Abstract
During development, the hematopoietic lineage transits through hemogenic endothelium, but the signaling pathways effecting this transition are incompletely characterized. Although the Hedgehog (Hh) pathway is hypothesized to play a role in patterning blood formation, early embryonic lethality of mice lacking Hh signaling precludes such analysis. To determine a role for Hh signaling in patterning of hemogenic endothelium, we assessed the effect of altered Hh signaling in differentiating mouse ES cells, cultured mouse embryos, and developing zebrafish embryos. In differentiating mouse ES cells and mouse yolk sac cultures, addition of Indian Hh ligand increased hematopoietic progenitors, whereas chemical inhibition of Hh signaling reduced hematopoietic progenitors without affecting primitive streak mesoderm formation. In the setting of Hh inhibition, induction of either Notch signaling or overexpression of Stem cell leukemia (Scl)/T-cell acute lymphocytic leukemia protein 1 rescued hemogenic vascular-endothelial cadherin+ cells and hematopoietic progenitor formation. Together, our results reveal that Scl overexpression is sufficient to rescue the developmental defects caused by blocking the Hh and Notch pathways, and inform our understanding of the embryonic endothelial-to-hematopoietic transition.
Keywords: dorsal aorta, runx1, hematopoietic stem cell, AGM, Tie2
During murine development, hematopoietic progenitors first appear as blood islands in the extraembryonic yolk sac around embryonic day 7.5 (E7.5) (1). Hematopoietic cells capable of conferring lymphoid-myeloid engraftment in neonatal recipients can be detected in the yolk sac around E9 (2), but it is widely held that true definitive hematopoietic stem cells (HSCs) responsible for life-long lymphoid-myeloid hematopoiesis first appear at E10.5 in the aorta-gonad-mesonephros (AGM) region (3). Understanding the mechanisms by which hematopoietic cells are generated during embryonic development lends insight into how one might generate engraftable HSCs in vitro for use in clinical settings (4).
Recent evidence suggests that HSCs transition through a hemogenic endothelial intermediate (5–9). Hemogenic endothelial cells are thought to arise from Flk1 (VEGF receptor 2) -positive cells, which then differentiate into endothelial cells expressing vascular-endothelial cadherin (VE-cadherin) or Tie2 (5, 9). VE-cadherin is expressed on vascular endothelial cells and is required for vascular lumen formation (9, 10). The transcription factor Runx1 is required for the endothelial-to-hematopoietic transition (8, 11). However, other transcription factors that can convert such endothelium to hematopoietic cells and the signaling pathways involved in the generation of the hemogenic endothelium remain incompletely described.
Because of the role of Hedgehog (Hh) signaling in vascular remodeling, we hypothesized that it plays a role in the generation of hemogenic endothelium (12). The Hh pathway is well-conserved and implicated in the regulation of both embryonic and adult hematopoiesis (4, 13–16). The binding of Hh ligands to the receptor Patched derepresses the transmembrane protein Smoothened (Smo), resulting in the activation and nuclear translocation of the Gli transcription factor family. The mammalian genome encodes three isoforms, Sonic Hh (Shh), Indian Hh (Ihh), and Desert Hh, of which Ihh has been most closely linked to hematopoietic development (12–14). In early gastrulation, Ihh signaling from the visceral endoderm is deemed necessary and sufficient for primitive erythropoiesis in the yolk sacs of mouse embryo explants (12, 13). Because of early embryonic lethality of embryos that lack components of the Hh pathway, the role of Hh at later stages of embryonic hematopoiesis in mice is less well-understood (12). Although adult hematopoietic cells in mice do not require Hh signaling (17, 18), studies in zebrafish suggest that Hh and Bone morphogenic protein (BMP) signaling are required for the polarization of the dorsal aorta, which implies that Hh patterns the hemogenic endothelium (15, 16).
Here, we use complementary developmental systems (differentiating murine ES cells, midgestation murine embryos, and developing zebrafish embryos) to define the distinct relationship of Hh and Notch signaling to the generation of the hemogenic endothelium and endothelial-to-hematopoietic transition. We show that Hh signaling is required for the generation of hemogenic VE-cadherin+ endothelial cells, that Hh acts upstream of Notch, and that Scl/Tal1 induction mediates the conversion of hemogenic endothelial cells to hematopoietic cells. This relationship between Hh, Notch, and Scl is conserved between mice and zebrafish. Together, this Hh-Notch-Scl axis regulates distinct stages of the endothelial-to-hematopoietic transition.
Results
Hh Signaling Augments Hematopoiesis During Mouse ES Cell Differentiation.
To study the role of the Hh pathway in embryonic hematopoiesis, we manipulated differentiating mouse ES cells (mESCs), an accessible in vitro model of murine blood development (19). Aggregated mESCs can be aggregated into embryoid bodies (EBs), forming primitive streak-like mesoderm on day 2, hemangioblasts on day 3.25 and hematopoietic precursors after day 5 (Fig. 1A) (20). To define the timing of endogenous Hh signaling in relation to these stages of hematopoietic development, we monitored the expression of Hh ligands (Ihh and Shh) and their transcriptional target (Gli1) during EB differentiation (Fig. 1B). Expression of Ihh and Shh as well as Gli1 increased from day 3, suggesting that Hh pathway activity in whole EBs is prominent after day 3 of differentiation (Fig. 1B).
Fig. 1.

Hh treatment augments hematopoiesis at a critical time point in EBs. (A) Experimental schema for EB development. (B) RNA expression of Hh ligands (Shh and Ihh) and target gene (Gli1) in whole EBs as assessed by quantitative PCR. Values were normalized to day 6 RNA levels (n ≥ 3). (C) CFUs from day 6 whole EBs in response to 1-d pulse induction of IHH during days 2–6 of EB differentiation (n = 6). (D) Flow cytometric quantification of CD41+c-Kit+ hematopoietic precursors cells in day 6 whole EBs that were treated with 1-d IHH pulses during days 2–6 of differentiation. Horizontal bars indicate mean values (n = 4). (E) Gli1 RNA levels in VE-cadherin+ and VE-cadherin− cell populations isolated on day 5 of EB differentiation (n = 3). (F) CFUs from day 6 VE-cadherin+ sorted cells after days 4–5 IHH treatment (n = 6). (G) Images of hemato-endothelial culture at 10× objective (Upper) and flow cytometry of CD45+ cells from hemato-endothelial culture (Lower). Whole EBs were treated with IHH on days 4–5, and VE-cadherin+CD41−CD45− cells were sorted on day 6 and cultured in hemato-endothelial medium for 4–6 d.
To determine whether increased Hh signaling augments hematopoiesis in EBs, we treated whole EBs with recombinant human IHH protein for 1-d intervals from day 2 to 6. IHH treatment from day 4 to 5 increased the number of hematopoietic CFUs and the percentage of cells marked by CD41+c-Kit+, a population that represents the earliest hematopoietic colony-forming cells in this culture system (Fig. 1 C and D and Fig. S1A) (21, 22). IHH treatment up-regulated downstream Hh targets at the end of each pulse (Fig. S1B). To activate Hh signaling by alternative means, we created a transgenic ESC line that expresses a constitutively active form of the Hh signaling mediator SMO (SMO-M2) in response to doxycycline (dox) induction (Fig. S2 A–C) (23, 24). Similar to IHH treatment, we found that SMO-M2 induction of 20 ng/mL dox on days 3–4 and 100 ng/mL dox on days 4–5 enhanced hematopoiesis from whole EBs (Fig. S2D), indicating concentration- and time-dependent effects of Hh signaling. Taken together, our data indicate that modulating the Hh pathway with either recombinant IHH protein or transgenic expression of SMO-M2 promotes hematopoiesis between days 3 and 5 of EB differentiation.
