Abstract
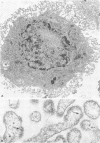
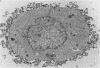
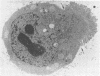
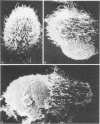
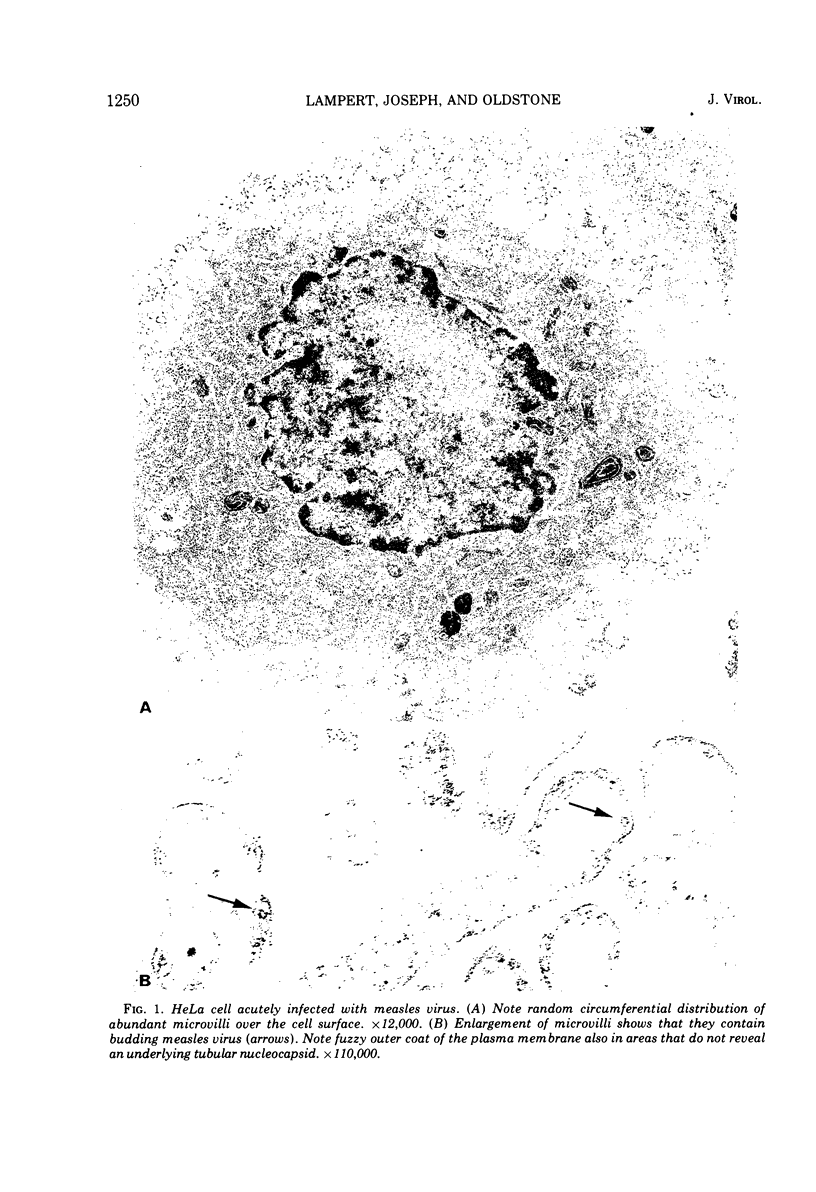
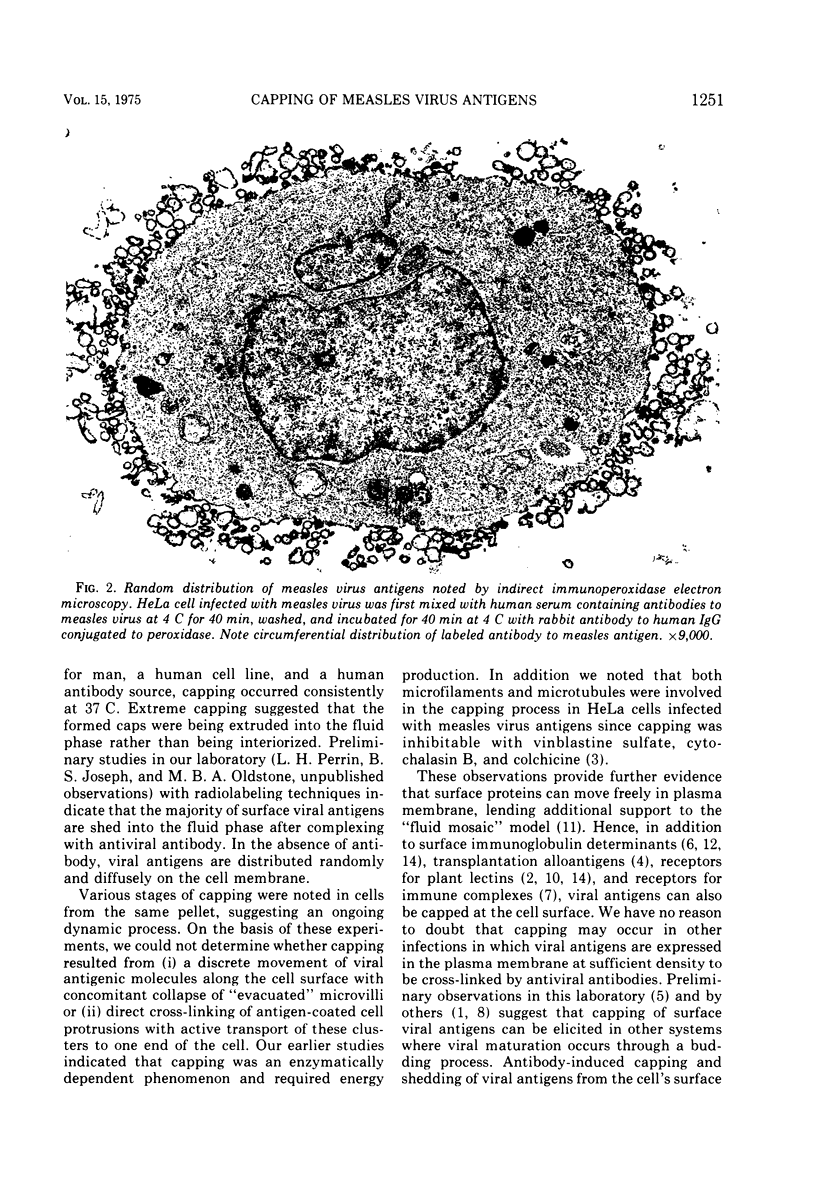
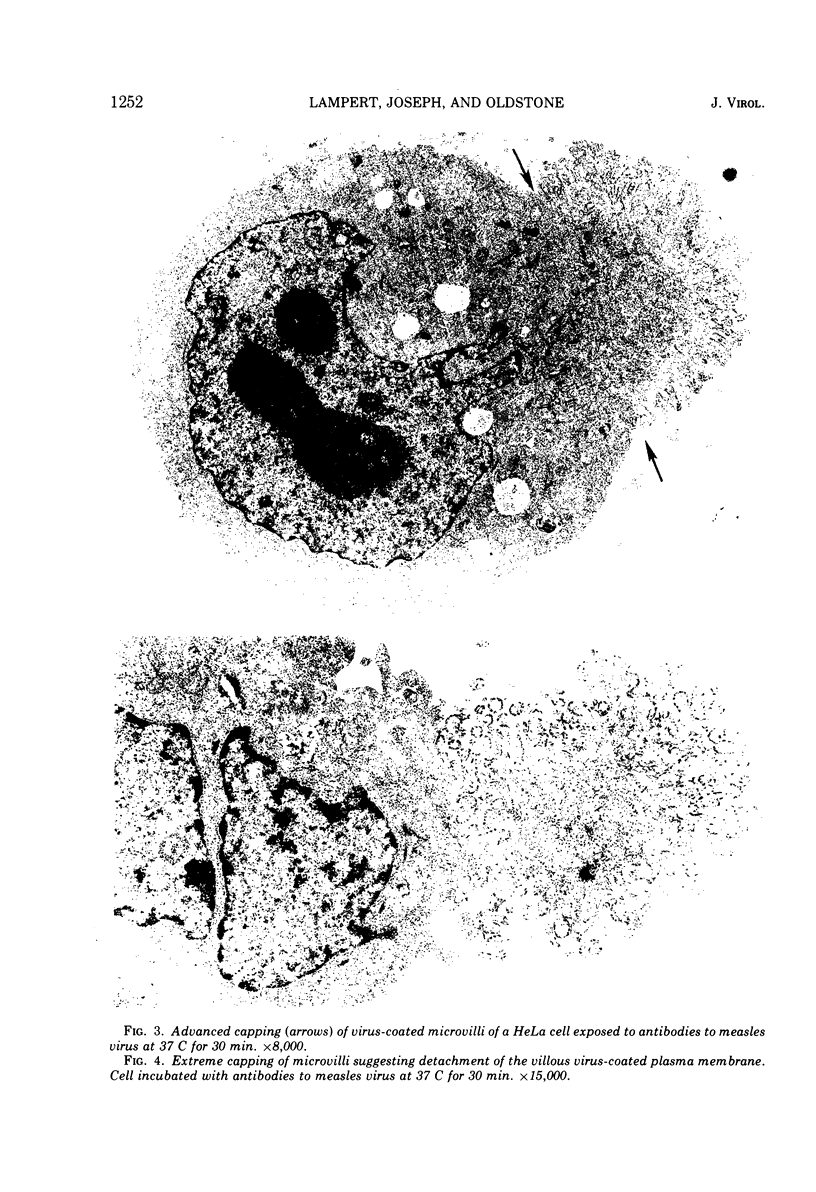
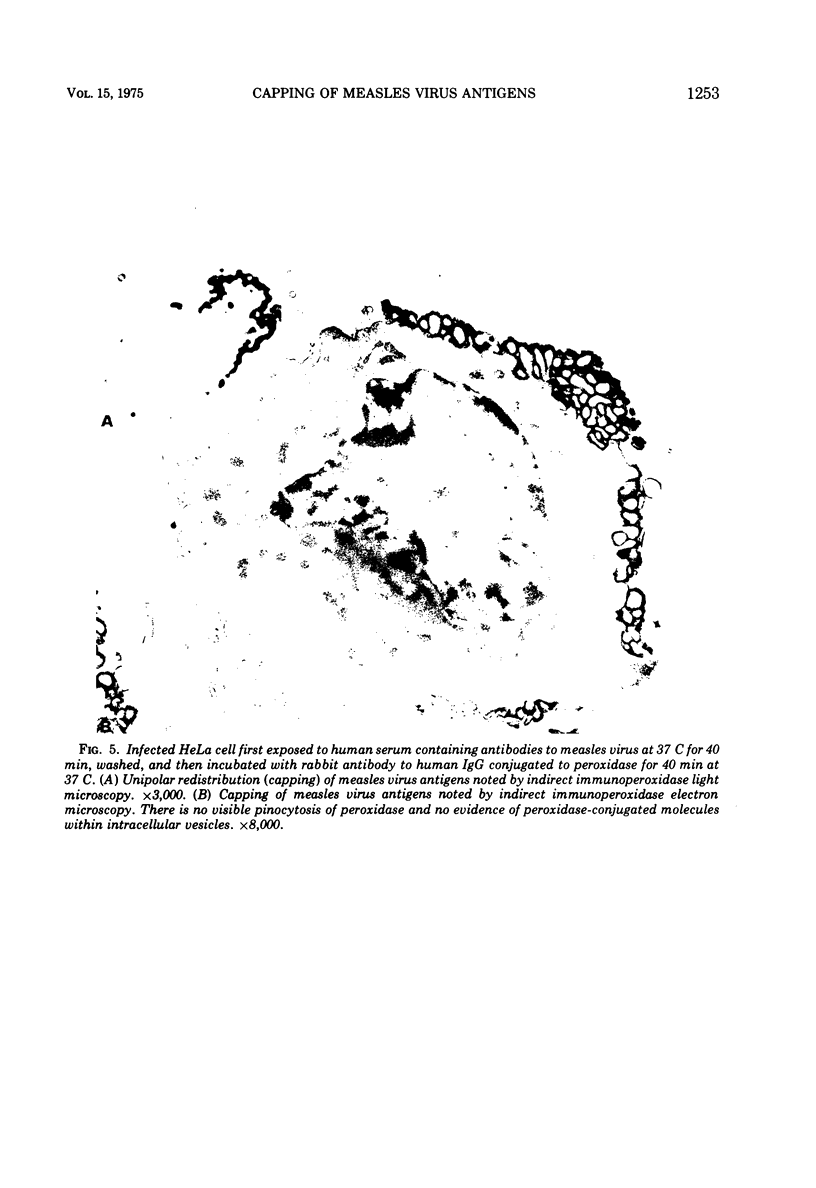
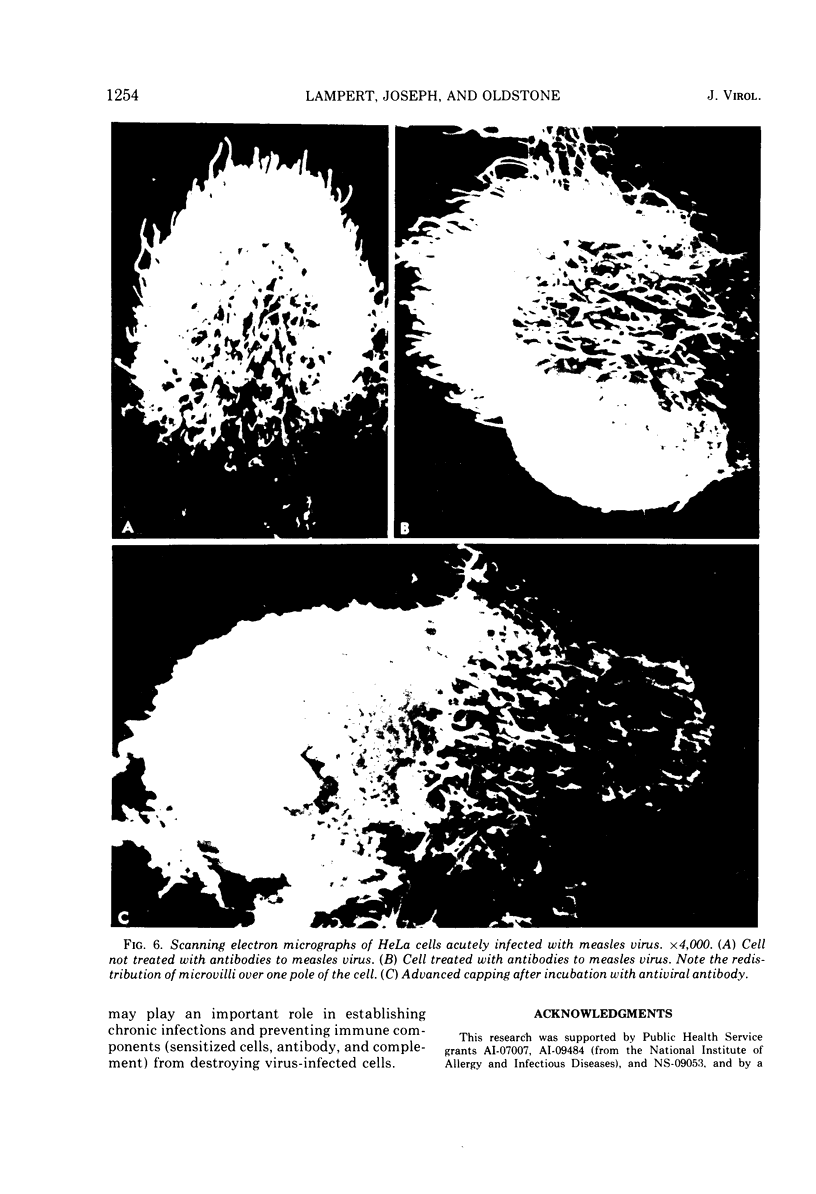
Antibodies specific for measles virus could redistribute ("cap") virus antigens on infected HeLa cells as shown by transmission and scanning electron microscopy. Using an indirect immunoperoxidase technique, infected cells showed diffuse, circumferential distribution of virus antigens over the cell surface when mixed with antibody at 4 C. At 37 C, virus-coated microvilli concentrated on one pole of the cell, leaving the remainder of the plasma membrane devoid of both