Abstract
The coagulation protease activated protein C (aPC) confers cytoprotective effects in various in vitro and in vivo disease models, including diabetic nephropathy. The nephroprotective effect may be related to antioxidant effects of aPC. However, the mechanism through which aPC may convey these antioxidant effects and the functional relevance of these properties remain unknown. Here, we show that endogenous and exogenous aPC prevents glomerular accumulation of oxidative stress markers and of the redox-regulating protein p66Shc in experimental diabetic nephropathy. These effects were predominately observed in podocytes. In vitro, aPC inhibited glucose-induced expression of p66Shc mRNA and protein in podocytes (via PAR-1 and PAR-3) and various endothelial cell lines, but not in glomerular endothelial cells. Treatment with aPC reversed glucose-induced hypomethylation and hyperacetylation of the p66Shc promoter in podocytes. The hyperacetylating agent sodium butyrate abolished the suppressive effect of aPC on p66Shc expression both in vitro and in vivo. Moreover, sodium butyrate abolished the beneficial effects of aPC in experimental diabetic nephropathy. Inhibition of p66Shc expression and mitochondrial translocation by aPC normalized mitochondrial ROS production and the mitochondrial membrane potential in glucose-treated podocytes. Genetic ablation of p66Shc compensated for the loss of protein C activation in vivo, normalizing markers of diabetic nephropathy and oxidative stress. These studies identify a unique mechanism underlying the cytoprotective effect of aPC. Activated PC epigenetically controls expression of the redox-regulating protein p66Shc, thus linking the extracellular protease aPC to mitochondrial function in diabetic nephropathy.
It is well established that coagulation proteases have functions extending well beyond the regulation of intravascular hemostasis (1). In addition to their role in hemostasis, some coagulation proteases, such as thrombin and activated protein C (aPC), have important functions in regulating cellular homeostasis. In this context, the serine protease aPC, which has emerged as a panacea for various diseases in animal models, has been intensively studied. The zymogen protein C (PC, PROC) is efficiently activated to aPC by thrombin if the latter is bound to the transmembrane and cell-surface associated protein thrombomodulin (TM, Thbd). aPC mediates its anticoagulant effects by inactivating the coagulation cofactors Va and VIIIa. In addition, aPC confers a number of cytoprotective effects, which are largely independent of its anticoagulant function and depend on the regulation of intracellular signaling pathways through receptor-dependent mechanisms (1).
The cytoprotective effects of aPC have been linked to altered gene expression (2) and regulation of transcription factors (3, 4). In cytokine-stimulated endothelial cells, aPC inhibits the binding activity of the redox-sensitive transcription factor, NF-κB (3). In a transgenic animal model of amyotrophic lateral sclerosis, aPC suppresses the expression of mutant SOD1 through SP-1, also a redox-sensitive transcription factor (4). These studies suggest that aPC regulates gene expression, at least in part, through redox-sensitive transcription factors, raising the question of the mechanism by which aPC modulates the activity of these redox-sensitive transcription factors.
Direct antioxidant effects of aPC have been described in murine macrophage-like cells (RAW264.7), in which aPC suppressed lipopolysaccharide-induced reactive oxygen species (ROS) generation and NF-κB binding activity (5). The potential relevance of the antioxidant properties of aPC is further supported by its potent nephroprotective effects in a mouse model of diabetic nephropathy (6, 7). Mitochondrial dysfunction resulting in the generation of ROS is considered to be a unifying mechanism underlying diabetic vascular complications, including diabetic nephropathy (8, 9). aPC efficiently suppresses the glucose-induced release of cytochrome c and Smac/Diablo from mitochondria and mitochondrial apoptosis in vitro and reduces peroxynitrite formation (ONOO−, resulting from a reaction of NO− with O2−) in diabetic kidneys in vivo (7). These results raised the question of whether the protective effect of aPC in diabetic nephropathy is mechanistically linked with mitochondrial ROS formation. However, the intracellular targets that mediate the antioxidant effects of aPC and the causal relationship of aPC-mediated ROS inhibition and cytoprotection remain to be elucidated.
In this context, the redox enzyme p66Shc constitutes a potential target of aPC. p66Shc is one of three isoforms derived by alternative splicing from the Shc locus, resulting in proteins with relative molecular masses of 46, 52, and 66 kDa (10). These proteins share a Src-homology 2 domain, a collagen-homology region, and a phosphotyrosine-binding domain (10). p66Shc differs from the smaller isoforms by the presence of an additional N-terminal region, which is required for its redox activity (10). The distinct nature of this structural feature is reflected by the diverse functions of the Shc isoforms; the p46Shc and p52Shc isoforms have been linked to the transmission of mitogenic signals from tyrosine kinases to RAS proteins, whereas the larger p66Shc isoform is primarily associated with mitochondrial ROS generation and apoptosis (11). p66Shc is partially localized within the mitochondrial fraction, where it reduces equivalents of the mitochondrial electron transfer chain through the oxidation of cytochrome c (12, 13). Cytochrome c release is reduced in the absence of p66Shc, and loss of p66Shc protects against glucose-induced cellular dysfunction (12). Separate promoter regions control expression of these structurally and functionally distinct Shc splicing variants. Interestingly, the p66Shc promoter is subject to epigenetic modifications (14), and glucose-induced hypomethylation and hyperacetylation of the p66Shc promoter results in high levels of p66Shc expression and ROS generation in endothelial cells (15). In agreement with these data, p66Shc-deficient mice are protected against diabetic nephropathy (16, 17). However, the mechanisms that control p66Shc expression in diabetic nephropathy remain unknown. Considering the cytoprotective effects of aPC and of p66Shc deficiency in diabetic nephropathy and the involvement of aPC and p66Shc in mitochondrial dysfunction, we evaluated whether aPC modulates mitochondrial ROS generation and diabetic nephropathy through a p66Shc-dependent mechanism.
