Abstract
Enteritis caused by Clostridium difficile toxin (Tx) is a nosocomial disease of increasing clinical concern, but the local mediators of C. difficile TxA inflammation are unknown. The potent vasodilator calcitonin gene-related peptide mediates neurogenic inflammation via the calcitonin receptor-like receptor (CLR). Here we examined the ileum-specific effects of reducing CLR on TxA ileitis by local preinjection of double-stranded RNAs. Treatment with CLR dsRNA for 7 d decreased CLR immunoreactivity, whereas treatment with non-CLR dsRNA did not. Subsequent injection of TxA in the same location increased CLR in rats treated with non-CLR dsRNA but not in rats treated with CLR dsRNA, documenting that local injection of dsRNA is effective in preventing the increase in CLR immunoreactivity in response to local TxA. After non-CLR dsRNA pretreatment, TxA induced robust intestinal secretion, myeloperoxidase activity, and histopathologic indications of inflammation including epithelial damage, congestion, neutrophil infiltration, loss of mucin from goblet cells, and increase in mast cell numbers. After CLR dsRNA pretreatment, TxA-induced changes in intestinal secretion and histopathologic inflammation were improved, including normal mucin staining and fewer resident mast cells. Loss of CLR prevented TxA-mediated activation of NF-κB and concomitant increases in pERK1/2 and TNF-α mRNA. Locally produced CLR plays a proinflammatory role in TxA ileitis via MAPK signaling and TNF-α. The results reported here strongly suggest that a local injection of dsRNA targeting CLR could be an effective local therapeutic approach at the inflammation site in the treatment of a growing, clinically relevant hospital-acquired disease, C. difficile infection.
Keywords: RNAi, nuclear translocation
The leading cause of diarrhea in hospitalized patients is Clostridium difficile infection due to antibiotic use (1). Toxin A (TxA) produced by C. difficile activates nuclear factor κB (NF-κB) and induces transcription of proinflammatory cytokines, including TNF-α and IL-8 (2). Intestinal inflammation induced by C. difficile TxA is characterized by neutrophil infiltration, intestinal secretion, and epithelial disruption, with a prominent neurogenic component (2–4).
A key player in neurogenic inflammation, calcitonin gene-related peptide (CGRP), is one of the most potent known vasodilators (5). The CGRP receptor is composed of calcitonin receptor-like receptor (CLR) and receptor activity-modifying protein 1 (RAMP1) (6). We developed highly specific antibodies that recognize CLR and RAMP1 and colocalized these components in the gastrointestinal tract and the spinal cord in rats (7, 8). CGRP and its receptors are expressed in distinct cell types in peripheral vs. central tissues, emphasizing the complexity of the CGRP/CLR/RAMP1 system (9). Identifying the peripheral function of these components is complex, because the peptide and its receptor are present both in intrinsic and extrinsic primary sensory afferent neurons (10, 11). Moreover, CGRP is produced by and activates migratory cells such as lymphocytes, granulocytes, and monocytes (12–14). Receptors for CGRP may be induced by intestinal inflammation, as is the substance P neurokinin 1 receptor in T cells and in intestinal epithelial cells (3, 15). Although systemic use of the peptide receptor antagonist CGRP-37 was shown to decrease TxA-induced inflammation (16), that study could not discriminate between the contributions made by central and ileal CGRP receptors to disease genesis or resolution.
In this report, to study whether ileally produced CLR has a role in C. difficile TxA enteritis, we injected dsRNA that targets CLR (CLR dsRNA) into the ileum of rats to transiently decrease the expression of CLR in the precise ileal segment where TxA would be injected at a later time. We determined whether local CLR is necessary and sufficient for C. difficile TxA to cause ileitis. We examined loss of mucin from goblet cells, mast cell recruitment, and the NF-κB, MAPK, and TNF-α signaling pathways, all of which are indices of C. difficile enteritis.
Results
Local Injection of CLR dsRNA in the Ileum Prevents TxA-Induced Increase in CLR Expression.
To determine the role of local CLR in TxA enteritis, we decreased CLR expression in the rat ileum by RNA interference (RNAi) and verified this decrease by loss of immunoreactivity for CLR. Treatment with non-CLR dsRNA did not alter CLR immunoreactivity (CLR-IR), whereas, as expected, treatment with CLR dsRNA markedly decreased CLR-IR in the ileum (Fig. 1). The use of dsRNA caused no adverse effects, and body weight gains and losses were similar among all rats during the course of the study (Fig. S1).
