Abstract
Parkinson disease (PD) is a neurodegenerative disorder particularly characterized by the loss of dopaminergic neurons in the substantia nigra. Pesticide exposure has been associated with PD occurrence, and we previously reported that the fungicide benomyl interferes with several cellular processes potentially relevant to PD pathogenesis. Here we propose that benomyl, via its bioactivated thiocarbamate sulfoxide metabolite, inhibits aldehyde dehydrogenase (ALDH), leading to accumulation of the reactive dopamine metabolite 3,4-dihydroxyphenylacetaldehyde (DOPAL), preferential degeneration of dopaminergic neurons, and development of PD. This hypothesis is supported by multiple lines of evidence. (i) We previously showed in mice the metabolism of benomyl to S-methyl N-butylthiocarbamate sulfoxide, which inhibits ALDH at nanomolar levels. We report here that benomyl exposure in primary mesencephalic neurons (ii) inhibits ALDH and (iii) alters dopamine homeostasis. It induces selective dopaminergic neuronal damage (iv) in vitro in primary mesencephalic cultures and (v) in vivo in a zebrafish system. (vi) In vitro cell loss was attenuated by reducing DOPAL formation. (vii) In our epidemiology study, higher exposure to benomyl was associated with increased PD risk. This ALDH model for PD etiology may help explain the selective vulnerability of dopaminergic neurons in PD and provide a potential mechanism through which environmental toxicants contribute to PD pathogenesis.
Parkinson disease (PD) is second only to Alzheimer disease as a prevalent neurodegenerative disorder, affecting millions worldwide. Symptoms result from the progressive degeneration of neurons, most notably dopaminergic neurons in the substantia nigra pars compacta. More than half of these neurons are lost by the time symptoms manifest themselves (1). Despite the identification of several genetic variants associated with familial as well as idiopathic PD, only a small fraction of total risk can be accounted for genetically (2). Thus, environmental factors almost certainly play an important role in PD. Understanding the relevant mechanisms particularly behind the selective loss of dopaminergic neurons may provide important clues to explain PD pathogenesis so that therapies can be developed to slow or reverse disease progression.
Over the past few decades, epidemiologic studies have consistently reported associations between PD occurrence and rural living, well water consumption, farming occupations, and pesticide exposure (3–12). Pesticides include diverse chemotypes that differ greatly in structures and mechanisms through which they act on target pests or produce chronic low-level exposure effects more relevant to human disease. Fungicides are among the pesticide classes warranting further investigation as potential contributors to PD pathogenesis (13); therefore, benomyl was selected for the present study.
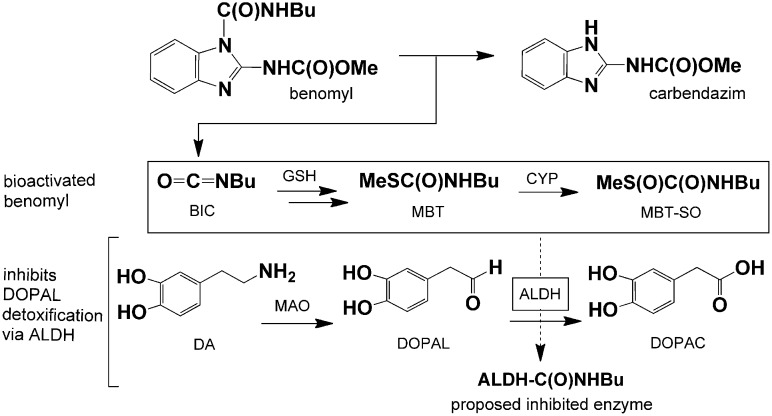
Benomyl was widely used for three decades until accumulating toxicological evidence in laboratory mammals of liver tumors, brain malformations, reproductive effects, and possible carcinogenesis led the US Environmental Protection Agency to cancel its registration in 2001 (14), although some countries continue to use this fungicide. Several relevant mechanisms suggest benomyl may also contribute to PD pathogenesis. The fungicidal action of benomyl is thought to result from microtubule assembly impairment (15), a mechanism that has been implicated in PD (16). Microtubule inhibitors disrupt the ubiquitin-proteasome system (UPS) (17) and cause selective dopaminergic cell damage and aggregation of α-synuclein, the predominant component of an intracytosolic Lewy body, which is the pathologic hallmark of PD (16). Furthermore, benomyl inhibits aldehyde dehydrogenase (ALDH) activity in liver and brain mitochondria (18, 19), although ALDH inhibition has not been measured directly in brains in vivo. The mitochondrial-associated ALDH2 is of particular interest because it metabolizes toxic aldehydes in brain tissue, including the dopamine (DA) metabolite 3,4-dihydroxyphenylacetaldehyde (DOPAL) (Fig. 1). DOPAL has been shown to be neurotoxic and has been suggested to contribute to PD pathogenesis (20–23), although a link with an environmental toxicant has not been established. The accumulation of DOPAL resulting from ALDH inhibition offers potential relevance to the preferential loss of dopaminergic neurons observed in PD.
Fig. 1.
ALDH inhibition as the proposed mechanism of benomyl-induced Parkinson disease. Benomyl is efficiently metabolized to potent ALDH inhibitors—BIC, MBT, and particularly MBT-SO—so exposure leads to the accumulation of the toxic dopamine metabolite DOPAL. This offers a possible explanation for the selective toxicity to dopaminergic neurons observed in PD pathogenesis. GSH, glutathione.
Benomyl decomposes spontaneously, creating a reservoir for slow release of carbendazim and butyl isocyanate (BIC) (Fig. 1). We previously showed that benomyl inhibits ALDH activity in vivo with indirect evidence in brains as elevated acetaldehyde levels upon ethanol challenge (18). The ALDH inhibitory activity is caused by BIC and its downstream metabolites including S-methyl N-butylthiocarbamate (MBT), which is further converted by cytochrome P450 (CYP) enzymes to MBT sulfoxide (MBT-SO), a very potent ALDH inhibitor (18). We have also shown that benomyl is an inhibitor of 26S UPS activity (24), but here we find that this occurs at micromolar concentrations. The present study shows that nanomolar concentrations of benomyl metabolite(s) inhibit ALDH activity, resulting in accumulation of toxic aldehydes (e.g., DOPAL) and dopaminergic neuronal loss in vitro and in vivo. Furthermore, we report an association between benomyl exposure and PD occurrence in a human population. This investigation integrates cellular and in vivo models with human patients and environmental exposure data in the study of PD. These findings identify ALDH dysfunction as a plausible pathway in PD pathogenesis and potential therapeutic target for developing disease-modifying therapies.
