Abstract
Nicotine addiction is a major public health problem, resulting in primary glutamatergic dysfunction. We measured the glutamate receptor binding in the human brain and provided direct evidence for the abnormal glutamate system in smokers. Because antagonism of the metabotropic glutamate receptor 5 (mGluR5) reduced nicotine self-administration in rats and mice, mGluR5 is suggested to be involved in nicotine addiction. mGluR5 receptor binding specifically to an allosteric site was observed by using positron emission tomography with [11C]ABP688. We found a marked global reduction (20.6%; P < 0.0001) in the mGluR5 distribution volume ratio (DVR) in the gray matter of 14 smokers. The most prominent reductions were found in the bilateral medial orbitofrontal cortex. Compared with 14 nonsmokers, 14 ex-smokers had global reductions in the average gray matter mGluR5 DVR (11.5%; P < 0.005), and there was a significant difference in average gray matter mGluR5 DVR between smokers and ex-smokers (9.2%; P < 0.01). Clinical variables reflecting current nicotine consumption, dependence and abstinence were not correlated with mGluR5 DVR. This decrease in mGluR5 receptor binding may be an adaptation to chronic increases in glutamate induced by chronic nicotine administration, and the decreased down-regulation seen in the ex-smokers could be due to incomplete recovery of the receptors, especially because the ex-smokers were abstinent for only 25 wk on average. These results encourage the development and testing of drugs against addiction that directly target the glutamatergic system.
Keywords: substance use disorders, thalamus, caudate, relapse
Addiction is a complex disorder characterized by continued drug intake despite negative consequences, repeated unsuccessful attempts to stop or reduce drug use, and the emergence of symptoms of tolerance and withdrawal. Although the dopamine system plays a key role in the acute reward and the initiation of addiction (1), there is growing evidence implicating adaptations in glutamatergic neurotransmission in the maintenance of addictive behavior (2).
Glutamate receptors can be classified into two types: ionotropic and metabotropic. Strong evidence for a specific role of the metabotropic glutamate receptor type 5 (mGluR5) was shown by Chiamulera et al. in 2001 (3), when they demonstrated that mGluR5 gene knockout mice do not respond to acute administration of various doses of cocaine and fail to acquire i.v. self-administration of cocaine. Since then, multiple studies by others have demonstrated that negative allosteric modulators of the mGluR5 receptor, such as MPEP and MTEP, reduce the self-administration of addictive drugs such as cocaine and nicotine (4–10). Therefore, these studies strongly suggested a key role for mGluR5 in mediating addiction and identified it as a potential target for the treatment of substance use disorders.
Direct evidence that mGluR5 plays a role in human addiction is lacking. To elucidate the potential role of mGluR5 in human addictive disorders, as suggested by the preclinical studies cited above, we assessed the availability of mGluR5 in smokers. However, such a study design cannot answer the question whether potential changes in mGluR5 availability are a precondition or the transitory effects of nicotine consumption. Given the evidence that the development of addiction may alter mGluR5 expression, we also examined ex-smokers. We used positron emission tomography (PET) with the radiolabeled mGluR5 antagonist 3-(6-methyl-pyridin-2-ylethynyl)-cyclohex-2-enone-O-11C-methyl-oxime ([11C]ABP688) (11), which binds with high selectivity to an allosteric site, to measure mGluR5 availability in healthy subjects (nonsmokers), smokers, and ex-smokers.
Results
The clinical characteristics of 14 smokers, 14 age- and sex-matched nonsmokers, and 14 ex-smokers are shown in Table 1. Age did not differ across the groups [F2,39 = 0.01, not significant (n.s.)], and there was no significant age difference between male and female subjects in the total sample or in the smoker, nonsmoker, and ex-smoker groups (P > 0.5). The groups did not differ with respect to Beck Depression Inventory (BDI) scores (F2,39 = 0.72, n.s.). However, there was a trend that the groups differed in highest educational qualification (F2,39 = 3.21, P < 0.1). Additionally, smokers and ex-smokers had higher Beck Anxiety Inventory (BAI) scores on the day of scanning than nonsmokers (t40 = 2.78, P < 0.05). Smokers and ex-smokers did not differ regarding number of cigarettes smoked per day (t26 = −0.46, n.s.) or the number of years smoking (t26 = −0.92, n.s.).
Table 1.
Clinical characteristics of study samples
| Clinical characteristic | Nonsmokers (n = 14) | Smokers (n = 14) | Ex-smokers (n = 14) |
| Sex, female/male | 8/6 | 8/6 | 6/8 |
| Age, yr | 36.8 (9.6) | 36.1 (10.2) | 37.7 (10.1) |
| No. of cigarettes smoked per day | — | 17.0 (4.5) | 17.9 (6.0) |
| No. of years smoking | — | 16.8 (7.6) | 19.6 (8.8) |
| Duration of nicotine abstinence, wk | — | — | 25.0 (19.4) |
| FTND score | — | 4.5 (2.0) | — |
| BAI score | 1.5 (1.2) | 4.3 (4.3) | 4.6 (3.3) |
| BDI score | 1.8 (2.1) | 2.7 (2.6) | 2.6 (2.0) |
| Comorbid diagnosis (history) | Alcohol abuse, n = 1 | Alcohol abuse, n = 1 | Major depression, n = 1 |
| Major depression, n = 2 | |||
| Major depression, n = 1 | Anorexia nervosa, n = 1 | ||
| Highest educational qualification | High school, completed, n = 0 | High school, completed, n = 2 | High school, completed, n = 4 |
| College, completed, n = 13 | College, completed, n = 12 | College, completed, n = 10 | |
| Academic completed, n = 1 | Academic completed, n = 0 | Academic completed, n = 0 |
Data shown are the mean (SD), unless indicated otherwise.
The mean [11C]ABP688 activity administered did not differ significantly among nonsmokers, smokers, and ex-smokers (729 ± 71, 765 ± 80, and 740 ± 34 MBq, respectively). In the smoker group, the time between the last cigarette smoked and scanning (mean, 69.3 min; SD ± 31.6 min) was not correlated with mGluR5 DVR in the 24 regions of interest (ROIs) examined.