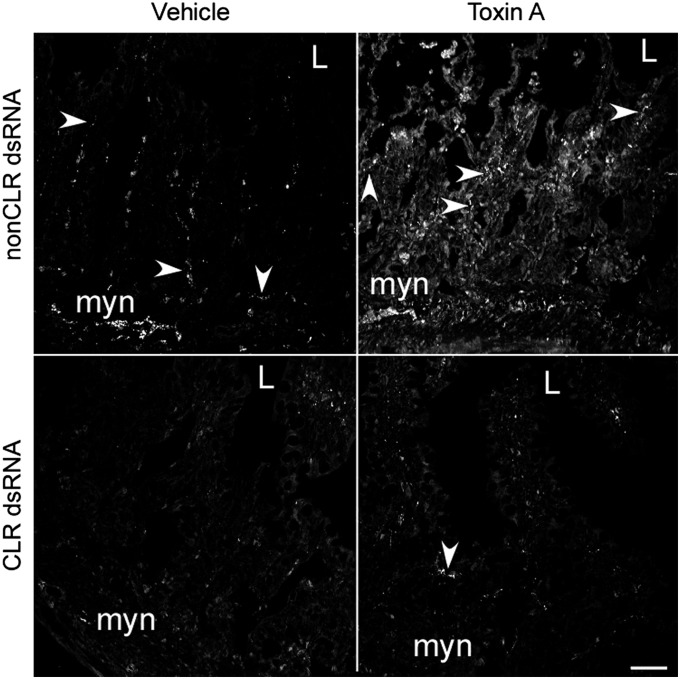
Fig. 1.
CLR dsRNA decreases basal CLR-IR and prevents TxA-induced increase in CLR. Seven days after pretreatment with vehicle, non-CLR dsRNA, or CLR dsRNA, the rats received an injection of vehicle or TxA into ileal loops and were killed after 4 h. Loop tissue from rats treated with non-CLR dsRNA and vehicle had basal CLR-IR as determined by confocal microscopy; pretreatment with non-CLR dsRNA had no effect. TxA increased staining for CLR in the ileum of rats treated with non-CLR dsRNA. Loop tissue from rats injected with CLR dsRNA had minimal CLR-IR. TxA did not increase CLR-IR in the ileum of rats pretreated with CLR dsRNA. Arrowheads point to CLR-IR. L, lumen; myn, myenteric neurons. (Scale bar, 50 µm.)
After treatment for 7 d with CLR and non-CLR dsRNAs, we induced TxA ileitis. After 4 h of exposure to TxA, CGRP-IR in nerve fibers in the lamina propria increased strikingly (Figs. S2 and S3). At the same time, CLR-IR increased in nerve varicosities located near CGRP-IR in non-CLR dsRNA–treated rats (Fig. 1 and Fig. S2). Thus, the ligand and its receptor increased similarly during TxA-induced ileitis, and is a host response to the inflammatory insult. After pretreatment with non-CLR dsRNA and TxA treatment, CLR-IR was increased in nerve fibers and in neurons of the myenteric plexus, where it occasionally colocalized with the neuronal marker PGP9.5 (Fig. S3 D–O). The most prominent increase in CLR-IR occurred in nerve fibers of the lamina propria, where CLR-IR was colocalized with PGP9.5 (Fig. S3 P–T). Interestingly, CLR-IR was also detected in epithelial cells (Fig. 1 and Fig. S3 S–T). Remarkably, TxA treatment did not increase CLR-IR after treatment with CLR dsRNA (Fig. 1). Thus, even in the presence of TxA, dsRNA prevented TxA-induced local increases in CLR, allowing us to document the contribution of local CLR in TxA-induced ileitis.
Local Injection of CLR dsRNA Decreased TxA-Induced Intestinal Secretion.
To determine whether decreased CLR expression before induction of TxA ileitis affects TxA-associated secretory responses, we compared the weight of the closed loop (mg/cm), as an index of intestinal secretion, among naïve, non-CLR dsRNA–injected controls, and CLR dsRNA-injected rats. Local injection of dsRNA did not alter basal intestinal secretion, which was similar among the naïve and non-CLR dsRNA– and CLR dsRNA-treated rats (170 ± 21 vs. 168 ± 19 vs. 139 ± 2 mg/cm, respectively) (Fig. 2A). As expected, in the naïve and non-CLR dsRNA groups, TxA induced robust intestinal secretion (to 317 ± 68 and 345 ± 30, respectively). Thus, non-CLR dsRNA did not alter the inflammatory response to TxA. An interesting observation is that, although baseline secretion was not altered by the local injection of CLR dsRNA, when the tissue was challenged by injection of TxA there was a loss of function, with intestinal secretion decreased by 50% (to 226 ± 35 mg/cm) (Fig. 2A).
Fig. 2.
Local injection of CLR dsRNA decreases ileitis mediated by TxA. After 7 d of pretreatment with non-CLR dsRNA or CLR dsRNA, an ileal loop spanning the dsRNA injection site was infused with vehicle or TxA (5 µg). (A) After 4 h of treatment, intestinal secretion was determined as the weight/length (mg/cm) of the excised loop. Secretion was significantly decreased (P < 0.05) with CLR dsRNA pretreatment. (B) MPO activity was also decreased in loop fluid (U/L) after CLR dsRNA pretreatment. Values are mean ± SEM per loop, n = 4–12 loops per group. *P < 0.05 vs. vehicle, #P < 0.05 vs. rats pretreated with non-CLR dsRNA and injected with TxA.