viral antigens and microvillus projections. Whereas extreme polar displacement of virus-antibody complexes frequently occurred, endocytosis was rarely seen. The findings indicate that antiviral antibodies can move and cluster virus on plasma membranes and suggest that virus-antibody complexes are stripped and shed from the cell surface.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ehrnst A., Weiner L., Norrby E. Fluctuations and distribution of measles virus antigens in chronically infected cells. Nature. 1974 Apr 19;248(5450):691–693. doi: 10.1038/248691a0. [DOI] [PubMed] [Google Scholar]
- Inbar M., Sachs L. Mobility of carbohydrate containing sites on the surface membrane in relation to the control of cell growth. FEBS Lett. 1973 May 15;32(1):124–128. doi: 10.1016/0014-5793(73)80753-7. [DOI] [PubMed] [Google Scholar]
- Joseph B. S., Oldstone M. B. Antibody-induced redistribution of measles virus antigens on the cell surface. J Immunol. 1974 Oct;113(4):1205–1209. [PubMed] [Google Scholar]
- Kourilsky F. M., Silvestre D., Neauport-Sautes C., Loosfelt Y., Dausset J. Antibody-induced redistribution of HL-A antigens at the cell surface. Eur J Immunol. 1972 Jun;2(3):249–257. doi: 10.1002/eji.1830020311. [DOI] [PubMed] [Google Scholar]
- Lerner R. A., Oldstone M. B., Cooper N. R. Cell cycle-dependent immune lysis of Moloney virus-transformed lymphocytes: presence of viral antigen, accessibility to antibody, and complement activation. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2584–2588. doi: 10.1073/pnas.68.10.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loor F., Forni L., Pernis B. The dynamic state of the lymphocyte membrane. Factors affecting the distribution and turnover of surface immunoglobulins. Eur J Immunol. 