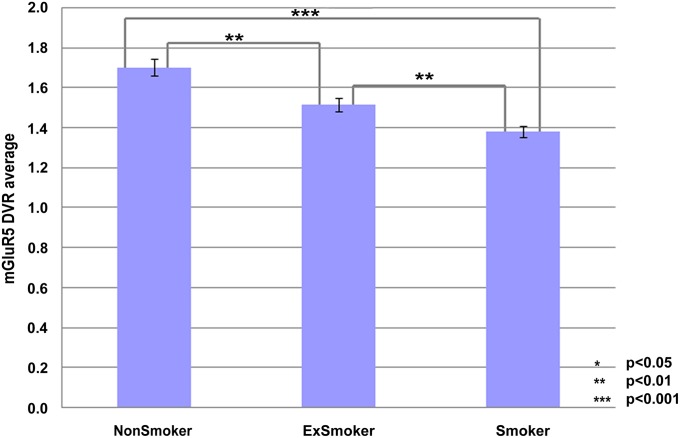
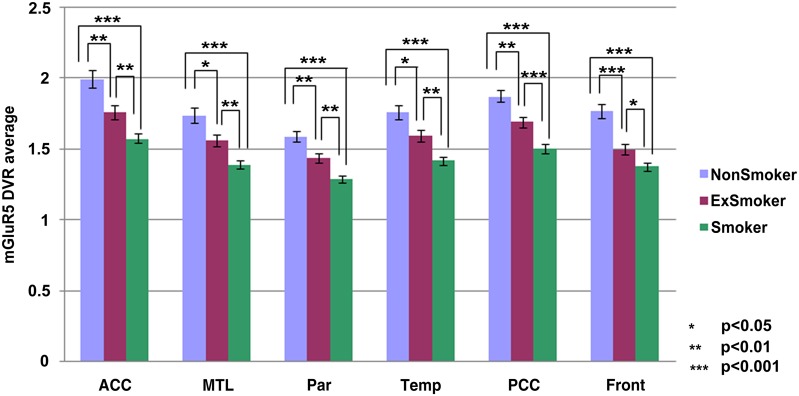
Table 2 and Figs. 1 and 2 summarize the results of the comparisons of the mGluR5 DVR across the 3 clinical groups in the 24 ROIs examined. Fig. 3 displays mGluR5 DVR in the whole brain. There were highly significant global reductions in mGluR5 DVR in smokers relative to nonsmokers and in ex-smokers relative to nonsmokers. Comparing smokers with nonsmokers, the most prominent reductions were found in the medial orbitofrontal cortex (left, 31%; right, 30%) and the caudate (left, 28%; right, 26%). Comparing ex-smokers with nonsmokers, the most prominent reductions were in the thalamus (right, 23%; left, 22%) and the medial orbitofrontal cortex (right, 21%; left, 20%); differences in the orbitofrontal cortex were not significant after Bonferroni correction. The mGluR5 DVR reductions in smokers were on average more prominent than those in ex-smokers; these differences were most clearly observed in the temporal cortex (right, 12%; left, 12%) in the medial temporal cortex (right, 12%; left, 12%) and the cingulate cortex (posterior, 12%; anterior, 11%). The differences in the right temporal cortex, the right medial temporal cortex, and the posterior cingulate cortex remained statistically significant after Bonferroni correction.
Table 2.
Comparison of mGluR5 DVR between smokers, nonsmokers, and ex-smokers
| Regions of interest | Nonsmoker vs. smoker | Nonsmoker vs. ex-smoker | Ex-smoker vs. smoker |
| Gray matter | |||
| P value | <0.0001 | <0.005 | <0.01 |
| padjusted | <0.0001 | <0.05 | n.s. |
| % difference | 20.6 | 11.5 | 9.2 |
| ACC | |||
| P value | <0.0001 | <0.01 | <0.01 |
| padjusted | <0.0001 | n.s. | n.s. |
| % difference | 23.5 | 12.3 | 11.3 |
| Amgdala left | |||
| P value | <0.0001 | <0.0005 | <0.05 |
| padjusted | <0.0001 | <0.05 | n.s. |
| % difference | 25.4 | 15.3 | 10.2 |
| Amygdala right | |||
| P value | <0.0001 | <0.001 | <0.05 |
| padjusted | <0.0001 | <0.01 | n.s. |
| % difference | 24.1 | 15.1 | 9.1 |
| Brainstem | |||
| P value | <0.01 | <0.005 | n.s. |
| padjusted | n.s. | <0.005 | n.s. |
| % difference | 8.9 | 13.9 | −5.0 |
| Caudatus left | |||
| P value | <0.0001 | <0.0005 | <0.05 |
| padjusted | <0.0001 | <0.005 | n.s. |
| % difference | 27.9 | 18.1 | 9.9 |
| Caudatus right | |||
| P value | <0.0001 | <0.005 | <0.05 |
| padjusted | <0.0001 | <0.05 | n.s. |
| % difference | 25.5 | 15.7 | 9.9 |
| Frontal left | |||
| P value | <0.0000 | <0.0005 | <0.05 |
| padjusted | <0.0001 | <0.01 | n.s. |
| % difference | 24.8 | 16.9 | 8.0 |
| Frontal right | |||
| P value | <0.0001 | <0.0005 | <0.05 |
| padjusted | <0.0001 | <0.01 | n.s. |
| % difference | 25.0 | 16.2 | 8.8 |
| Medial temporal left | |||
| P value | <0.0001 | <0.01 | <0.005 |
| padjusted | <0.0001 | n.s. | n.s. |
| % difference | 22.4 | 11.0 | 11.5 |
| Medial temporal right | |||
| P value | <0.0001 | <0.05 | <0.005 |
| padjusted | <0.0001 | n.s. | <0.05 |