Mouse KO models of Hh signaling suggest that vascular progenitors are present but not properly specified into vessels (12). We hypothesized that VE-cadherin+ cells would be responsive to Hh signaling, because VE-cadherin mediates contacts between VE cells. Indeed, VE-cadherin+ cells sorted on day 5 of EB differentiation showed elevated endogenous Gli1 expression (Fig. 1E). We analyzed microarray gene expression data in sorted populations of cells from day 6 of differentiating murine EBs. Gli1 expression was high in VE-cadherin+CD41− endothelial cells and low in CD41+ populations in day 6 EBs (Fig. S3). Moreover, EBs with overexpression of SMO-M2 (GFP+) had relatively greater percentages of VE-cadherin+ cells (Fig. S2 E–G). Finally, VE-cadherin+ cells isolated on day 6 from IHH-treated whole EBs were enriched in hematopoietic activity (Fig. 1F), suggesting that Hh signaling promotes hematopoietic fate through VE-cadherin+ cell intermediates.
To investigate whether Hh promotes hemogenic endothelial cells, we sorted VE-cadherin+CD41−CD45− cells from day 6 EBs (Fig. S4 A and B), which excludes colony-forming blood progenitors (Fig. S4C). These cells are endothelial cells capable of metabolizing DiI-acetylated-low density lipoprotein (DiI-Ac-LDL) and stain positive for von Willebrand Factor (Fig. S4D). When cultured in hemato-endothelial media, these cells gave rise to semiadherent blood cells (Fig. S4 E and F). To test the effect of IHH, whole EBs were exposed to IHH during days 4–5 of differentiation, and VE-cadherin+CD41−CD45− cells were sorted from day 6 EBs into hemato-endothelial culture. IHH treatment increased the number of semiadherent cells that were CD45+ (Fig. 1G), suggesting that activation of Hh pathway promotes the formation of hemogenic endothelium. Supporting this finding, treatment of isolated CD41+ with IHH did not result in expansion of CD41+ cells in hemato-endothelial media, suggesting that VE-cadherin+ cells rather than CD41+ cells respond to HH signaling (Fig. S4G).
Hh Signaling Is Required for Hematopoietic Differentiation in EBs.
To determine whether endogenous Hh signaling is required for hematopoiesis in differentiating EBs, we chemically inhibited Smo with cyclopamine to block Hh signaling. When EBs were treated with cyclopamine from day 2 to 5 of EB differentiation, we observed a dose-dependent reduction in hematopoietic CFUs (Fig. 2A), CD41+c-Kit+ hematopoietic progenitors (Fig. 2B and Fig. S5A), and hematopoietic gene expression (adult globin β-major, Gata3, and Scl) on day 6 (Fig. 2C). Confirming inhibition of the pathway, Gli1 was significantly down-regulated from day 3 of EB differentiation in response to cyclopamine treatment (Fig. 2D). Cyclopamine treatment at this dosage was not associated with a decrease in the percentage of live cells (Fig. S5B) and preserved morphology comparable with the control (Fig. S5C), showing low nonspecific toxicity effects. Collectively, these data indicate that Hh signaling is required for blood formation in EBs.
Fig. 2.
Inhibition of Hh signaling through cyclopamine (cyc) blocks hematopoietic development in EBs. (A) CFUs measured from day 6 whole EBs in response to cyc treatment during days 2–5 of differentiation (n = 4). (B) Flow cytometric quantification of CD41+c-Kit+ hematopoietic precursors cells from day 6 whole EBs in response to cyc treatment during days 2–5 of differentiation. Horizontal bars indicate mean values (n = 5). (C) Hematopoietic gene expression measured in day 6 whole EBs after cyc treatment from days 2 to 5 of differentiation as assessed by quantitative PCR (n = 3). (D) Gene expression time course of Hedgehog target Gli1 and mesoderm-related genes Brachyury and Cerberus during days 2–5 of cyc treatment using whole EBs (n = 2). P values were derived from one-way ANOVA for correlated samples. (E) Flk1+ levels (shown in red) on days 3.25–3.75 after cyc treatment from day 2 of differentiation as assessed by flow cytometry. Black lines represent the isotype controls (n = 2). (F) Percentage of beating EBs on days 7–8 in response to cyc treatment during days 2–5 of differentiation (n = 3). (G) Reduction in cardiac troponin T (cTnT) gene expression in whole EBs in response to cyc treatment from day 2 to 5 (n = 3). (H) Blast colony formation during treatment of day 3.5 Flk1+ with cyc for 4 d in BL-CFC media (n = 3). (I) Core colony formation during treatment of day 3.5 Flk1+ with cyc for 4 d in BL-CFC media (n = 3). (J) Diagram of cell subpopulations affected by cyclopamine treatment.
Hh Inhibition Disrupts Differentiation from the Flk1+ Mesoderm in EBs.
To assess the effect of Hh inhibition on mesodermal patterning, we profiled gene expression levels of several primitive streak genes over the course of EB differentiation in the presence or absence of cyclopamine. Cyclopamine treatment did not significantly alter the expression of the pan-mesodermal marker Brachyury (20) (Fig. 2D), Cerberus (a marker of the anterior boundary of the primitive streak) (Fig. 2D), Gsc and Foxa2 (markers of the anterior primitive streak) or Mesp1 and Evx (markers of ventral–posterior mesoderm) (Fig. S5 D and E) (25, 26). These results show that blocking Hh signaling with cyclopamine reduces hematopoietic differentiation without compromising early mesoderm formation.
Flk1+ cells represent a subset of mesodermal tissue that gives rise to hematopoietic, cardiac, and endothelial cells (27–29). Cyclopamine treatment from day 2 to 5 did not change the percentage of Flk1+ cells as assessed by flow cytometry on days 3.25–3.75 (Fig. 2E). However, cyclopamine treatment reduced the number of beating EBs and the expression of cardiac troponin T (Fig. 2 F and G). When we performed blast colony-forming cell (BL-CFC) assays using sorted Flk1+ cells to determine the direct effect of cyclopamine, the number of blast colonies, which can give rise to both primitive and definitive hematopoietic precursors in methylcellulose, was decreased (Fig. 2H and Fig. S6A) (27). The number of core colonies, which are the inner vascular core of blast colonies with definitive hematopoietic potential (5, 30), was also reduced in the presence of cyclopamine (Fig. 2I and Fig. S6 B and C). Hh signaling is, therefore, required for the formation of multiple mesodermal lineages, such as endothelial cells from Flk1+ cells (Fig. 2J).
Notch Signaling Rescues Hematopoiesis by Restoring VE-Cadherin+ Cells.
Notch signaling has been implicated in the formation of hemogenic endothelium, because defective Notch signaling in E9.5 mouse paraaortic splanchnopleura (P-Sp) results in defects in both vascular remodeling and hematopoiesis (31). Thus, we explored whether both Hh and Notch signaling were active in hemogenic VE-cadherin+ cells. In sorted VE-cadherin+ cells from day 4 EBs, IHH treatment increased the expression of the Notch downstream target Hes1 in adherent cells, whereas treatment with both IHH and the Notch inhibitor N-[N-(3,5-Difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester (DAPT) abrogated this effect (Fig. 3A). IHH treatment also increased the number of cells in each cluster, whereas combined IHH and DAPT treatment reversed this effect (Fig. 3B). Therefore, Hh and Notch pathways likely interact in VE-cadherin+ cells.
Fig. 3.
