Abstract
The ability of terrestrial microorganisms to grow in the near-surface environment of Mars is of importance to the search for life and protection of that planet from forward contamination by human and robotic exploration. Because most water on present-day Mars is frozen in the regolith, permafrosts are considered to be terrestrial analogs of the martian subsurface environment. Six bacterial isolates were obtained from a permafrost borehole in northeastern Siberia capable of growth under conditions of low temperature (0 °C), low pressure (7 mbar), and a CO2-enriched anoxic atmosphere. By 16S ribosomal DNA analysis, all six permafrost isolates were identified as species of the genus Carnobacterium, most closely related to C. inhibens (five isolates) and C. viridans (one isolate). Quantitative growth assays demonstrated that the six permafrost isolates, as well as nine type species of Carnobacterium (C. alterfunditum, C. divergens, C. funditum, C. gallinarum, C. inhibens, C. maltaromaticum, C. mobile, C. pleistocenium, and C. viridans) were all capable of growth under cold, low-pressure, anoxic conditions, thus extending the low-pressure extreme at which life can function.
Keywords: astrobiology, planetary protection
A central goal of astrobiology is to explore the limits at which life can occur and to search for life and habitable locations outside Earth (1). The planet Mars is considered a promising astrobiological exploration target owing to its relative proximity and similarity to Earth, coupled with increasing evidence pointing to the past and present existence of liquid water at the surface and near subsurface (2–4). The current surface environment of Mars presents formidable challenges to life, such as a scarcity of liquid water and organic nutrients, extreme low temperatures, a low-pressure CO2-dominated atmosphere, harsh solar and galactic cosmic radiation, and a lack of organic nutrients (5). To address the question of whether terrestrial life could survive and grow in the martian environment, several researchers have turned to Mars environmental simulations conducted in chambers that replicate the temperature, pressure, atmospheric composition, regolith composition, and solar radiation environment of the Mars surface and near subsurface (6). Results from past experiments testing the ability of dozens of terrestrial microorganisms to grow in one such simulator demonstrated that the combination of low pressure (P; 7 mbar), low temperature (T; 0 °C), and anoxic atmosphere (A), called here low-PTA conditions, posed significant barriers to growth (5, 7, 8).
Because much of the water on present-day Mars exists in a permanently frozen state mixed with mineral matrix, permafrosts on Earth are considered to be terrestrial analogs of the martian environment (9). On the basis of similarities between martian regolith and terrestrial permafrost, we reasoned that permafrost might contain microbes capable of growth under low-PTA conditions. In this communication we report that screening of ca. 10,000 microbes obtained from four Siberian permafrost samples resulted in the isolation of six bacterial strains capable of growth under low-PTA conditions, and that all of these isolates belonged to the Gram-positive genus Carnobacterium.
Results and Discussion
Isolation of Microorganisms from Siberian Permafrost.
Samples of permafrost obtained from the Siberian arctic (Fig. 1) were suspended and plated on trypticase soy broth yeast extract salt (TSBYS) medium and incubated at room temperature (ca. 23 °C) for up to 28 d. Colonies were either picked or replica-plated onto fresh TSBYS plates and incubated for 30 d under low-PTA conditions. Out of a total of ∼9.3 × 103 colonies tested from four different permafrost soil samples, 6 colonies were observed to grow under low-PTA conditions (Table 1). Five isolates, (strains WN1359 and WN1370–WN1373) were obtained from permafrost soil sample 4, and a single isolate (strain WN1374) was obtained from soil sample 9 (Table 1).
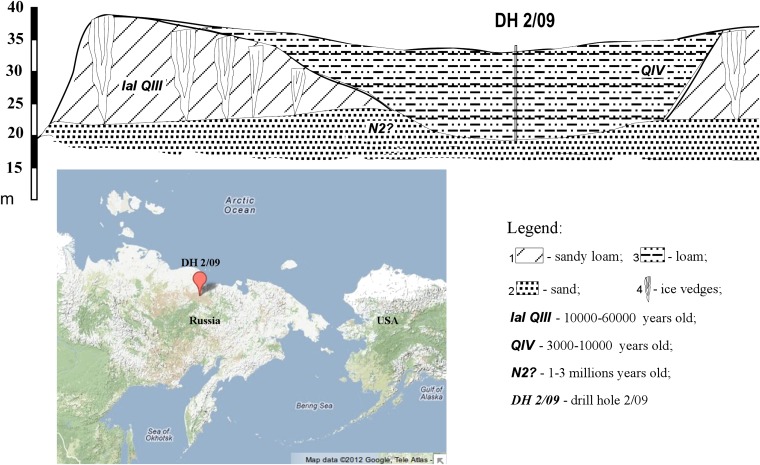
Fig. 1.