| % difference | 22.4 | 10.6 | 11.6 |
| mOFC left | |||
| P value | <0.0001 | <0.05 | n.s. |
| padjusted | <0.0005 | n.s. | n.s. |
| % difference | 30.9 | 19.8 | 11.3 |
| mOFC right | |||
| P value | <0.0001 | <0.01 | n.s. |
| padjusted | <0.0005 | n.s. | n.s. |
| % difference | 30.2 | 20.9 | 9.4 |
| Putamen left | |||
| P value | <0.0001 | <0.0005 | <0.05 |
| padjusted | <0.0001 | <0.01 | n.s. |
| % difference | 20.6 | 13.4 | 7.2 |
| Putamen right | |||
| P value | <0.0001 | <0.005 | <0.05 |
| padjusted | <0.0005 | n.s. | n.s. |
| % difference | 18.4 | 11.9 | 6.5 |
| Temporal left | |||
| P value | <0.0001 | <0.05 | <0.005 |
| padjusted | <0.0001 | n.s. | n.s. |
| % difference | 20.0 | 9.2 | 10.9 |
| Temporal right | |||
| P value | <0.0001 | <0.005 | <0.005 |
| padjusted | <0.0001 | n.s. | n.s. |
| % difference | 21.9 | 10.7 | 11.3 |
| Thalamus left | |||
| P value | <0.0001 | <0.005 | <0.005 |
| padjusted | <0.0001 | n.s | 0.024 |
| % difference | 21.8 | 10.2 | 11.7 |
| Thalamus right | |||
| P value | <0.0001 | <0.01 | <0.05 |
| padjusted | <0.0001 | n.s. | n.s. |
| % difference | 19.8 | 11.2 | 8.6 |
| PCC | |||
| P value | <0.0001 | <0.005 | <0.01 |
| padjusted | <0.0001 | <0.05 | n.s. |
| % difference | 21.1 | 12.9 | 8.2 |
| Putamen left | |||
| P value | <0.0001 | <0.05 | <0.005 |
| padjusted | <0.0001 | n.s. | n.s. |
| % difference | 21.8 | 10.3 | 11.6 |
| Putamen right | |||
| P value | <0.0001 | <0.05 | <0.005 |
| padjusted | <0.0001 | n.s. | <0.05 |
| % difference | 21.4 | 9.5 | 12.0 |
| Temporal left | |||
| P value | <0.0001 | <0.0001 | n.s. |
| padjusted | <0.0001 | <0.0001 | n.s. |
| % difference | 20.2 | 21.5 | −1.3 |
| Temporal right | |||
| P value | <0.0001 | <0.0001 | n.s. |
| padjusted | <0.0001 | <0.0001 | n.s. |
| % difference | 20.0 | 22.8 | −2.8 |
ACC, anterior cingulate cortex; mOFC, medial orbitofrontal cortex; n.s., not significant; PCC, posterior cingulate cortex.
Fig. 1.
Comparison of mGluR5 DVR averaged over all regions between the three groups (nonsmokers, ex-smokers, and smokers). Error bars indicate SEs. Asterisks indicate the uncorrected P values from the t statistics.
Fig. 2.
Average mGluR5 DVR values in selected brain regions (left and right sites together). Error bars indicate SEs. Asterisks indicate the uncorrected P values from the t statistics. ACC, anterior cingulate cortex; MTL, medial temporal cortex; Par, parietal cortex; Temp, temporal cortex; PCC, posterior cingulate cortex; Front, frontal cortex.
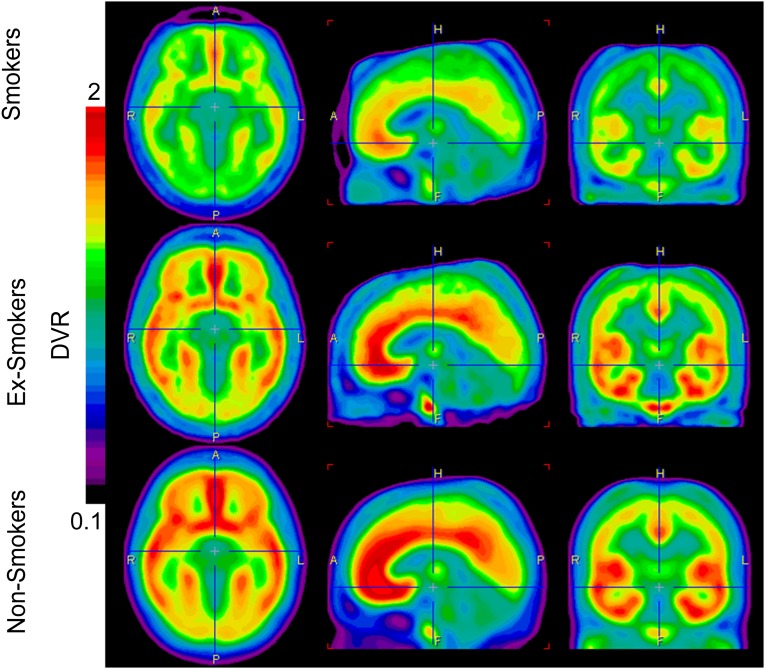
Fig. 3.
Images display the average brain uptake of mGluR5 DVR in the three diagnostic groups. The brain uptake is visibly reduced in smoker and ex-smoker groups. Images are calculated by PMOD software, version 3 (PMOD Technologies).
In the entire sample, age was correlated with mGluR5 DVR in the left putamen (r = 0.38, P < 0.05), the right putamen (r = 0.31, P < 0.05), and the left occipital lobe (r = 0.31, P < 0.05).