NICD induction rescues hematopoiesis from Hh inhibition in EBs. (A) Immunoflurescence showing Notch downstream target Hes1 (red) and DAPI (blue) in VE-cadherin+ cells sorted from day 4 EBs and treated with IHH, DAPT, or both for 36 h. Lower magnifies representative clusters that are highlighted with red arrows in Upper. (B) Box plot for the number of cells in each VE-cadherin+ cluster shown in A. (C) NICD expression in whole EBs on dox induction (n = 2). (D) CFUs from day 6 whole EBs that were NICD-overexpressed and/or cyc-treated during days 2–5 of differentiation (n = 4). (E) Flow cytometric quantification of VE-cadherin and CD41 populations from day 6 whole EBs that were NICD-overexpressed and/or cyc-treated during days 2–5 of differentiation (n = 2). (F) Blast and core colony formation during NICD overexpression and/or cyc treatment as assessed by BL-CFC assay (n = 3). (G) CFU potential of sorted VE-cadherin+ cells from day 6 EBs that were NICD-overexpressed and/or cyc-treated during days 2–5 of differentiation (n = 5). (H) Images of cells from hemato-endothelial culture at 10× objective (Upper) and flow cytometry for CD45+ cells from hemato-endothelial culture (Lower). Whole EBs were NICD-overexpressed from day 2 to 5; then, VE-cadherin+CD41−CD45− cells were sorted on day 6 and cultured in hemato-endothelial medium for 4 d.
To explore the role of Notch signaling in hematopoiesis, we used an mESC line in which expression of the human NOTCH1 intracellular domain (NICD) is controlled by a dox-responsive promoter (Fig. 3C) (32). Overexpression of NICD alone from day 3 to 5 of EB differentiation increased the total CFU activity by twofold and partially rescued the effects of cyclopamine treatment (Fig. 3D). These data establish that Notch signaling can rescue CFUs in the absence of Hh signaling.
Although some factors mediating the endothelial-to-hematopoietic transition are known (e.g., Runx1) (8), signals required for the formation of hemogenic endothelium are not well-understood. We sought to determine whether Hh and Notch signaling had an effect on the quantity of VE-cadherin+ endothelial cells during EB differentiation. Inhibition of Hh signaling with cyclopamine resulted in a decrease in both VE-cadherin+ and CD41+ cells (Fig. 3E). NICD induction combined with cyclopamine treatment more prominently rescued VE-cadherin+ than CD41+ cells (Fig. 3E), suggesting that Hh and Notch signaling are involved in the formation of hemogenic VE-cadherin+ endothelium. To determine whether NICD induction directly promotes progression of Flk1+ cells to vascular core colonies containing tightly adherent endothelial cells (30), we performed BL-CFC assays using sorted Flk1+ cells in the presence of dox and/or cyclopamine. In BL-CFC assays, blast colonies can give rise to both primitive and definitive hematopoietic precursors (27). Core colonies, however, are the inner vascular core of blast colonies with definitive hematopoietic potential (5, 30). NICD overexpression reduced the number of blast colonies while promoting the number of vascular core colonies, and it rescued the number of vascular core colonies in the presence of cyclopamine (Fig. 3F), suggesting that NICD induction and cyclopamine directly change Flk1+ potential. In agreement, NICD induction modestly rescued CFU potential of VE-cadherin+ in the setting of Hh inhibition (Fig. 3G).
To assess the effect of NICD overexpression on the hemogenic endothelium, we sorted VE-cadherin+CD41−CD45− cells from day 6 EBs that were NICD-induced from day 2 to 5. When these NICD-induced endothelial cells are cultured in hemato-endothelial media, the number of semiadherent CD45+ cells significantly increased (Fig. 3H), suggesting that Notch induction promotes the formation of the hemogenic endothelium. As the number of seeded hemogenic endothelial cells was normalized, day 2–5 cyclopamine-treated cells were still able to give rise to semiadherent CD45+ cells at quantities comparable with the vehicle control (Fig. S5F), which suggests that blocking Hh signaling after hemogenic endothelium has formed has little effect on hematopoietic output. Similarly, NICD induction after formation of hemogenic endothelial cells did not result in additional increase in CD45+ cells (Fig. S7). Therefore, both NICD and Hh signaling have a role during the formation of hemogenic endothelial cells but not afterward.
Scl Induction Bypasses Hh Inhibition by Restoring VE-Cadherin+CD41+ Cells.
We sought to determine the relationship between Hh and Notch signaling and the master hematopoietic transcriptional regulator Scl in the formation of the hemogenic endothelium and the endothelial-to-hematopoietic transition. Cyclopamine treatment of EBs reduced Scl expression (Fig. 4A), suggesting that Scl acts downstream of Hh signaling. We used an mESC line, in which Scl expression is under the control of a dox-responsive promoter (33), to determine whether overexpression of Scl would rescue hematopoiesis in the presence of cyclopamine. Scl overexpression alone increased CFUs (Fig. 4B) and the percentage of CD41+c-Kit+ hematopoietic progenitors (Fig. 4C). In the setting of cyclopamine treatment, overexpression of Scl rescued the number of CFUs and the percentage of CD41+c-Kit+ cells to levels comparable with Scl overexpression alone (Fig. 4 B and C). When cyclopamine treatment and Scl induction were combined, the number of blast colonies was restored (Fig. 4D), suggesting that Scl acts downstream of Hh signaling.
Fig. 4.
Scl induction rescues hematopoiesis from Hh inhibition in EBs. (A) Gene expression time course measured by quantitative PCR for Scl, Gata2, and Runx1 during the course of cyc treatment during days 2–5 of differentiation (n = 2). P values were derived from one-way ANOVA for correlated samples. (B) CFUs from day 6 whole EBs that were Scl-overexpressed and/or cyc-treated during days 2–5 or 3–5 of differentiation (n = 4). (C) Flow cytometric quantification of CD41+c-Kit+ hematopoietic precursors from day 6 whole EBs after Scl induction and/or cyc treatment. Horizontal bars indicate mean values (n = 4). (D) Blast and core colony formation during Scl overexpression and/or cyc treatment as assessed by BL-CFC assay (n = 2). (E) Gene expression profile by quantitative PCR in day 6 whole EBs that were Scl-overexpressed and/or cyc-treated during days 2–5 of differentiation (n = 2). (F) Flow cytometric quantification of VE-cadherin+CD41+ double-positive and VE-cadherin+CD41− single-positive cells over the course of EB differentiation (n = 2). (G) CFU potential of sorted VE-cadherin+ cells from day 6 EBs that were Scl-overexpressed and/or cyc-treated during days 2–5 of differentiation (n = 4). (H) Images of cells from hemato-endothelial culture at 10× objective (Upper) and flow cytometry for CD45+ cells from hemato-endothelial culture (Lower). VE-cadherin+CD41−CD45− cells were sorted from day 6 EBs and grown in hemato-endothelial culture in conjunction with Scl induction for 3–4 d. (I) Effect of Scl overexpression via dox treatment and/or cyc treatment on sorted VE-cadherin+ cells from E9 to E10 yolk sacs that were infected with lentiviruses for dox-inducible Scl. (n = 5).
To further dissect the interaction of the Hh pathway and Scl signaling, we examined the expression profile of several Scl target genes in day 6 whole EBs, including βh1, Pu.1, Gata1, Fli1, Gata2, LMO2, and Ikaros (34). We found that, although target genes were down-regulated by cyclopamine treatment (Fig. 4E), overexpression of Scl restored their expression in cyclopamine-treated samples (Fig. 4E). The rescue of gene expression suggests that Scl overexpression rescues defective hematopoietic gene expression caused by Hh inhibition. However, Scl overexpression did not significantly rescue endothelial gene expression, such as Tie2 and Ets1 (Fig. 4E) (35), suggesting that Scl acts on a preformed endothelial cell population.