Results
ALDH Inhibition in Primary Neurons and Mitochondrial Preparations.
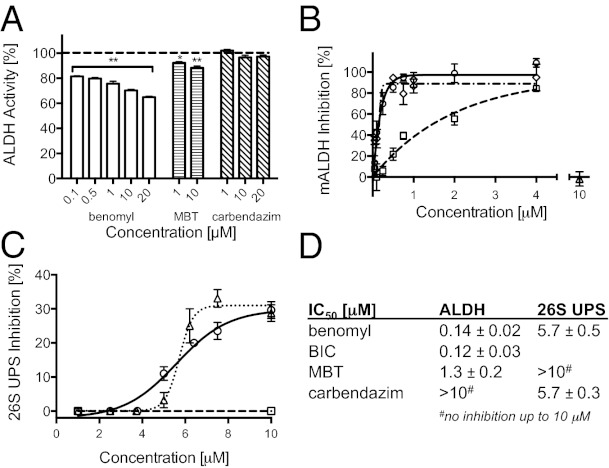
We previously reported that benomyl inhibits ALDH activity in mouse mitochondria (18); we extend the study here to direct measurement in primary neurons using a cell-based assay in which fluorescence increases with ALDH activity. Exposure of ex vivo suspensions of nigral neurons to benomyl for 30 min resulted in concentration-dependent ALDH inhibition (Fig. 2A). ALDH activity was inhibited by 19 ± 0.4% (P = 4.6 × 10−7, n = 3) at the lowest concentration tested (0.1 μM), progressively increasing to 35 ± 0.6% inhibition at the highest concentration tested (20 μM; P = 2.7 × 10−20, n = 10). MBT exposure (10 μM) inhibited ALDH activity by 12 ± 1% (P = 2.9 × 10−5, n = 5). In contrast, carbendazim was ineffective at concentrations up to 20 μM (Fig. 2A). We also quantitated the ALDH IC50 values of benomyl and its metabolites using mitochondria isolated from rat liver (Fig. 2 B and D). Benomyl and BIC were essentially equipotent with IC50 values of 0.12–0.14 μM. MBT had an IC50 of 1.3 μM, whereas carbendazim did not inhibit ALDH activity (Fig. 2 B and D). These results are consistent with those of Staub et al. for hepatic mitochondria prepared from mice (18).
Fig. 2.
Inhibitory actions of benomyl and its metabolites. (A) Exposure to benomyl or MBT, but not carbendazim, inhibited ex vivo ALDH activity in mesencephalic neurons dissociated from 2-d-old rat pups (n = 3–11). (B) Benomyl, BIC, and MBT inhibited in vitro mitochondrial ALDH activity (n = 2–8 at each concentration tested). Carbendazim did not significantly inhibit mALDH activity at up to 20 μM (n = 4). (C) Benomyl and carbendazim inhibited 26S UPS activity in SK-N-MC neuroblastoma cells (n = 4–14 at each concentration tested), whereas MBT exposure up to 10 μM had no effect (n = 5). (D) Benomyl/BIC/MBT inhibit ALDH activity at lower concentrations than benomyl/carbendazim inhibit the 26S UPS. Data are expressed as percent relative to vehicle controls (0.01% DMSO), except for 26S UPS inhibition which is relative to treatment with the 26S UPS inhibitor lactacystin (5 μM). *P < 0.01, **P < 0.0001; benomyl (○), BIC (◇), MBT (☐), carbendazim (Δ). mALDH, mitochondrial aldehyde dehydrogenase; 26S UPS, ubiquitin-proteasome system.
UPS Inhibition.
We previously reported that benomyl is a UPS inhibitor (24), but the inhibiting moiety was not determined. Benomyl inhibited the 26S UPS with an IC50 of 5.7 μM after a 48-h exposure in an SK-N-MC neuroblastoma cell line (Fig. 2 C and D). Carbendazim had the same effect (IC50 = 5.7 μM), whereas MBT did not inhibit the UPS, suggesting that the carbendazim moiety is responsible for benomyl’s inhibition of the UPS at micromolar concentrations.
Selective Dopaminergic Neurotoxicity in Vitro.
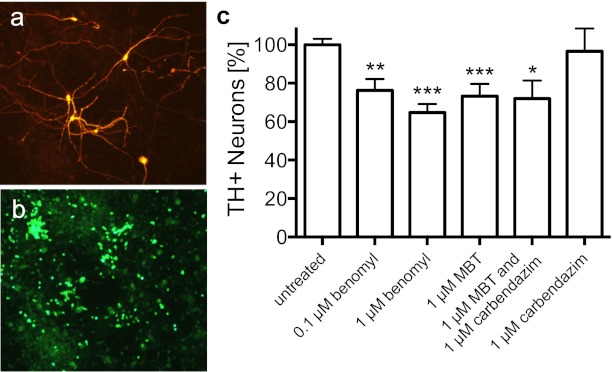
Immunocytochemistry against tyrosine hydroxylase (TH+) and neuronal nuclei (NeuN+) was conducted on primary mesencephalic cultures to determine the toxicities of benomyl and metabolites on dopaminergic and total neurons (Fig. 3 A and B). Benomyl was selectively toxic to dopaminergic neurons, resulting in a 24 ± 6% decrease in TH+ cells at 0.1 μM and a 35 ± 4% decrease at 1 μM after 48-h exposures (Fig. 3C) with no significant loss of total NeuN+ neurons at either concentration (e.g., 1 μM, 3 ± 14% change, P = 0.79), demonstrating the selective toxicity of benomyl. The total number of NeuN+ neurons (i.e., total neurons) did not significantly change despite the loss of TH+ neurons because TH+ cells only contributed on the order of 1% of total neurons counted. MBT exposure (1 μM) resulted in a 27 ± 6% decrease in TH+ cells (Fig. 3C), comparable to benomyl exposure, and similarly had no effect on NeuN+ cells. In contrast, carbendazim exposure alone had no significant effect on TH+ or NeuN+ neuron counts, and there was no synergistic effect when cells were exposed simultaneously to MBT and carbendazim. These results suggest that benomyl’s neurotoxicity is due to MBT, and therefore ALDH inhibition, at the concentrations tested.
Fig. 3.