In smokers, age was positively correlated with mGluR5 DVR in some brain regions. The strongest correlations were found in the putamen (left, r = 0.82, P < 0.0005; right, r = 0.73, P < 0.005), posterior cingulate cortex (r = 0.74, P < 0.005), amygdala (right, r = 0.71, P < 0.005; left, r = 0.70, P < 0.01), medial temporal lobes (right, r = 0.73, P < 0.005; left, r = 0.71, P < 0.005), and the right parietal cortex (r = 0.71, P < 0.005). The number of years of smoking was positively correlated with mGluR5 DVR in the left putamen (r = 0.74, P < 0.005) and the amygdala (right, r = 0.67, P < 0.01; left, r = 0.64, P < 0.05). When age and the number of years of smoking were both included in the regression model of mGluR5 DVR in smokers, the numbers of years of smoking was not a significant predictor in any ROI, whereas age remained a significant predictor of mGluR5 DVR in the right putamen (P < 0.01).
In ex-smokers, age was only correlated with mGluR5 DVR in the right thalamus (r = 0.54, P < 0.05), number of years smoking was correlated with mGluR5 DVR in the left amygdala (r = 0.55, P < 0.05) and the right temporal lobe (r = 0.56, P < 0.05), and the duration of nicotine abstinence was not correlated with mGluR5 DVR in the 24 regions of interest (ROIs) examined.
In smokers, the number of cigarettes smoked per day and the score on the Fagerstrom test for nicotine dependence (FTND) did not correlate with mGluR5 DVR in any ROI. In ex-smokers, the number of cigarettes smoked per day was correlated with mGluR5 DVR only in the brainstem (r = −0.65, P < 0.05). In smokers and nonsmokers, BDI and BAI scores did not correlate with mGluR5 DVR in any ROI. In ex-smokers, the BAI score correlated with mGluR5 DVR in the left putamen (r = 0.54, P < 0.05) and the left thalamus (r = 0.63, P < 0.05).
Among the smokers and ex-smokers, mGluR5 DVR did not differ between male and female subjects (P > 0.05 in all ROIs). Female nonsmokers had a significantly lower mGluR5 DVR than male nonsmokers in all ROIs (24.7% vs. 8.5%; P < 0.05) except the anterior cingulate cortex (P < 0.1), posterior cingulate cortex (P < 0.1), left frontal cortex, and right putamen. The only regions that were significant after Bonferroni correction were the left caudate (padjusted < 0.05), right caudate (padjusted < 0.05), right medial temporal lobe (padjusted < 0.05), right medial orbitofrontal cortex (padjusted < 0.05), and right thalamus (padjusted < 0.05).
Discussion
The present study analyzes mGluR5 availability in smokers, ex-smokers, and nicotine-naïve controls by using PET. We found marked global reductions in mGluR5 binding in smokers and ex-smokers compared with controls. These findings provide human evidence of a potential role for mGluR5 in nicotine addiction and possibly other substance use disorders.
The reduction in mGluR5 binding associated with nicotine dependence affected all brain regions except the brainstem. Current nicotine consumption and estimates of current nicotine dependence did not correlate with mGluR5 binding. Female nonsmokers had significantly lower mGluR5 binding than male nonsmokers; however, these sex differences were not observed in smokers and ex-smokers. Age and mGluR5 binding were not correlated in nonsmokers but were positively correlated in most ROIs in smokers; age at smoking onset was positively correlated with mGluR5 binding in the basal ganglia, bilateral parietal cortex, and anterior and posterior cingulate cortex.
Previous preclinical studies have provided evidence of distinct roles for mGluR5 and dopamine receptors in addiction. Although dopamine receptor stimulation mediated cocaine and morphine reward but not sensitization, mGluR5 antagonism specifically affected cocaine reward and sensitization (12). A previous study explored whether the mGluR5 antagonist MPEP decreased nicotine and cocaine self-administration, reflecting attenuation of the reinforcing and incentive motivational effects of these drugs (13). This study found that MPEP decreased the breakpoints for nicotine and cocaine in rats, implicating mGluR5 in the reinforcing properties of nicotine. In contrast, other studies have suggested that negative allosteric modulators of mGluR5 reduce nicotine seeking but do not interfere with the reinforcement enhancement, mood enhancement, and cognitive-function enhancement effects of nicotine (14, 15). In a rat model of cocaine escalation, functional up-regulation of mGluR2/3 and down-regulation of mGluR5 were found to be likely factors in the transition from cocaine use to cocaine dependence (16). In addition, mGluR1/5 and their intracellular binding protein Homer1 were down-regulated in the nucleus accumbens after withdrawal from chronic cocaine administration (17–20). Although these findings consistently suggest that mGluR5 plays an important role in the development of substance dependence, the functional role of mGluR5 down-regulation remains unclear. It has been suggested that mGluR5 down-regulation represents a compensatory neuroadaptation (21), an enhancer of drug-induced reward acquisition (22), and a factor mediating the effects of contextual cues in the conditioned behavioral responses to nicotine (23).
In our PET study, the reductions in DVR indicate reduced binding of [11C]ABP688, which may reflect reduced mGluR5 density or a change in the affinity of the binding site. In a previous study of mGluR5 in depression, we examined mGluR5 protein expression in postmortem tissue preparations to facilitate better interpretation of our imaging data (24). The postmortem data showed a decrease in mGluR5 protein expression in the prefrontal cortex of depressed individuals, suggesting that the reduced mGluR5 DVR observed in depression most likely reflects a reduced density of functional receptors due to decreased protein concentration. Alternatively, a nicotine-related increase in basal glutamate could potentially trigger receptor internalization and lead to reduced binding to the receptor (25). Here, we can only speculate about the mechanisms underlying the widespread decrease in [11C]ABP688 binding to mGluR5 in smokers and ex-smokers. Reduced mGluR5 affinity for [11C]ABP688 may represent a biological trait associated with an increased risk for nicotine dependence, possibly due to genetic factors. Alternatively, reduced mGlu5 receptor availability may be the result of mGluR5 down-regulation, possibly because of the nicotine-induced increase in glutamate activity (26, 27). The relatively decreased reductions of mGluR5 affinity or density seen in the ex-smokers may reflect an incomplete recovery of mGluR5 receptors, especially since the ex-smokers were abstinent for only 25 wk on average, and supports the hypothesis of a lasting effect of regular nicotine consumption, possibly associated with the risk of relapse. The positive correlation between mGluR5 DVR and age at smoking onset in some brain regions suggests that early onset of nicotine dependence has a particularly strong effect on mGluR5 down-regulation or, alternatively, that reduced mGluR5 may predispose individuals to earlier onset of addiction. This correlation may reflect the preclinical finding that nicotine exposure in adolescent rats led to lasting reductions in mGluR function (28).