Results
Activated Protein C Reduces ROS Formation in Podocytes.
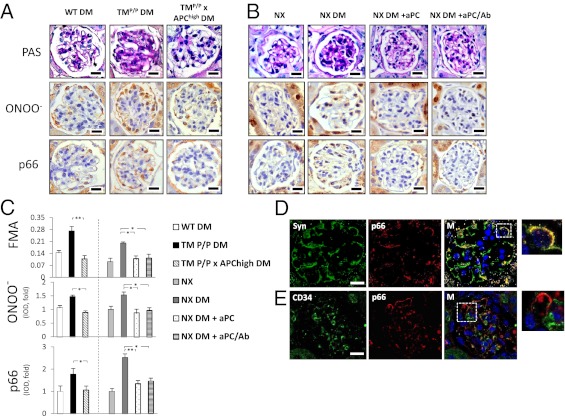
Increased levels of peroxynitrite, which reflect enhanced ROS formation, have been detected by immunoblotting renal cortex extracts of diabetic mice with genetically impaired thrombomodulin (TM)-dependent protein C (PC) activation (TMP/P mice) (7). To determine whether peroxynitrite formation occurs in glomeruli, the presence of nitrotyrosine, a marker of peroxynitrite formation, was immunohistochemically analyzed. In parallel to increased fractional mesangial area (FMA), nitrotyrosine staining intensity was significantly enhanced in glomeruli of diabetic TMP/P mice (Fig. 1 A and C). Restitution of aPC in diabetic TMP/P mice by crossing them with transgenic mice with increased plasma levels of aPC (TMP/P × APChigh mice) was sufficient to prevent the increase in FMA and glomerular nitrotyrosine formation (Fig. 1 A and C).
Fig. 1.
aPC prevents glucose-induced nitrotyrosine formation and p66Shc expression in podocytes. (A–C) Genetically compensating for aPC deficiency (TMP/P x APChigh DM mice) is sufficient to reverse enhanced extracellular matrix deposition (FMA), glomerular nitrotyrosine (ONOO−) formation, and glomerular p66Shc expression in diabetic TMP/P (TMP/P DM) mice (A and C). In diabetic uninephrectomized wild-type mice (NX DM), therapeutic application of both native aPC (NX DM+aPC) or aPC lacking its anticoagulant properties (NX DM+aPC/Ab) reduced FMA, glomerular nitrotyrosine formation, and glomerular p66Shc expression to the levels observed in nondiabetic uninephrectomized wild-type mice (NX; B and C). Representative images of PAS, nitrotyrosine, and p66shc stained glomeruli (A and B) and bar graphs reflecting the overall results [C; integrated optical density (IOD) shown as the mean value ± SEM] are shown. *P < 0.05, **P < 0.005. p66Shc and nitrotyrosine were detected with HRP-DAB (brown) and hematoxylin counterstain (blue). (D and E) Representative confocal immunofluorescence images showing predominant colocalization (yellow) of p66Shc (red) with the podocyte marker synaptopodin (green; D), but not with the endothelial cell marker CD34 (green; E). Hoechst 33258 was used for nuclear counter staining (blue). (Scale bars: 20 µm.)
To evaluate the therapeutic applicability of aPC, we used a model of diabetic nephropathy in unilaterally nephrectomized C57BL/6 mice. As shown in recent reports, i.p. injections of aPC ameliorated diabetic nephropathy without improving blood glucose levels (Fig. S1 A and B) (6). In addition to reducing FMA, treatment with exogenous aPC decreased glomerular nitrotyrosine formation (Fig. 1 B and C). Administration of aPC preincubated with the antibody HAPC1573, which specifically blocks the anticoagulant properties of aPC (18), had the same effect (Fig. 1 B and C), demonstrating that the effect of aPC is independent of its anticoagulant properties.
Of note, within renal glomeruli, peripheral cells predominantly stained for nitrotyrosine (Fig. 1 A and B), suggesting that podocytes are prone to nitrotyrosine formation. Nitrotyrosine formation in podocytes was confirmed by immunohistochemical colocalization studies using the podocyte marker synaptopodin (Fig. S1D). Similar results were obtained with immunohistochemical analysis with another marker of oxidative stress, 8-hydroxydeoxyguanosine (8-OH-dG; Fig. S1 E–G). Hydrogen peroxide-induced nitrotyrosine accumulation in podocytes was prevented in vitro by aPC, but not by aPC with the active site blocked (DEGR-aPC), demonstrating that the proteolytic activity of aPC is required for its antioxidant effect (Fig. S1 H–J). These data imply that insufficient activation of PC promotes glomerular nitrotyrosine formation in podocytes and that exogenous aPC inhibits glomerular nitrotyrosine accumulation in a mouse model of diabetic nephropathy.
Activated PC Suppresses Glomerular p66Shc Expression in Diabetic Mice.
p66Shc is an important modulator of mitochondrial ROS generation and has an established role in diabetic nephropathy (16). To evaluate the potential interaction of the extracellular TM-PC system with p66Shc in regulating diabetic glomerulopathy, p66Shc expression was analyzed. Expression of p66Shc was increased in diabetic TMP/P mice compared with diabetic wild-type mice (Fig. 1 A and C). Reconstitution of aPC in diabetic TMP/P mice (TMP/P × APChigh DM) reduced the expression levels of p66Shc (Fig. 1 A and C). In uninephrectomized diabetic mice, exogenous application of either aPC (NX DM+aPC) or anticoagulant-blocked aPC (NX DM+aPC/Ab) reduced p66Shc expression equally in the renal glomeruli of diabetic mice (Fig. 1 B and C). Like nitrotyrosine, expression of p66Shc was localized predominantly to peripheral cells within the renal glomeruli (Fig. 1 A and B), suggesting that p66Shc expression is primarily induced in podocytes.