Dopaminergic neuronal damage in primary mesencephalic cultures exposed to benomyl or its metabolites. Representative field of view (20×) shows untreated immunoreactive (A) dopaminergic neurons (TH+) and (B) neuronal nuclei (NeuN+). (C) Benomyl neurotoxicity was recapitulated by MBT exposure, whereas carbendazim was not toxic to TH+ neurons at 1 μM. NeuN+ counts were unaffected by any treatment. Because MBT is either the proximal or penultimate benomyl metabolite that inhibits ALDH activity, there appears to be an association between neuronal damage and ALDH inhibition; proteasomal inhibition by the carbendazim moiety is not sufficient to kill cells under the same conditions. Data are expressed as percent relative to vehicle controls (0.01% DMSO). *P < 0.05, **P < 0.01, ***P < 0.0001.
ALDH Inhibition as a Neurotoxic Mechanism.
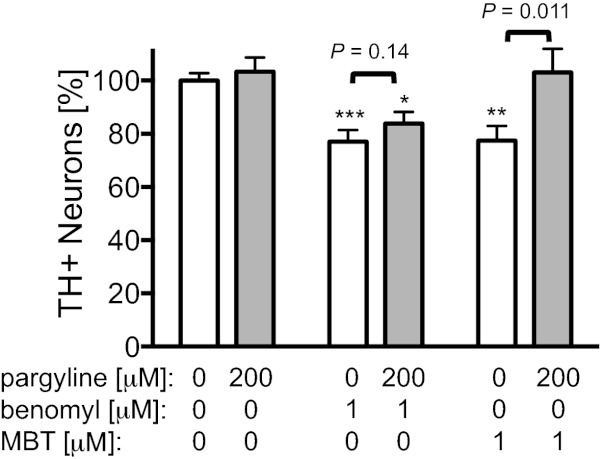
We hypothesized that benomyl’s selective toxicity to TH+ neurons was caused by its effects on DA metabolism. DA is oxidized by monoamine oxidase (MAO) to form DOPAL, which is then further oxidized to 3,4-dihydroxyphenylacetic acid (DOPAC) by ALDH (Fig. 1). We were unable to measure DOPAL concentration ([DOPAL]) directly because of its instability and very low concentrations in cultures, so we measured [DA] and [DOPAC] to determine if DA homeostasis shifted with benomyl treatment. A subset of primary cultures treated with benomyl was sacrificed at 3 h. [DOPAC] was 42 ± 11% less in benomyl-treated cultures (P = 0.034, n = 16), and [DA] remained relatively unchanged (1% decrease, P = 0.44), so [DOPAC]/[DA] was 38 ± 13% less (P = 0.035), consistent with ALDH inhibition in these neurons. To test if accumulation of ALDH substrates (i.e., DOPAL) caused benomyl’s neurotoxicity, DOPAL formation was inhibited with the MAO inhibitor pargyline. TH+ neuronal loss was attenuated by 30 ± 9% (P = 0.14, n = 13–14; Fig. 4) in cultures cotreated with pargyline (200 μM) and benomyl (1 μM). Pargyline completely prevented neurotoxicity in cultures treated with MBT (1 μM), a less potent ALDH inhibitor (P = 0.011, n = 14–15). Pargyline alone had no effect on TH+ neuronal counts at this concentration.
Fig. 4.
Monoamine oxidase (MAO) inhibitor protects against neurotoxicity because of DOPAL accumulation. Neuronal loss resulting from 1 μM benomyl or MBT exposure was mitigated by cotreatment with the MAO inhibitor pargyline (200 μM, n = 13–28). Because MAO inhibition reduces the metabolism of dopamine to DOPAL, this suggests that DOPAL is toxic to dopaminergic neurons and that benomyl is toxic via DOPAL accumulation as a result of ALDH inhibition. Data are expressed as percent relative to vehicle controls (0.01% DMSO). *P = 0.0027, **P = 2.4 × 10−4, ***P = 6.1 × 10−5.
α-Synuclein Levels.
The major pathologic hallmark of PD is the formation of Lewy bodies which are comprised primarily of α-synuclein aggregates. α-Synuclein levels measured using immunocytochemistry in surviving dopaminergic neurons did not change significantly in TH+ neurons exposed to benomyl, MBT, carbendazim, or a combination of MBT and carbendazim.
Selective Aminergic Neurotoxicity in Vivo.
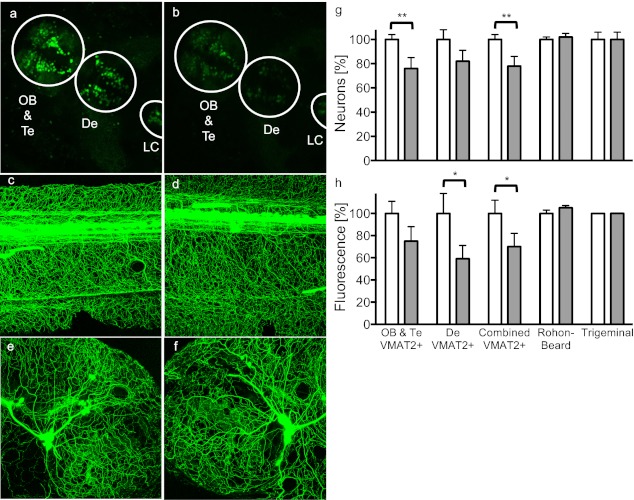
Zebrafish have been used in developmental toxicology studies, and they are now being used to investigate neurotoxicity (25). To test the specificity of benomyl neurotoxicity in a vertebrate system, a model was developed using transgenic zebrafish (Danio rerio) lines that label specific neuronal populations with GFP that can be visualized because embryos are transparent. Zebrafish have a well-developed dopaminergic system. The anterior clusters shown in Fig. 5 A and B correspond to aminergic neurons in the olfactory bulb and telencephalon of ETvmat2:GFP zebrafish (26) embryos; the posterior clusters contain the diencephalon. These clusters are predominantly dopaminergic, although they also include some (nor)adrenergic neurons (27). Exposure to 1 μM benomyl from 5 h until 120 h postfertilization resulted in a 24 ± 9% decrease in VMAT2+ (vesicular monoamine transporter) neuronal counts in anterior clusters (P = 0.041, n = 19; Fig. 5G) and an 18 ± 9% decrease in diencephalic clusters (P = 0.15), constituting an overall 22 ± 8% decrease in VMAT2+ neurons (P = 0.043). Fluorescence similarly trended lower by 25 ± 13% (anterior, P = 0.16; Fig. 5H), 38 ± 13% (diencephalon, P = 0.061), and 27 ± 12% (overall, P = 0.089). Tg(isl1[ss]:Gal4-VP16,UAS:EGFP)zf154 embryos (28) that were exposed to the same conditions exhibited no significant differences in neuron counts or fluorescence in Rohon-Beard (Fig. 5 C and D) or trigeminal (Fig. 5 E and F) sensory neurons. Even the complex axons were unaffected, adding further evidence that benomyl is toxic to dopaminergic neurons in a selective manner (Fig. 5 G and H). Benomyl exposure under these conditions was not lethal to zebrafish embryos, but swimming behavior was altered. After 2 wk of exposure, benomyl-treated zebrafish exhibited a 45 ± 10% deficit in spontaneous swimming distance versus unexposed zebrafish (P = 0.006, n = 11–15), suggesting that benomyl’s selective toxicity to dopaminergic neurons has functional significance in vivo.