(Upper) Geological cross-section of drilling site DH 2/09 on the right bank of the Kolyma River. Scale bar at left denotes meters above sea level. (Lower) Map location of drill site DH 2/09 at 68.633°N, 159.079°E.
Table 1.
Isolation from Siberian permafrost of bacterial strains capable of growing under simulated Mars conditions
| Permafrost sample | Total colonies tested | No. of isolates | Strain designations |
| 4 | 2.1 × 103 | 5 | WN1359 WN1370 WN1371 WN1372 WN1373 |
| 5 | 3.1 × 103 | 0 | n/a |
| 8 | 3.3 × 103 | 0 | n/a |
| 9 | 7.9 × 102 | 1 | WN1374 |
n/a, not applicable.
Growth of Permafrost Isolates Under Low-PTA Conditions.
To further understand the effects of temperature, atmospheric composition, and pressure on permafrost isolates, the following experiment was performed, depicted in Fig. 2. One hundred isolates from permafrost samples 4, 5, 8, and 9 were picked onto triplicate TSBYS plates. All plates were incubated at 0 °C for 30 d under different combinations of atmosphere and pressure. The TSBYS plate incubated at Earth atmospheric composition and pressure (i.e., low-T conditions) showed luxuriant colony development with a variety of different colony morphotypes, including distinctive orange colonies (Fig. 2A). On the replicate TSBYS plate incubated under anoxic atmosphere but Earth-normal pressure of 1,013 mbar (i.e., low-TA conditions) only one colony, number 43, was able to grow (Fig. 2B). This same colony was also able to grow at 7 mbar pressure under full low-PTA conditions (Fig. 2C); in fact, it seemed that colony 43 actually grew best under low-PTA conditions (compare in Fig. 2 panels A, B, and C). Isolate 43 was streak-purified and designated strain WN1359. Despite the fact that growth of the other 99 colonies was severely inhibited by 30 d of exposure to low-PTA conditions, all colonies recovered and grew rapidly (within 24 h) upon return to laboratory benchtop conditions (Fig. 2D). This result is in good agreement with previous observations that growth inhibition of microbes caused by exposure to low pressure is apparently nonlethal and rapidly reversible (7, 10).
Fig. 2.
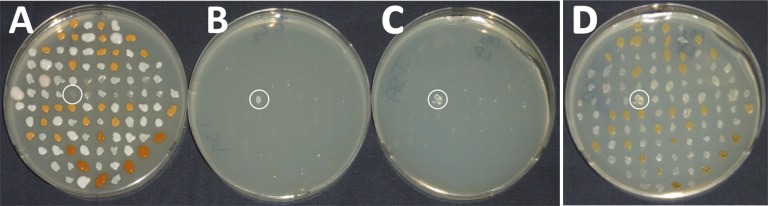
Growth of 100 isolates from Siberian permafrost samples on TSBYS plates. Isolates are from permafrost sample 5 (colonies 1–25), 4 (colonies 26–50), 8 (colonies 51–75), and 9 (colonies 76–100). (A–C) All isolates were cultivated for 30 d at 0 °C under the following conditions: (A) Earth atmosphere and pressure. (B) Simulated Mars atmosphere, Earth pressure. (C) Simulated Mars atmosphere and pressure. Colony 43 from permafrost sample 4 was designated strain WN1359. (D) Same plate as in C, but after 1 additional day of incubation on the laboratory bench (i.e., at room temperature, Earth atmospheric composition and pressure).
Identification of Permafrost Isolates as Carnobacterium spp.
