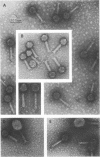
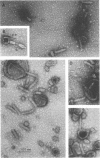
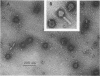
Abstract
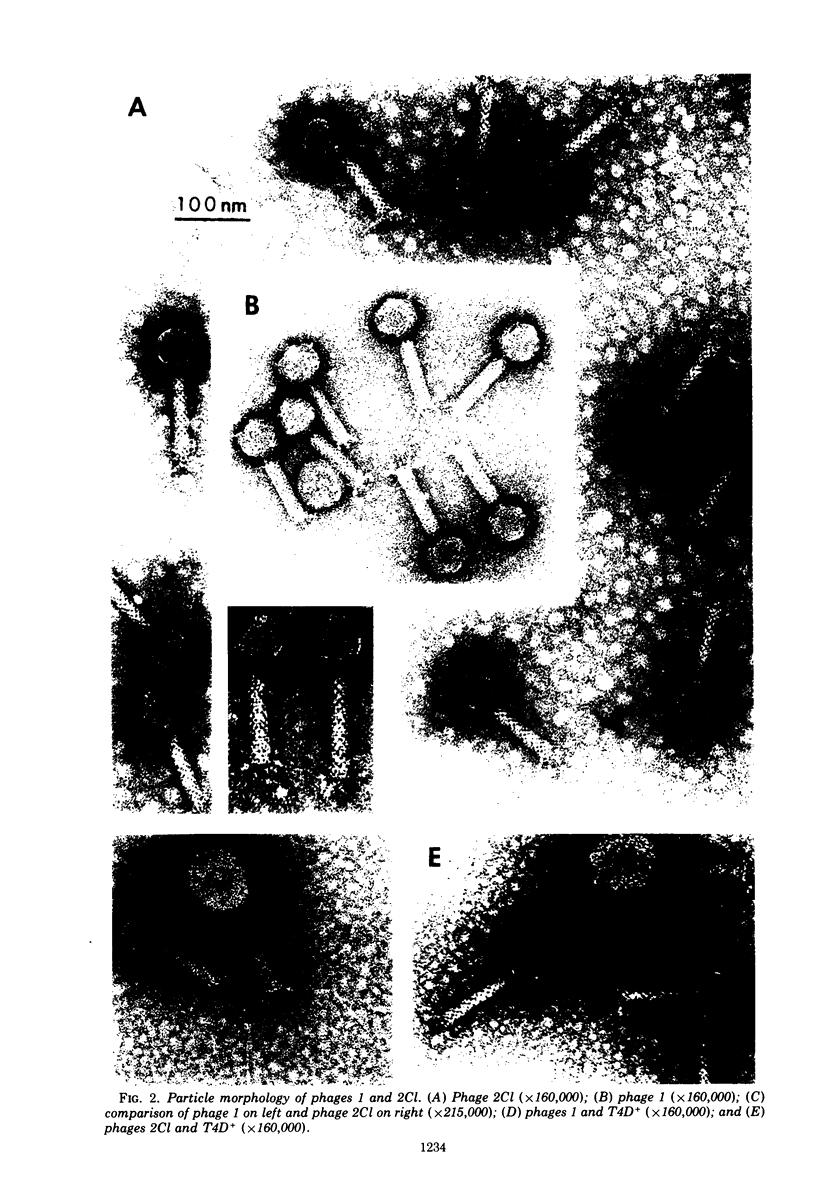
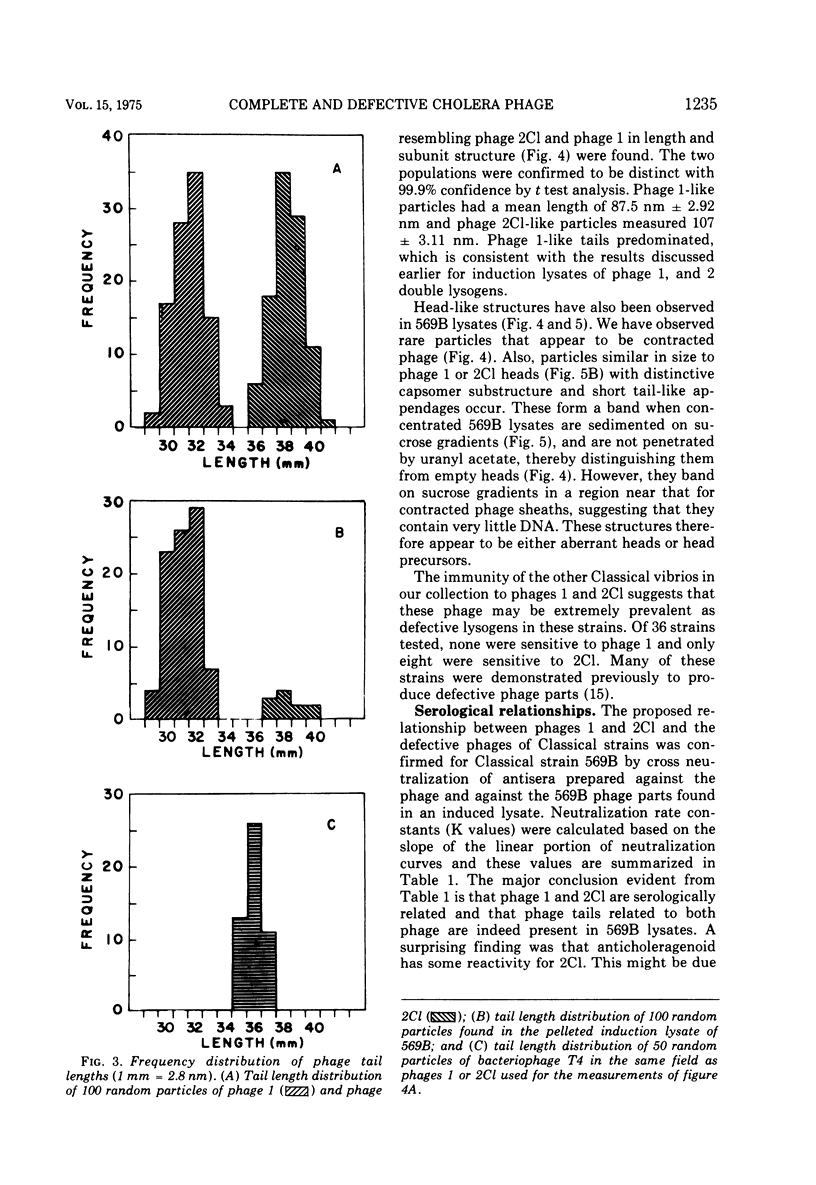
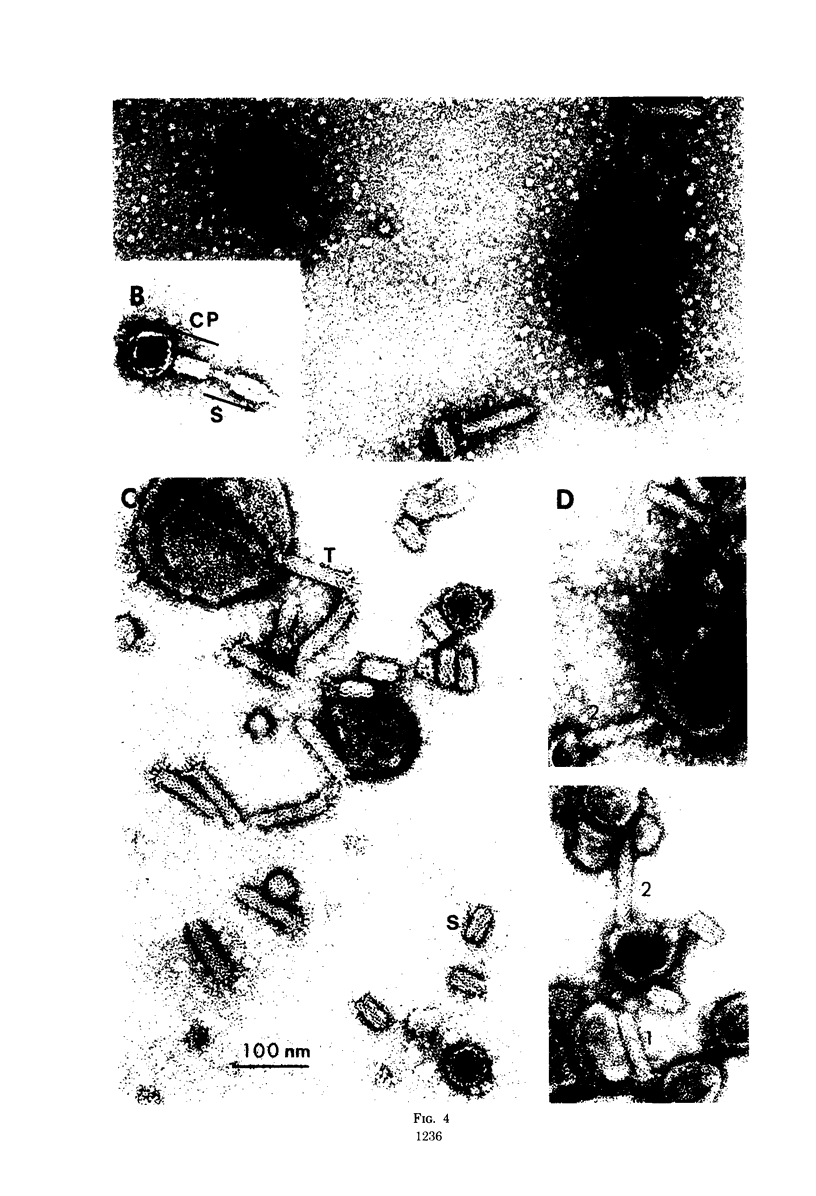
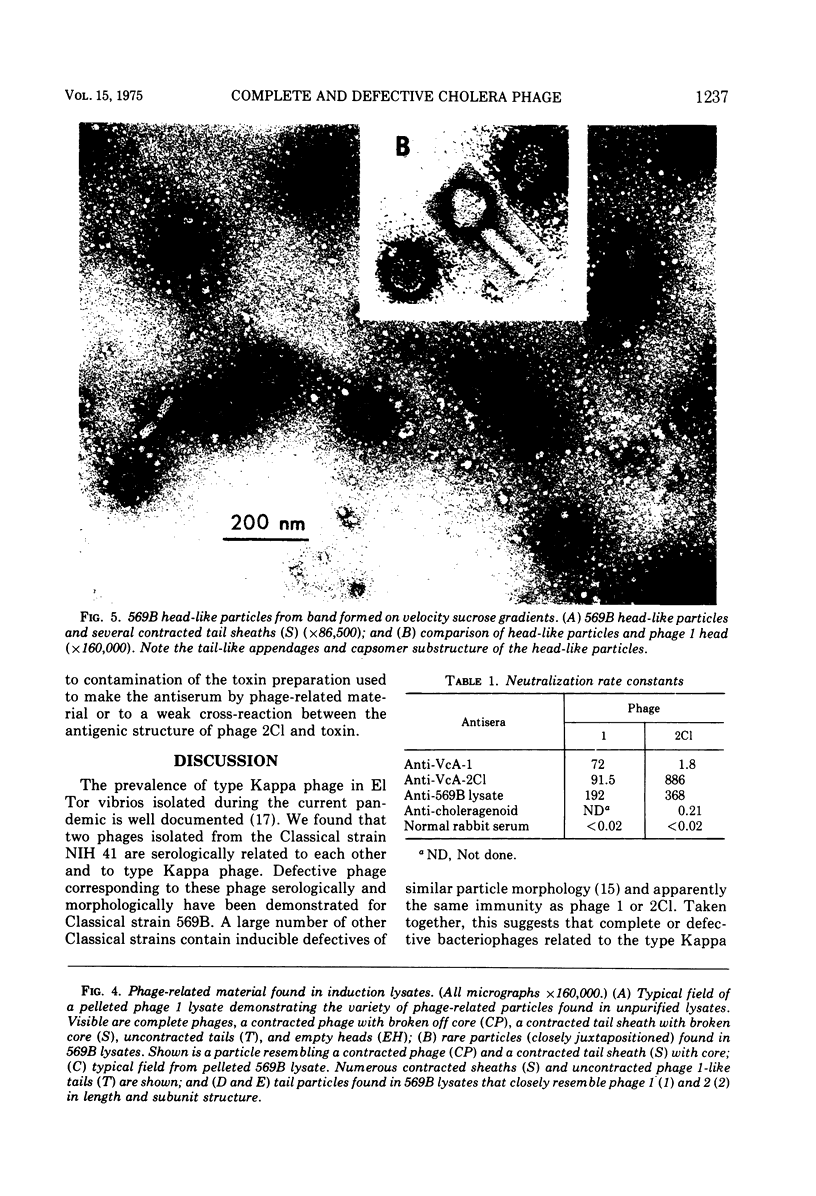
The Classical Vibrio cholerae strain NIH 41 contains two temperate bacteriophages, designated VcA-1 and VcA-2, that are distinguished by immunity, plaque morphology, induction kinetics, and particle morphology. Both phage are serologically related to phage Kappa. However, only phage VcA-2 has the Kappa type host range and immunity. The induction kinetics and immunity patterns of Classical vibrios suggest that these strains may contain defective phage related to the phages isolated from NIH 41. Classical strain 569B releases phage-tail structures upon induction that are morphologically and serologically