Fig. 5.
Aminergic neuronal damage in Danio rerio embryos exposed to benomyl. Representative confocal images of zebrafish embryos (A, C, and E) unexposed or (B, D, and F) bathed in 1 μM benomyl from 5 h until 120 h postfertilization are shown. (G) Neuronal counts (A and B) decreased in VMAT2+ anterior and diencephalic clusters of ETvmat2:GFP zebrafish exposed to benomyl (solid bars) but were unaffected in (C and D) Rohon-Beard and (E and F) trigeminal neurons in Tg(isl1[ss]:Gal4-VP16,UAS:EGFP)zf154 zebrafish. (H) Measurement of total fluorescence yielded similar results. Data are expressed as percent relative to vehicle controls (0.01% DMSO). *P < 0.1, **P < 0.05. De, diencephalon; LC, locus coeruleus; OB, olfactory bulb; Te, telencephalon.
Epidemiologic Association.
Potential association between benomyl exposure and PD occurrence was investigated in an epidemiologic study to determine the possible relevance of these findings that benomyl exposure resulted in selective dopaminergic neuronal damage in vitro and in vivo. These analyses included 360 PD patients (“cases”) and 754 neurologically normal subjects (“controls”) (Table S1). PD risk increased 67% for individuals with estimated ambient benomyl exposure at their workplace equal to or above the median level (OR = 1.67, 95%CI: 1.19–2.34; P = 0.0027; Table S2). This risk increased to almost twofold for those with estimated exposures in the highest quartile (OR = 1.97, 95%CI: 1.29–3.02; P = 0.0017), revealing a dose–response trend (P = 0.0019; Table 1). There was no trend between benomyl exposure and PD based on residential addresses. These analyses had been adjusted for known PD risk factors (e.g., age, sex), and the findings did not change in sensitivity analyses adjusting for race/ethnicity or family history of PD, or when excluding subjects potentially overrepresenting particular residential clusters or missing 1 or more years of occupational data.
Table 1.
Associations between estimated ambient occupational or residential benomyl exposures and PD risk in the UCLA PEG Study
| Exposure, n (%)* | Cases (n = 360) | Controls (n = 754) | OR (95%CI)† | P value‡ |
| Occupational | ||||
| No | 217 (60.3) | 531 (70.4) | Reference | |
| First quartile | 23 (6.4) | 55 (7.3) | 0.90 (0.53–1.53) | 0.69 |
| Second quartile | 26 (7.2) | 51 (6.8) | 1.07 (0.64–1.79) | 0.81 |
| Third quartile | 40 (11.1) | 62 (8.2) | 1.38 (0.88–2.17) | 0.16 |
| Fourth quartile | 54 (15.0) | 55 (7.3) | 1.97 (1.29–3.02) | 0.0017 |
| Trend P value | 0.0019 | |||
| Residential | ||||
| No | 209 (58.1) | 467 (61.9) | Reference | |
| First quartile | 31 (8.6) | 71 (9.4) | 0.87 (0.54–1.40) | 0.56 |
| Second quartile | 41 (11.4) | 72 (9.5) | 1.14 (0.73–1.77) | 0.57 |
| Third quartile | 31 (8.6) | 73 (9.7) | 0.79 (0.49–1.27) | 0.34 |
| Fourth quartile | 48 (13.3) | 71 (9.4) | 1.20 (0.78–1.86) | 0.40 |
| Trend P value | 0.071 |
CI, confidence interval; OR, unconditional logistic odds ratio.
*Exposure quartiles defined using distributions of exposed controls, separately for occupational or residential estimates. Two cases and one control with incomplete occupational data and one case with incomplete residential data were assumed to be unexposed.
†Adjusted for age (continuous), sex (male/female), smoking status (current/former/never), county (Fresno/Kern/Tulare, California), and education (<12 y/=12 y/>12 y).
‡For multiple testing considerations, 8 tests were performed so a P value of 0.006 was considered statistically significant.
Discussion
PD etiology has proven very difficult to determine using various genetic models and epidemiologic studies. It likely includes a combination of genetic and environmental contributions, so investigations involving environmental toxicants may help elucidate the complex mechanisms at play. The recognition of new epidemiologic contributors to PD etiology expands the consideration of possible mechanisms involved. The proposed environmental contributor here is benomyl, recognized in an epidemiologic investigation, and this suggests ALDH inhibition in humans contracting PD. Because direct causality of environmental risk factors cannot be tested in humans, this investigation sought to determine if exposure in experimental models could recapitulate some of the pathologic features of PD. Here we report selective dopaminergic neuronal damage in both an in vitro system (primary cultures) and an in vivo zebrafish model. There was significant loss in zebrafish aminergic neurons measured both by neuron counts and fluorescence, whereas the nonaminergic trigeminal and Rohon-Beard sensory neurons remained intact after benomyl exposure. A selective loss of dopaminergic neurons was similarly observed in mesencephalic cultures exposed to benomyl, reflecting the preferential loss of dopaminergic neurons characteristic of PD.