The most prominent reductions in mGluR5 binding in smokers were found in the medial orbitofrontal cortex and the caudate. The most prominent reductions of mGluR5 binding in ex-smokers were in the medial orbitofronal cortex and the thalamus. These findings are consistent with the results of functional imaging studies, which suggest that cortico-basal ganglia-thalamic brain circuits play an important role in nicotine addiction (29). Specifically, the dorsal striatum, including the caudate, has appeared to be recruited during the development of compulsive drug seeking (30). The orbitofrontal cortex with heavy projections to the dorsal striatum has been implicated in salience attribution and motivation, the disruption of which results in compulsivity (31). In smokers exposed to nicotine, the thalamus showed abnormal activity, possibly contributing not only to impairments in sensory processing and attention, but also to craving and the risk of relapse (32).
In animal models of Parkinson’s disease (PD), activation of mGluR5 contributed to the development of nigrostriatal damage (33), whereas blocking mGluR5 reduced nigrostriatal damage (34). As a result, the reduced mGluR5 binding observed in smokers and ex-smokers may explain the epidemiological finding that cigarette smokers and former heavy smokers (who stopped 1–20 y before PD onset) are 50% less likely to have PD than are age- and sex-matched nonsmokers (35).
This imaging study reports sex differences in mGluR5 binding in healthy, nonsmoking volunteers. Preclinical studies have suggested an interaction between estrogen receptors and mGluR5 (36). Interestingly, mGluR5 may play a role in the sex differences in nicotine action (37); women are less successful in quitting smoking and benefit less from nicotine replacement therapy than men. In rats, nicotine induced conditioned place preferences in males but not females, and a selective mGluR5 antagonist inhibited nicotine-induced place preferences only in male rats (38).
Several limitations of our methods merit comment. First, the design of this study cannot distinguish whether abnormalities in mGluR5 receptor binding reflect a biological vulnerability for nicotine dependence or are a consequence of nicotine intake. A second limitation is the potentially confounding effect of previous or undetected psychiatric or neurological conditions. However, exclusion of subjects with a history of psychiatric disorders did not alter the results of this study. Finally, we used a bolus-infusion technique and normalized the PET images to the cerebellar radioactivity concentration to avoid the need for potentially painful arterial cannulation. This reference tissue method was based on previous in vivo and in vitro evidence, demonstrating that mGluR5 levels are extremely low in the cerebellum relative to the predefined ROIs in other brain areas (39). A recent study demonstrated that quantification of mGluR5 receptor with [18F]FPEB using noninvasive modeling with the cerebellum as a reference region may be feasible (40). In support of this method, recent in vitro and in vivo studies suggested negligible ABP688 binding in the cerebellum and validated the use of the cerebellum as a reference region (39, 41). In addition, in a recent postmortem study, mGluR5 protein expression was not observed in the cerebellum (24). Previous studies on cerebellar mRNA expression have demonstrated both the presence (42) and absence (43) of mGluR5 mRNA or weak mRNA expression exclusively in Bergmann glia (44). In summary, in all studies of cerebellar mGluR5 concentration that collected data from more than one subject (45) found negligible mGluR5 expression in the cerebellum, suggesting that the cerebellum can be used as a reference region.
In conclusion, we demonstrated marked global reductions in mGluR5 binding in smokers and ex-smokers. These findings combined with the correlational analyses of clinical variables suggest that neurotransmission at mGluR5 is reduced as a precondition of nicotine dependence and/or because of long-term compensatory changes in the activity of the glutamate system. The implications of these findings are that mGluR5 receptor binding might be an effective biomarker in smoking and a good target for the discovery of novel medications against nicotine dependence. These findings in living subjects encourage future studies of the genetic and environmental influences on mGluR5 and the interactions among mGluR5, glutamate homeostasis (46), ionotropic glutamate receptors, and dopamine and opioid receptor systems (47) in drug abuse disorders.
Patients and Methods
Subjects.
Participants were recruited through advertisements in local newspapers and posters at Zurich University Hospital and were evaluated during screening visits to the outpatient psychiatry clinic of the Zurich University Hospital. The inclusion criterion for smokers was the consumption of at least 11 cigarettes per day. Ex-smokers had to smoke at least 11 cigarettes per day in the past and had to be nicotine abstinent for at least 3 mo. The inclusion criterion for nonsmokers was lifetime nicotine abstinence. To exclude subjects with current psychiatric conditions, research subjects were evaluated with an unstructured clinical interview by a psychiatrist and a structured interview using the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (48, 49). The clinical evaluation also included a physical examination, laboratory tests and, in some cases, electrocardiography. Exclusion criteria included current psychiatric, neurological, or medical disorders, pregnancy, breastfeeding, a history of psychosis, manic episodes, substance dependence (except for nicotine dependence), autism, and present or past regular use of psychotropic medications. Magnetic resonance imaging (MRI) was performed on each subject and assessed by a radiologist to exclude any structural brain pathology. Subjects were enrolled in the study after they received a full explanation of the study purpose and procedures, and after written consent was obtained as approved by the local ethics committee (KantonaleEthikkommission Zürich). Clinical evaluations on the day of the PET scan included FTND (50), BAI (51), and BDI (52) assessments.
PET.