1972 Jun;2(3):203–212. doi: 10.1002/eji.1830020304. [DOI] [PubMed] [Google Scholar]
- Miller G. W., Saluk P. H., Nussenzweig V. Complement-dependent release of immune complexes from the lymphocyte membrane. J Exp Med. 1973 Sep 1;138(3):495–507. doi: 10.1084/jem.138.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips E. R., Perdue J. F. Ultrastructural distribution of cell surface antigens in avian tumor virus-infected chick embryo fibroblasts. J Cell Biol. 1974 Jun;61(3):743–756. doi: 10.1083/jcb.61.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter K. R., Fonte V., Weiss G. A scanning microscope study of the topography of HeLa cells. Cancer Res. 1974 Jun;34(6):1385–1394. [PubMed] [Google Scholar]
- Rosenblith J. Z., Ukena T. E., Yin H. H., Berlin R. D., Karnovsky M. J. A comparative evaluation of the distribution of concanavalin A-binding sites on the surfaces of normal, virally-transformed, and protease-treated fibroblasts. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1625–1629. doi: 10.1073/pnas.70.6.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Unanue E. R., Karnovsky M. J. Redistribution and fate of Ig complexes on surface of B lymphocytes: functional implications and mechanisms. Transplant Rev. 1973;14:184–210. doi: 10.1111/j.1600-065x.1973.tb00107.x. [DOI] [PubMed] [Google Scholar]
- Unanue E. R., Perkins W. D., Karnovsky M. J. Ligand-induced movement of lymphocyte membrane macromolecules. I. Analysis by immunofluorescence and ultrastructural radioautography. J Exp Med. 1972 Oct 1;136(4):885–906. doi: 10.1084/jem.136.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]