To investigate whether Scl has a role in the endothelial-to-hematopoietic transition, we examined the effect of Scl overexpression on VE-cadherin+ cells during EB differentiation. Whereas Scl overexpression did not increase the percentage of total VE-cadherin+ cells during the course of EB differentiation, it increased the hematopoietic subpopulation of VE-cadherin+CD41+ cells compared with the vehicle control (Fig. 4F). Cyclopamine treatment decreased percentages of both VE-cadherin+CD41+ and VE-cadherin+CD41−, whereas Scl overexpression rescued the VE-cadherin+CD41+ subpopulation in cyclopamine-treated EBs (Fig. 4F). Consistent with this finding, Scl overexpression in whole EBs resulted in a sixfold increase in hematopoietic CFUs in VE-cadherin+ cells (Fig. 4G). The expression of the endothelial marker VE-cadherin in CD41+ subpopulation suggests that these blood progenitors have an endothelial origin.
To determine whether Scl can promote conversion of hemogenic endothelium to blood cells, we sorted VE-cadherin+CD41−CD45− endothelial cells from day 6 EBs and cultured them in hemato-endothelial conditions with Scl overexpression. Hemogenic endothelial cells plated in hemato-endothelial culture with Scl induction gave rise to a 60% increase in semiadherent CD45+ cells (Fig. 4H). Cyclopamine treatment during hemato-endothelial culture was still able to give rise to semiadherent CD45+ cells at quantities comparable with the vehicle control (Fig. S5G), again suggesting that blocking Hh signaling has little effect on hematopoietic output after the hemogenic endothelium has formed. Moreover, Scl overexpression did not result in large differences in percentages of dead cells (Fig. S5H) or cell division, which was assessed by dilution of Carboxyfluorescein succinimidyl ester (CFSE) dye in CD41+ or CD45+ cells (Fig. S5I). Overall, our data favor the interpretation that Scl can compensate for loss of Hh signaling by promoting the conversion of VE-cadherin+ endothelial cells to hematopoietic cells rather than expanding hematopoietic progenitors.
Scl Induction Increases Hematopoietic Potential in VE-Cadherin+ Cells from E9 to E10 Mouse Embryos.
With information gleaned from mESC manipulation, we sought to confirm that Scl can rescue hematopoiesis in the absence of Hh signaling in midgestation mouse embryos. Treatment of whole yolk sac cells isolated at E9–E10 with IHH (25 ng/mL) increased CFUs (Fig. S8A), increased CD41+c-Kit+ cells (Fig. S8B), and up-regulated Gli1 expression (Fig. S8C). Interestingly, Hh treatment of whole E9–E10 P-Sp (an immediate precursor of AGM) did not significantly alter numbers of CFUs, although cyclopamine treatment decreased CFUs (Fig. S8D). Similarly, CD41+c-Kit+ percentage in P-Sp cells did not change appreciably to IHH signaling (Fig. S8E), although cells responded with increased Gli1 expression (Fig. S8F). One possible explanation is that hematopoietic progenitors at different stages of development are responsive to different doses of Hh signaling.
To determine whether Scl induction could directly act on VE-cadherin+ cells to promote their commitment to hematopoietic fates, we isolated VE-cadherin+ cells from the E9–E10 yolk sac and P-Sp/AGM, infected the cells with the dox-inducible Scl virus and evaluated the effect of dox induction or cyclopamine treatment on hematopoietic CFUs. Scl induction by dox increased CFUs from VE-cadherin+ cells isolated from yolk sac in the presence of cyclopamine (Fig. 4I), and Scl induction showed a similar trend toward rescue in P-Sp/AGM (Fig. S8G). These results suggest that the hematopoietic activity of Hh is largely conserved at both primitive and definitive sites of embryonic hematopoiesis with distinct dosage or timing requirements.
Notch Signaling Rescues Hematopoiesis from Hh Inhibition in Zebrafish Aorta.
To test whether Hh and Notch signaling are conserved during definitive hematopoiesis among vertebrates and to better visualize the response to signaling changes in the context of an anatomic endothelial-to-hematopoietic transition, we conducted analogous experiments in zebrafish embryos. In zebrafish, definitive HSCs positive for the prototypical hemogenic endothelium marker Runx1 can be detected in the dorsal aorta 36 h postfertilization (hpf) (36). Furthermore, the suppression of Hh and Notch signaling both result in decreased Runx1+ cells (15). To test whether ectopic Notch signaling can restore hematopoiesis in the absence of Hh signaling, we obtained hsp70:gal4;uas:NICD embryos that allow heat shock-inducible overexpression of NICD (37). Cyclopamine treatment beginning at the 10-somite stage eliminated Runx1+ cells in the trunk at 36 hpf (Fig. S9 A and B). NICD induction through heat shock at either 12- (15 hpf) (Fig. S9C) or 17-somite stages (17.5 hpf) (Fig. S9E) increased Runx1+ cells in the aorta (37). When embryos were treated with cyclopamine from the 10-somite stage, NICD induction at either time point restored Runx1+ cells in the aorta (14 hpf) (Fig. S9 D and F). These experiments confirm that the fundamental components of both Hh and Notch signaling pathways are conserved between mice and zebrafish, consistent with the known conservation of many aspects of vertebrate hematopoiesis (25, 34, 38).
Scl mRNA Injection Rescues Definitive Hematopoiesis in Cyclopamine-Treated Zebrafish.
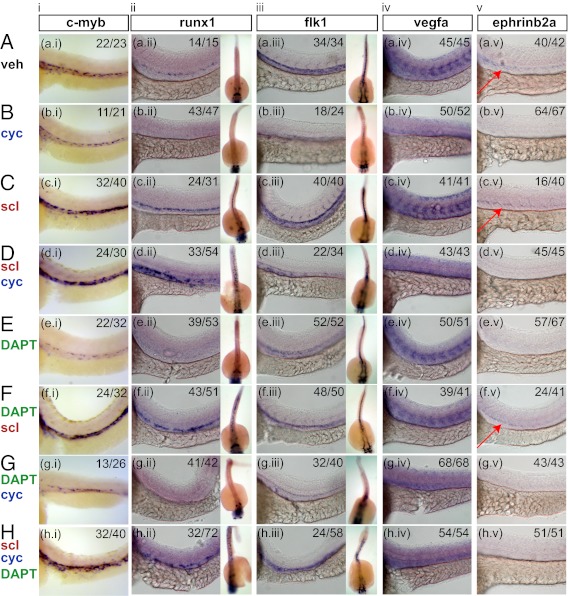
We next tested whether a similar relationship between Hh signaling and Scl exists in the establishment of definitive hematopoiesis in the AGM region of the zebrafish. We chemically inhibited Hh signaling using cyclopamine from 70% epiboly, 4-somite stage, or 10-somite stage to decrease Runx1+ cells in the zebrafish aorta at 36 hpf (Fig. 5B, ii and Table 1) (15). To test whether Scl acts downstream of Hh signaling to regulate hematopoiesis in the AGM, we combined cyclopamine treatment at indicated stages with injection of scl mRNA into the 1- to 8-cell stage yolk, which allows scl to be expressed constitutively. Injection of scl mRNA rescued the definitive hematopoietic markers c-myb and runx1 at 36 hpf when cyclopamine treatment was initiated at the 10-somite stage (Fig. 5 B, i and ii and D, i and ii) (37). However, earlier treatment did not rescue runx1 expression (Table 1 and Fig. S10). We also found that scl injection into zebrafish embryos rescued c-myb and runx1 expression when Notch signaling was inhibited by DAPT treatment (Fig. 5 E, i and ii and F, i and ii) as well as in the absence of both Hh and Notch (Fig. 5H, i and ii). Finally, we observed that, when the embryos were cyclopamine-treated or induced with Notch signaling, the expression of scl is affected at the level of the dorsal aorta at 36 hpf and not at the lateral plate mesoderm stage at 19.5 hpf (21 somites) (Fig. S11). Taken together, these results show that Scl acts downstream of both Hh and Notch signaling to specify definitive HSC formation in zebrafish embryos.