To examine the expression of p66Shc in podocytes and glomerular endothelial cells of diabetic TMP/P mice, confocal double immunofluorescent analyses were performed. Indeed, p66Shc was localized predominately to podocytes and not to CD34-positive endothelial cells (Fig. 1 D and E). Taken together, these results demonstrate that insufficient PC activation enhances p66Shc expression in podocytes and that restoration of aPC levels in diabetic TMP/P mice or application of exogenous aPC in diabetic wild-type mice prevents the induction of p66Shc.
aPC Inhibits Glucose-Induced p66Shc Expression in Podocytes via PAR-1 and PAR-3 in Vitro.
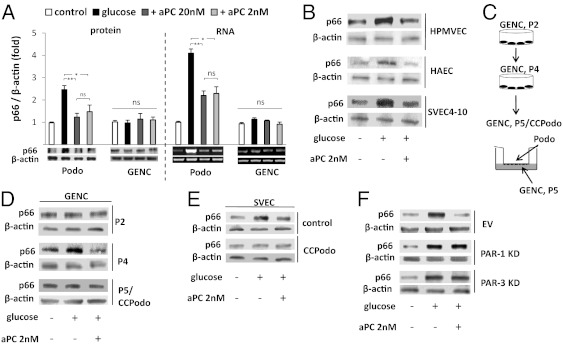
Next, the effect of glucose and aPC on p66Shc expression in glomerular cells was investigated in vitro. Exposure of podocytes to glucose (25 mM), but not mannitol (25 mM), induced p66Shc expression in podocytes at both the protein and the mRNA levels (Fig. 2A and Fig. S2A). Treatment with aPC normalized p66Shc expression in glucose-stressed podocytes (Fig. 2A).
Fig. 2.
aPC prevents glucose-dependent p66Shc induction in podocytes in vitro. (A) Glucose (25 mM, 24 h) induces p66Shc protein and mRNA expression in mouse podocytes (Podo), but not in mouse glomerular endothelial cells (GENC). aPC prevents the glucose-mediated p66Shc induction in podocytes. Representative images of immunoblots (IB) and RT-PCR with bar graphs (>three independent repeat experiments; mean value ± SEM. *P < 0.05, **P < 0.005; ns, not significant). (B) Glucose-induced p66Shc expression in human pulmonary microvascular endothelial cells (HPMVEC), human aortic endothelial cells (HAEC), and the murine endothelial cell line SVEC4-10 is prevented by aPC. (C and D) Glucose induces p66Shc in late (P4) but not early (P2) passage GENCs. Coculture of P5 GENCs with murine podocytes (24 h, P5/CCPodo) renders GENCs once again unresponsive to glucose-induced expression of p66Shc. Scheme illustrating the experimental approach (C) and representative immunoblots (D). (E) Coculture of SVEC4-10 cells with mouse podocytes (24 h) prevents glucose-dependent p66Shc induction. (F) aPC-mediated inhibition of glucose-induced p66Shc expression requires PAR-1 and PAR-3. Representative immunoblots of p66Shc in control cells transfected with empty vector (EV) and in PAR-1 and PAR-3 knockdown (KD) podocytes.
However, glucose failed to induce p66Shc expression in primary mouse glomerular endothelial cells (GENCs), whereas, in agreement with previous work (15), glucose induced p66Shc expression in various non-GENCs (Fig. 2 A and B). Thus, GENCs and non-GENCs differ with respect to glucose-dependent induction of p66Shc. The lack of p66Shc induction by glucose in GENCs was apparent at early passages (P2), but not at later passages (P4; Fig. 2D). Coculture of passage 5 GENCs with podocytes rendered the endothelial cells once again unresponsive to glucose-induced p66Shc expression, indicating that the failure of glucose to induce p66Shc expression in GENCs is podocyte dependent. To determine whether podocyte-dependent modulation of the p66Shc response is specific for GENCs, we next cocultured SVEC4-10 cells with podocytes. SVEC4-10 cells are lymph node-derived endothelial cells and, as shown above, express p66Shc in response to glucose. Coculture of SVEC4-10 cells with podocytes for 24 h abolished the glucose-mediated induction of p66Shc expression, indicating that podocytes render SVEC4-10 cells unresponsive to glucose in regard to p66Shc expression. Thus, with regard to p66Shc expression, SVEC4-10 cells adopt a GENC-like phenotype (Fig. 2E). These in vitro data are in agreement with the above-described induction of p66Shc in podocytes, but not glomerular endothelial cells, in vivo (Fig. 1 D and E).
We next explored whether the inhibitory effect of aPC on glucose-induced p66Shc expression in podocytes depends on PAR-1 and PAR-3, the receptors that mediate the cytoprotective effect of aPC, in podocytes (18). Indeed, specific activation of PAR-1 or PAR-3 was sufficient to prevent glucose-induced p66Shc expression in podocytes, whereas activation of PAR-2 failed to inhibit p66Shc (Fig. S2B). After knockdown of either PAR-1 or PAR-3, aPC failed to normalize glucose-induced p66Shc expression in podocytes (Fig. 2F and Fig. S2 C and D). Hence, PAR-1 and PAR-3 are required for aPC-dependent inhibition of glucose-induced p66Shc expression in podocytes.
p66Shc Expression in Podocytes Is Epigenetically Inhibited by aPC.