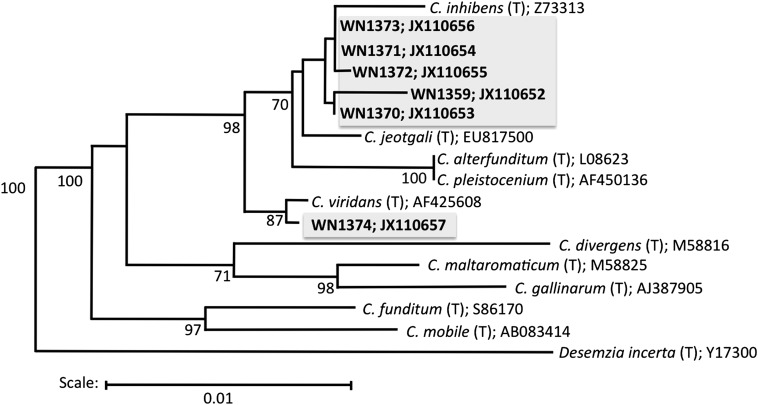
The 16S ribosomal DNA (rDNA) sequences were determined for all six isolates that could grow under low-PTA conditions (Table 1) and were compared with the16S rDNA database in the Ribosomal Database Project (Fig. 3). All five isolates from permafrost sample 4 were found to be most closely related to Carnobacterium inhibens type strain DSM13024T, with the following % identities: WN1359 (94.9%), WN1370 (95.7%), WN1371 (95.9%), WN1372 (96.4%), and WN1373 (96.6%). The sole isolate from permafrost sample 9, strain WN1374, was found to be most closely related to Carnobacterium viridans type strain DSM14451T (96.7%). Using the molecular criteria that bacterial species and genera demonstrate >97% and >95% 16S rDNA sequence identity, respectively (11, 12), the permafrost isolates likely represent unique species of the genus Carnobacterium; however, definitive designation of species status must await more rigorous taxonomic characterization (13). It is quite interesting to note that the 16S rDNA sequences from all five Carnobacterium spp. isolates obtained from permafrost sample 4 were found to be >97% identical to Carnobacterium sp. strain LV62:W1 isolated from Lake Vanda, Antarctica (GenBank accession no. AF076637) (14). Furthermore, 16S rDNA from the single strain isolated from permafrost sample 9 was >97% identical to that of Carnobacterium sp. strain ARCTIC-P35 (GenBank accession no. AY573048 (15). Thus, all of the permafrost isolates obtained in this study were most closely related to other Carnobacterium spp. isolated from Arctic and Antarctic environments.
Fig. 3.
Phylogenetic tree depicting the relatedness of permafrost isolates (gray boxes) to Carnobacterium type strains. The tree was constructed using the Tree Builder program on the Ribosomal Database Project server, using Desemzia incerta as the outgroup. Numbers to the right of strain names are the GenBank accession numbers of the corresponding 16S rDNA sequences.
Several of the distinctive orange colonies (Fig. 2) were also characterized by 16S rDNA sequence analysis as most closely related to the Gram-positive psychrophile Exiguobacterium sibiricum strain 7-3, which was previously reported to have been isolated from permafrost samples obtained from the Kolyma region by one of the authors (D.G.) (16). Although these isolates were unable to grow under low-PTA conditions (Fig. 2), this observation lent greater confidence that the Carnobacterium spp. isolates described here indeed originated from permafrost and were not contaminants.
Growth Kinetics of Permafrost Isolates and Carnobacterium Type Species Under Low-PTA Conditions.
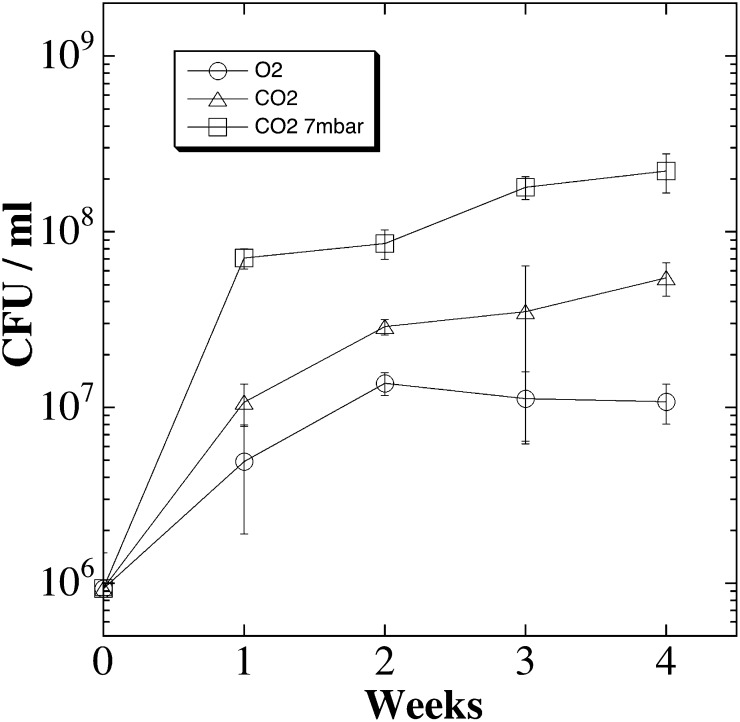
At present, the genus Carnobacterium consists of ten recognized species: C. alterfunditum, C. divergens, C. funditum, C. gallinarum, C. inhibens, C. jeotgali, C. maltaromaticum, C. mobile, C. pleistocenium, and C. viridans. All Carnobacterium species to date are capable of growing at low temperatures and have been isolated from a wide variety of environments, including the Arctic and Antarctic (Table 2). This observation prompted us to quantify the growth kinetics under low-PTA conditions of both the permafrost isolates and nine type species of Carnobacterium. A typical experiment performed on triplicate samples of permafrost strain WN1359 (Fig. 4) showed that strain WN1359 grew better under low-TA conditions (triangles) than low-T conditions (circles), and grew best under low-PTA conditions (squares).
