Abstract
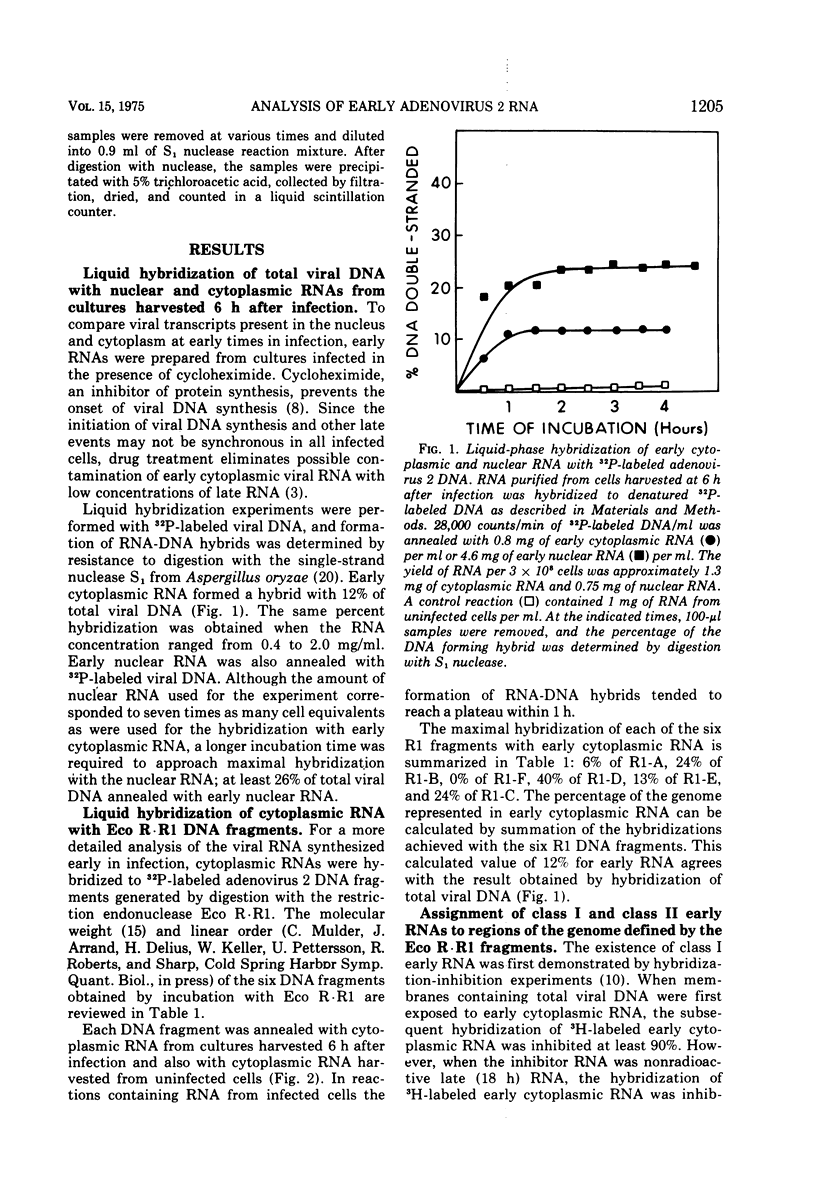
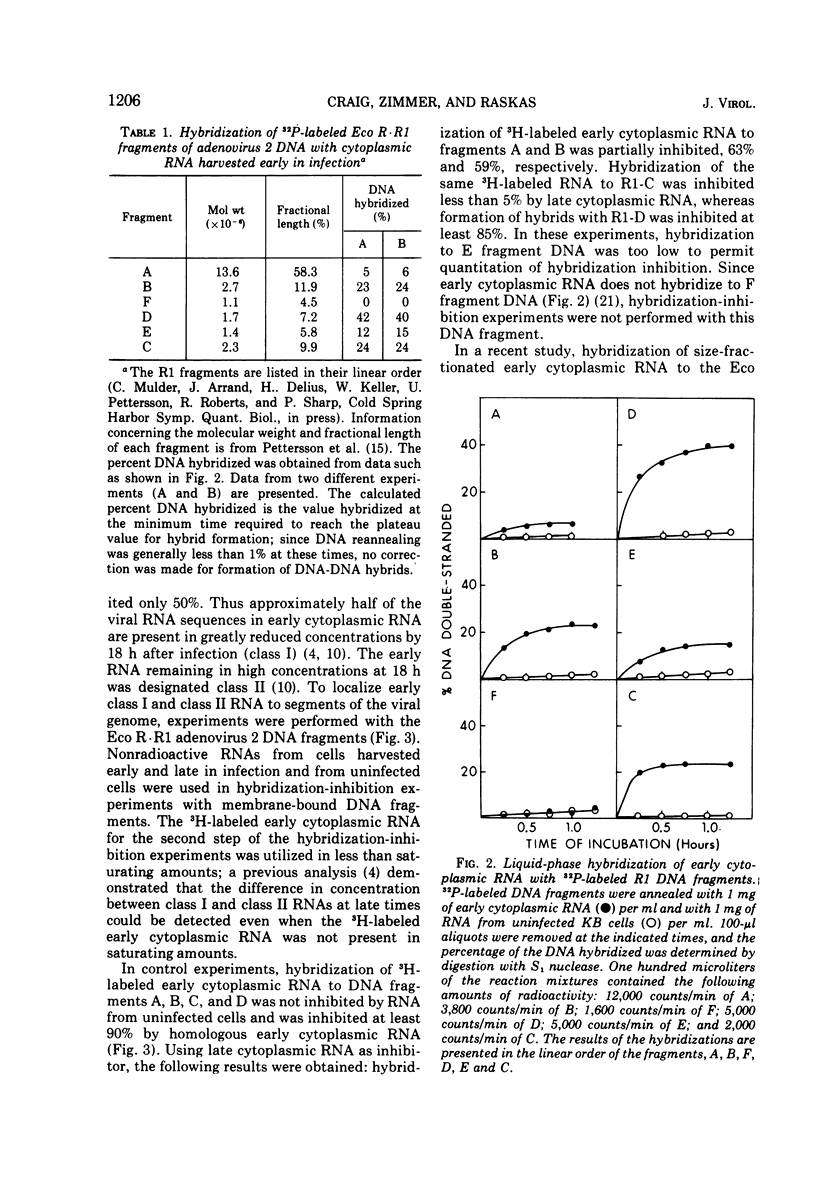
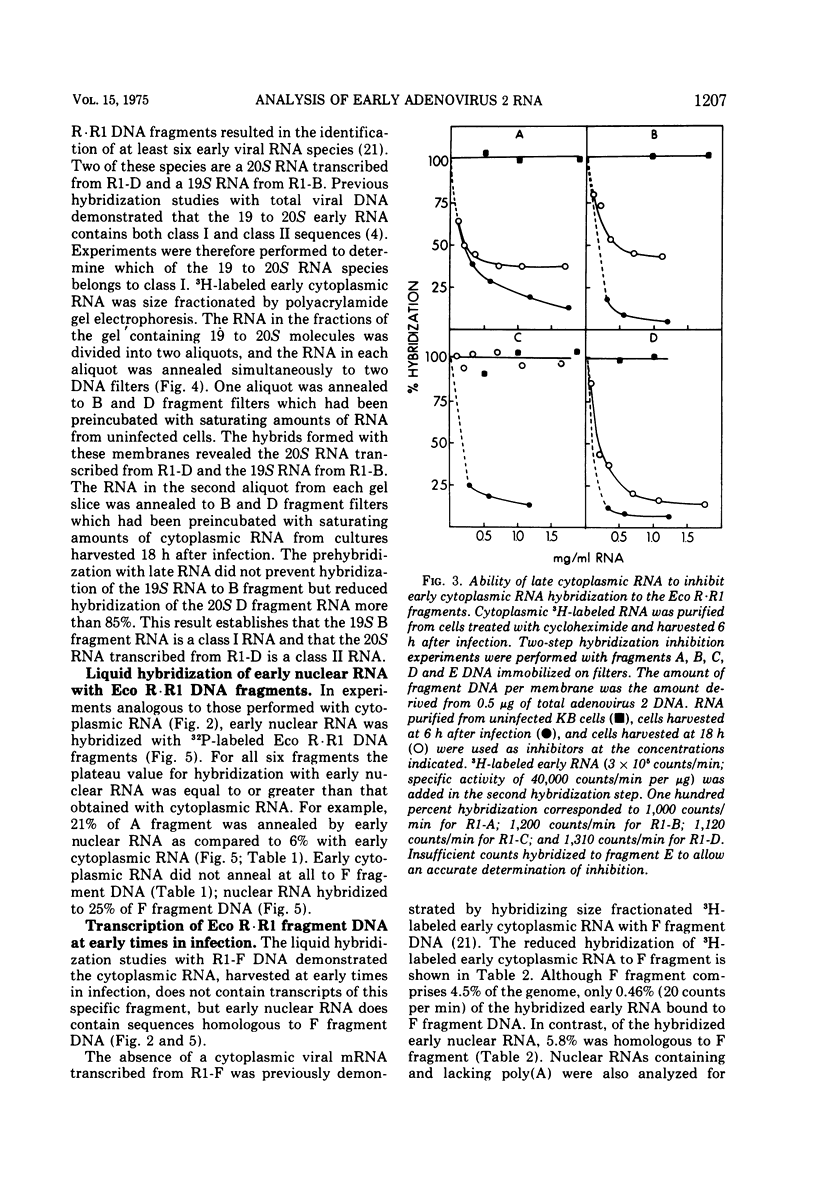
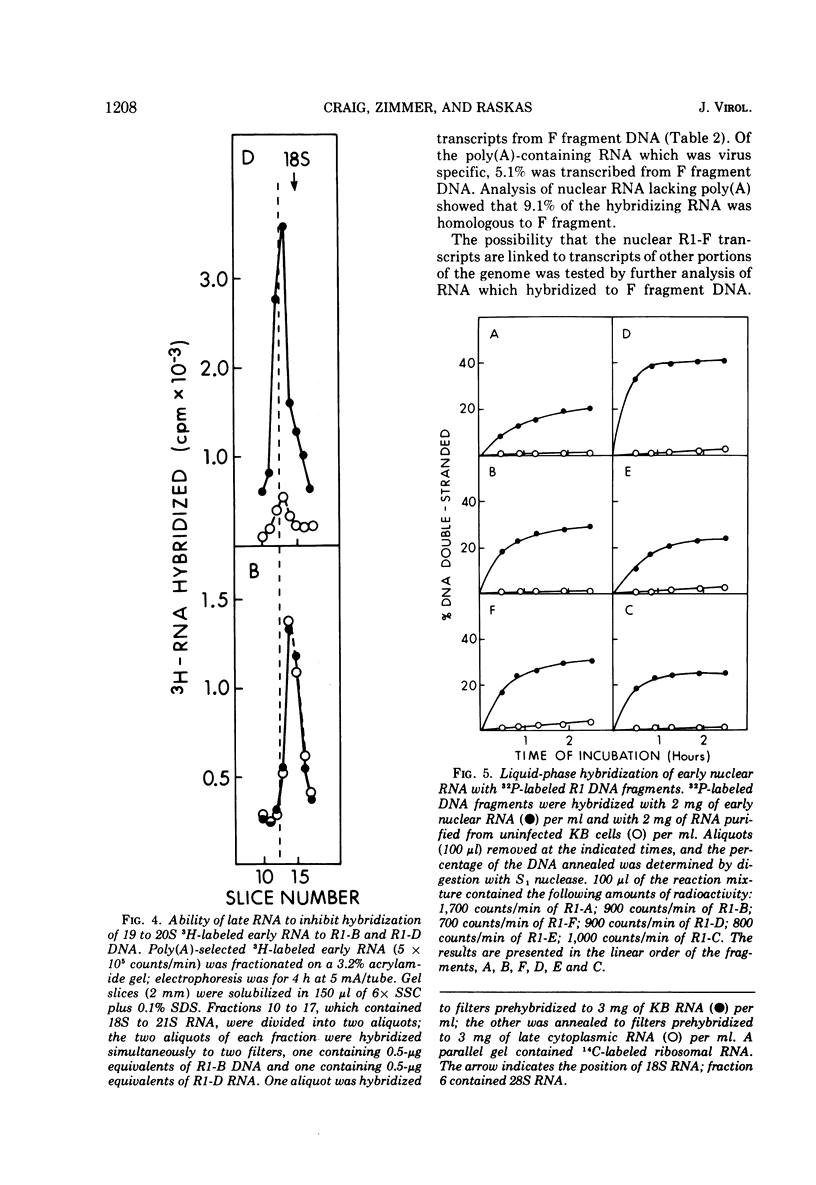
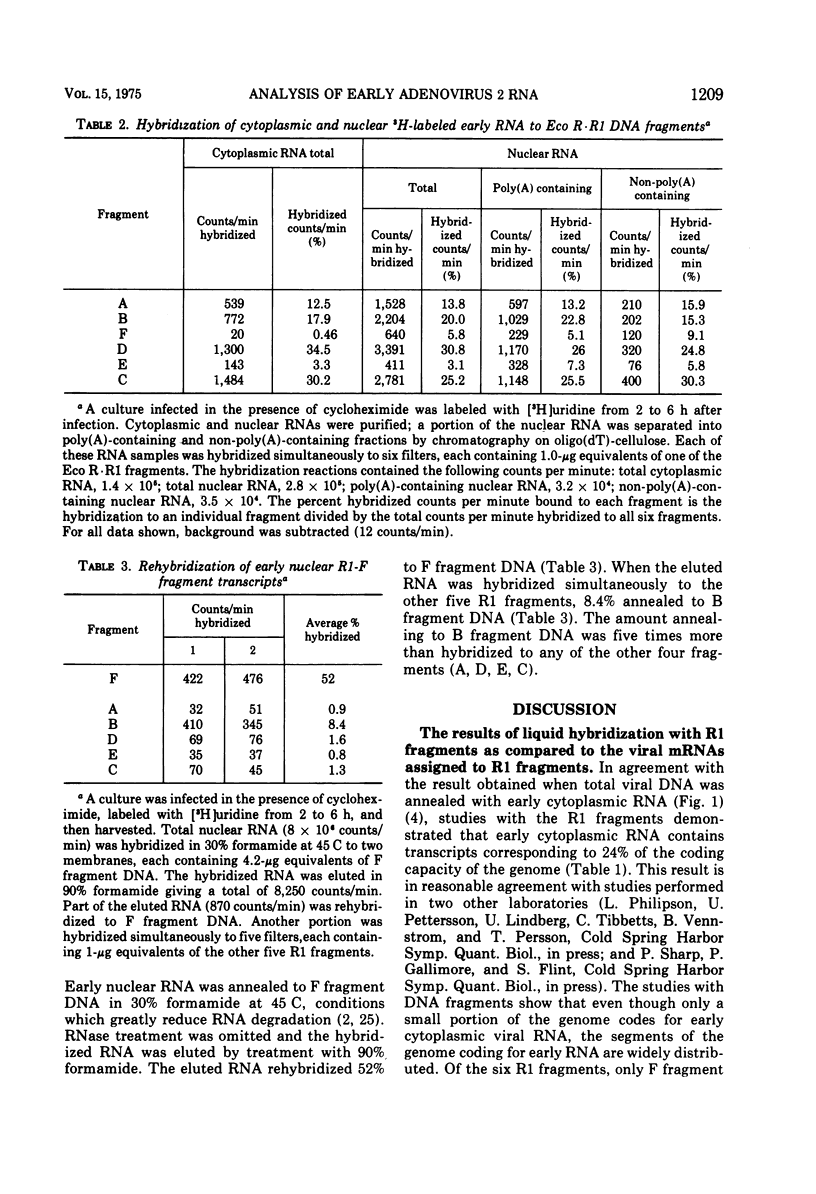
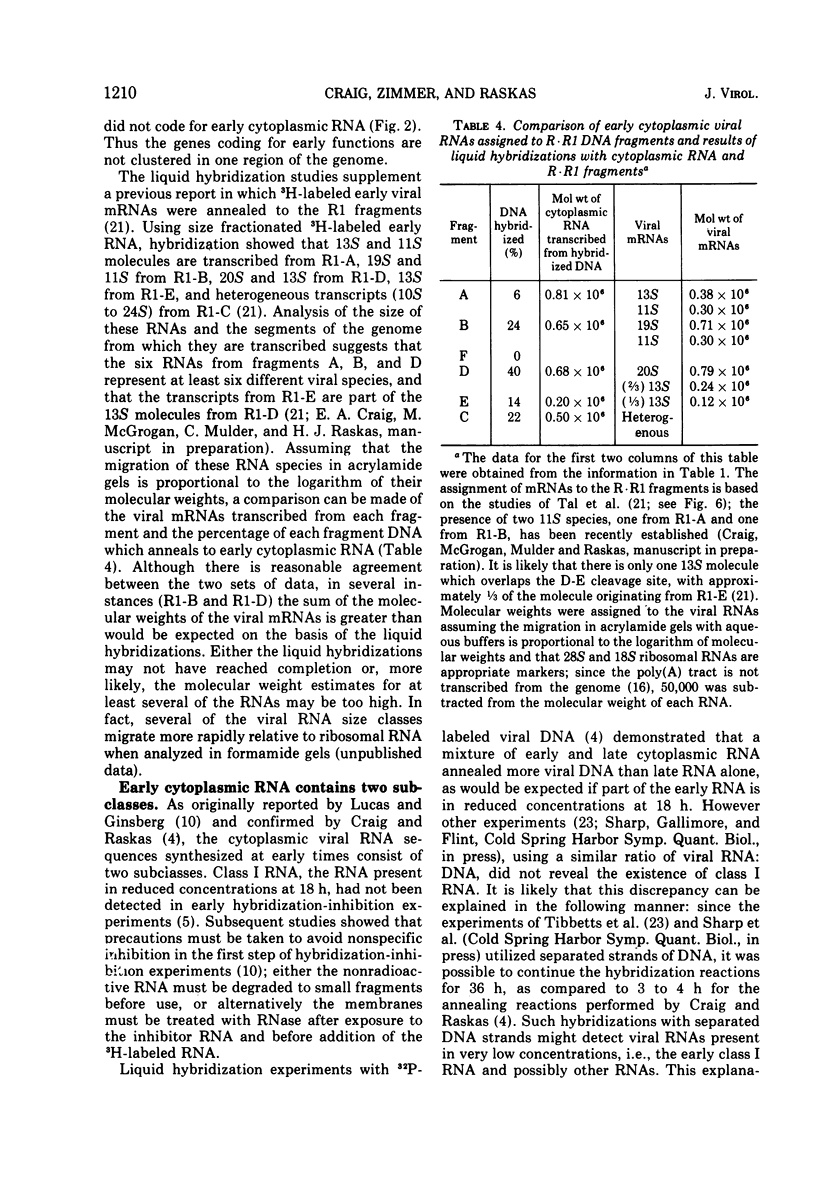
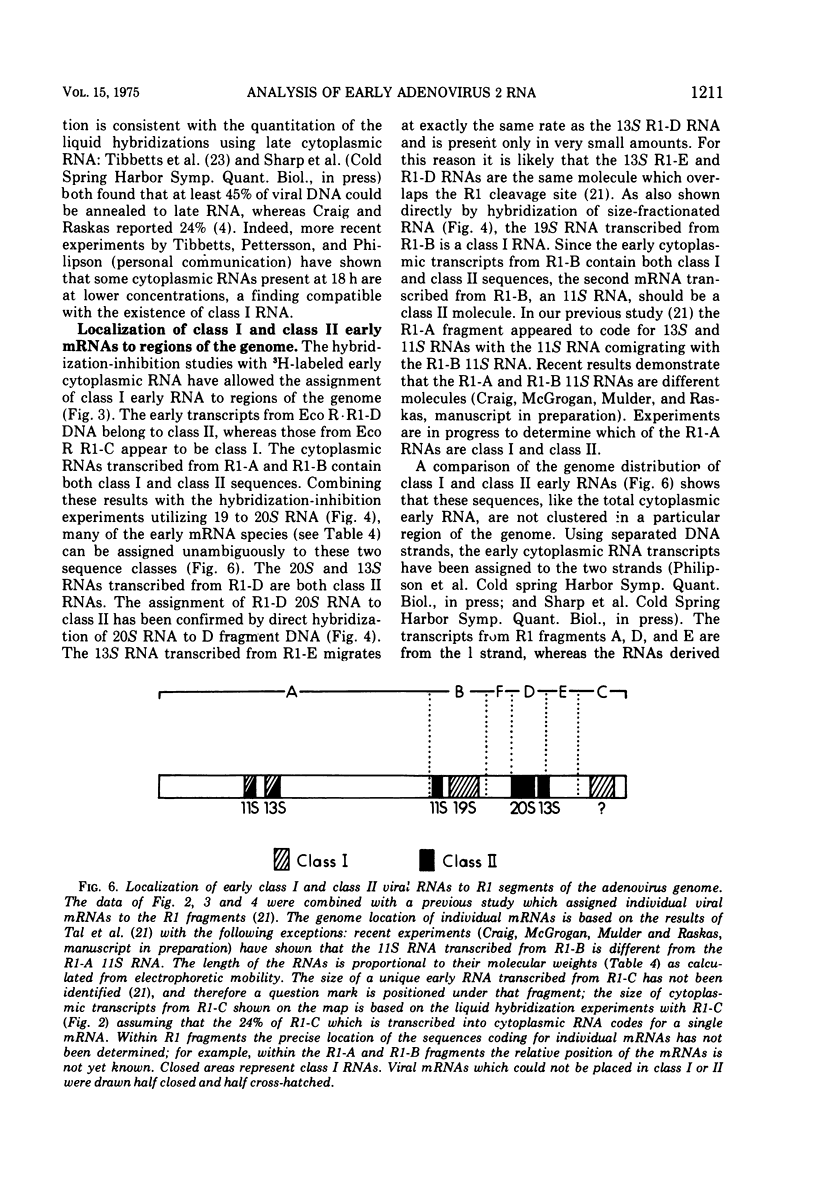
Adenovirus 2 RNA synthesized early in productive infection was analyzed by RNA-DNA hybridization. Hybridization experiments were performed with adenovirus 2 DNA and wit, the six adenovirus 2 DNA fragments generated 0y digestion with the restriction endonuclease Eco R.R1. Duplex formation between RNA and -32P-labeled viral DNA was assayed by S(1) nuclease digestion. RNA from the cytoplasm annealed 12 percent of the total viral DNA and the following percentage of each of the R.R1 fragments: 6 percent of R1-A, 24 percent of R1-B, 0 percent of R1-F, 40 percent of R1-D, 13 percent of R1-E, and 22 percent of R1-C. The early cytoplasmic RNA is composed of two sequence classes: class I, present in greatly reduced quantities at late times in infection (18 