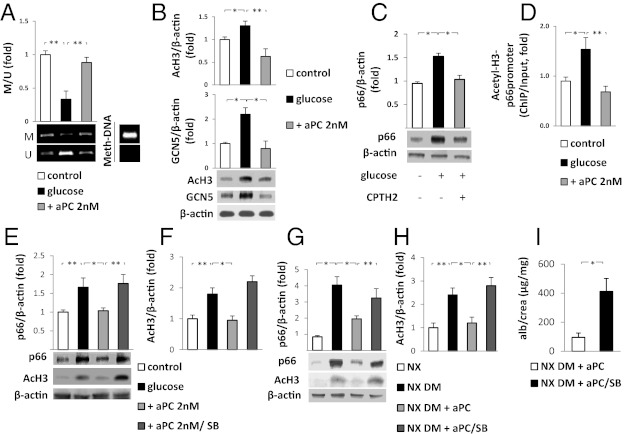
Changes in p66Shc expression in podocytes induced by glucose and aPC were apparent at both the protein and mRNA levels, indicating that p66Shc is regulated primarily at the transcriptional level. Because p66Shc expression is epigenetically controlled, the methylation and acetylation of the p66Shc promoter by aPC were investigated. Glucose-induced hypomethylation of CpG dinucleotides within the p66Shc promoter was prevented by aPC (Fig. 3A). Hypomethylation of the p66Shc promoter has been shown to be associated with histone 3 (H3) hyperacetylation (15). Indeed, glucose was also found to induce H3 acetylation, an effect that was prevented by aPC (Fig. 3B). Glucose-induced H3 hyperacetylation is mediated—at least in par—by the H3 acetyltransferase GCN5 (15). Consistently, glucose-dependent induction of GCN5 was observed in podocytes, and this induction was prevented by aPC (Fig. 3B). Inhibition of GCN5 using the GCN5-inhibitor CPTH2 prevented glucose-mediated p66Shc induction, demonstrating that the inhibition of GCN5 is sufficient to prevent glucose-stimulated p66Shc induction in podocytes (Fig. 3C). In addition, ChIP analyses demonstrated that glucose induces and aPC suppresses H3 acetylation within the p66Shc promoter (Fig. 3D). Finally, the hyperacetylating agent sodium butyrate, which increases H3 acetylation (19), abolished the effect of aPC in glucose-treated podocytes, increasing H3 acetylation and p66Shc expression in the presence of aPC (Fig. 3 E and F). These results establish a unique function of the serine protease aPC in the epigenetic control of p66Shc expression.
Fig. 3.
aPC prevents glucose-induced p66Shc hypomethylation and H3 acetylation. (A) Glucose (25 mM) reduces methylation of the p66Shc promoter in podocytes, which is reversed by addition of 2 nM aPC. Representative image of methylated (M) and unmethylated (U) p66Shc promoter DNA revealed by methylation-specific -PCR(MSP) and a bar graph showing the ratio of methylated to unmethylated p66Shc promoter DNA (M/U, fold change) are shown. Universal methylated mouse DNA (Meth-DNA) was used as a control. (B) Glucose (25 mM)-induced H3 acetylation (AcH3) and increased H3 acetyltransferase (GCN5) expression is prevented by the addition of aPC in podocytes. (C) Treatment of podocytes with the GCN5 inhibitor CPTH2 (50 µM) is sufficient to prevent glucose-induced p66Shc expression. (D) H3 acetylation within the p66Shc promoter is induced by glucose and prevented by aPC (2 nM). Acetylated H3 was immunoprecipitated with an AcH3 antibody, and the p66shc promoter was quantitated by qRT-PCR (ChIP data were normalized to input). (E and F) Sodium butyrate (SB) increases H3 acetylation even in the presence of aPC (+aPC 2nM/SB) and abolishes the suppressive effects of aPC on H3 acetylation and p66Shc expression in glucose-treated podocytes (25 mM glucose). (G and H) The suppressive effect of aPC on H3 acetylation and p66Shc expression in the renal cortex of uninephrectomized diabetic wild-type mice (NX DM+aPC) is abolished by concomitant treatment with SB (NX DM+aPC/SB). (I) SB abolishes the aPC-mediated reduction in albuminuria in uninephrectomized wild-type diabetic mice (NX DM+aPC/SB). Representative images of MSP (A) or immunoblots (B, C, E, and G) and bar graphs (mean value ± SEM) summarizing the results of at least three repeat experiments or five different mice are shown. *P < 0.05; **P < 0.005.
To gain insight into whether aPC may epigenetically constrain glucose-induced p66Shc expression in vivo, uninephrectomized diabetic wild-type mice were treated with aPC without or with sodium butyrate. Sodium butyrate abolished the protective effect of aPC in diabetic mice, increasing renal H3 acetylation, PAS-positive staining, nitrotyrosine accumulation, p66Shc expression, and albuminuria, without affecting blood glucose levels or albuminuria in nondiabetic control animals (Fig. 3 F–I and Fig. S3). Taken together, these in vitro and in vivo data demonstrate that aPC epigenetically inhibits glucose-induced expression of p66Shc, thus protecting against diabetic nephropathy.
aPC Maintains the Mitochondrial Membrane Potential and Inhibits Glucose-Induced Mitochondrial p66Shc Translocation and ROS Generation.