Fig. 5.
scl mRNA injection rescues hematopoietic deficiency caused by reduction in Hh signaling (cyc) and/or reduction in notch signaling (DAPT) from the 10-somite stage. Embryos were fixed at 36 hpf and analyzed by whole-mount in situ hybridization for (i) c-myb, (ii) runx1, (iii) flk1, (iv) vegfa, and (v) ephrinb2a. For runx1 and flk1 expressions, lateral views of the trunk are accompanied by dorsal views on the right. The ratios indicate the number of embryos out of the total that show similar staining to the representative picture shown. The ratio within the scl injection represents the number of embryos that show an increase in staining over the vehicle control. The red arrows highlight ephrinb2a expression.
Table 1.
Rescue of runx1 in cyclopamine-treated embryos by scl mRNA injection
| Stages of cyclopamine treatment | Vehicle | cyc | scl (runx1 increased over vehicle) | scl + cyc |
| From 70% epiboly | 73/73 (100%) | 0/85 (0%) | 29/35 (83%) | 4/46 (9%) |
| From four-somite stage | 47/47 (100%) | 3/70 (4%) | 32/43 (74%) | 10/35 (29%) |
| From 10-somite stage | 14/15 (93%) | 4/47 (9%) | 24/31 (77%) | 33/54 (61%) |
The fractions represent the number of embryos that have runx1 positive stains near the aorta over the total number of embryos, except for the scl injection control, which shows the efficiency of mRNA injection.
Previous studies suggest that arterial endothelial cells of the dorsal aorta and definitive HSCs in zebrafish arise from a common precursor (15, 16). It is known that Hh signaling is required for vascular assembly and therefore, dorsal aorta formation (12, 15), and we confirmed this finding by observing the lack of organized flk1 expression in cyclopamine-treated embryos (Fig. 5B, iii, dorsal view). Notch signaling promotes arterial fate from preexisting vessels (37, 39), and as a result, DAPT-treated embryos retain expression of flk1 and aorta vessel morphology (Fig. 5E, iii). In embryos treated with cyclopamine alone (Fig. 5B, iii) or cyclopamine and DAPT together (Fig. 5G, iii), scl injection did not result in midline flk1 expression but rather, diffuse truncal staining reminiscent of midline angioblast migration (Fig. 5 D, iii and H, iii). Similar truncal staining is also evident when examining the dorsal views of runx1 expression in embryos that were both scl injected and cyclopamine-treated (Fig. 5 D, ii and H, ii). Together, these results suggest that Scl can drive hemogenic endothelial cells to hematopoietic cells in the absence of a functional dorsal aorta.
To ensure that the effects of cyclopamine and DAPT are consistent with existing evidence on Hh signaling, we examined the expression of vegfa in chemically treated embryos. Similar to previous studies (39), Vegf signaling functions downstream of Hh signaling and upstream of Notch, because the somitic patterning of vegfa was unaltered in DAPT-treated embryos (Fig. 5E, iv) but absent in cyclopamine-treated embryos (Fig. 5B, iv). Consequently, scl mRNA injections alone did not alter the pattern of vegfa expression (Fig. 5C, iv), and they did not restore somitic vegfa pattern in the presence of cyclopamine or DAPT (Fig. 5 D, iv; F, iv; and H, iv).
Because arterial development has been linked to blood formation (37, 39), we questioned whether scl injection would restore the Ephrinb2a-dependent arterial program in the absence of Hh or Notch signaling. Inhibition of either Hh or Notch signaling with cyclopamine or DAPT, respectively, resulted in the absence of ephrinb2a expression (Fig. 5 B, v and E, v). Injection of scl restored ephrinb2a expression in the presence of DAPT, showing that scl can orchestrate arterial fate in the absence of Notch signaling in zebrafish (Fig. 5F, v). In combined cyclopamine-treated and scl-injected embryos, the arterial program was not rescued (Fig. 5D, v). As a result, even the arterial program can be decoupled from the emergence of Runx1 in zebrafish (Fig. 5H, i, ii, and v).
Discussion
Taken together, our results suggest that Hh, Notch, and Scl act in various stages of the endothelial-to-hematopoietic transition (Fig. 6). Hh is required for proper endothelial patterning, activation of Notch signaling confers arterial identity to endothelial cells, and Scl promotes blood formation from hemogenic endothelial cells. Importantly, our combined analysis of differentiating mESCs, mouse embryo cultures, and zebrafish embryos suggests that this pathway governing hemogenic endothelium is evolutionarily conserved among vertebrates.
Fig. 6.
A model representing distinct stages of the endothelial-to-hematopoietic transition. The arrows indicate rescue of cell types but not necessarily direct transcriptional regulation.
In the hierarchy of signals necessary for murine HSC/progenitor cell formation, the Hh pathway assumes a primary role in patterning a functional dorsal aorta, the harbinger of hemogenic endothelium. Hh mutants are unable to form a functional and remodeled vasculature, including the dorsal aorta (12, 39, 40), which at E10.5, becomes the initial site of definitive hematopoiesis (3). Hh signaling patterns blood formation in a variety of embryonic hematopoietic organs, but its role in hemogenic endothelium has not been explored. For example, Hh is important for blood island formation in the yolk sac (12, 13) and promotion of engraftment of HSCs derived from AGM explant culture (14). In zebrafish, Hh signaling interacts with the Notch and BMP pathways to promote HSC formation in the dorsal aorta (15, 16). In our study, we have defined a link between the vascular and hematopoietic patterning aspects of Hh signaling, providing evidence that the signaling axis involving Hh, Notch, and Scl act in various stages of the endothelial-to-hematopoietic transition.
Recently, it has been recognized that embryonic hemogenic endothelial intermediates give rise to all adult hematopoietic cells (8, 11). The identification of markers of hemogenic endothelium, such as VE-cadherin or Tie2, from imaging and lineage tracing studies has facilitated the study of this phenomenon (5, 6), and much of the current focus has been on the role of transcription factor Runx1. Cre-mediated Runx1 deletion in VE-cadherin+ cells abrogates definitive hematopoietic cells in all embryonic hematopoietic organs, such as the dorsal aorta, liver, yolk sac, and placenta (8), and restoration of Runx1 in Tie2+ cells showed complementary rescue of definitive hematopoiesis (11).
The major role that Hh plays in the heart and the close functional relationship shared between the myocardium and endothelium adds to the challenge of dissecting the role of Hh signaling within the endothelium in vivo. Specifically, Hh signaling is critical for development of the cardiac tissues (Fig. 2 F and G), and disruption of VE-cadherin results in vastly disorganized endo/myocardium and arrest by E9.5 (41). Our approach of using complementary experimental systems—mESCs and murine embryo culture, and zebrafish embryos—has enabled the dissection of these pathways with better precision. Taking advantage of the conservation between murine and zebrafish hematopoiesis (25, 31, 36, 37), we combined the efficient study of the signaling pathways using mESCs and murine embryos with anatomic context provided by zebrafish embryos to study the hemogenic endothelium.
