Int J Psychophysiol. 2012;85(2):270–273. doi: 10.1016/j.ijpsycho.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 31.de Rooij SR, Painter RC, Holleman F, Bossuyt PM, Roseboom TJ. The metabolic syndrome in adults prenatally exposed to the Dutch famine. Am J Clin Nutr. 2007;86(4):1219–1224. doi: 10.1093/ajcn/86.4.1219. [DOI] [PubMed] [Google Scholar]
- 32.Painter RC, et al. 2006. Early onset of coronary artery disease after prenatal exposure to the Dutch famine. Am J Clin Nutr 84(2):322–327; quiz 466–467.
- 33.Painter RC, et al. Transgenerational effects of prenatal exposure to the Dutch famine on neonatal adiposity and health in later life. BJOG. 2008;115(10):1243–1249. doi: 10.1111/j.1471-0528.2008.01822.x. [DOI] [PubMed] [Google Scholar]
- 34.van Abeelen AF, et al. Survival effects of prenatal famine exposure. Am J Clin Nutr. 2012;95(1):179–183. doi: 10.3945/ajcn.111.022038. [DOI] [PubMed] [Google Scholar]
- 35.Brennan KA, Olson DM, Symonds ME. Maternal nutrient restriction alters renal development and blood pressure regulation of the offspring. Proc Nutr Soc. 2006;65(1):116–124. doi: 10.1079/pns2005484. [DOI] [PubMed] [Google Scholar]
- 36.Holemans K, Aerts L, Van Assche FA. Fetal growth restriction and consequences for the offspring in animal models. J Soc Gynecol Investig. 2003;10(7):392–399. doi: 10.1016/s1071-5576(03)00134-5. [DOI] [PubMed] [Google Scholar]
- 37.Vieau D, et al. HPA axis programming by maternal undernutrition in the male rat offspring. Psychoneuroendocrinology. 2007;32(Suppl 1):S16–S20. doi: 10.1016/j.psyneuen.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 38.Adewale HB, Jefferson WN, Newbold RR, Patisaul HB. Neonatal bisphenol-A exposure alters rat reproductive development and ovarian morphology without impairing activation of gonadotropin-releasing hormone neurons. Biol Reprod. 2009;81(4):690–699. doi: 10.1095/biolreprod.109.078261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Markey CM, Wadia PR, Rubin BS, Sonnenschein C, Soto AM. Long-term effects of fetal exposure to low doses of the xenoestrogen bisphenol-A in the female mouse genital tract. Biol Reprod. 2005;72(6):1344–1351. doi: 10.1095/biolreprod.104.036301. [DOI] [PubMed] [Google Scholar]
- 40.Xi W, et al. Effect of perinatal and postnatal bisphenol A exposure to the regulatory circuits at the hypothalamus-pituitary-gonadal axis of CD-1 mice. Reprod Toxicol. 2011;31(4):409–417. doi: 10.1016/j.reprotox.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 41.Jašarević E, et al. Disruption of adult expression of sexually selected traits by developmental exposure to bisphenol A. Proc Natl Acad Sci USA. 2011;108(28):11715–11720. doi: 10.1073/pnas.1107958108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lamartiniere CA, Jenkins S, Betancourt AM, Wang J, Russo J. Exposure to the endocrine disruptor bisphenol A alters susceptibility for mammary cancer. Horm Mol Biol Clin Investig. 2011;5(2):45–52. doi: 10.1515/HMBCI.2010.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prins GS, Tang WY, Belmonte J, Ho SM. Developmental exposure to bisphenol A increases prostate cancer susceptibility in adult rats: Epigenetic mode of action is implicated. Fertil Steril. 2008;89(2, Suppl):e41. doi: 10.1016/j.fertnstert.2007.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dolinoy DC. The agouti mouse model: An epigenetic biosensor for nutritional and environmental alterations on the fetal epigenome. Nutr Rev. 2008;66(Suppl 1):S7–S11. doi: 10.1111/j.1753-4887.2008.00056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frye CA, et al. Endocrine disruptors: A review of some sources, effects, and mechanisms of actions on behaviour and neuroendocrine systems. J Neuroendocrinol. 2011;24:144–159. doi: 10.1111/j.1365-2826.2011.02229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ryan BC, Vandenbergh JG. Developmental exposure to environmental estrogens alters anxiety and spatial memory in female mice. Horm Behav. 2006;50(1):85–93. doi: 10.1016/j.yhbeh.