We applied a bolus/infusion protocol (53) that we had evaluated for PET with [11C]ABP688 (24, 54). Previous studies showed that equilibrium between the tracer in tissue and blood is achieved 40 min after the start of radioligand infusion (53, 55). Before scanning, catheters were placed in the right antecubital vein for tracer injection. Subjects were scanned in a single session by using [11C]ABP688 and a whole body PET scanner (Discovery VCT; GE Healthcare) run in three-dimensional mode with an axial field of view of 14.6 cm and an in-plane resolution of 7.0 mm. Low-dose CT was acquired before tracer injection for attenuation correction. Using a previously evaluated equilibrium protocol (54, 56), a total of 500–650 MBq of [11C]ABP688 in a 50-mL volume was administered by using an infusion pump. Half of the activity was given as a bolus over 2 min, and the other half was infused over the next 58 min. At the start of the bolus injection, the emission scan was initiated, which consisted of 20 frames: 10 frames of 60 s followed by 10 frames of 300 s, leading to a total duration of 60 min per acquisition. Transaxial images were reconstructed to a 128 × 128 matrix with 35 slices of 2.34 × 2.34 × 3.27 mm voxel size by using filtered back projection. In each subject, we verified that equilibrium was reached by analyzing the tissue time–activity curve in a cingular (high receptor density) and a cerebellar (low receptor density) region. The activity reached equilibrium at 30 min. The ratio of tissue activity divided by the cerebellar activity at equilibrium (CT/Ccer at 45–60 min) was chosen as a measure of receptor availability. At equilibrium, CT/Ccer is equal to the ratio of the distribution volumes of the tracer in target and reference tissue, (CT/Ccer)eq = VT/VND, where VND is the distribution volume of the nondisplaceable compartment (55), in our case the cerebellum. VT/VND is referred to as the DVR. It can be further demonstrated that the DVR equals BPT + 1, where BPT is the binding potential in the target tissue, which is often used as measure for receptor density divided by affinity (53).
Statistical Analysis.
All calculations were performed by using dedicated PMOD software, version 3 (PMOD Technologies). The DVR images were then transformed to a common space, the Montreal Neurological Institute template, by using PMOD.
Predefined ROIs were defined over various brain structures and applied to the normalized DVR PET images by using PMOD. The ROIs included four cortical regions (frontal, parietal, temporal, and occipital), two regions within the cingulate gyrus (anterior and posterior), three regions in the prosencephalon (caudate, putamen, and thalamus), three regions in the limbic systems (medial orbitofrontal cortex, amygdala, and medial temporal lobe), and one brainstem region. In addition, we calculated the average gray matter mGluR5 DVR by using a gray matter mask with a pixel value cutoff of 12. Two-sample two-tailed t tests were used to test the differences in mGluR5 DVR between the clinical groups. Bonferroni adjustment (padjusted) was used to correct for the multiple comparisons used when testing group differences in the 24 predefined ROIs. Pearson correlations were used to test associations between continuous variables and mGluR5 DVR within each clinical group. For these correlational analyses, no Bonferroni correction was applied.
Acknowledgments
We thank the PET team (Zurich University) for help with data acquisition. PET data were analyzed at Zurich University Hospital and at Psychiatric University Hospital Bern by Alfred Buck, Valerie Treyer, Funda Akkus, and Gregor Hasler. The PET study was supported by Novartis Pharma, OPO Foundation, Zurich; Olga Mayenfisch Foundation, Zurich; Vontobel Foundation, Zurich; and Hartmann Muller Foundation, Zurich.
Footnotes
Conflict of interest statement: B.G.M. and J.S. work for Novartis Pharma, which is developing and testing drugs targeting the mGlu5 receptor.
This article is a PNAS Direct Submission.
References
- 1.Kalivas PW, Volkow ND. The neural basis of addiction: A pathology of motivation and choice. Am J Psychiatry. 2005;162(8):1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 2.Olive MF. Metabotropic glutamate receptor ligands as potential therapeutics for addiction. Curr Drug Abuse Rev. 2009;2(1):83–98. doi: 10.2174/1874473710902010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiamulera C, et al. Reinforcing and locomotor stimulant effects of cocaine are absent in mGluR5 null mutant mice. Nat Neurosci. 2001;4(9):873–874. doi: 10.1038/nn0901-873. [DOI] [PubMed] [Google Scholar]
- 4.Kenny PJ, et al. Metabotropic glutamate 5 receptor antagonist MPEP decreased nicotine and cocaine self-administration but not nicotine and cocaine-induced facilitation of brain reward function in rats. Ann N Y Acad Sci. 2003;1003:415–418. doi: 10.1196/annals.1300.040. [DOI] [PubMed] [Google Scholar]
- 5.Paterson NE, Semenova S, Gasparini F, Markou A. The mGluR5 antagonist MPEP decreased nicotine self-administration in rats and mice. Psychopharmacology (Berl) 2003;167(3):257–264. doi: 10.1007/s00213-003-1432-z. [DOI] [PubMed] [Google Scholar]
- 6.Tessari M, Pilla M, Andreoli M, Hutcheson DM, Heidbreder CA. Antagonism at metabotropic glutamate 5 receptors inhibits nicotine- and cocaine-taking behaviours and prevents nicotine-triggered relapse to nicotine-seeking. Eur J Pharmacol. 2004;499(1-2):121–133. doi: 10.1016/j.ejphar.2004.07.056. [DOI] [PubMed] [Google Scholar]
- 7.Bespalov AY, et al. Metabotropic glutamate receptor (mGluR5) antagonist MPEP attenuated cue- and schedule-induced reinstatement of nicotine self-administration behavior in rats. Neuropharmacology. 2005;49(Suppl 1):167–178. doi: 10.1016/j.neuropharm.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 8.Kenny PJ, Boutrel B, Gasparini F, Koob GF, Markou A. Metabotropic glutamate 5 receptor blockade may attenuate cocaine self-administration by decreasing brain reward function in rats. Psychopharmacology (Berl) 2005;179(1):247–254. doi: 10.1007/s00213-004-2069-2. [DOI] [PubMed] [Google Scholar]