Table 2.
Bacterial strains used in this study
| Strain | Genus and species | Isolated from: | Source [ref(s).] |
| WN1343 | Serratia liquefaciens ATCC27592 | n/a | ATCC |
| WN1359 | Carnobacterium sp. | Permafrost sample 4 | This study |
| WN1370 | Carnobacterium sp. | Permafrost sample 4 | This study |
| WN1371 | Carnobacterium sp. | Permafrost sample 4 | This study |
| WN1372 | Carnobacterium sp. | Permafrost sample 4 | This study |
| WN1373 | Carnobacterium sp. | Permafrost sample 4 | This study |
| WN1374 | Carnobacterium sp. | Permafrost sample 9 | This study |
| WN1360 (DSM 4847T) | C. gallinarum | Refrigerated, vacuum-packed meat | DSMZ (25) |
| WN1361 (DSM 4848T) | C. mobile | Irradiated chicken meat | DSMZ (25) |
| WN1362 (DSM 13024T) | C. inhibens | Intestine of Atlantic salmon | DSMZ (26) |
| WN1363 (DSM 14451T) | C. viridans | Refrigerated, vacuum-packed bologna | DSMZ (27) |
| WN1364 (DSM 20342T) | C. maltaromaticum | Milk with malty flavor | DSMZ (28) |
| WN1366 (DSM 20623T) | C. divergens | Refrigerated, vacuum-packed meat | DSMZ (25, 29) |
| WN1367 (DSM 5970T) | C. funditum | Anoxic waters, Ace Lake, Antarctica | DSMZ (30) |
| WN1368 (DSM 5972T) | C. alterfunditum | Anoxic waters, Ace Lake, Antarctica | DSMZ (30) |
| WN1369 (DSM 17715T) | C. pleistocenium | Permafrost, Fox Tunnel, Alaska | DSMZ (18) |
ATCC, American Type Culture Collection; n/a, not applicable.
Fig. 4.
Growth kinetics of permafrost isolate WN1359 on TSBYS at 0 °C and Earth atmosphere and pressure (circles); simulated Mars atmosphere and Earth pressure (triangles); and simulated Mars atmosphere and pressure (squares). Data are averages and SDs of triplicate determinations.
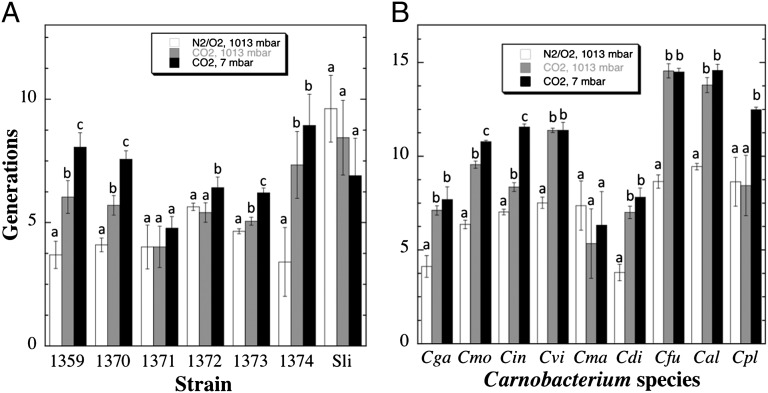
The same experiment depicted for strain WN1359 in Fig. 4 was also conducted on all six permafrost isolates (Fig. 5A) and nine Carnobacterium spp. type strains (Fig. 5B); to conserve space, the data were converted to number of generations each strain grew over the 28-d course of the experiment (Materials and Methods). All bacteria were capable of growth under low-PTA conditions, but several distinct patterns of growth under different environmental conditions emerged (Fig. 5). For convenience, the growth pattern observed by strain WN1359 is designated here as abc, following its statistical grouping (Fig. 5A). In other words, growth followed the pattern low-PTA > low-TA > low-T (Fig. 5A). In addition to strain WN1359, strains WN1370, WN1373, C. mobile, and C. inhibens also exhibited the abc pattern of growth (Fig. 5). Other growth patterns were also discernible. For example, several bacteria demonstrated an abb growth pattern (i.e., their growth followed the pattern low-TA = low-PTA > low-T). These strains included permafrost strain WN1374, C. gallinarum, C. viridans, C. divergens, C. funditum, and C. alterfunditum. Strains WN1372 and C. pleistocenium exhibited an aab growth pattern, in which they only grew better under low-PTA conditions (Fig. 5). Finally, strains WN1371 and C. maltaromaticum exhibited an aaa growth pattern in which no statistical difference in growth was detected under the three conditions tested. For comparative purposes, the same experiment was performed on S. liquefaciens strain ATCC7529, also recently shown to grow at 7 mbar, which exhibited an aaa type growth pattern.