Benomyl’s action as a fungicide is thought to be due to its ability to impair microtubule assembly, interfering with mitosis and other processes (29, 30). Microtubule inhibitors such as nocodazole and rotenone also inhibit the UPS (17). We found that the carbendazim moiety of benomyl, a known microtubule inhibitor, confers benomyl’s UPS inhibitory activity. However, it is unlikely that microtubule or UPS dysfunction is responsible for benomyl’s toxicity observed in primary cultures or zebrafish, given the low concentrations used relative to its IC50 (5.7 μM). [Because concentrations in the range of 1.3–50 μM are necessary for benomyl’s use as a fungicide in the environment (31–33), we used a conservative concentration range (0.1–1 μM) in primary cultures and zebrafish to enhance relevance to actual exposures for humans.] Furthermore, carbendazim had no effect on neuronal survival at 1 μM, whereas MBT recapitulated benomyl’s toxicity. Because benomyl and its metabolite MBT, but not carbendazim, inhibit ALDH activity at the concentrations used, ALDH inhibition likely confers benomyl’s neurotoxicity. Additional support for the potential role of toxicant-induced ALDH inhibition in the pathogenesis of PD comes from a report from Wey et al. that describes TH+ neuronal loss, DOPAL accumulation, and motobehavioral deficits in mice lacking cytosolic and mitochondrial ALDH (Aldh1a1−/−xAldh2−/−) (34). Their work demonstrated that ALDH inhibition can recapitulate some pathologic aspects of PD in an in vivo model. It is remarkable that the environmental impact of benomyl exposure in the present work yielded similar results in vitro and in vivo as their targeted genetic approach. This is in contrast to another fungicide, ziram, which increases α-synuclein levels and damages dopaminergic cells by inhibiting E1 ligase activity in the UPS (35). Benomyl appears to act via a novel mechanism (ALDH inhibition), damaging dopaminergic cells via alterations in DA homeostasis, independent of α-synuclein.
Our observed alterations in DA metabolites after benomyl exposure add further evidence that ALDH inhibition is the cause of benomyl’s neurotoxicity. Goldstein et al. similarly found that striatal [DOPAL]:[DA] was four times higher in postmortem PD brains (36). They also observed decreased VMAT2 activity (37). Burke et al. reported that DOPAL is 400-fold more toxic to dopaminergic neurons in vivo than its precursor (i.e., DA) or its ALDH product (i.e., DOPAC) (21–23), potentially because of the formation of reactive radicals and quinones (38). VMAT2 inhibition could provide a larger reservoir of cytosolic DA, making neurons more susceptible to DOPAL toxicity. Thus, benomyl’s selective toxicity to dopaminergic neurons can be explained by ALDH inhibition leading to toxic DOPAL accumulation. Consistent with this hypothesis, reducing DOPAL synthesis with an MAO inhibitor mitigated benomyl’s toxicity.
The present work reveals ALDH inhibition as a toxicant-induced neurotoxic mechanism and adds an epidemiologic association that suggests the relevance of this mechanism in a human population. The University of California Los Angeles (UCLA) Parkinson’s Environment and Genes (PEG) Study is unique in its ability to use data from state-mandated commercial agricultural Pesticide Use Reporting forms to overcome subject recall bias and to determine associations with specific toxicants in a heavy pesticide use region of California. Here we show that chronic exposure to ambient benomyl at the workplace was associated with 67% to almost twofold increases in risk of developing PD after adjusting for known PD risk factors. Analyses based on workplace addresses potentially incorporate the best estimates of subjects’ ambient benomyl exposures because pesticide applications occur during the workday when subjects would have been at their workplace rather than their residence. Notably, this model only estimates relative ambient exposures and does not take into account other routes of exposures such as occupational handling of pesticides and drinking well water, both associated with increased risk of PD, which could exacerbate the estimated risk even further (39–41). We have used this method to report positive associations between PD onset and exposures to ziram, maneb, and paraquat (42, 43). In a logistic regression model that includes our benomyl variable and a variable for the combination of ziram, maneb, and paraquat, the benomyl association remains robust and the confidence interval excludes the null.
Concluding Remarks
Benomyl was widely used in the United States for three decades until toxicological evidence revealed its potential to elicit liver tumors, brain malformations, reproductive effects, and carcinogenesis. In 2001, the registrants of benomyl voluntarily canceled their commercial use registrations, and the US Environmental Protection Agency has not granted additional registrations (14). The present work adds a PD association to these toxicological investigations and demonstrates the possibility for long-lasting toxicological effects of pesticide use even a decade after chronic exposure. This work suggests that exposure to an environmental toxicant may inhibit ALDH sufficiently to damage dopaminergic neurons and increase the risk of exposed humans developing PD. This finding may be generalizable to all PD patients, so augmenting ALDH activity may be a potential therapeutic target for designing disease-modifying therapies for PD regardless of environmental exposures.
Methods
ALDH Activity Assays.
All procedures using animals were approved by the UCLA Animal Research Committee. Mesencephalic neurons (postnatal day 2) were dissected and dissociated as described for primary cultures below. Instead of plating, neurons were resuspended in buffer from the Aldefluor kit (STEMCELL Technologies). Aldefluor was added (1 μL/mL) to the neuronal suspension, and 300-μL aliquots were immediately transferred to culture tubes containing 3 μL of test compound solutions, resulting in final concentrations of 100 nM–20 μM. Culture tubes were incubated at 37 °C for 30 min with periodic gentle shaking. Using FACS (XL-MCL, Beckman), cells were gated by forward- and side-scatter, and intracellular green fluorescence was measured on channel FL1. ALDH inhibition was determined by comparing fluorescence in the presence or absence of test compounds.
Rat hepatic mitochondria were isolated using published protocols (44, 45). These preparations (10 μL) were exposed to test compounds for 5 min in 170 μL of 50 mM pyrophosphate buffer (pH 9.0), 50 mM NAD+, 0.1 mM pyrazole, 0.5% (wt/vol) sodium deoxycholate, and 2 μM rotenone (added in 2.7 μL methanol). Absorbance at 340 nm was monitored for 10 min at 5-s intervals after 1 mM acetaldehyde (20 μL) was added (SpectraMax 340PC384 Absorbance Microplate Reader, SoftMax Pro software, Molecular Devices). ALDH activity was determined from the slope as the increase in absorbance over time from 1 to 3 min.
26S UPS Activity Assay.
The 26S UPS activity was determined by FACS as previously described (24). Briefly, neuroblastoma SK-N-MC cells transfected with an EGFP–degron fusion protein and passaged multiple times were exposed to test compounds for 48 h at 2 mL/well before FACS analysis (Beckman XL-MCL). UPS inhibition was inferred from high fluorescence corresponding to the level of EGFP–degron fusion protein that was not selectively degraded by the proteasome (46).
Primary Neuronal Cultures.
Primary neuronal cultures were prepared using a protocol adapted from Rayport et al. and previously described (35, 47). Briefly, mesencephalic cells containing the substantia nigra pars compacta (excluding the ventral tegmental area) were dissected from coronal sections of brains from postnatal day 1 or 2 rat pups, dissociated in papain, and plated onto glial cells at densities of 4 × 105 per coverslip. Cultures were grown for 6–8 d and then treated by exchanging 1 mL of the media in each plate with 1 mL of fresh media amended with test compound(s) and vehicle (0.01% DMSO).