Distinct from previous studies focusing on Runx1, our study focuses on the formation of VE-cadherin+ hemogenic endothelium from the early Flk1+ mesoderm. Flk1+ cells from the primitive streak in early embryogenesis represent one of the earliest mesoderm precursors capable of forming cardiac (29), endothelial, and primitive erythroid cells (42). We hypothesize that the disorganized endothelial phenotype observed previously in Hh mutants was caused by loss of VE-cadherin (12), because VE-cadherin plays a critical role in cell–cell adhesion and lumen formation (10). In our study, inhibition of Hh signaling halts differentiation from Flk1+ to VE-cadherin+ hemogenic endothelium and reduces hematopoiesis in both cultured mouse embryos and zebrafish, which is unlikely due to reduced survival or proliferation of hemogenic endothelium; cyclopamine treatment after formation of hemogenic endothelium does not reduce CD45 output (Fig. S5G), and blast colony forming assays show that the number rather than the size of vascular colonies decreases dramatically with cyclopamine treatment (Figs. 2I and 3F). After formation of the hemogenic endothelium, there may be the additional effect of proliferation, because IHH treatment also increases endothelial cluster formation (Fig. 3B). Consistent with our findings, other groups have shown that Hh ligand treatment of early but not late E10 AGM explants from mouse embryos can improve their blood engraftment potential, showing that Hh signaling acts before endothelial tissues have acquire their hematopoietic potential (14). The evidence that Hh signaling is not required for normal adult hematopoietic cell homeostasis is consistent with the role of prototypical hemogenic endothelial transcription factor Runx1 (17, 18), which plays a specific role in the endothelial-to-hematopoietic transition period but is dispensable thereafter (8).
The role of Notch signaling in definitive hematopoiesis is widely appreciated (15, 31, 37), but the direct connection between Notch signaling and VE-cadherin+ hemogenic endothelium has not previously been explored. In Notch signaling, Δ-like/Jagged ligands trigger the cleavage of the NICD, which then translocates to the nucleus to alter gene transcription (43). Previous data on the role of Notch signaling in promoting VE-cadherin+ cells are difficult to interpret, because Notch1−/− mouse embryos show three- to fourfold increased VE-cadherin+ cells but markedly reduced definitive hematopoiesis (31). Evidence from the zebrafish, in contrast, suggests that the loss of Notch signaling results in failure to form the arterialized dorsal aorta (8, 37), which would be VE-cadherin+ (8). In our study, hemogenic VE-cadherin+ endothelium isolated from EBs responds to IHH by forming dense endothelial clusters with higher Notch activity. When Hh signaling is inhibited, NICD induction rescues the hemogenic VE-cadherin+CD41− cell populations as well as vascular core colonies, which suggests that Notch signaling can at least partially compensate for loss of Hh signaling to increase formation of hemogenic VE-cadherin+ cells. The rescue of hematopoietic cells from Hh inhibition is also supported by our zebrafish experiments.
Scl is best known as a basic helix–loop–helix transcription factor that is essential for many aspects of hematopoiesis, but its role in the endothelial-to-hematopoietic transition has not previously been documented. Scl KO mice, like Smo KO mice, die between E8.5 and E10 with lack of hematopoietic development (44). We have shown that expression of Scl can restore CD41+VE-cadherin+ cells in the context of Hh inhibition, thus establishing that Scl acts downstream of Hh signaling during embryonic hematopoiesis. Our study refines and extends the model offered in the work by Lancrin et al. (5), which proposed that Scl forms the hemogenic endothelium (defined as expressing Tie2 but not CD41) from the Flk1 mesoderm after observing the lack of adherent endothelial clusters in differentiating Scl null mESCs. We find that Scl induction also promotes the transition of VE-cadherin+ endothelial cells to CD41+ hematopoetic cells. As shown by our direct culture of Scl-overexpressed CD41 or CD45 cells in hemato-endothelial culture, this phenomenon seems to be distinct from survival or expansion of committed CD41 progenitors (Fig. S5 H and I). The results are consistent with previous data on Scl-transfected mESCs, which showed increased definitive hematopoiesis in the VE-cadherin+ compartment (45). In VE-cadherin+ cells derived from murine yolk sac, Scl can significantly rescue hematopoiesis in the setting of Hh inhibition, showing that the pathway operates on the primitive wave of hematopoiesis in vivo. Moreover, in zebrafish, we observe that Scl can rescue definitive hematopoiesis in the absence of both Hh and Notch signaling. This finding shows that Scl acts downstream of both Hh and Notch pathways in the endothelial-to-hematopoietic transition.
Because Scl expression is ubiquitous throughout hematopoietic development, clarifying the distinct role of Scl in the endothelium is especially important. It is accepted that Scl plays an early role in hemangioblast development (28, 45), but additional roles of Scl have been difficult to interpret. For example, the work by Endoh et al. (45) used an inducible Scl (loxP-STOP-loxP-Scl system with tamoxifen-inducible Cre) in an Scl null background to conclude that restoring SCL permanently between days 2 and 4 of ES differentiation rescues both primitive and definitive hematopoiesis. This window corresponds to the period of hemangioblast specification. Because Cre excision results in permanent genetic alterations, the work by Endoh et al. (45) could not tease out additional roles of Scl in the hemogenic endothelium. Additionally, the work by Schlaeger et al. (46) used Sclfl/flTie2Cre embryos to delete Scl in Tie2-expressing endothelial cells. The work by Schlaeger et al. (46) showed that long-term HSCs in the E12.5 fetal liver were not affected, although erythropoiesis and megakaryopoiesis were substantially reduced. Although the decreased number of hematopoietic progenitors is consistent with our study, the data on the lack of effect on long-term HSCs are intriguing. However, it is unclear when their Tie2 excision became active, and their data likely represent incomplete or delayed excision inherent in the Cre-based system. A similar report by Li et al. also deleted Runx1 in Tie2-expressing cells and found that hematopoietic block was, indeed, incomplete in the fetal liver (47). Two of their Runx1-deleted embryos even survived to the postnatal period, which suggests that their hematopoietic system was intact. Meanwhile, there is significant evidence that Runx1 plays a critical role in the endothelial-to-hematopoietic transition (8, 11, 36). Because Scl activates Runx1 (5, 34) and chimeric mice generated using Scl null ES cells show no contributions from input cells to definitive hematopoiesis (48), there is much evidence to suggest that Scl plays a role in the endothelial-to-hematopoietic transition.
Although current dogma holds that Runx1+ cells require both prior arterial specification and functional dorsal aorta formation, we show in the context of zebrafish definitive hematopoiesis that formation of Runx1+ cells by Scl can be decoupled from both of those requirements. In cyclopamine-treated zebrafish embryos, which lack normal expression of the arterial marker ephrinb2a, injection of scl mRNA results in Runx1-positive cells that cluster around the region of the dorsal aorta, suggesting that the migrating dorsal aorta angioblasts became Runx1+. However, the window in which Scl is able to do this task is quite narrow, because cyclopamine treatment before 10-somite stages does not result in a rescue of Runx1. The most likely reason is that Hh inhibition prevents cellular development to a stage that is competent to receive Scl signals for the induction of hemogenic endothelium. In agreement, Scl loss-of-function experiments show that only hematopoietic tissues are affected, sparing the angioblasts and somatic mesoderm (49). More recent evidence suggests that, in etsrp morphants, scl injection can specify HSC fate independent of ephrinb2a arterial specification (50). In the context of dual Hh and Notch inhibition, such conversion speaks to the potent cell-autonomous effect that Scl has to mediate conversion of hemogenic endothelium to hematopoietic cells.