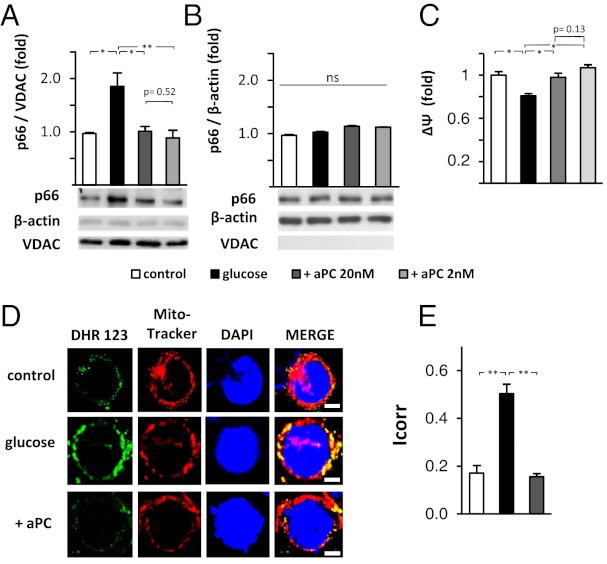
Regulation of ROS, mitochondrial dysfunction, and mitochondrial membrane potential by p66Shc depends on the expression and mitochondrial translocation of p66Shc (13). Hence, p66Shc levels were measured in cytosolic and mitochondrial fractions of podocytes. In glucose-stressed (25 mM, 24 h) podocytes, translocation of p66Shc into the mitochondria was evident (Fig. 4A). The cytosolic p66Shc fraction remained unchanged in glucose-treated podocytes (Fig. 4B). This result reflects the concomitant increase of both total p66Shc expression (as shown in Fig. 2A) and p66Shc translocation in glucose-stressed podocytes (Fig. 4 A and B). Treatment of glucose-stressed podocytes with aPC completely prevented the mitochondrial increase of p66Shc in glucose-stressed podocytes (Fig. 4A).
Fig. 4.
aPC prevents glucose-induced mitochondrial translocation of p66Shc, maintains mitochondrial membrane potential, and reduces mitochondrial ROS-generation in podocytes. (A and B) Glucose-induced (25 mM, 24 h) translocation of p66Shc into mitochondria is efficiently prevented by aPC. Representative immunoblots of p66Shc, VDAC (mitochondrial marker), and β-actin (cytosolic marker) in mitochondrial (A) or cytosolic (B) cellular subfractions. (C) Glucose reduces the MMP (Mito-Probe JC-1) in podocytes. aPC treatment of glucose-exposed podocytes maintains the MMP. (D and E) Representative fluorescence microscopy images showing single mouse podocytes (D). Podocytes were left untreated (5 mM glucose) or stimulated with glucose (25 mM, 24 h) without or with aPC (20 nM). ROS formation was monitored by using dihydrorhodamine (DHR, green) and localized to mitochondria by using Mitotracker CMX (red). Hoechst 33258 was used for nuclear counter staining (blue). Bar graph (E) summarizing the results (mean value ± SEM) of four independent repeat experiments using automated digital colocalization analyses, yielding the Icorr index. p66, p66Shc; *P < 0.05 and **P < 0.005; ns, not significant. (Scale bars: 5 µm.)
Glucose-induced ROS generation is associated with mitochondrial dysfunction and impairment of the mitochondrial membrane potential (MMP). Indeed, exposure of podocytes to 25 mM glucose reduced the MMP (Fig. 4C). This glucose-dependent impairment of the MMP was efficiently prevented by treatment with aPC (Fig. 4C).
In agreement with the changes observed regarding mitochondrial translocation of p66Shc and impairment of the MMP, glucose induced ROS production in podocytes, which localized predominately to the mitochondria (Fig. 4D). Treatment of glucose-stressed podocytes with aPC prevented mitochondrial ROS induction (Fig. 4 D and E). Thus, glucose induces mitochondrial translocation of p66Shc in association with a decline in the MMP and increased mitochondrial ROS formation in podocytes. These glucose-dependent effects in podocytes are prevented by aPC.
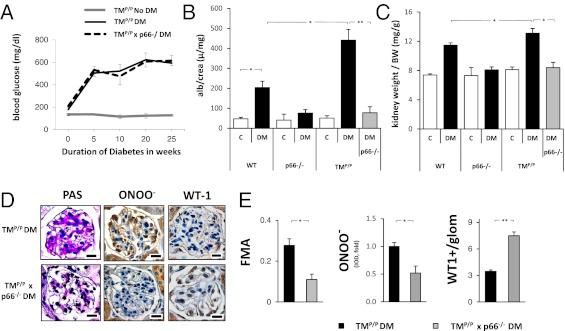
p66Shc Deficiency Corrects for Impaired PC Activation in Experimental Diabetic Nephropathy.
To evaluate the pathophysiological relevance of TM-dependent p66Shc regulation in vivo, TMP/P mice were crossed with p66Shc deficient (p66Shc−/−) mice, thus genetically correcting for induced p66Shc expression in diabetic TMP/P mice. Persistent hyperglycemia was then induced in wild-type control, TMP/P, p66Shc−/−, and double mutant TMP/P × p66Shc−/− mice, and the mice were followed for 26 wk. Blood glucose levels did not differ between diabetic mice (Fig. 5A and Fig. S4A). In wild-type mice, p66Shc deficiency protected against diabetic nephropathy, as has been shown (ref. 16; Fig. 5B). Albuminuria and normalized kidney weight, which were significantly increased in diabetic TMP/P mice (Fig. 5 B and C), remained normal in diabetic TMP/P × p66Shc−/− mice (Fig. 5 B and C). Of note, albuminuria and normalized kidney weight in diabetic TMP/P × p66Shc−/− mice did not differ from the results obtained in nondiabetic wild-type control mice (Fig. 5 B and C).
Fig. 5.