Local Injection of CLR dsRNA Decreases TxA-Induced Granulocyte Infiltration and Myeloperoxidase Activity.
Pronounced neutrophil infiltration is a hallmark of inflammation induced by TxA. We determined whether the lack of CLR expression affected granulocyte infiltration. Treatment with vehicle in the non-CLR dsRNA and CLR dsRNA groups did not increase myeloperoxidase (MPO) activity, confirming that dsRNA treatment was not proinflammatory (Fig. 2B). As expected, treatment with TxA increased MPO activity fivefold in the loop fluid of the non-CLR dsRNA rats (622 ± 82 vs. basal levels of 128 ± 35 mU/mL). Pretreatment with CLR dsRNA decreased TxA-induced MPO activity by 40% compared with controls (367 ± 65 vs. basal levels of 113 ± 29 mU/mL) (Fig. 2B). Thus, locally produced CLR participates in the accumulation of granulocytes during TxA ileitis.
Local Injection of CLR dsRNA Decreases TxA-Induced Histopathologic Indication of Inflammation.
We quantified changes in epithelial damage, edema and congestion, and granulocyte infiltration induced by TxA by scoring hematoxylin and eosin-stained sections. Pretreatment with non-CLR or CLR dsRNA did not change baseline histologic scores (Fig. 3A). TxA treatment increased epithelial damage, edema, and granulocyte infiltration in non-CLR controls (Fig. 3A). However, CLR dsRNA pretreatment reduced TxA-induced histopathologic inflammation compared with non-CLR controls (3.6 ± 0.5 vs. 6 ± 0.6, P < 0.05) (Fig. 3A).
Fig. 3.
Local injection of CLR dsRNA decreases histopathologic changes induced by TxA. A portion of loop tissue was sectioned and stained with hematoxylin and eosin. (A) Graphical representation of the histologic score is shown. Duplicate sections were scored for epithelial damage, edema and congestion, and neutrophil infiltration (0–5 per end point) (17). *P < 0.05 vs. vehicle, #P < 0.05 vs. rats treated with non-CLR dsRNA, followed by TxA treatment 7 d later. Values are mean ± SEM per loop, n = 8–12 loops per group. PMNs, polymorphonuclear leukocytes. (B) Pretreatment with CLR dsRNA minimized histopathologic indications of inflammation induced by TxA. In the non-CLR dsRNA controls, TxA induced severe damage (note dilated blood vessels; arrows). Pretreatment with CLR dsRNA preserved epithelial integrity even after TxA treatment (arrowheads). lp, lamina propria (n = 6–8 per group). (Scale bar, 100 µm.)
Pretreatment with non-CLR or CLR dsRNA did not significantly affect baseline histologic architecture (Fig. 3B). As expected, TxA treatment induced histopathologic indications of inflammation in non-CLR dsRNA controls. Dilated blood vessels were prominent (Fig. 3B), with adherent neutrophils. Polymorphonuclear leukocytes and single epithelial cells were present in the lumen, compromising the epithelial integrity and exposing the lamina propria. Cellular infiltration markedly increased in the lamina propria. In contrast, pretreatment with CLR dsRNA decreased the TxA-induced histopathologic indications of inflammation (Fig. 3B). Preservation of epithelial architecture was the most significant change after CLR dsRNA pretreatment.
Local Injection of CLR dsRNA Decreases TxA-Induced Mucin Secretion.
We previously found that loss of mucin in goblet cells accompanied TxA-mediated epithelial damage (17). Because epithelial architecture was preserved in rats pretreated with CLR dsRNA before TxA treatment (Fig. 3B), we examined mucin retention at the epithelial surface after TxA treatment. After vehicle treatment, intact goblet cells containing abundant mucin were evident in the epithelium of tissue from rats treated with non-CLR dsRNA or CLR dsRNA (Fig. 4A). TxA treatment induced a marked loss of mucin from goblet cells in the non-CLR dsRNA controls, most frequently at the tips of the villi (Fig. 4A). Secreted mucin was often detectable in the intestinal lumen. CLR dsRNA pretreatment prevented TxA-induced activation of goblet cells and loss of mucin, with little mucin evident in the lumen (Fig. 4A).
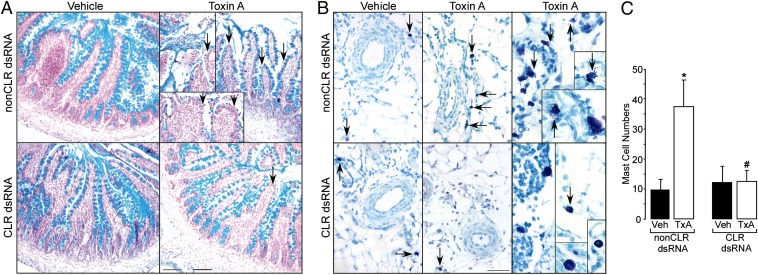
Fig. 4.