Our results reveal a critical signaling network that advances the understanding of hematopoietic stem progenitor formation during embryogenesis. Additional study of the heterogeneity in this hemogenic endothelial population as well as the combinatorial effects of various transcription factors in patterning the endothelial-to-hematopoietic transition will facilitate the future development of methods to convert pluripotent stem cells into therapeutically relevant hematopoietic lineages.
Materials and Methods
Detailed methods can be found in SI Materials and Methods. Briefly, mESCs were differentiated by aggregation of cells in hanging drops in serum-containing media in the absence of Leukemia inhibitory factor (LIF). Manipulated whole EBs or sorted cells of interest were subject to methylcellulose CFU assay (24), flow cytometry, or gene expression analysis through quantitative PCR. Sorted Flk1+ cells were subject to BL-CFC assay as described previously (27). Sorted VE-cadherin+CD41−CD45− cells were used for hemato-endothelial culture as described previously (27). Inducible SMO-M2–IRES–eGFP (23) and NICD (32) mESC lines were generated using the Ainv15 mESC line (24). For mouse embryo culture, staged mouse embryos were obtained from pregnant C57/Bl6 females and yolk sacs, and P-Sp regions were dissected and cultured in modified hemangioblast media (27). For Scl mRNA injection into zebrafish yolk sacs, we used full-length scl mRNA that was transcribed from linearized pCS2+-SCLA2.1 (GenBank accession no. AF045432). For NICD induction, zebrafish embryos from hsp70:gal4 and uas:NICD matings were used for heat shock (37). Antisense riboprobes to c-myb, runx1, flk1, vegfa, ephrinb2a, and scl were used for in situ hybridization as described previously (36, 39, 49). Primers are listed in Table S1. Statistical significance is indicated by *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001.
Supplementary Material
Acknowledgments
The inducible Scl mESC line is a gift from Michael Kyba. G.Q.D. is an investigator of the Howard Hughes Medical Institute and the Manton Center for Orphan Disease Research. G.Q.D. is supported by grants from the US National Institutes of Health (NIH Heart, Lung and Blood Institute Progenitor Cell Biology Consortium), the Roche Foundation for Anemia Research, and the Doris Duke Medical Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. H.K.M. is a guest editor invited by the Editorial Board.
See Author Summary on page 398 (volume 110, number 2).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1214361110/-/DCSupplemental.
References
- 1.Moore MA, Metcalf D. Ontogeny of the haemopoietic system: Yolk sac origin of in vivo and in vitro colony forming cells in the developing mouse embryo. Br J Haematol. 1970;18(3):279–296. doi: 10.1111/j.1365-2141.1970.tb01443.x. [DOI] [PubMed] [Google Scholar]
- 2.Yoder MC, et al. Characterization of definitive lymphohematopoietic stem cells in the day 9 murine yolk sac. Immunity. 1997;7(3):335–344. doi: 10.1016/s1074-7613(00)80355-6. [DOI] [PubMed] [Google Scholar]
- 3.Medvinsky A, Dzierzak E. Definitive hematopoiesis is autonomously initiated by the AGM region. Cell. 1996;86(6):897–906. doi: 10.1016/s0092-8674(00)80165-8. [DOI] [PubMed] [Google Scholar]
- 4.Kim PG, Daley GQ. Application of induced pluripotent stem cells to hematologic disease. Cytotherapy. 2009;11(8):980–989. doi: 10.3109/14653240903348319. [DOI] [PubMed] [Google Scholar]
- 5.Lancrin C, et al. The haemangioblast generates haematopoietic cells through a haemogenic endothelium stage. Nature. 2009;457(7231):892–895. doi: 10.1038/nature07679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zovein AC, et al. Fate tracing reveals the endothelial origin of hematopoietic stem cells. Cell Stem Cell. 2008;3(6):625–636. doi: 10.1016/j.stem.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taoudi S, et al. Extensive hematopoietic stem cell generation in the AGM region via maturation of VE-cadherin+CD45+ pre-definitive HSCs. Cell Stem Cell. 2008;3(1):99–108. doi: 10.1016/j.stem.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Chen MJ, Yokomizo T, Zeigler BM, Dzierzak E, Speck NA. Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature. 2009;457(7231):887–891. doi: 10.1038/nature07619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eilken HM, Nishikawa S, Schroeder T. Continuous single-cell imaging of blood generation from haemogenic endothelium. Nature. 2009;457(7231):896–900. doi: 10.1038/nature07760. [DOI] [PubMed] [Google Scholar]
- 10.Strilić B, et al. The molecular basis of vascular lumen formation in the developing mouse aorta. Dev Cell. 2009;17(4):505–515. doi: 10.1016/j.devcel.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 11.Liakhovitskaia A, et al. Restoration of Runx1 expression in the Tie2 cell compartment rescues definitive hematopoietic stem cells and extends life of Runx1 knockout animals until birth. Stem Cells. 2009;27(7):1616–1624. doi: 10.1002/stem.71. [DOI] [PubMed] [Google Scholar]
- 12.Byrd N, et al. Hedgehog is required for murine yolk sac angiogenesis. Development. 2002;129(2):361–372. doi: 10.1242/dev.129.2.361. [DOI] [PubMed] [Google Scholar]
- 13.Dyer MA, Farrington SM, Mohn D, Munday JR, Baron MH. Indian hedgehog activates hematopoiesis and vasculogenesis and can respecify prospective neurectodermal cell fate in the mouse embryo. Development. 2001;128(10):1717–1730. doi: 10.1242/dev.128.10.1717. [DOI] [PubMed] [Google Scholar]
- 14.Peeters M, et al. Ventral embryonic tissues and Hedgehog proteins induce early AGM hematopoietic stem cell development. Development. 2009;136(15):2613–2621. doi: 10.1242/dev.034728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gering M, Patient R. Hedgehog signaling is required for adult blood stem cell formation in zebrafish embryos. Dev Cell. 2005;8(3):389–400. doi: 10.1016/j.devcel.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 16.Wilkinson RN, et al. Hedgehog and Bmp polarize hematopoietic stem cell emergence in the zebrafish dorsal aorta. Dev Cell. 2009;16(6):909–916. doi: 10.1016/j.devcel.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hofmann I, et al. Hedgehog signaling is dispensable for adult murine hematopoietic stem cell function and hematopoiesis. Cell Stem Cell. 2009;4(6):559–567. doi: 10.1016/j.stem.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao J, et al. Hedgehog signaling is dispensable for adult hematopoietic stem cell function. Cell Stem Cell. 2009;4(6):548–558. doi: 10.1016/j.stem.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doetschman TC, Eistetter H, Katz M, Schmidt W, Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines: Formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol. 1985;87:27–45. [PubMed] [Google Scholar]
- 20.Fehling HJ, et al. Tracking mesoderm induction and its specification to the hemangioblast during embryonic stem cell differentiation. Development. 2003;130(17):4217–4227. doi: 10.1242/dev.00589. [DOI] [PubMed] [Google Scholar]