h), and class II, which remains at high concentrations at 18 h. In hybridization-inhibition experiments, hybridization of class II RNA is inhibited by late cytoplasmic RNA, whereas hybridization of class I RNA is not blocked by late cytoplasmic RNA (J. J. Lucas and H. S. Ginsberg, 1971; E.A. Craig and H. J. Raskas, 1971). To determine the location of class I and II sequences on the genome, membrane bound DNA fragments were used in hybridization-inhibition experiments. These studies demonstrated that the early cytoplasmic transcripts of R1-D belong to class II, whereas R1-C transcripts are class I sequences. The cytoplasmic RNAs transcribed from fragments A and B contain both class I and class II sequences. Analysis of cytoplasmic RNA fractionated by size demonstrated that the class I sequences include a 19 S RNA transcribed from R1-B and class II sequences include a 20S RNA derived from R1-D. Nuclear RNA purified from cultures early in infection was annealed with -32P-labeled R1 fragments. With all six fragments the nuclear RNA annealed as much or more of the DNA than did cytoplasmic RNA. Eco R1-F annealed at least 25 percent with early nuclear RNA, whereas no sequences homologous to R1-F were detected in early cytoplasmic RNA. When cultures were labeled from 2 to 6 h after infection, at least 5 percent of the -3H-labeled early nuclear viral RNA annealed to Eco R1-F. Some of these nuclear transcripts from R1-F appear to be covalently linked to sequences transcribed from a contiguous region of the genome (Eco R1-B). 8.4 percent of the RNA selected by hybridization of R1-F reannealed to R1-B, whereas no more than 1.5 percent reannealed to R1 fragments A, D, E, or C.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büttner W., Veres-Molnár Z., Green M. Isolation of DNA Strand-specific early messenger RNA species in cells infected by human adenovirus 2. Proc Natl Acad Sci U S A. 1974 Aug;71(8):2951–2955. doi: 10.1073/pnas.71.8.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig E. A., Raskas H. J. Effect of cycloheximide on RNA metabolism early in productive infection with adenovirus 2. J Virol. 