Local injection of CLR dsRNA blocks goblet and mast cell involvement in TxA-induced enteritis. Triplicate sections were stained with Alcian Blue and Fast Red to identify goblet cell mucin, or toluidine blue to identify mast cells. (A) Pretreatment with CLR dsRNA decreased mucin loss due to TxA treatment. In areas adjacent to epithelial cell loss, TxA, but not vehicle, induced marked loss of mucin in goblet cells of rats pretreated with non-CLR dsRNA (arrows). (Scale bar, 100 µm.) (B) Pretreatment with CLR dsRNA prevented a TxA-induced change in mast cell numbers and appearance. In rats pretreated with non-CLR dsRNA, TxA induced mast cell accumulation in the mesenteric fat adjacent to the ileal loop (arrows). Often these cells were associated with dispersed metachromatic granules suggestive of degranulation. (Scale bar, 54 µm.) (C) Quantitation of mast cells showed basal levels occurred after CLR dsRNA and TxA treatment. *P < 0.05 vs. vehicle, #P < 0.05 vs. rats pretreated with non-CLR dsRNA and treated with TxA. Values are mean ± SEM, n = 8–12 per group.
Local Injection of CLR dsRNA Decreases TxA-Induced Mast Cell Recruitment.
Responses to TxA involve the recruitment of mast cells (2) that can secrete proteases and other inflammatory mediators that participate in the host response to TxA. Mast cell numbers were low after pretreatment with non-CLR or CLR dsRNA and vehicle (Fig. 4B). TxA increased mast cell numbers in the adjacent connective tissue in rats pretreated with non-CLR dsRNA (32.8 ± 9 vs. 9.7 ± 4 per field), and these cells were often degranulated (Fig. 4 B and C). TxA failed to recruit mast cells after CLR dsRNA pretreatment; numbers were similar to those at baseline (12.4 ± 4 vs. 12.5 ± 5).
Local Injection of CLR dsRNA Decreased TxA-Induced NF-κB and IκBα Expression.
The transcription factor NF-κB complexed with IκBα is present in the cytosol in an inactive state (18). Phosphorylation of IκBα results in the release of active NF-κB that translocates from the cytosol to the nucleus to activate transcription of a plethora of genes, including TNF-α. Western blot analysis showed that both IκBα and NF-κB were present at baseline (or after vehicle treatment of non-CLR dsRNA ileum) in the cytosolic fraction (Fig. 5A). Immunofluorescence confirmed the presence of NF-κB (p65) diffuse throughout the cytosol (Fig. 5B). Interestingly, loss of CLR resulted in NF-κB being sequestered in discrete cytosolic areas, even at baseline. After pretreatment with non-CLR dsRNA, TxA treatment decreased cytosolic levels of both IκBα (63%, P = 0.029) and NF-κB (50%, P = 0.003) (Fig. 5 A and C). Importantly, pretreatment with CLR dsRNA abolished TxA-induced decreases in IκBα and NF-κB proteins, and the levels remained comparable to baseline even after TxA treatment (Fig. 5 A and C). Moreover, immunostaining confirmed that loss of CLR prevented activation of NF-κB (p65), as it did not translocate to the nucleus even after TxA treatment (Fig. 5B).
Fig. 5.
Local injection of CLR dsRNA modifies TxA-induced signaling pathways. (A–C) Western blot analysis of the cytosolic fraction showed decreases in NF-κB (50%, *P = 0.003) and IκBα (63%, #P = 0.029) protein levels (actin, a housekeeping gene, was used as a normalization control) after TxA treatment in non-CLR dsRNA controls (A and C). Pretreatment with CLR dsRNA appears to prevent phosphorylation of IκBα, and thus protein levels of both IκBα and NF-κB in the cytosol remain unchanged even after TxA treatment (B). Confocal images of ileal villi showed diffuse cytoplasmic staining of NF-κB (p65) in buffer-treated rats that were pretreated with non-CLR dsRNA. TxA treatment results in nuclear translocation of NF-κB where it colocalizes with DAPI, a nuclear stain, and loss of CLR prevents activation of NF-κB and translocation to the nucleus. (B) Dashed box area of the tissue is shown in adjacent box at 3× magnification. Arrows show nuclear localization of NF-κB in nonCLRdsRNA tissue and arrowheads show cytoplasmic retention of NF-κB after pretreatment with CLR dsRNA. (D) Agarose gel showed TNF-α mRNA products amplified by RT-PCR of ileal tissue; cyclophilin, a housekeeping gene, was used as a normalization control. TxA treatment increased TNF-α mRNA levels in non-CLR dsRNA controls, whereas pretreatment with CLR dsRNA decreased this effect. Quantitation of RT-PCR data showed that TxA increased TNF-α mRNA levels over that seen with vehicle treatment in controls. Pretreatment with CLR dsRNA decreased this effect. (E) Western blot analysis of the cytosolic fraction from ileal tissue showed increases in pERK1/2 but not total ERK levels after treatment with TxA. TxA treatment increased pERK1/2 levels in controls, whereas pretreatment with CLR dsRNA reduced activation of total ERK to pERK1/2. Quantitation of pERK1/2 normalized to total ERK showed that TxA increased pERK1/2 over vehicle in controls, and pretreatment with CLR dsRNA decreased this effect. *P < 0.05 vs. vehicle, #P < 0.05 vs. rats pretreated with non-CLR dsRNA and injected with TxA. Values are mean ± SEM, n = 8–12 per group, two to four experiments.