related to both phages VcA-1 and VcA-2. The possible reason for the defectiveness of these phages in 569B is discussed. It is concluded that complete or defective bacteriophages of the Kappa type morphology and serology are extremely prevalent in V. cholerae, regardless of biotype.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradley D. E. Ultrastructure of bacteriophage and bacteriocins. Bacteriol Rev. 1967 Dec;31(4):230–314. doi: 10.1128/br.31.4.230-314.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casjens S., King J. P22 morphogenesis. I: Catalytic scaffolding protein in capsid assembly. J Supramol Struct. 1974;2(2-4):202–224. doi: 10.1002/jss.400020215. [DOI] [PubMed] [Google Scholar]
- Chatterjee S. N., Das J., Barua D. Electron microscopy of cholera phages. Indian J Med Res. 1965 Oct;53(10):934–937. [PubMed] [Google Scholar]
- Finkelstein R. A. Monospecific equine antiserum against cholera exo-enterotoxin. Infect Immun. 1970 Dec;2(6):691–697. doi: 10.1128/iai.2.6.691-697.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garro A. J., Marmur J. Defective bacteriophages. J Cell Physiol. 1970 Dec;76(3):253–263. doi: 10.1002/jcp.1040760305. [DOI] [PubMed] [Google Scholar]
- Jayawardene A., Farkas-Himsley H. Particulate nature of vibriocin: a bacteriocin from Vibrio comma. Nature. 1968 Jul 6;219(5149):79–80. doi: 10.1038/219079a0. [DOI] [PubMed] [Google Scholar]
- King J. Assembly of the tail of bacteriophage T4. J Mol Biol. 1968 Mar 14;32(2):231–262. doi: 10.1016/0022-2836(68)90007-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Mölbert E., Showe M., Kellenberger E. Form-determining function of the genes required for the assembly of the head of bacteriophage T4. J Mol Biol. 1970 Apr 14;49(1):99–113. doi: 10.1016/0022-2836(70)90379-7. [DOI] [PubMed] [Google Scholar]
- Luftig R. B., Lundh N. P. Bacteriophage T4 head morphogenesis. Isolation, partial characterization, and fate of gene 21-defective tau-particles. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1636–1640. doi: 10.1073/pnas.70.6.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEWMAN F. S., EISENSTARK A. PHAGE-HOST RELATIONSHIPS IN VIBRIO CHOLERAE. J Infect Dis. 1964 Jun;114:217–225. doi: 10.1093/infdis/114.3.217. [DOI] [PubMed] [Google Scholar]
- Parker C., Richardson S. H., Romig W. R. Production of Bacteriophage-Associated Materials by Vibrio cholerae: Possible Correlation with Pathogenicity. Infect Immun. 1970 Apr;1(4):417–420. doi: 10.1128/iai.1.4.417-420.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston L., Drexler H., Richardson S. H. Characterization of vibriophage VA-1. J Gen Virol. 1973 Oct;21:155–158. doi: 10.1099/0022-1317-21-1-155. [DOI] [PubMed] [Google Scholar]