Fig. 5.
Growth of permafrost isolates (A) and Carnobacterium spp. type strains (B) after 28 d of incubation on TSBYS plates at 0 °C and the following conditions: Earth atmosphere and pressure (open bars); simulated Mars atmosphere and Earth pressure (shaded bars); and simulated Mars atmosphere and pressure (black bars). Generations were calculated as described in Materials and Methods. Data shown are averages and SDs of triplicate determinations. Lowercase letters denote significant differences compared with the Earth control of each strain (ANOVA, P ≤ 0.05). Cga, C. gallinarum; Cmo, C. mobile; Cin, C. inhibens; Cvi, C. viridans; Cma, C. maltaromaticum; Cdi, C. divergens; Cfu, C. funditum; Cal, C. alterfunditum; Cpl, C. pleistocenium; Sli, Serratia liquefaciens.
Conclusions.
From this work it can be seen that microbial communities from Siberian permafrost contain organisms capable of growing under conditions of low temperature, low pressure, and anoxic CO2-dominated atmosphere. It should be stressed, however, that low-PTA conditions are only a subset of the total potentially biotoxic physical factors constraining the survival or growth of terrestrial microbes on Mars, such as solar UV, extreme desiccation, solar particle events, and galactic cosmic rays. In addition, the martian regolith itself contains numerous potentially biotoxic factors, such as salinity, pH, and Eh of available liquid water; oxidizing soils created by UV-induced processes and soil chemical reactions; or the presence of heavy metals (discussed extensively in refs. 7 and 17).
Not all of the tested permafrost soil samples yielded isolates, perhaps because the initial incubations were performed at room temperature (ca. 23 °C), which would inhibit the growth of strict psychrophiles; however, all isolates obtained from this set of experiments belonged to the genus Carnobacterium. As its name suggests, the first isolates of the genus were obtained as putative spoilage organisms of meat products. In this regard it is interesting to note that three species, C. gallinarum, C. viridans, and C. divergens, were all isolated from refrigerated vacuum-packed meats (Table 2), suggesting the ability of these microbes to grow under cold, low-pressure conditions. Several Carnobacterium spp. have also been isolated from permanently cold environments (C. alterfunditum, C. funditum), including arctic permafrost (C. pleistocenium) (Table 2). The extreme age of the Siberian permafrost isolates reported here (∼6,000–8,000 y) and the Alaskan permafrost isolate C. pleistocenium (∼32,000 y) (18) indicates that these species are capable of long periods of survival in permanently frozen environments.
Terrestrial life has evolved mechanisms to tolerate and indeed flourish in extreme high-pressure habitats such as the deep ocean (19), but very little is understood regarding microbial responses to low pressure, a physical constraint not found in nature on the Earth’s surface. The discovery of terrestrial bacteria that can inhabit cold, low-pressure, anoxic environments has implications for the fields of extremophile biology, astrobiology, the search for extraterrestrial life, and planetary protection. Permafrosts seem to be promising locations for the isolation of microbes capable of growth under cold, anoxic, and hypobaric conditions such as are encountered on Mars. It is anticipated that further sampling of permafrost from other locations or depths will yield additional microbes exhibiting this unique set of properties.
Materials and Methods
Permafrost Samples.