Local Injection of CLR dsRNA Decreased TxA-Induced TNF-α Expression.
TxA mediates its inflammatory effects through induction of NF-κB, thereby increasing transcription of proinflammatory cytokines, including TNF-α (2). CLR’s role in TNF-α induction is unknown. TxA, but not vehicle, increased TNF-α mRNA levels after pretreatment with non-CLR dsRNA (1.6 ± 0.17 vs. 0.52 ± 0.22) (Fig. 5D). CLR dsRNA decreased TxA-induced TNF-α expression, but did not alter basal TNF-α expression (0.49 ± 0.11 vs. 0.50 ± 0.22). Thus, a local decrease in CLR is sufficient to prevent TxA induction of TNF-α expression.
Local Injection of CLR dsRNA Decreased TxA-Induced MAP Kinase Activation.
TxA activates MAP kinase signaling pathways during inflammatory insults, with ERK and p38 mediating monocyte necrosis, IL-1β release, and IL-8 gene expression (19). We examined pERK1/2 and ERK1/2 levels by Western blot analysis. TxA increased pERK1/2 levels over vehicle treatment in the non-CLR dsRNA–pretreated rats (1.01 ± 0.07 vs. 0.23 ± 0.06, respectively) (Fig. 5E). Pretreatment with CLR dsRNA decreased the effect of TxA on pERK1/2, but did not alter basal levels of pERK1/2 (0.33 ± 0.09 vs. 0.23 ± 0.05, respectively). Thus, local CLR participates in TxA-induced MAP kinase activation, a signaling pathway that promotes inflammation in response to TxA.
Discussion
In this study, we examined the role of local CLR in C. difficile TxA ileitis, a clinically relevant model of neurogenic intestinal inflammation. We previously showed that CLR is required for functional responses to CGRP (20). CLR and RAMP1 comprise the CGRP receptor (6), and are colocalized throughout the gastrointestinal tract in nerve fibers and soma that may participate in TxA enteritis (7). We now show that TxA treatment increases CLR-IR as well as CGRP, and that local CLR is sufficient to mediate a host response to TxA. Local injection of dsRNA targeting CLR ameliorates the inflammatory responses to TxA by all variables examined, including intestinal secretion, MPO activity, loss of mucin, and mast cell infiltration. Pretreatment with CLR dsRNA decreases TxA-mediated histopathologic damage to about 50% of that seen in rats pretreated with non-CLR dsRNA; epithelial damage, edema/congestion, and neutrophil infiltration all decrease. The change in intestinal secretion, edema, and congestion suggests that locally produced CLR is sufficient to participate in vascular responses to TxA. Our results build upon the findings obtained with repeated systemic injections of the peptide receptor antagonist CGRP8-37, and document a local site of action for CLR (16). Because the systemic approach did not yield an even greater effect, our results support the hypothesis that the major site of action of CLR in the response to TxA is local. Data also suggest that the TxA-induced increase in CLR-IR in the ileum promotes pathologic changes in the host, and that a local decrease of CLR can alter the outcome of TxA ileitis.
We found that a local decrease in CLR does not significantly alter basal levels of some end points examined, because there are factors other than CLR to mediate mucosal function. Differences in responses between rats pretreated with non-CLR dsRNA and rats pretreated with CLR dsRNA readily become manifest only after challenge with locally injected TxA, thereby documenting that the loss of CLR played a role in the response to TxA. Other experiments have shown that in diet-induced obesity, changes in macrophage function were revealed only after bacterial challenge (21). Lamina propria macrophages that were isolated from inflamed tissue had heightened TNF-α secretion, even without stimulation, suggesting that their environment altered their function (22). Thus, our finding that a role for CLR was only clearly demonstrated after inflammatory responses is paralleled by similar findings in other systems.
Because CGRP is a potent vasodilator (5), the effect of CLR dsRNA on vascular congestion was expected. It was unclear why CLR dsRNA decreased TxA-induced epithelial damage and neutrophil migration. After TxA binds to its receptor on colonocytes, ERK1/2, NF-κB, and p38 MAP kinase are activated, leading to increased expression of proinflammatory cytokines that activate sensory nerves and recruit monocytes, mast cells, and neutrophils (2, 19). After a local injection of CLR dsRNA, TxA was unable to increase pERK1/2 or decrease NF-κB and IκBα protein expression in the cytosol. Decreased expression of phosphorylated IκBα in the cytosol is required to release NF-κB from the inactive complex. Our finding that loss of local CLR prevented TxA-mediated decrease in cytosolic NF-κB and IκBα suggests that an increase in CLR expression is key for inflammatory mediators to initiate signaling cascades (such as MAPK/ERK) that in turn regulate NF-κB activation and cytokine production. Inability of NF-κB to translocate to the nucleus in turn prevented TxA-mediated increases in TNF-α. Thus, the proinflammatory pathway that leads to epithelial destruction and cell recruitment was decreased. These results clarify the mechanism of decreased epithelial cell damage and recruitment of monocytes and mast cells in rats pretreated with CLR dsRNA and injected with TxA.