Genetic p66Shc deficiency compensates for the loss of TM-dependent PC activation in experimental diabetic nephropathy. (A) No difference in the blood glucose levels was apparent between diabetic TMP/P and diabetic TMP/P x p66Shc−/− mice. (B and C) p66Shc deficiency normalizes albuminuria (B) and kidney weight (C) in mice with genetically impaired PC activation (TMP/P mice). (D and E) p66Shc deficiency in diabetic TMP/P mice reduces histological indices of diabetic nephropathy. PAS staining, fractional mesangial area (FMA; E), and immunohistochemical analyses of nitrotyrosine and the podocyte protein WT-1 are shown. Representative images (D) and bar graphs summarizing the IODs (E). Data are presented as the mean ± SEM; at least six mice per group (A–C) or ≥30 glomeruli per genotype and mouse (D and E) were analyzed. *P < 0.05, **P < 0.005. (Scale bars: 20 µm.)
Histological evaluation of glomeruli also revealed improved pathological indices. Thus, the FMA and nitrotyrosine staining intensity were markedly reduced in glomeruli of diabetic TMP/P × p66Shc−/− mice compared with diabetic TMP/P mice (Fig. 5 D and E). In line with these results, p66Shc deficiency also reduced the staining intensity of the oxidative stress marker 8-OH-dG in the glomeruli of diabetic TMP/P mice (Fig. S4B). These changes were associated with a higher number of WT-1–positive glomerular cells in diabetic TMP/P × p66Shc−/− mice (Fig. 5 D and E), indicating amelioration of podocyte injury by p66Sch deficiency in diabetic TMP/P mice. Taken together, these findings demonstrate that genetic inactivation of p66Shc is sufficient to compensate for the loss of TM-dependent PC activation in experimental diabetic nephropathy.
Discussion
The current report identifies a unique mechanism underlying the cytoprotective effects of aPC. We identified p66Shc as an intracellular target of aPC in both an experimental model of diabetic nephropathy and glucose-stressed podocytes. aPC epigenetically suppresses glucose-induced p66Shc expression by enhancing methylation while diminishing acetylation of the p66Shc promoter. The suppression of glucose-induced p66Shc expression is paralleled by an aPC-mediated reduction of glucose-induced mitochondrial p66Shc translocation and ROS generation. In vivo, genetic p66Shc deficiency corrects for aPC deficiency. Conversely, increasing H3 acetylation with sodium butyrate abolishes the nephroprotective and p66Shc suppressive effects of exogenously administered aPC. This work establishes that the serine protease aPC ameliorates diabetic nephropathy by epigenetically constraining expression of the redox-enzyme p66Shc.
The aPC-mediated reversal of glucose-induced hypomethylation and hyperacetylation of the p66Shc promoter establishes that aPC modulates gene expression not only by regulating transcription factors, such as NF-κB or SP-1 (20), but in addition through epigenetic mechanisms. Epigenetic control of gene expression may explain the profound effects of intermittently administered exogenous aPC in various animal models despite its half-life of only ∼25 min (4, 6).
aPC suppressed expression of p66Shc at concentrations as low as 2 nM, which is ∼100-fold lower than the concentrations of aPC previously used by Yamaji et al. (5) to study the antioxidant effects of aPC (10 µg/mL; approximately 200 nM). Although Yamaji et al. suggested that aPC exerts a direct antioxidant effect, which was detectable at these high concentrations, we propose that aPC at much lower concentrations conveys an indirect antioxidant effect by epigenetically suppressing the expression of p66Shc. Whether the regulation of the redox-sensitive transcription factors NF-κB and SP-1 by aPC depends on its effect on p66Shc remains to be evaluated.
A recent study by Paneni et al. (15) demonstrated that epigenetically sustained p66Shc expression mediates hyperglycemic memory in glucose-stressed endothelial cells and experimental models of diabetes mellitus. Considering the effect of aPC on the epigenetic regulation of p66Shc in podocytes and nonglomerular endothelial cells, we speculate that aPC signaling may modify hyperglycemic memory in diabetes mellitus. Of note, reduced endothelial expression of cytoprotective TM, an established observation of endothelial dysfunction in diabetes mellitus, may depend on p66Shc, because p66Shc has been shown to inhibit TM expression by suppressing Kruppel like factor-2 (21). These observations, together with the current findings, imply the existence of a self-regulatory feed-forward mechanism, which may be disrupted in diseases like diabetes mellitus. Glucose-induced hypomethylation and hyperacetylation of the p66Shc promoter, resulting in increased p66Shc expression (15), may disrupt this protective feed-forward system, inhibiting TM expression and initiating a self-propagating cycle of diabetic vascular complications. Fortunately, the observed effects of exogenously administered aPC indicate that this protective feed-forward pathway may be restored by therapeutic interventions. Considering potential side effects of aPC (e.g., hemorrhage), unique therapeutic approaches mimicking specifically the nephroprotective mechanism of aPC need to be identified.
The lack of glucose-mediated induction of p66Shc expression in GENCs appears at first to contradict previous reports demonstrating that glucose induces p66Shc expression in (nonglomerular) endothelial cells (15). However, this lack of glucose-induced p66Shc expression depends on the presence of podocytes, as prolonged culture of GENCs in the absence of podocytes enabled these cells to induce p66Shc expression in response to glucose. Moreover, similar to GENCs, nonglomerular cells cultured in the presence of podocytes lose the ability to induce p66Shc expression after glucose treatment, similar to GENCs (Fig. 2). These results illustrate that podocytes, and potentially other perivascular cells, can determine the phenotype and heterogeneity of endothelial cells (22).
Podocytes, like pericytes, cover the outer surface of the filtering capillaries. Podocyte injury is considered to convey a predominantly pathogenic role in glomerular disease and has been linked to ROS generation in various diseases, such as diabetic nephropathy and puromycinaminonucleoside-induced experimental glomerulopathy (17, 23, 24). Some work suggests that podocytes primarily depend on mitochondria for energy homeostasis (25), which may render podocytes particularly sensitive to oxidative damage. The inducible expression of p66Shc in podocytes, but not in glomerular endothelial cells, constitutes an additional mechanism predisposing podocytes to ROS-induced injury.