Permafrost samples with a mean annual temperature of −7 °C were taken in the forest-tundra zone of northeastern Siberia (68.633°N, 159.079°E, the right bank of the Kolyma River) (Fig. 1) using a dry drilling rig that operates without fluids and prevents down-hole contamination (20). Four frozen soil samples, 4, 5, 8, and 9, were collected from the depths of 12.7, 16.5, 20.1, and 21.8 m below the surface, respectively. The strict protocols for drilling and the subsequent handling of cores were designed to ensure that uncontaminated material was retrieved (20). After removal from the corer, the surfaces of the cores (68- to 107-mm diameter) were cleaned by shaving off the outer layers with an alcohol-sterilized knife. For microbiological studies the cores were split into 5-cm-long segments, placed into sterile plastic bags, stored frozen in the field within a hole excavated in the permafrost, transported frozen to the Soil Cryology Laboratory in Pushchino, Russia, and then transported frozen by air to Kennedy Space Center, FL. The samples represent epigenetically frozen layers of Holocene age (6–8 kyBP) formed by refreezing of sediments that had melted and drained. They are hydrocarbonate-calcium fine-grained peaty sandy-loams, firmly bound by ice (ice content 25–37%), with pH close to neutral (6.8–7.2) and containing organic carbon (0.5–2.0%).
Microbiological Techniques.
Medium used throughout was TSBYS medium containing (per L): trypticase soy broth, 30 g; yeast extract, 3 g; NaCl, 13 g; KCl, 0.34 g; MgCl2 × 6H2O, 4 g; MgSO4 × 7 H2O, 3.45 g; NH4Cl, 0.25 g; CaCl2 × 2H2O, 0.14 g. For plates, TSBYS was solidified with agar (15 g/L). Permafrost samples (0.9–1.4 g) were diluted serially 10-fold in double-distilled water, plated on TSBYS agar, and incubated at room temperature (ca. 23 °C) for times ranging from 1 to 30 d. Colonies arising were either replica-plated or picked onto fresh TSBYS plates in 100-colony grid patterns and exposed to environmental simulations (example in Fig. 2). Each colony able to grow under simulated Mars conditions was picked, streak-purified, grown overnight in TSBYS liquid medium, and stored as a frozen glycerol stock at −70 °C. Carnobacterium type strains were obtained from the Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ)-German Collection of Microorganisms and Cell Cultures (www.dsmz.de). All strains used are listed in Table 2.
Low-PTA Simulations.
The hypobaric system used for attaining low-PTA conditions has been described in detail (7). In brief, a 4-L polystyrene desiccator was placed in a low-temperature incubator set at 0 °C and connected to a low-pressure controller and vacuum pump (model PU-842; KNF Neuberger). Inoculated TSBYS plates were inserted into the desiccator, agar surface upward (i.e., not inverted). Six anaerobic pouches (model R681001 AnaeroPacks, Remel, Thermo Fisher Scientific) were placed inside with the vertically stacked plates. The system was then flushed with ultra-high-purity CO2 and the desiccator sealed; this constituted anoxic atmosphere. The pressure inside the desiccator was either left at Earth-normal level (1,013 mbar) or pumped down to 7 mbar, the global average Mars surface pressure (5). At 7-d intervals the hypobaric chamber was vented to laboratory atmosphere, samples removed for assay, the anaerobic pouches replaced, and the system returned to test conditions. The anaerobic pouches have been shown to reduce the O2 concentration to <0.1% within 1 h (21). All plates were maintained for up to 30 d at 0 °C.
Quantitative Growth Assays.
Two microliters of a fresh overnight culture made in liquid TSBYS (containing from 5 × 104 to 5 × 106 cells) were spotted in triplicate onto TSBYS plates and incubated under low-PTA conditions. At weekly intervals, agar blocks containing the cell spots were cut out of the plates with a sterile scalpel and placed individually into 1 mL of sterile PBS (10 mM potassium phosphate and 150 mM NaCl, pH 7.5). Cells were suspended by vortexing, diluted serially 10-fold, and plated for viable cells on TSBYS plates incubated at room temperature. Numbers of generations (n) were calculated using the equation
where N1 and N2 are the number of cells per spot at 0 and 4 wk, respectively (22).
Molecular Taxonomy of Strains by 16S rDNA Analysis.
Chromosomal DNA was purified and 16S rDNAs were amplified and sequenced as described previously (23). 16S rDNA sequences were used to query the Ribosomal Database Project (RDP) database (24) (http://rdp.cme.msu.edu/). The phylogenetic tree was built with the RDP Tree Builder using the Weighbor weighted neighbor-joining tree-building algorithm. All 16S rDNA sequences have been deposited in GenBank under the accession numbers denoted in Fig. 3.