C. difficile TxA induces a profound host response within 4 h, with a marked influx of granulocytes and profound epithelial changes. The TxA-induced increase in CLR-IR did not occur in rats pretreated with CLR dsRNA, providing a tool to study tissue-specific roles of genes in animals that are not easily amenable to genetic manipulation and without the caveat of developmental compensations. Compared with pharmacologic therapies that have varied metabolic profiles and excretion rates, local decrease of gene expression enables a defined, clearly targeted therapy that minimizes potential side effects. As CGRP has diverse roles in different tissues (23–25), a targeted local delivery of dsRNA for CLR or CGRP is preferable. In agreement with previous findings, our dsRNA was not proinflammatory, because it did not alter weight gain or basal levels of intestinal secretion or cytokines and MPO activity (20, 26) and, importantly, CLR dsRNA specifically ameliorated TxA-induced ileitis. Because local injection of CLR dsRNA universally improved all end points of TxA-induced ileitis examined, our current findings suggest efficacy for encapsulated or luminal delivery of dsRNA to alter local inflammation in the gastrointestinal tract. This approach may be highly relevant, because C. difficile infections extend hospitalization (27).
A striking observation of the current study is the retention of normal epithelial architecture after TxA treatment in rats pretreated with CLR dsRNA. An early event in epithelial damage induced by TxA is the loss of mucin from goblet cells (2), which did not occur after pretreatment with CLR dsRNA. We have found that in TxA ileitis, protease-activated receptor 2−/− mice have an improved outcome and a minimal loss of mucin (17). CGRP can recruit mast cells to sites of inflammation (28) and, in response to TxA, mast cells can alter microvascular tone and promote fluid secretion (2). These results agree with our finding that locally produced CLR participates in TxA-induced mast cell recruitment and intestinal secretion.
In summary, we found that local injection of CLR dsRNA decreases CLR expression, which in turn is sufficient to prevent TxA-mediated increases in CLR and pathophysiologic responses to C. difficile TxA. Thus, local CLR has a striking proinflammatory role in host responses to TxA, and a local decrease of CLR offers a unique targeted therapy for C. difficile enteritis.
Materials and Methods
For antibodies and detailed experimental procedures, please refer to SI Materials and Methods.
Animals.
Male Sprague–Dawley rats (n = 4–8 per group) weighing 250–280 g were individually housed in temperature- and light-controlled rooms, with ad libitum access to chow and water. All procedures were in accordance with the Institutional Animal Care and Use Committee at the University of California, San Francisco.
Synthesis of dsRNA.
dsRNA for CLR and control (globin) was prepared as described (20). Cloned cDNAs for CLR and globin were used as templates to transcribe sense and antisense RNAs in vitro using a MEGAscript RNA Kit (Ambion) as specified.
Ileal Decrease of CLR with RNA Interference.
Rats underwent anesthesia with isofluorane and a midline laparotomy. The terminal ileum was exteriorized and a marking suture was placed in the ileum wall (2 cm from the cecum). dsRNA (20 µg) for either β-globin (non-CLR dsRNA) or CLR (CLR dsRNA) was mixed with 1.5 µL of Lipofectamine 2000 (Invitrogen) and injected into the lumen as described by us previously (20).
C. difficile TxA Enteritis Model and Assessment of Inflammation.
Seven days after dsRNA injections, purified TxA (5 µg) or vehicle (saline) was injected into a 4-cm ileal loop comprising the dsRNA injection site. Rats were euthanized 4 h later. Loops were weighed and intestinal secretion was recorded (mg loop weight/cm loop length). Loop fluid was frozen for assay of neutrophil infiltration (myeloperoxidase, mU/mL). Tissue was frozen for RNA and protein analyses, or fixed in 10% (vol/vol) formalin and paraffin-embedded for histologic analysis or 4% (wt/vol) paraformaldehyde for immunoreactivity analysis. Naïve rats and rats injected with non-CLR dsRNA (20μg in 200μL vol) had similar responses to TxA, with identical intestinal secretion (Fig. 2). Rats injected with non-CLR dsRNA were used as controls for all other end points examined.
Assessment of Histopathologic Changes.
Paraffin-embedded sections (6-µm) were stained with hematoxylin and eosin. A histologist scored coded tissue sections for epithelial damage, congestion and edema, and neutrophil infiltration (0–5) (17).