- 9.Lee B, Platt DM, Rowlett JK, Adewale AS, Spealman RD. Attenuation of behavioral effects of cocaine by the Metabotropic Glutamate Receptor 5 Antagonist 2-Methyl-6-(phenylethynyl)-pyridine in squirrel monkeys: Comparison with dizocilpine. J Pharmacol Exp Ther. 2005;312(3):1232–1240. doi: 10.1124/jpet.104.078733. [DOI] [PubMed] [Google Scholar]
- 10.van der Kam EL, de Vry J, Tzschentke TM. Effect of 2-methyl-6-(phenylethynyl) pyridine on intravenous self-administration of ketamine and heroin in the rat. Behav Pharmacol. 2007;18(8):717–724. doi: 10.1097/FBP.0b013e3282f18d58. [DOI] [PubMed] [Google Scholar]
- 11.Ametamey SM, et al. Radiosynthesis and preclinical evaluation of 11C-ABP688 as a probe for imaging the metabotropic glutamate receptor subtype 5. J Nucl Med. 2006;47(4):698–705. [PubMed] [Google Scholar]
- 12.Veeneman MM, et al. Dissociable roles of mGlu5 and dopamine receptors in the rewarding and sensitizing properties of morphine and cocaine. Psychopharmacology (Berl) 2011;214(4):863–876. doi: 10.1007/s00213-010-2095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paterson NE, Markou A. The metabotropic glutamate receptor 5 antagonist MPEP decreased break points for nicotine, cocaine and food in rats. Psychopharmacology (Berl) 2005;179(1):255–261. doi: 10.1007/s00213-004-2070-9. [DOI] [PubMed] [Google Scholar]
- 14.Palmatier MI, Liu X, Donny EC, Caggiula AR, Sved AF. Metabotropic glutamate 5 receptor (mGluR5) antagonists decrease nicotine seeking, but do not affect the reinforcement enhancing effects of nicotine. Neuropsychopharmacology. 2008;33(9):2139–2147. doi: 10.1038/sj.npp.1301623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harrison AA, Gasparini F, Markou A. Nicotine potentiation of brain stimulation reward reversed by DH beta E and SCH 23390, but not by eticlopride, LY 314582 or MPEP in rats. Psychopharmacology (Berl) 2002;160(1):56–66. doi: 10.1007/s00213-001-0953-6. [DOI] [PubMed] [Google Scholar]
- 16.Hao Y, Martin-Fardon R, Weiss F. Behavioral and functional evidence of metabotropic glutamate receptor 2/3 and metabotropic glutamate receptor 5 dysregulation in cocaine-escalated rats: Factor in the transition to dependence. Biol Psychiatry. 2010;68(3):240–248. doi: 10.1016/j.biopsych.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghasemzadeh MB, Vasudevan P, Mueller C, Seubert C, Mantsch JR. Neuroadaptations in the cellular and postsynaptic group 1 metabotropic glutamate receptor mGluR5 and Homer proteins following extinction of cocaine self-administration. Neurosci Lett. 2009;452(2):167–171. doi: 10.1016/j.neulet.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ary AW, Szumlinski KK. Regional differences in the effects of withdrawal from repeated cocaine upon Homer and glutamate receptor expression: A two-species comparison. Brain Res. 2007;1184:295–305. doi: 10.1016/j.brainres.2007.09.035. [DOI] [PubMed] [Google Scholar]
- 19.Swanson CJ, Baker DA, Carson D, Worley PF, Kalivas PW. Repeated cocaine administration attenuates group I metabotropic glutamate receptor-mediated glutamate release and behavioral activation: A potential role for Homer. J Neurosci. 2001;21(22):9043–9052. doi: 10.1523/JNEUROSCI.21-22-09043.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szumlinski KK, et al. Homer isoforms differentially regulate cocaine-induced neuroplasticity. Neuropsychopharmacology. 2006;31(4):768–777. doi: 10.1038/sj.npp.1300890. [DOI] [PubMed] [Google Scholar]
- 21.Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10(8):561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- 22.Rutten K, Van Der Kam EL, De Vry J, Bruckmann W, Tzschentke TM. The mGluR5 antagonist 2-methyl-6-(phenylethynyl)-pyridine (MPEP) potentiates conditioned place preference induced by various addictive and non-addictive drugs in rats. Addict Biol. 2010;16(1):108–115. doi: 10.1111/j.1369-1600.2010.00235.x. [DOI] [PubMed] [Google Scholar]
- 23.Tronci V, Vronskaya S, Montgomery N, Mura D, Balfour DJ. The effects of the mGluR5 receptor antagonist 6-methyl-2-(phenylethynyl)-pyridine (MPEP) on behavioural responses to nicotine. Psychopharmacology (Berl) 2010;211(1):33–42. doi: 10.1007/s00213-010-1868-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deschwanden A, et al. Reduced metabotropic glutamate receptor 5 density in major depression determined by [11C]ABP688 PET and postmortem study. Am J Psychiatry. 2011;168(7):727–734. doi: 10.1176/appi.ajp.2011.09111607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyake N, et al. Imaging changes in glutamate transmission in vivo with the metabotropic glutamate receptor 5 tracer [11C] ABP688 and N-acetylcysteine challenge. Biol Psychiatry. 2011;69(9):822–824. doi: 10.1016/j.biopsych.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 26.Mansvelder HD, Keath JR, McGehee DS. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron. 2002;33(6):905–919. doi: 10.1016/s0896-6273(02)00625-6. [DOI] [PubMed] [Google Scholar]
- 27.Mansvelder HD, McGehee DS. Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron. 2000;27(2):349–357. doi: 10.1016/s0896-6273(00)00042-8. [DOI] [PubMed] [Google Scholar]
- 28.Counotte DS, et al. Lasting synaptic changes underlie attention deficits caused by nicotine exposure during adolescence. Nat Neurosci. 2011;14(4):417–419. doi: 10.1038/nn.2770. [DOI] [PubMed] [Google Scholar]