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 47.Cao J, Mickens JA, McCaffrey KA, Leyrer SM, Patisaul HB. Neonatal bisphenol A exposure alters sexually dimorphic gene expression in the postnatal rat hypothalamus. Neurotoxicology. 2012;33(1):23–36. doi: 10.1016/j.neuro.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eilam-Stock T, Serrano P, Frankfurt M, Luine VN. Bisphenol-A impairs memory and reduces dendritic spine density in adult male rats. Behav Neurosci. 2012;126(1):175–185. doi: 10.1037/a0025959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.MacLusky NJ, Hajszan T, Leranth C. The environmental estrogen bisphenol A inhibits estradiol-induced hippocampal synaptogenesis. Environ Health Perspect. 2005;113(6):675–679. doi: 10.1289/ehp.7633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Poimenova A, Markaki E, Rahiotis C, Kitraki E. Corticosterone-regulated actions in the rat brain are affected by perinatal exposure to low dose of bisphenol A. Neuroscience. 2010;167(3):741–749. doi: 10.1016/j.neuroscience.2010.02.051. [DOI] [PubMed] [Google Scholar]
- 51.Perera F, et al. Prenatal bisphenol a exposure and child behavior in an inner-city cohort. Environ Health Perspect. 2012;120(8):1190–1194. doi: 10.1289/ehp.1104492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carlson S, Peng N, Prasain JK, Wyss JM. 2008. Effects of botanical dietary supplements on cardiovascular, cognitive, and metabolic function in males and females. Gend Med 5(Suppl A):S76–S90.
- 53.Fitzpatrick LA. Soy isoflavones: Hope or hype? Maturitas. 2003;44(Suppl 1):S21–S29. doi: 10.1016/s0378-5122(02)00345-6. [DOI] [PubMed] [Google Scholar]
- 54.Badger TM, et al. Soy protein isolate reduces hepatosteatosis in yellow Avy/a mice without altering coat color phenotype. Exp Biol Med (Maywood) 2008;233(10):1242–1254. doi: 10.3181/0802-RM-60. [DOI] [PubMed] [Google Scholar]
- 55.Wolff GL. Influence of maternal phenotype on metabolic differentiation of agouti locus mutants in the mouse. Genetics. 1978;88(3):529–539. doi: 10.1093/genetics/88.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bauer E, Williams BA, Smidt H, Mosenthin R, Verstegen MW. Influence of dietary components on development of the microbiota in single-stomached species. Nutr Res Rev. 2006;19(1):63–78. doi: 10.1079/NRR2006123. [DOI] [PubMed] [Google Scholar]
- 57.Canani RB, et al. Epigenetic mechanisms elicited by nutrition in early life. Nutr Res Rev. 2011;24(2):198–205. doi: 10.1017/S0954422411000102. [DOI] [PubMed] [Google Scholar]
- 58.Fåk F, Karlsson CL, Ahrné S, Molin G, Weström B. Effects of a high-fat diet during pregnancy and lactation are modulated by E. coli in rat offspring. Int J Obes (Lond) 2012;36(5):744–751. doi: 10.1038/ijo.2011.118. [DOI] [PubMed] [Google Scholar]
- 59.Sanz Y. Gut microbiota and probiotics in maternal and infant health. Am J Clin Nutr. 2011;94(6, Suppl):2000S–2005S. doi: 10.3945/ajcn.110.001172. [DOI] [PubMed] [Google Scholar]
- 60.Schaible TD, Harris RA, Dowd SE, Smith CW, Kellermayer R. Maternal methyl-donor supplementation induces prolonged murine offspring colitis susceptibility in association with mucosal epigenetic and microbiomic changes. Hum Mol Genet. 2011;20(9):1687–1696. doi: 10.1093/hmg/ddr044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bermejo-Alvarez P, Roberts RM, Rosenfeld CS. Effect of glucose concentration during in vitro culture of mouse embryos on development to blastocyst, success of embryo transfer, and litter sex ratio. Mol Reprod Dev. 2012;79(5):329–336. doi: 10.1002/mrd.22028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Edwards MJ. Genetic selection of embryos that later develop the metabolic syndrome. Med Hypotheses. 2012;78(5):621–625. doi: 10.1016/j.mehy.2012.01.040. [DOI] [PubMed] [Google Scholar]
- 63.Ong KK, Dunger DB. Developmental aspects in the pathogenesis of type 2 diabetes. Mol Cell Endocrinol. 2001;185(1–2):145–149. doi: 10.1016/s0303-7207(01)00625-6. [DOI] [PubMed] [Google Scholar]