1974 Jul;14(1):26–32. doi: 10.1128/jvi.14.1.26-32.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig E. A., Raskas H. J. Two classes of cytoplasmic viral RNA synthesized early in productive infection with adenovirus 2. J Virol. 1974 Oct;14(4):751–757. doi: 10.1128/jvi.14.4.751-757.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujinaga K., Green M. Mechanism of viral carcinogenesis by DNA mammalian viruses. VII. Viral genes transcribed in adenovirus type 2 infected and transformed cells. Proc Natl Acad Sci U S A. 1970 Feb;65(2):375–382. doi: 10.1073/pnas.65.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN M., DAESCH G. E. Biochemical studies on adenovirus multiplication. II. Kinetics of nucleic acid and protein synthesis in suspension cultures. Virology. 1961 Feb;13:169–176. doi: 10.1016/0042-6822(61)90051-4. [DOI] [PubMed] [Google Scholar]
- GREEN M., PINA M. BIOCHEMICAL STUDIES ON ADENOVIRUS MULTIPLICATION, VI. PROPERTIES OF HIGHLY PURIFIED TUMORIGENIC HUMAN ADENOVIRUSES AND THEIR DNA. Proc Natl Acad Sci U S A. 1964 Jun;51:1251–1259. doi: 10.1073/pnas.51.6.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. S., Brayton C., Baum S. G. Synthesis of type 2 adenovirus DNA in the presence of cycloheximide. J Virol. 1973 Apr;11(4):544–551. doi: 10.1128/jvi.11.4.544-551.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg U., Persson T., Philipson L. Isolation and characterization of adenovirus messenger ribonucleic acid in productive infection. J Virol. 1972 Nov;10(5):909–919. doi: 10.1128/jvi.10.5.909-919.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas J. J., Ginsberg H. S. Synthesis of virus-specific ribonucleic acid in KB cells infected with type 2 adenovirus. J Virol. 1971 Aug;8(2):203–214. doi: 10.1128/jvi.8.2.203-214.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas J. J., Ginsberg H. S. Transcription and transport of virus-specific ribonucleic acids in African green monkey kidney cells abortively infected with type 2 adenovirus. J Virol. 1972 Dec;10(6):1109–1117. doi: 10.1128/jvi.10.6.1109-1117.