- 21.Mikkola HK, Fujiwara Y, Schlaeger TM, Traver D, Orkin SH. Expression of CD41 marks the initiation of definitive hematopoiesis in the mouse embryo. Blood. 2003;101(2):508–516. doi: 10.1182/blood-2002-06-1699. [DOI] [PubMed] [Google Scholar]
- 22.McKinney-Freeman SL, et al. Modulation of murine embryonic stem cell-derived CD41+c-kit+ hematopoietic progenitors by ectopic expression of Cdx genes. Blood. 2008;111(10):4944–4953. doi: 10.1182/blood-2007-11-124644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu C, et al. Direct and indirect effects of hedgehog pathway activation in the mammalian retina. Mol Cell Neurosci. 2006;32(3):274–282. doi: 10.1016/j.mcn.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 24.Kyba M, Perlingeiro RC, Daley GQ. HoxB4 confers definitive lymphoid-myeloid engraftment potential on embryonic stem cell and yolk sac hematopoietic progenitors. Cell. 2002;109(1):29–37. doi: 10.1016/s0092-8674(02)00680-3. [DOI] [PubMed] [Google Scholar]
- 25.Lengerke C, et al. BMP and Wnt specify hematopoietic fate by activation of the Cdx-Hox pathway. Cell Stem Cell. 2008;2(1):72–82. doi: 10.1016/j.stem.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 26.Gadue P, Huber TL, Paddison PJ, Keller GM. Wnt and TGF-beta signaling are required for the induction of an in vitro model of primitive streak formation using embryonic stem cells. Proc Natl Acad Sci USA. 2006;103(45):16806–16811. doi: 10.1073/pnas.0603916103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi K, Kennedy M, Kazarov A, Papadimitriou JC, Keller G. A common precursor for hematopoietic and endothelial cells. Development. 1998;125(4):725–732. doi: 10.1242/dev.125.4.725. [DOI] [PubMed] [Google Scholar]
- 28.Chung YS, et al. Lineage analysis of the hemangioblast as defined by FLK1 and SCL expression. Development. 2002;129(23):5511–5520. doi: 10.1242/dev.00149. [DOI] [PubMed] [Google Scholar]
- 29.Kattman SJ, Huber TL, Keller GM. Multipotent flk-1+ cardiovascular progenitor cells give rise to the cardiomyocyte, endothelial, and vascular smooth muscle lineages. Dev Cell. 2006;11(5):723–732. doi: 10.1016/j.devcel.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Cheng X, Huber TL, Chen VC, Gadue P, Keller GM. Numb mediates the interaction between Wnt and Notch to modulate primitive erythropoietic specification from the hemangioblast. Development. 2008;135(20):3447–3458. doi: 10.1242/dev.025916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumano K, et al. Notch1 but not Notch2 is essential for generating hematopoietic stem cells from endothelial cells. Immunity. 2003;18(5):699–711. doi: 10.1016/s1074-7613(03)00117-1. [DOI] [PubMed] [Google Scholar]
- 32.Carlesso N, Aster JC, Sklar J, Scadden DT. Notch1-induced delay of human hematopoietic progenitor cell differentiation is associated with altered cell cycle kinetics. Blood. 1999;93(3):838–848. [PubMed] [Google Scholar]
- 33.Ismailoglu I, Yeamans G, Daley GQ, Perlingeiro RC, Kyba M. Mesodermal patterning activity of SCL. Exp Hematol. 2008;36(12):1593–1603. doi: 10.1016/j.exphem.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 34.Patterson LJ, Gering M, Patient R. Scl is required for dorsal aorta as well as blood formation in zebrafish embryos. Blood. 2005;105(9):3502–3511. doi: 10.1182/blood-2004-09-3547. [DOI] [PubMed] [Google Scholar]
- 35.Ema M, et al. Primitive erythropoiesis from mesodermal precursors expressing VE-cadherin, PECAM-1, Tie2, endoglin, and CD34 in the mouse embryo. Blood. 2006;108(13):4018–4024. doi: 10.1182/blood-2006-03-012872. [DOI] [PubMed] [Google Scholar]
- 36.Kalev-Zylinska ML, et al. Runx1 is required for zebrafish blood and vessel development and expression of a human RUNX1-CBF2T1 transgene advances a model for studies of leukemogenesis. Development. 2002;129(8):2015–2030. doi: 10.1242/dev.129.8.2015. [DOI] [PubMed] [Google Scholar]
- 37.Burns CE, Traver D, Mayhall E, Shepard JL, Zon LI. Hematopoietic stem cell fate is established by the Notch-Runx pathway. Genes Dev. 2005;19(19):2331–2342. doi: 10.1101/gad.1337005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patterson LJ, et al. The transcription factors Scl and Lmo2 act together during development of the hemangioblast in zebrafish. Blood. 2007;109(6):2389–2398. doi: 10.1182/blood-2006-02-003087. [DOI] [PubMed] [Google Scholar]
- 39.Lawson ND, Vogel AM, Weinstein BM. sonic hedgehog and vascular endothelial growth factor act upstream of the Notch pathway during arterial endothelial differentiation. Dev Cell. 2002;3(1):127–136. doi: 10.1016/s1534-5807(02)00198-3. [DOI] [PubMed] [Google Scholar]
- 40.Vokes SA, et al. Hedgehog signaling is essential for endothelial tube formation during vasculogenesis. Development. 2004;131(17):4371–4380. doi: 10.1242/dev.01304. [DOI] [PubMed] [Google Scholar]
- 41.Carmeliet P, et al. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell. 1999;98(2):147–157. doi: 10.1016/s0092-8674(00)81010-7. [DOI] [PubMed] [Google Scholar]
- 42.Nishikawa SI, Nishikawa S, Hirashima M, Matsuyoshi N, Kodama H. Progressive lineage analysis by cell sorting and culture identifies FLK1+VE-cadherin+ cells at a diverging point of endothelial and hemopoietic lineages. Development. 1998;125(9):1747–1757. doi: 10.1242/dev.125.9.1747. [DOI] [PubMed] [Google Scholar]
- 43.Itoh M, et al. Mind bomb is a ubiquitin ligase that is essential for efficient activation of Notch signaling by Delta. Dev Cell. 2003;4(1):67–82. doi: 10.1016/s1534-5807(02)00409-4. [DOI] [PubMed] [Google Scholar]
- 44.Shivdasani RA, Mayer EL, Orkin SH. Absence of blood formation in mice lacking the T-cell leukaemia oncoprotein tal-1/SCL. Nature. 1995;373(6513):432–434. doi: 10.1038/373432a0. [DOI] [PubMed] [Google Scholar]
- 45.Endoh M, Ogawa M, Orkin S, Nishikawa S. SCL/tal-1-dependent process determines a competence to select the definitive hematopoietic lineage prior to endothelial differentiation. EMBO J. 2002;21(24):6700–6708. doi: 10.1093/emboj/cdf674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schlaeger TM, Mikkola HK, Gekas C, Helgadottir HB, Orkin SH. Tie2Cre-mediated gene ablation defines the stem-cell leukemia gene (SCL/tal1)-dependent window during hematopoietic stem-cell development. Blood. 2005;105(10):3871–3874. doi: 10.1182/blood-2004-11-4467. [DOI] [PubMed] [Google Scholar]
- 47.Li Z, Chen MJ, Stacy T, Speck NA. Runx1 function in hematopoiesis is required in cells that express Tek. Blood. 2005;107(1):106–110. doi: 10.1182/blood-2005-05-1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robb L, et al. The scl gene product is required for the generation of all hematopoietic lineages in the adult mouse. EMBO J. 1996;15(16):4123–4129. [PMC free article] [PubMed] [Google Scholar]
- 49.Dooley KA, Davidson AJ, Zon LI. Zebrafish scl functions independently in hematopoietic and endothelial development. Dev Biol. 2005;277(2):522–536. doi: 10.1016/j.ydbio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 50.Ren X, Gomez GA, Zhang B, Lin S. Scl isoforms act downstream of etsrp to specify angioblasts and definitive hematopoietic stem cells. Blood. 2010;115(26):5338–5346. doi: 10.1182/blood-2009-09-244640. [DOI] [PMC free article] [PubMed] [Google Scholar]