Acknowledgments
We thank Krystal Kerney, Rafael Rodrigues de Oliveira, Samantha Waters, and Vasiliy Mironov for excellent technical assistance and the three reviewers for their insightful comments. This work was supported in part by Grant NNX08AO15G from the National Aeronautics and Space Administration (NASA) Astrobiology: Exobiology and Evolutionary Biology program (to W.L.N. and A.C.S.). Michael Dolan and the Marine Biological Laboratory’s NASA Planetary Biology Internship program supported K.K. during part of this work.
Footnotes
References
- 1.Chyba CF, Hand KP. Astrobiology: The study of the living universe. Annu Rev Astron Astrophys. 2005;43:31–74. [Google Scholar]
- 2.Jones EG, Lineweaver CH, Clarke JD. An extensive phase space for the potential martian biosphere. Astrobiology. 2011;11(10):1017–1033. doi: 10.1089/ast.2011.0660. [DOI] [PubMed] [Google Scholar]
- 3.McCoy TJ, Corrigan CM, Herd CDK. Combining meteorites and missions to explore Mars. Proc Natl Acad Sci USA. 2011;108(48):19159–19164. doi: 10.1073/pnas.1013478108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horneck G. The microbial case for Mars and its implication for human expeditions to Mars. Acta Astronaut. 2008;63(7-10):1015–1024. [Google Scholar]
- 5.Schuerger AC. Microbial ecology of the surface exploration of Mars with human-operated vehicles. In: Cockell CS, editor. Martian Expedition Planning. Santa Barbra, CA: Univelt; 2004. pp. 363–386. [Google Scholar]
- 6.Jensen LL, et al. A facility for long-term Mars simulation experiments: The Mars Environmental Simulation Chamber (MESCH) Astrobiology. 2008;8(3):537–548. doi: 10.1089/ast.2006.0092. [DOI] [PubMed] [Google Scholar]
- 7.Schuerger AC, Nicholson WL. Interactive effects of hypobaria, low temperature, and CO2 atmospheres inhibit the growth of mesophilic Bacillus spp. under simulated martian conditions. Icarus. 2006;185(1):143–152. [Google Scholar]
- 8.Berry BJ, Jenkins DG, Schuerger AC. Effects of simulated Mars conditions on the survival and growth of Escherichia coli and Serratia liquefaciens. Appl Environ Microbiol. 2010;76(8):2377–2386. doi: 10.1128/AEM.02147-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frolov AD. A review of the nature and geophysical studies of the thick permafrost in Siberia: Relevance to exploration on Mars. J Geophys Res Planets. 2003;108(E4):7. [Google Scholar]
- 10.Nicholson WL, et al. Exploring the low-pressure growth limit: Evolution of Bacillus subtilis in the laboratory to enhanced growth at 5 kilopascals. Appl Environ Microbiol. 2010;76(22):7559–7565. doi: 10.1128/AEM.01126-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schloss PD, Handelsman J. Introducing DOTUR, a computer program for defining operational taxonomic units and estimating species richness. Appl Environ Microbiol. 2005;71(3):1501–1506. doi: 10.1128/AEM.71.3.1501-1506.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stackebrandt E, Goebel BM. A place for DNA-DNA reassociation and 16S ribosomal RNA sequence analysis in the present species definition in bacteriology. Int J Syst Bacteriol. 1994;44(4):846–849. [Google Scholar]
- 13.Tindall BJ, Rosselló-Móra R, Busse HJ, Ludwig W, Kämpfer P. Notes on the characterization of prokaryote strains for taxonomic purposes. Int J Syst Evol Microbiol. 2010;60(Pt 1):249–266. doi: 10.1099/ijs.0.016949-0. [DOI] [PubMed] [Google Scholar]
- 14.Bratina BJ, Stevenson BS, Green WJ, Schmidt TM. Manganese reduction by microbes from oxic regions of the lake vanda (Antarctica) water column. Appl Environ Microbiol. 1998;64(10):3791–3797. doi: 10.1128/aem.64.10.3791-3797.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee HK, Lee YK, Sung KC. Phylogenetic analysis of protease-producing Arctic bacteria, Carnobacterium sp. ARCTIC-P35. 2004. Available at www.ncbi.nlm.nih.gov/nuccore/45826492. Accessed November 26, 2012.