Cultures after 48-h treatment were fixed in paraformaldehyde (4% wt/vol) for 30 min, washed with PBS, blocked with normal donkey serum (5% vol/vol) and Triton X-100 (0.5% vol/vol) in PBS for 1–2 h, incubated with antibodies against TH (1:1,500, anti-rabbit, Calbiochem) and NeuN (1:200, anti-mouse, Millipore) overnight at 4 °C, washed with 3× and 1× PBS, incubated with Alexa Fluor 488 (1:200, Invitrogen) and 555 (1:1,500, Invitrogen) secondary antibodies at room temperature for 2 h, and washed with Tween-20 (0.1%) in PBS and then with PBS before coverslipping. All TH-immunoreactive neurons were counted, and NeuN-immunoreactive neuron counts were estimated for each coverslip from neurons quantified in five representative fields of view using a 20× objective. Quantification was determined by blinded raters. Some cultures were incubated with antibodies against TH and α-synuclein (1:500, anti-mouse, BD Biosciences). Relative levels of α-synuclein in TH+ cells were determined as previously described (35), using the GNU Image Manipulation Program (GIMP 2.6).
To determine cell levels of dopamine and its metabolites, some cultures were extracted with perchloric acid (100 mM) and EDTA (0.1% wt/vol) and stored at −80 °C until HPLC analysis. Samples were separated on a C18 reverse phase column (TSKgel Super ODS 2 μm particle size, 10 × 2.1 mm, maintained at 33 °C, Tosoh Bioscience) using a mobile phase (2.5% methanol, 100 mg/L sodium-1-octane sulfonate, 42 mM citric acid, 38 mM sodium acetate, 50 mg/L EDTA) pumped at 0.2 mL/min (LC-10AD pump, Shimadzu). Monoamines and metabolites were oxidized on a glassy carbon electrode against an Ag/AgCl reference (Antec Leyden) with an applied potential of 0.75V. Data were collected using EzChrom software (Agilent).
In Vivo Studies.
Zebrafish embryos were bathed in 1 μM benomyl or vehicle (0.01% DMSO) in E3 medium for zebrafish embryos from 5 h until 120 h postfertilization, anesthetized using tricaine methanesulfonate (0.02%), fixed in paraformaldehyde (4%) overnight, and mounted in agarose (1.2% wt/vol) for confocal imaging at 20× magnification. Zebrafish expressing GFP tagged to vesicular monoamine transporter protein (ETvmat2:GFP) were used to identify VMAT2+ (dopaminergic, (nor)adrenergic, serotonergic) neurons in whole embryos (26). Approximately 100 optical sections were gathered for each embryo, spaced 1.34 µM apart, using a Zeiss LSM 5 Pascal inverted microscope. Images were reconstituted, and clusters of anterior (including olfactory bulb and telencephalon) and diencephalic neurons were identified for analyses. Peripheral sensory neurons (trigeminal and Rohon-Beard) were visualized using the Tg(isl1[ss]:Gal4-VP16,UAS:EGFP)zf154 transgenic line, which has been referred to as Tg(sensory:GFP) (28). Approximately 60 optical sections were spaced 3 µM apart, using a Zeiss LSM 510 inverted microscope. Cells were counted blindly in 3D projections, and fluorescence in composite images was measured blindly using ImageJ (National Institutes of Health). Spontaneous zebrafish movement was monitored with ZebraLab (Viewpoint Life Sciences, Inc.). Total distance was measured by tracking individual embryos for 30 min. Embryos were considered immobile at 0–2 mm/s.
Epidemiologic Study.
This investigation was conducted as part of the UCLA PEG Study (Table S1). Written informed consent was obtained from all enrolled subjects, and all procedures were approved by the UCLA Human Subjects Committee. Subject recruitment methods and case definition criteria have been described in detail (42). Pesticide exposure assessments were performed using a geographic information system–based computer model incorporating Pesticide Use Reporting forms that have been mandated by the California Department of Pesticide Regulation since 1974 and provide information on the location, date, and amount of pesticide in each commercial application (43, 48–50). Geocoded lifetime occupational and residential addresses were considered separately. Ambient exposure was assumed to be proportional to the amount of pesticide applied to crop acreage within a 500-m radius surrounding the subject’s address averaged over the 26-y period (1974–1999). Exposure was categorized by the median in controls, resulting in a three-level variable (unexposed, exposure below the median, exposure equal to or above the median). For quartile analyses, exposure was categorized by quartiles in exposed controls, resulting in a five-level variable (unexposed, four quartiles).
Statistical Analyses.
Demographic characteristics were compared for deviation from expected by χ2 test (categorical variables) or for difference in mean by t test (age). Logistic regression analyses were performed on the epidemiologic data using SAS 9.1 (SAS Institute Inc.). Odds ratios and 95% confidence intervals were estimated after adjusting for age (continuous variable), sex (male/female), county of residence (Fresno/Kern/Tulare, California), education (less than 12 y of schooling/12 y or completion of general education development test/more than 12 y), and smoking status (current/former/never). Sensitivity analyses were performed to assess the effects of adjustments for race (Caucasian/non-Caucasian) and family history of PD (first-degree family history present/absent). Additional sensitivity analyses were performed excluding 23 controls who had lived in the same cluster as another control at the time of recruitment for at least 2 y before 1999, and excluding 44 cases and 95 controls who were missing one or more occupational addresses from 1974 to 1999. For trend tests, quartile categories were assigned scores of 0, 1, 2, 3, or 4 and entered into the logistic regression equation as a linear term. The Wald statistic was used as a test for linear trend of the odds ratio. In biochemical assays, IC50 values were determined using sigmoidal curve fits of percent inhibition at varying concentrations (PRISM 5, GraphPad). For all other analyses, statistical significance was determined using a paired t test.
Supplementary Material
Acknowledgments
We thank Dr. Neil Harris for use of the Zeiss LSM 5 Pascal microscope. This work was funded in part by National Institute of Environmental Health Sciences Grants P01ES016732, R01ES010544, 5R21ES16446-2, and U54ES012078; National Institute of Neurological Disorders and Stroke Grant NS038367; the Veterans Affairs Healthcare System (Southwest Parkinson’s Disease Research, Education, and Clinical Center); the Michael J. Fox Foundation; the Levine Foundation; and the Parkinson Alliance. A.G.F. was supported in part by a National Defense Science and Engineering Graduate Fellowship and US Department of Health and Human Services Ruth L. Kirschstein Institutional National Research Service Award T32ES015457 in Molecular Toxicology (to the University of California, Los Angeles).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1220399110/-/DCSupplemental.