Detection of Mucin and Mast Cells.
Paraffin-embedded sections were stained with Alcian Blue pH 2.5 and Nuclear Fast Red (American MasterTech Scientific) to detect changes in goblet cell mucin. To detect connective tissue mast cells, sections were stained with 0.1% toluidine blue (pH 2.3) (Sigma) for 3 min.
Immunofluorescence Staining and Confocal Microscopy.
A cross-section of loop tissue from rats treated with TxA or vehicle was fixed in 4% paraformaldehyde and stained for CLR using a rabbit antibody recognizing rat CLR (RK11) and a mouse antibody recognizing CGRP (4901), as previously described (7); see SI Materials and Methods for details. Optical sections were acquired on a Zeiss LSM510 Meta microscope.
RNA Analysis.
A portion of the ileal loop was homogenized, and RNA was isolated and analyzed as previously described (20); details of PCR primers are in SI Materials and Methods.
Western Blot Analysis.
Total ileal protein (40 µg of total protein) was separated by SDS/PAGE, transferred to PVDF membranes (Immobilon-FL; Millipore), and incubated with antibodies to pERK1/2, ERK2, NF-κB, and IκBα and analyzed with the Odyssey Infrared Imaging System (LI-COR). See SI Materials and Methods for details.
Statistical Analysis.
Data are shown as mean ± SEM. Differences among groups were analyzed by analysis of variance and Student–Newman–Keuls test or Bonferroni t tests, as appropriate. Histologic assessment was analyzed by Mann–Whitney rank-sum test. A P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
This project was supported by DK52387 (to E.F.G.), CCFA1730 (to E.F.G.), DK080787 (to A.B.), DK080787-S2 (to A.B.), and DK47343 (to C.P.). M.S.C. was supported by National Institutes of Health Training Grant T32 DK07573.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1219733110/-/DCSupplemental.
References
- 1.Loo VG, et al. Host and pathogen factors for Clostridium difficile infection and colonization. N Engl J Med. 2011;365(18):1693–1703. doi: 10.1056/NEJMoa1012413. [DOI] [PubMed] [Google Scholar]
- 2.Pothoulakis C, Lamont JT. Microbes and microbial toxins: Paradigms for microbial-mucosal interactions II. The integrated response of the intestine to Clostridium difficile toxins. Am J Physiol Gastrointest Liver Physiol. 2001;280(2):G178–G183. doi: 10.1152/ajpgi.2001.280.2.G178. [DOI] [PubMed] [Google Scholar]
- 3.Pothoulakis C, et al. Substance P receptor expression in intestinal epithelium in Clostridium difficile toxin A enteritis in rats. Am J Physiol. 1998;275(1 Pt 1):G68–G75. doi: 10.1152/ajpgi.1998.275.1.G68. [DOI] [PubMed] [Google Scholar]
- 4.Kirkwood KS, et al. Deletion of neutral endopeptidase exacerbates intestinal inflammation induced by Clostridium difficile toxin A. Am J Physiol Gastrointest Liver Physiol. 2001;281(2):G544–G551. doi: 10.1152/ajpgi.2001.281.2.G544. [DOI] [PubMed] [Google Scholar]
- 5.Brain SD, Grant AD. Vascular actions of calcitonin gene-related peptide and adrenomedullin. Physiol Rev. 2004;84(3):903–934. doi: 10.1152/physrev.00037.2003. [DOI] [PubMed] [Google Scholar]
- 6.McLatchie LM, et al. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393(6683):333–339. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- 7.Cottrell GS, et al. Localization of calcitonin receptor-like receptor and receptor activity modifying protein 1 in enteric neurons, dorsal root ganglia, and the spinal cord of the rat. J Comp Neurol. 2005;490(3):239–255. doi: 10.1002/cne.20669. [DOI] [PubMed] [Google Scholar]
- 8.Marvizón JC, et al. Calcitonin receptor-like receptor and receptor activity modifying protein 1 in the rat dorsal horn: Localization in glutamatergic presynaptic terminals containing opioids and adrenergic alpha2C receptors. Neuroscience. 2007;148(1):250–265. doi: 10.1016/j.neuroscience.2007.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lennerz JK, et al. Calcitonin receptor-like receptor (CLR), receptor activity-modifying protein 1 (RAMP1), and calcitonin gene-related peptide (CGRP) immunoreactivity in the rat trigeminovascular system: Differences between peripheral and central CGRP receptor distribution. J Comp Neurol. 2008;507(3):1277–1299. doi: 10.1002/cne.21607. [DOI] [PubMed] [Google Scholar]
- 10.Wong HC, et al. Monoclonal antibody to rat alpha-CGRP: Production, characterization, and in vivo immunoneutralization activity. Hybridoma. 1993;12(1):93–106. doi: 10.1089/hyb.1993.12.93. [DOI] [PubMed] [Google Scholar]