- 16.Rodrigues DF, et al. Characterization of Exiguobacterium isolates from the Siberian permafrost. Description of Exiguobacterium sibiricum sp. nov. Extremophiles. 2006;10(4):285–294. doi: 10.1007/s00792-005-0497-5. [DOI] [PubMed] [Google Scholar]
- 17.Schuerger AC, Golden DC, Ming DW. Biotoxicity of Mars soils: 1. Dry deposition of analog soils on microbial colonies and survival under martian conditions. Planet Space Sci. 2012 doi: 10.1016/j.pss.2012.07.026. [DOI] [Google Scholar]
- 18.Pikuta EV, et al. Carnobacterium pleistocenium sp. nov., a novel psychrotolerant, facultative anaerobe isolated from permafrost of the Fox Tunnel in Alaska. Int J Syst Evol Microbiol. 2005;55(Pt 1):473–478. doi: 10.1099/ijs.0.63384-0. [DOI] [PubMed] [Google Scholar]
- 19. Meersman F, Heremans K (2008) High-Pressure Microbiology, eds Michiels C, Bartlett DH, Aertsen A (ASM Press, Washington, DC), pp 1-17.
- 20.Shi T, Reeves RH, Gilichinsky DA, Friedmann EI. Characterization of viable bacteria from Siberian permafrost by 16S rDNA sequencing. Microb Ecol. 1997;33(3):169–179. doi: 10.1007/s002489900019. [DOI] [PubMed] [Google Scholar]
- 21.Van Horn KG, Warren K, Baccaglini EJ. Evaluation of the AnaeroPack system for growth of anaerobic bacteria. J Clin Microbiol. 1997;35(8):2170–2173. doi: 10.1128/jcm.35.8.2170-2173.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neidhardt FC, Ingraham JL, Schaechter M. Physiology of the Bacterial Cell: A Molecular Approach. Sunderland, MA: Sinauer Associates; 1990. [Google Scholar]
- 23.Fajardo-Cavazos P, Nicholson W. Bacillus endospores isolated from granite: Close molecular relationships to globally distributed Bacillus spp. from endolithic and extreme environments. Appl Environ Microbiol. 2006;72(4):2856–2863. doi: 10.1128/AEM.72.4.2856-2863.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cole JR, et al. The Ribosomal Database Project: Improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 2009;37(Database issue):D141–D145. doi: 10.1093/nar/gkn879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collins MD, Farrow JAE, Phillips BA, Ferusu S, Jones D. Classification of Lactobacillus divergens, Lactobacillus piscicola, and some catalase-negative, asporogenous, rod-shaped bacteria from poultry in a new genus, Carnobacterium. Int J Syst Bacteriol. 1987;37(4):310–316. [Google Scholar]
- 26.Jöborn A, Dorsch M, Olsson JC, Westerdahl A, Kjelleberg S. Carnobacterium inhibens sp. nov., isolated from the intestine of Atlantic salmon (Salmo salar) Int J Syst Bacteriol. 1999;49(Pt 4):1891–1898. doi: 10.1099/00207713-49-4-1891. [DOI] [PubMed] [Google Scholar]
- 27.Holley RA, Guan TY, Peirson M, Yost CK. Carnobacterium viridans sp. nov., an alkaliphilic, facultative anaerobe isolated from refrigerated, vacuum-packed bologna sausage. Int J Syst Evol Microbiol. 2002;52(Pt 5):1881–1885. doi: 10.1099/00207713-52-5-1881. [DOI] [PubMed] [Google Scholar]
- 28.Mora D, Scarpellini M, Franzetti L, Colombo S, Galli A. Reclassification of Lactobacillus maltaromicus (Miller et al. 1974) DSM 20342(T) and DSM 20344 and Carnobacterium piscicola (Collins et al. 1987) DSM 20730(T) and DSM 20722 as Carnobacterium maltaromaticum comb. nov. Int J Syst Evol Microbiol. 2003;53(Pt 3):675–678. doi: 10.1099/ijs.0.02405-0. [DOI] [PubMed] [Google Scholar]
- 29.Holzapfel WH, Gerber ES. Lactobacillus divergens sp. nov., a new heterofermentative Lactobacillus species producing L(+)-lactate. Syst Appl Microbiol. 1983;4:522–534. doi: 10.1016/S0723-2020(83)80010-1. [DOI] [PubMed] [Google Scholar]
- 30.Franzmann PD, Höpfl P, Weiss N, Tindall BJ. Psychrotrophic, lactic acid-producing bacteria from anoxic waters in Ace Lake, Antarctica; Carnobacterium funditum sp. nov. and Carnobacterium alterfunditum sp. nov. Arch Microbiol. 1991;156(4):255–262. doi: 10.1007/BF00262994. [DOI] [PubMed] [Google Scholar]