References
- 1.Fearnley JM, Lees AJ. Ageing and Parkinson’s disease: Substantia nigra regional selectivity. Brain. 1991;114(Pt 5):2283–2301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- 2.Nalls MA, et al. International Parkinson Disease Genomics Consortium Imputation of sequence variants for identification of genetic risks for Parkinson’s disease: A meta-analysis of genome-wide association studies. Lancet. 2011;377(9766):641–649. doi: 10.1016/S0140-6736(10)62345-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Priyadarshi A, Khuder SA, Schaub EA, Priyadarshi SS. Environmental risk factors and Parkinson’s disease: A metaanalysis. Environ Res. 2001;86(2):122–127. doi: 10.1006/enrs.2001.4264. [DOI] [PubMed] [Google Scholar]
- 4.Ascherio A, et al. Pesticide exposure and risk for Parkinson’s disease. Ann Neurol. 2006;60(2):197–203. doi: 10.1002/ana.20904. [DOI] [PubMed] [Google Scholar]
- 5.Firestone JA, et al. Pesticides and risk of Parkinson disease: A population-based case-control study. Arch Neurol. 2005;62(1):91–95. doi: 10.1001/archneur.62.1.91. [DOI] [PubMed] [Google Scholar]
- 6.Moisan F, et al. The relation between type of farming and prevalence of Parkinson’s disease among agricultural workers in five French districts. Mov Disord. 2011;26(2):271–279. doi: 10.1002/mds.23370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petrovitch H, et al. Plantation work and risk of Parkinson disease in a population-based longitudinal study. Arch Neurol. 2002;59(11):1787–1792. doi: 10.1001/archneur.59.11.1787. [DOI] [PubMed] [Google Scholar]
- 8.Kamel F, et al. Pesticide exposure and self-reported Parkinson’s disease in the agricultural health study. Am J Epidemiol. 2007;165(4):364–374. doi: 10.1093/aje/kwk024. [DOI] [PubMed] [Google Scholar]
- 9.Tanner CM, et al. Rotenone, paraquat, and Parkinson’s disease. Environ Health Perspect. 2011;119(6):866–872. doi: 10.1289/ehp.1002839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dhillon AS, et al. Pesticide/environmental exposures and Parkinson’s disease in East Texas. J Agromed. 2008;13(1):37–48. doi: 10.1080/10599240801986215. [DOI] [PubMed] [Google Scholar]
- 11.Hancock DB, Martin ER, Vance JM, Scott WK. Nitric oxide synthase genes and their interactions with environmental factors in Parkinson’s disease. Neurogenetics. 2008;9(4):249–262. doi: 10.1007/s10048-008-0137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elbaz A, et al. CYP2D6 polymorphism, pesticide exposure, and Parkinson’s disease. Ann Neurol. 2004;55(3):430–434. doi: 10.1002/ana.20051. [DOI] [PubMed] [Google Scholar]
- 13.Hatcher JM, Pennell KD, Miller GW. Parkinson’s disease and pesticides: a toxicological perspective. Trends Pharmacol Sci. 2008;29(6):322–329. doi: 10.1016/j.tips.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Environmental Protection Agency Benomyl's cancellation order. Fed Regist. 2001;66:41589–41591. [Google Scholar]
- 15.Gupta K, et al. Antimitotic antifungal compound benomyl inhibits brain microtubule polymerization and dynamics and cancer cell proliferation at mitosis, by binding to a novel site in tubulin. Biochemistry. 2004;43(21):6645–6655. doi: 10.1021/bi036112v. [DOI] [PubMed] [Google Scholar]
- 16.Ren Y, Liu W, Jiang H, Jiang Q, Feng J. Selective vulnerability of dopaminergic neurons to microtubule depolymerization. J Biol Chem. 2005;280(40):34105–34112. doi: 10.1074/jbc.M503483200. [DOI] [PubMed] [Google Scholar]
- 17.Chou AP, Li S, Fitzmaurice AG, Bronstein JM. Mechanisms of rotenone-induced proteasome inhibition. Neurotoxicology. 2010;31(4):367–372. doi: 10.1016/j.neuro.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Staub RE, Quistad GB, Casida JE. Mechanism for benomyl action as a mitochondrial aldehyde dehydrogenase inhibitor in mice. Chem Res Toxicol. 1998;11(5):535–543. doi: 10.1021/tx980002l. [DOI] [PubMed] [Google Scholar]
- 19.Leiphon LJ, Picklo MJ., Sr Inhibition of aldehyde detoxification in CNS mitochondria by fungicides. Neurotoxicology. 2007;28(1):143–149. doi: 10.1016/j.neuro.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 20.Marchitti SA, Deitrich RA, Vasiliou V. Neurotoxicity and metabolism of the catecholamine-derived 3,4-dihydroxyphenylacetaldehyde and 3,4-dihydroxyphenylglycolaldehyde: The role of aldehyde dehydrogenase. Pharmacol Rev. 2007;59(2):125–150. doi: 10.1124/pr.59.2.1. [DOI] [PubMed] [Google Scholar]
- 21.Burke WJ, et al. Neurotoxicity of MAO metabolites of catecholamine neurotransmitters: Role in neurodegenerative diseases. Neurotoxicology. 2004;25(1-2):101–115. doi: 10.1016/S0161-813X(03)00090-1. [DOI] [PubMed] [Google Scholar]
- 22.Burke WJ, Li SW, Williams EA, Nonneman R, Zahm DS. 3,4-Dihydroxyphenylacetaldehyde is the toxic dopamine metabolite in vivo: Implications for Parkinson’s disease pathogenesis. Brain Res. 2003;989(2):205–213. doi: 10.1016/s0006-8993(03)03354-7. [DOI] [PubMed] [Google Scholar]
- 23.Panneton WM, Kumar VB, Gan Q, Burke WJ, Galvin JE. The neurotoxicity of DOPAL: Behavioral and stereological evidence for its role in Parkinson disease pathogenesis. PLoS ONE. 2010;5(12):e15251. doi: 10.1371/journal.pone.0015251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang XF, Li S, Chou AP, Bronstein JM. Inhibitory effects of pesticides on proteasome activity: Implication in Parkinson’s disease. Neurobiol Dis. 2006;23(1):198–205. doi: 10.1016/j.nbd.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 25.Levin ED, Tanguay RL. Introduction to zebrafish: Current discoveries and emerging technologies for neurobehavioral toxicology and teratology. Neurotoxicol Teratol. 2011;33(6):607. doi: 10.1016/j.ntt.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wen L, et al. Visualization of monoaminergic neurons and neurotoxicity of MPTP in live transgenic zebrafish. Dev Biol. 2008;314(1):84–92. doi: 10.1016/j.ydbio.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 27.Holzschuh J, Ryu S, Aberger F, Driever W. Dopamine transporter expression distinguishes dopaminergic neurons from other catecholaminergic neurons in the developing zebrafish embryo. Mech Dev. 2001;101(1-2):237–243. doi: 10.1016/s0925-4773(01)00287-8. [DOI] [PubMed] [Google Scholar]