- 29.Brody AL. Functional brain imaging of tobacco use and dependence. J Psychiatr Res. 2006;40(5):404–418. doi: 10.1016/j.jpsychires.2005.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Everitt BJ, et al. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363(1507):3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35(1):217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharma A, Brody AL. In vivo brain imaging of human exposure to nicotine and tobacco. Handb Exp Pharmacol. 2009;192:145–171. doi: 10.1007/978-3-540-69248-5_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Battaglia G, et al. Endogenous activation of mGlu5 metabotropic glutamate receptors contributes to the development of nigro-striatal damage induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in mice. J Neurosci. 2004;24(4):828–835. doi: 10.1523/JNEUROSCI.3831-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Armentero MT, Fancellu R, Nappi G, Bramanti P, Blandini F. Prolonged blockade of NMDA or mGluR5 glutamate receptors reduces nigrostriatal degeneration while inducing selective metabolic changes in the basal ganglia circuitry in a rodent model of Parkinson’s disease. Neurobiol Dis. 2006;22(1):1–9. doi: 10.1016/j.nbd.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 35.Fratiglioni L, Wang HX. Smoking and Parkinson’s and Alzheimer’s disease: Review of the epidemiological studies. Behav Brain Res. 2000;113(1-2):117–120. doi: 10.1016/s0166-4328(00)00206-0. [DOI] [PubMed] [Google Scholar]
- 36.Grove-Strawser D, Boulware MI, Mermelstein PG. Membrane estrogen receptors activate the metabotropic glutamate receptors mGluR5 and mGluR3 to bidirectionally regulate CREB phosphorylation in female rat striatal neurons. Neuroscience. 2010;170(4):1045–1055. doi: 10.1016/j.neuroscience.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pogun S, Yararbas G. Sex differences in nicotine action. Handb Exp Pharmacol. 2009;192:261–291. doi: 10.1007/978-3-540-69248-5_10. [DOI] [PubMed] [Google Scholar]
- 38.Yararbas G, Keser A, Kanit L, Pogun S. Nicotine-induced conditioned place preference in rats: Sex differences and the role of mGluR5 receptors. Neuropharmacology. 2010;58(2):374–382. doi: 10.1016/j.neuropharm.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 39.Elmenhorst D, et al. In vivo and in vitro validation of reference tissue models for the mGluR(5) ligand [(11)C]ABP688. J Cereb Blood Flow Metab. 2010;30(8):1538–1549. doi: 10.1038/jcbfm.2010.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barret O, et al. Quantitation of glutamate mGluR5 receptor with 18F-FPEB PET in humans. J Nucl Med. 2010;51(Supplement 2):215. [Google Scholar]
- 41.Ametamey SM, et al. Human PET studies of metabotropic glutamate receptor subtype 5 with 11C-ABP688. J Nucl Med. 2007;48(2):247–252. [PubMed] [Google Scholar]
- 42.Malherbe P, et al. Identification and characterization of a novel splice variant of the metabotropic glutamate receptor 5 gene in human hippocampus and cerebellum. Brain Res Mol Brain Res. 2002;109(1-2):168–178. doi: 10.1016/s0169-328x(02)00557-0. [DOI] [PubMed] [Google Scholar]
- 43.Daggett LP, et al. Molecular and functional characterization of recombinant human metabotropic glutamate receptor subtype 5. Neuropharmacology. 1995;34(8):871–886. doi: 10.1016/0028-3908(95)00085-k. [DOI] [PubMed] [Google Scholar]
- 44.Berthele A, et al. Expression of metabotropic glutamate receptor subtype mRNA (mGluR1-8) in human cerebellum. Neuroreport. 1999;10(18):3861–3867. doi: 10.1097/00001756-199912160-00026. [DOI] [PubMed] [Google Scholar]
- 45.Patel S, et al. Species differences in mGluR5 binding sites in mammalian central nervous system determined using in vitro binding with [18F]F-PEB. Nucl Med Biol. 2007;34(8):1009–1017. doi: 10.1016/j.nucmedbio.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 46.Kupchik YM, et al. The effect of N-acetylcysteine in the nucleus accumbens on neurotransmission and relapse to cocaine. Biol Psychiatry. 2012;71(11):978–986. doi: 10.1016/j.biopsych.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berrendero F, Robledo P, Trigo JM, Martín-García E, Maldonado R. Neurobiological mechanisms involved in nicotine dependence and reward: Participation of the endogenous opioid system. Neurosci Biobehav Rev. 2010;35(2):220–231. doi: 10.1016/j.neubiorev.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) New York: Biometrics Res, NY State Psychiatr Inst; 2001. [Google Scholar]
- 49.First MB, Gibbon M, Spitzer RL, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders: Nonpatient Edition (SCID-I/NP) New York: NY State Psychiatr Inst; 1996. [Google Scholar]
- 50.Fagerstrom KO, Schneider NG. Measuring nicotine dependence: A review of the Fagerstrom Tolerance Questionnaire. J Behav Med. 1989;12(2):159–182. doi: 10.1007/BF00846549. [DOI] [PubMed] [Google Scholar]
- 51.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: Psychometric properties. J Consult Clin Psychol. 1988;56(6):893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 52.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 53.Carson RE, et al. Comparison of bolus and infusion methods for receptor quantitation: Application to [18F]cyclofoxy and positron emission tomography. J Cereb Blood Flow Metab. 1993;13(1):24–42. doi: 10.1038/jcbfm.1993.6. [DOI] [PubMed] [Google Scholar]
- 54.Burger C, et al. Evaluation of a bolus/infusion protocol for 11C-ABP688, a PET tracer for mGluR5. Nucl Med Biol. 2010;37(7):845–851. doi: 10.1016/j.nucmedbio.2010.04.107. [DOI] [PubMed] [Google Scholar]
- 55.Blasberg RG, et al. Strategies for the study of the opaite receptor in brain: Application to the opiate antagonist cyclofoxy. J Cereb Blood Flow Metab. 1989;9:S732. [Google Scholar]
- 56.Treyer V, et al. Evaluation of the metabotropic glutamate receptor subtype 5 using PET and 11C-ABP688: Assessment of methods. J Nucl Med. 2007;48(7):1207–1215. doi: 10.2967/jnumed.107.039578. [DOI] [PubMed] [Google Scholar]