- 64.Prentice AM. Early influences on human energy regulation: Thrifty genotypes and thrifty phenotypes. Physiol Behav. 2005;86(5):640–645. doi: 10.1016/j.physbeh.2005.08.055. [DOI] [PubMed] [Google Scholar]
- 65.Michaud EJ, et al. A molecular model for the genetic and phenotypic characteristics of the mouse lethal yellow (Ay) mutation. Proc Natl Acad Sci USA. 1994;91(7):2562–2566. doi: 10.1073/pnas.91.7.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huszar D, et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88(1):131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 67.Mynatt RL, Stephens JM. Agouti regulates adipocyte transcription factors. Am J Physiol Cell Physiol. 2001;280(4):C954–C961. doi: 10.1152/ajpcell.2001.280.4.C954. [DOI] [PubMed] [Google Scholar]
- 68.Xue BZ, et al. The agouti gene product stimulates pancreatic [beta]-cell Ca2+ signaling and insulin release. Physiol Genomics. 1999;1(1):11–19. doi: 10.1152/physiolgenomics.1999.1.1.11. [DOI] [PubMed] [Google Scholar]
- 69.Smith SR, et al. Agouti expression in human adipose tissue: Functional consequences and increased expression in type 2 diabetes. Diabetes. 2003;52(12):2914–2922. doi: 10.2337/diabetes.52.12.2914. [DOI] [PubMed] [Google Scholar]
- 70.Millar SE, Miller MW, Stevens ME, Barsh GS. Expression and transgenic studies of the mouse agouti gene provide insight into the mechanisms by which mammalian coat color patterns are generated. Development. 1995;121(10):3223–3232. doi: 10.1242/dev.121.10.3223. [DOI] [PubMed] [Google Scholar]
- 71.Linnen CR, Kingsley EP, Jensen JD, Hoekstra HE. On the origin and spread of an adaptive allele in deer mice. Science. 2009;325(5944):1095–1098. doi: 10.1126/science.1175826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mullen LM, Hoekstra HE. Natural selection along an environmental gradient: A classic cline in mouse pigmentation. Evolution. 2008;62(7):1555–1570. doi: 10.1111/j.1558-5646.2008.00425.x. [DOI] [PubMed] [Google Scholar]
- 73.Steiner CC, Weber JN, Hoekstra HE. Adaptive variation in beach mice produced by two interacting pigmentation genes. PLoS Biol. 2007;5(9):e219. doi: 10.1371/journal.pbio.0050219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Manceau M, Domingues VS, Mallarino R, Hoekstra HE. The developmental role of Agouti in color pattern evolution. Science. 2011;331(6020):1062–1065. doi: 10.1126/science.1200684. [DOI] [PubMed] [Google Scholar]
- 75.Shorter KR, et al. Peromyscus as a mammalian epigenetic model. Genet Res Int. 2012;2012:179159. doi: 10.1155/2012/179159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vom Saal FS, et al. The importance of appropriate controls, animal feed, and animal models in interpreting results from low-dose studies of bisphenol A. Birth Defects Res A Clin Mol Teratol. 2005;73(3):140–145. doi: 10.1002/bdra.20120. [DOI] [PubMed] [Google Scholar]
- 77.Sieli PT, et al. Comparison of serum bisphenol A concentrations in mice exposed to bisphenol A through the diet versus oral bolus exposure. Environ Health Perspect. 2011;119(9):1260–1265. doi: 10.1289/ehp.1003385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cox KH, Gatewood JD, Howeth C, Rissman EF. Gestational exposure to bisphenol A and cross-fostering affect behaviors in juvenile mice. Horm Behav. 2010;58(5):754–761. doi: 10.1016/j.yhbeh.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Padmanabhan V, et al. Maternal bisphenol-A levels at delivery: A looming problem? J Perinatol. 2008;28(4):258–263. doi: 10.1038/sj.jp.7211913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Teeguarden JG, et al. 2011. Twenty-four hour human urine and serum profiles of bisphenol A during high dietary exposure. Toxicol Sci 123(1):48–57.
- 81.Vandenberg LN, et al. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ Health Perspect. 2010;118(8):1055–1070. doi: 10.1289/ehp.0901716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ounpraseuth S, et al. A method to quantify mouse coat-color proportions. PLoS ONE. 2009;4(4):e5414. doi: 10.1371/journal.pone.0005414. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.