- 28.Sagasti A, Guido MR, Raible DW, Schier AF. Repulsive interactions shape the morphologies and functional arrangement of zebrafish peripheral sensory arbors. Curr Biol. 2005;15(9):804–814. doi: 10.1016/j.cub.2005.03.048. [DOI] [PubMed] [Google Scholar]
- 29.Morpurgo G, Bellincampi D, Gualandi G, Baldinelli L, Crescenzi OS. Analysis of mitotic nondisjunction with Aspergillus nidulans. Environ Health Perspect. 1979;31(1):81–95. doi: 10.1289/ehp.793181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oakley BR, Morris NR. Nuclear movement is beta—tubulin-dependent in Aspergillus nidulans. Cell. 1980;19(1):255–262. doi: 10.1016/0092-8674(80)90407-9. [DOI] [PubMed] [Google Scholar]
- 31.Boubaker H, Saadi B, Boudyach EH, Benaoumar AA. Resistance of Verticillium theobromae to benzimidazole fungicides in Morocco. J Appl Sci. 2008;8(21):3903–3909. [Google Scholar]
- 32.Potocnik I, et al. In vitro toxicity of selected fungicides from the groups of benzimidazoles and demethylation inhibitors to Cladobotryum dendroides and Agaricus bisporus. J Environ Sci Health B. 2009;44(4):365–370. doi: 10.1080/03601230902801059. [DOI] [PubMed] [Google Scholar]
- 33.Kataria HR, Grover RK. Effect of benomyl and thiophanate-methyl on metabolic activities of Rhizoctonia solani Kühn. Ann Microbiol (Paris) 1976;127A(2):297–306. [PubMed] [Google Scholar]
- 34.Wey MC, et al. Neurodegeneration and motor dysfunction in mice lacking cytosolic and mitochondrial aldehyde dehydrogenases: implications for Parkinson’s disease. PLoS ONE. 2012;7(2):e31522. doi: 10.1371/journal.pone.0031522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chou AP, et al. Ziram causes dopaminergic cell damage by inhibiting E1 ligase of the proteasome. J Biol Chem. 2008;283(50):34696–34703. doi: 10.1074/jbc.M802210200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldstein DS, et al. Catechols in post-mortem brain of patients with Parkinson disease. Eur J Neurol. 2011;18(5):703–710. doi: 10.1111/j.1468-1331.2010.03246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goldstein DS, Holmes C, Kopin IJ, Sharabi Y. Intra-neuronal vesicular uptake of catecholamines is decreased in patients with Lewy body diseases. J Clin Invest. 2011;121(8):3320–3330. doi: 10.1172/JCI45803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anderson DG, Mariappan SV, Buettner GR, Doorn JA. Oxidation of 3,4-dihydroxyphenylacetaldehyde, a toxic dopaminergic metabolite, to a semiquinone radical and an ortho-quinone. J Biol Chem. 2011;286(30):26978–26986. doi: 10.1074/jbc.M111.249532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gatto NM, Cockburn M, Bronstein J, Manthripragada AD, Ritz B. Well-water consumption and Parkinson’s disease in rural California. Environ Health Perspect. 2009;117(12):1912–1918. doi: 10.1289/ehp.0900852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goldman SM, et al. Solvent exposures and Parkinson disease risk in twins. Ann Neurol. 2012;71(6):776–784. doi: 10.1002/ana.22629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tanaka K, et al. Occupational risk factors for Parkinson's disease: A case-control study in Japan. BMC Neurol. 2011;11(83):1–6. doi: 10.1186/1471-2377-11-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Costello S, Cockburn M, Bronstein J, Zhang X, Ritz B. Parkinson’s disease and residential exposure to maneb and paraquat from agricultural applications in the central valley of California. Am J Epidemiol. 2009;169(8):919–926. doi: 10.1093/aje/kwp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang A, et al. Parkinson’s disease risk from ambient exposure to pesticides. Eur J Epidemiol. 2011;26(7):547–555. doi: 10.1007/s10654-011-9574-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Quistad GB, Sparks SE, Casida JE. Aldehyde dehydrogenase of mice inhibited by thiocarbamate herbicides. Life Sci. 1994;55(20):1537–1544. doi: 10.1016/0024-3205(94)00314-9. [DOI] [PubMed] [Google Scholar]
- 45.Tottmar SO, Pettersson H, Kiessling KH. The subcellular distribution and properties of aldehyde dehydrogenases in rat liver. Biochem J. 1973;135(4):577–586. doi: 10.1042/bj1350577a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bence NF, Sampat RM, Kopito RR. Impairment of the ubiquitin-proteasome system by protein aggregation. Science. 2001;292(5521):1552–1555. doi: 10.1126/science.292.5521.1552. [DOI] [PubMed] [Google Scholar]
- 47.Rayport S, et al. Identified postnatal mesolimbic dopamine neurons in culture: Morphology and electrophysiology. J Neurosci. 1992;12(11):4264–4280. doi: 10.1523/JNEUROSCI.12-11-04264.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ritz BR, et al. Dopamine transporter genetic variants and pesticides in Parkinson’s disease. Environ Health Perspect. 2009;117(6):964–969. doi: 10.1289/ehp.0800277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goldberg DW, Wilson JP, Knoblock CA, Ritz B, Cockburn MG. An effective and efficient approach for manually improving geocoded data. Int J Health Geogr. 2008;7(60):1–20. doi: 10.1186/1476-072X-7-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rull RP, Ritz B. Historical pesticide exposure in California using pesticide use reports and land-use surveys: An assessment of misclassification error and bias. Environ Health Perspect. 2003;111(13):1582–1589. doi: 10.1289/ehp.6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.