Hitherto, studies exploring vascular disease have focused on the effect of aPC or p66Shc in endothelial cells (26), but the effect of aPC on p66Shc in podocytes reported here implies that perivascular cells should be equally considered and evaluated. Along this line, a potential role for aPC outside the vascular compartment (in microglia and astrocytes) has been reported in a murine model of amyotrophic lateral sclerosis (4). Considering the role of p66Shc in both endothelial cells and podocytes, we suggest that mechanistic evaluations and therapeutic developments should not be limited to targeting endothelial cells, but should involve podocytes and, more generally, pericytes as well.
p66Shc has been linked not only to diabetic nephropathy, but also to various other conditions, such as other glomerulopathies, cardiomyopathy, ischemia/reperfusion injury, neurodegenerative diseases, and in vitro models of intestinal cell dysfunction (27–30). Likewise, aPC has evolved as a panacea, ameliorating a broad array of experimental diseases, including experimental cardiac or renal ischemia reperfusion injury, neurodegenerative diseases, inflammatory bowel disease, and diabetic glomerulopathy (1, 31). The functional role of both p66Shc and aPC in various partially overlapping disease models suggests that the mechanistic interaction described here between the extracellular, cytoprotective protease aPC, and the intracellular redox-regulator p66Shc may be of broader relevance.
Materials and Methods
For additional materials and methods, see SI Materials and Methods.
Diabetes Models.
Two streptozotocin (STZ)-dependent models of diabetic nephropathy were used in the current study. A long-term model (26 wk) following a previously published protocol (7) was used in genetically modified and control mice (see SI Materials and Methods for details). To evaluate the therapeutic applicability of exogenous aPC, a recently published model in which the mice underwent unilateral nephrectomy to aggravate the progression of hyperglycemia-induced nephropathy (6) was used. Mice were anesthetized with pentobarbital (1 mg/kg body weight, i.p.). A dorsolumbar incision (∼1 cm) was made, and the ureter, the renal artery, and renal vein were ligated and subsequently cut. The kidney was removed, and the incision was stitched. Two weeks after surgery, diabetes was induced by injections of STZ (i.p., 40 mg/kg body weight, freshly dissolved in 0.05 M sterile sodium citrate, pH 4.5) for 5 d consecutively. Control mice received 100 µL of PBS i.p. for 5 d consecutively. This model allowed the use of various therapeutic interventions. After 4 wk, STZ-treated mice received 1 mg/kg aPC i.p. (Xigris; Lilly) every other day for 4 wk. In a subgroup of mice, aPC was preincubated before injection with HAPC1573 antibody at a 1:1 ratio for 10 min under gentle agitation to block its anticoagulant activity (18). Sodium butyrate was supplemented in the drinking water at a concentration of 8 g/L, an oral dose that has been shown to increase H3 acetylation (19).
Statistical Analysis.
The data are summarized as the mean ± SEM. The Kolmogorov–Smirnov test was used to determine whether the data within are consistent with a Gaussian distribution. Statistical analyses were performed with the Student t test, ANOVA, or Mann–Whitney test, as appropriate. Post hoc comparisons of ANOVA were corrected with Tukey’s method. Prism 5 (GraphPad) software were used for statistical analyses. Statistical significance was accepted at values of P < 0.05.
Supplementary Material
Acknowledgments
This work was supported by Deutsche Forschungsgemeinschaft Grants IS 67/2-4 (to B.I.) and TH 1789/1-1 (to T.M.), and grants from the European Foundation for the Study of Diabetes (B.I.), the Deutsche Diabetes Stiftung (B.I.), the Stiftung für Pathobiochemie und Molekulare Diagnostik (B.I. and F.B.), and the Dietmar Hopp Stiftung (B.I. and P.P.N.). P.P.N. is supported by SFB938. C.T.E. is an investigator of the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. A.H.S. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1218667110/-/DCSupplemental.