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire P. M., Swart C., Hodge L. D. Adenovirus messenger RNA in mammalian cells: failure of polyribosome association in the absence of nuclear cleavage. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1578–1582. doi: 10.1073/pnas.69.6.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons J. T., Gardner J., Green M. Biochemical studies on adenovirus multiplication, XIX. Resolution of late viral RNA species in the nucleus and cytoplasm. Proc Natl Acad Sci U S A. 1971 Mar;68(3):557–560. doi: 10.1073/pnas.68.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons J. T., Green M. Biochemical studies on adenovirus multiplication. 18. Resolution of early virus-specific RNA species in Ad 2 infected and transformed cells. Virology. 1971 Jul;45(1):154–162. doi: 10.1016/0042-6822(71)90122-x. [DOI] [PubMed] [Google Scholar]
- Pettersson U., Mulder C., Deluis H., Sharp P. A. Cleavage of adenovirus type 2 DNA into six unique fragments by endonuclease R-RI. Proc Natl Acad Sci U S A. 1973 Jan;70(1):200–204. doi: 10.1073/pnas.70.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipson L., Wall R., Glickman G., Darnell J. E. Addition of polyadenylate sequences to virus-specific RNA during adenovirus replication. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2806–2809. doi: 10.1073/pnas.68.11.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskas H. J., Thomas D. C., Green M. Biochemical studies on adenovirus multiplication. XVII. Ribosome synthesis in uninfected and infected KB cells. Virology. 1970 Apr;40(4):893–902. doi: 10.1016/0042-6822(70)90135-2. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Sutton W. D. A crude nuclease preparation suitable for use in DNA reassociation experiments. Biochim Biophys Acta. 1971 Jul 29;240(4):522–531. doi: 10.1016/0005-2787(71)90709-x. [DOI] [PubMed] [Google Scholar]
- Tal J., Craig E. A., Zimmer S., Raskas H. J. Localization of adenovirus 2 messenger RNA's to segments of the viral genome defined by endonuclease R-R1. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4057–4061. doi: 10.1073/pnas.71.10.4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. C., Green M. Biochemical studies on adenovirus multiplication. XV. Transcription of the adenovirus type II genome during productive infection. Virology. 1969 Oct;39(2):205–210. doi: 10.1016/0042-6822(69)90040-3. [DOI] [PubMed] [Google Scholar]
- Tibbetts C., Pettersson U., Johansson K., Philpson L. Relationship of mRNA from productively infected cells to the complementary strands of adenovirus type 2 DNA. J Virol. 1974 Feb;13(2):370–377. doi: 10.1128/jvi.13.2.370-377.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall R., Philipson L., Darnell J. E. Processing of adenovirus specific nuclear RNA during virus replication. Virology. 1972 Oct;50(1):27–34. doi: 10.1016/0042-6822(72)90342-x. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A., Warnaar S. O., Winocour E. Isolation and characterization of simian virus 40 ribonucleic acid. J Virol. 1972 Aug;10(2):193–201. doi: 10.1128/jvi.10.2.193-201.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]