- 11.Holzer P, Holzer-Petsche U. Tachykinins in the gut. Part I. Expression, release and motor function. Pharmacol Ther. 1997;73(3):173–217. doi: 10.1016/s0163-7258(96)00195-7. [DOI] [PubMed] [Google Scholar]
- 12.Linscheid P, et al. Expression and secretion of procalcitonin and calcitonin gene-related peptide by adherent monocytes and by macrophage-activated adipocytes. Crit Care Med. 2004;32(8):1715–1721. doi: 10.1097/01.ccm.0000134404.63292.71. [DOI] [PubMed] [Google Scholar]
- 13.Mikami N, et al. Calcitonin gene-related peptide is an important regulator of cutaneous immunity: Effect on dendritic cell and T cell functions. J Immunol. 2011;186(12):6886–6893. doi: 10.4049/jimmunol.1100028. [DOI] [PubMed] [Google Scholar]
- 14.Kay AB. Calcitonin gene-related peptide- and vascular endothelial growth factor-positive inflammatory cells in late-phase allergic skin reactions in atopic subjects. J Allergy Clin Immunol. 2011;127(1):232–237. doi: 10.1016/j.jaci.2010.10.028. [DOI] [PubMed] [Google Scholar]
- 15.Weinstock JV, et al. Substance P regulates Th1-type colitis in IL-10 knockout mice. J Immunol. 2003;171(7):3762–3767. doi: 10.4049/jimmunol.171.7.3762. [DOI] [PubMed] [Google Scholar]
- 16.Keates AC, et al. CGRP upregulation in dorsal root ganglia and ileal mucosa during Clostridium difficile toxin A-induced enteritis. Am J Physiol. 1998;274(1 Pt 1):G196–G202. doi: 10.1152/ajpgi.1998.274.1.G196. [DOI] [PubMed] [Google Scholar]
- 17.Cottrell GS, et al. Protease-activated receptor 2, dipeptidyl peptidase I, and proteases mediate Clostridium difficile toxin A enteritis. Gastroenterology. 2007;132(7):2422–2437. doi: 10.1053/j.gastro.2007.03.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moynagh PN. The NF-kappaB pathway. J Cell Sci. 2005;118(Pt 20):4589–4592. doi: 10.1242/jcs.02579. [DOI] [PubMed] [Google Scholar]
- 19.Warny M, et al. p38 MAP kinase activation by Clostridium difficile toxin A mediates monocyte necrosis, IL-8 production, and enteritis. J Clin Invest. 2000;105(8):1147–1156. doi: 10.1172/JCI7545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clifton MS, et al. Role of calcitonin receptor-like receptor in colonic motility and inflammation. Am J Physiol Gastrointest Liver Physiol. 2007;293(1):G36–G44. doi: 10.1152/ajpgi.00464.2006. [DOI] [PubMed] [Google Scholar]
- 21.Amar S, Zhou Q, Shaik-Dasthagirisaheb Y, Leeman S. Diet-induced obesity in mice causes changes in immune responses and bone loss manifested by bacterial challenge. Proc Natl Acad Sci USA. 2007;104(51):20466–20471. doi: 10.1073/pnas.0710335105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bushell KN, et al. LITAF mediation of increased TNF-α secretion from inflamed colonic lamina propria macrophages. PLoS One. 2011;6(9):e25849. doi: 10.1371/journal.pone.0025849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moreno MJ, Abounader R, Hébert E, Doods H, Hamel E. Efficacy of the non-peptide CGRP receptor antagonist BIBN4096BS in blocking CGRP-induced dilations in human and bovine cerebral arteries: Potential implications in acute migraine treatment. Neuropharmacology. 2002;42(4):568–576. doi: 10.1016/s0028-3908(02)00008-4. [DOI] [PubMed] [Google Scholar]
- 24.Granholm S, Henning P, Lerner UH. Comparisons between the effects of calcitonin receptor-stimulating peptide and intermedin and other peptides in the calcitonin family on bone resorption and osteoclastogenesis. J Cell Biochem. 2011;112(11):3300–3312. doi: 10.1002/jcb.23256. [DOI] [PubMed] [Google Scholar]
- 25.Toda M, et al. Neuronal system-dependent facilitation of tumor angiogenesis and tumor growth by calcitonin gene-related peptide. Proc Natl Acad Sci USA. 2008;105(36):13550–13555. doi: 10.1073/pnas.0800767105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.la Fleur SE, Wick EC, Idumalla PS, Grady EF, Bhargava A. Role of peripheral corticotropin-releasing factor and urocortin II in intestinal inflammation and motility in terminal ileum. Proc Natl Acad Sci USA. 2005;102(21):7647–7652. doi: 10.1073/pnas.0408531102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Forster AJ, et al. The effect of hospital-acquired infection with Clostridium difficile on length of stay in hospital. CMAJ. 2012;184(1):37–42. doi: 10.1503/cmaj.110543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salisbury E, et al. Sensory nerve induced inflammation contributes to heterotopic ossification. J Cell Biochem. 2011;112(10):2748–2758. doi: 10.1002/jcb.23225. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.