References
- 1.Griffin JH, Zlokovic BV, Mosnier LO. Protein C anticoagulant and cytoprotective pathways. Int J Hematol. 2012;95(4):333–345. doi: 10.1007/s12185-012-1059-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riewald M, Petrovan RJ, Donner A, Mueller BM, Ruf W. Activation of endothelial cell protease activated receptor 1 by the protein C pathway. Science. 2002;296(5574):1880–1882. doi: 10.1126/science.1071699. [DOI] [PubMed] [Google Scholar]
- 3.Joyce DE, Gelbert L, Ciaccia A, DeHoff B, Grinnell BW. Gene expression profile of antithrombotic protein c defines new mechanisms modulating inflammation and apoptosis. J Biol Chem. 2001;276(14):11199–11203. doi: 10.1074/jbc.C100017200. [DOI] [PubMed] [Google Scholar]
- 4.Zhong Z, et al. Activated protein C therapy slows ALS-like disease in mice by transcriptionally inhibiting SOD1 in motor neurons and microglia cells. J Clin Invest. 2009;119(11):3437–3449. doi: 10.1172/JCI38476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamaji K, et al. Activated protein C, a natural anticoagulant protein, has antioxidant properties and inhibits lipid peroxidation and advanced glycation end products formation. Thromb Res. 2005;115(4):319–325. doi: 10.1016/j.thromres.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 6.Gil-Bernabe P, et al. Exogenous activated protein C inhibits the progression of diabetic nephropathy. J Thromb Haemost. 2012;10(3):337–346. doi: 10.1111/j.1538-7836.2012.04621.x. [DOI] [PubMed] [Google Scholar]
- 7.Isermann B, et al. Activated protein C protects against diabetic nephropathy by inhibiting endothelial and podocyte apoptosis. Nat Med. 2007;13(11):1349–1358. doi: 10.1038/nm1667. [DOI] [PubMed] [Google Scholar]
- 8.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 9.Forbes JM, Coughlan MT, Cooper ME. Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes. 2008;57(6):1446–1454. doi: 10.2337/db08-0057. [DOI] [PubMed] [Google Scholar]
- 10.Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012;24(5):981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trinei M, et al. P66Shc signals to age. Aging (Albany NY) 2009;1(6):503–510. doi: 10.18632/aging.100057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orsini F, et al. The life span determinant p66Shc localizes to mitochondria where it associates with mitochondrial heat shock protein 70 and regulates trans-membrane potential. J Biol Chem. 2004;279(24):25689–25695. doi: 10.1074/jbc.M401844200. [DOI] [PubMed] [Google Scholar]
- 13.Giorgio M, et al. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell. 2005;122(2):221–233. doi: 10.1016/j.cell.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 14.Ventura A, Luzi L, Pacini S, Baldari CT, Pelicci PG. The p66Shc longevity gene is silenced through epigenetic modifications of an alternative promoter. J Biol Chem. 2002;277(25):22370–22376. doi: 10.1074/jbc.M200280200. [DOI] [PubMed] [Google Scholar]
- 15.Paneni F, et al. Gene silencing of the mitochondrial adaptor p66(Shc) suppresses vascular hyperglycemic memory in diabetes. Circ Res. 2012;111(3):278–289. doi: 10.1161/CIRCRESAHA.112.266593. [DOI] [PubMed] [Google Scholar]
- 16.Menini S, et al. Deletion of p66Shc longevity gene protects against experimental diabetic glomerulopathy by preventing diabetes-induced oxidative stress. Diabetes. 2006;55(6):1642–1650. doi: 10.2337/db05-1477. [DOI] [PubMed] [Google Scholar]
- 17.Vashistha H, et al. 2012. Null mutations at the p66 and Bradykinin 2 receptor loci induce divergent phenotypes in the diabetic kidney. Am J Physiol Renal Physiol, 10.1152/ajprenal.00246.2012.
- 18.Madhusudhan T, et al. Cytoprotective signaling by activated protein C requires protease-activated receptor-3 in podocytes. Blood. 2012;119(3):874–883. doi: 10.1182/blood-2011-07-365973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Minamiyama M, et al. Sodium butyrate ameliorates phenotypic expression in a transgenic mouse model of spinal and bulbar muscular atrophy. Hum Mol Genet. 2004;13(11):1183–1192. doi: 10.1093/hmg/ddh131. [DOI] [PubMed] [Google Scholar]
- 20.White B, et al. Activated protein C inhibits lipopolysaccharide-induced nuclear translocation of nuclear factor kappaB (NF-kappaB) and tumour necrosis factor alpha (TNF-alpha) production in the THP-1 monocytic cell line. Br J Haematol. 2000;110(1):130–134. doi: 10.1046/j.1365-2141.2000.02128.x. [DOI] [PubMed] [Google Scholar]
- 21.Kumar A, et al. Transcriptional repression of Kruppel like factor-2 by the adaptor protein p66shc. FASEB J. 2009;23(12):4344–4352. doi: 10.1096/fj.09-138743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res. 2007;100(2):158–173. doi: 10.1161/01.RES.0000255691.76142.4a. [DOI] [PubMed] [Google Scholar]
- 23.Susztak K, Raff AC, Schiffer M, Böttinger EP. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes. 2006;55(1):225–233. [PubMed] [Google Scholar]
- 24.Husain M, et al. Inhibition of p66ShcA longevity gene rescues podocytes from HIV-1-induced oxidative stress and apoptosis. J Biol Chem. 2009;284(24):16648–16658. doi: 10.1074/jbc.M109.008482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abe Y, et al. Bioenergetic characterization of mouse podocytes. Am J Physiol Cell Physiol. 2010;299(2):C464–C476. doi: 10.1152/ajpcell.00563.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Camici GG, et al. Genetic deletion of p66(Shc) adaptor protein prevents hyperglycemia-induced endothelial dysfunction and oxidative stress. Proc Natl Acad Sci USA. 2007;104(12):5217–5222. doi: 10.1073/pnas.0609656104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spescha RD, et al. Deletion of the ageing gene p66Shc reduces early stroke size following ischaemia/reperfusion brain injury. Eur Heart J. 2012 doi: 10.1093/eurheartj/ehs331. 10.1093/eurheartj/ehs331. [DOI] [PubMed] [Google Scholar]
- 28.Su KG, et al. Genetic inactivation of the p66 isoform of ShcA is neuroprotective in a murine model of multiple sclerosis. Eur J Neurosci. 2012;35(4):562–571. doi: 10.1111/j.1460-9568.2011.07972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Menini S, et al. Ablation of the gene encoding p66Shc protects mice against AGE-induced glomerulopathy by preventing oxidant-dependent tissue injury and further AGE accumulation. Diabetologia. 2007;50(9):1997–2007. doi: 10.1007/s00125-007-0728-7. [DOI] [PubMed] [Google Scholar]
- 30.Giovannini C, et al. Apoptosis induced by oxidized lipids is associated with up-regulation of p66Shc in intestinal Caco-2 cells: Protective effects of phenolic compounds. J Nutr Biochem. 2008;19(2):118–128. doi: 10.1016/j.jnutbio.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 31.Vetrano S, et al. Unexpected role of anticoagulant protein C in controlling epithelial barrier integrity and intestinal inflammation. Proc Natl Acad Sci USA. 2011;108(49):19830–19835. doi: 10.1073/pnas.1107140108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.