Abstract
Interleukin (IL)-7 is a cytokine essential for T lymphocyte development and homeostasis. However, little is known about the roles of IL-7 receptor α-chain (IL-7Rα) in late stages of T-cell development. To address this question, we established IL-7Rα–floxed mice and crossed them with CD4-Cre transgenic mice. Resultant IL-7R conditional knockout (IL-7RcKO) mice exhibited marked reduction in CD8 single positive (SP) T cells, regulatory T cells (Tregs), and natural killer T (NKT) cells in thymus. The proportion and proliferation of both mature CD4SP and CD8SP thymocytes were decreased without affecting Runx expression. In addition, expression of the glucocorticoid-induced TNF receptor was reduced in CD4SP and CD8SP thymocytes, and expression of CD5 was decreased in CD8SP thymocytes. IL-7RcKO mice also showed impaired Treg and NKT cell proliferation and inhibition of NKT cell maturation. Bcl-2 expression was reduced in CD4SP and CD8SP thymocytes but not in Tregs and NKT cells, and introduction of a Bcl-2 transgene rescued frequency and CD5 expression of CD8SP thymocytes. Furthermore, IL-7RcKO mice exhibited greatly increased numbers of B cells and, to a lesser extent, γδ T and dendritic cells in thymus. Overall, this study demonstrates that IL-7Rα differentially controls development and maturation of thymocyte subpopulations in late developmental stages and suggests that IL-7R expression on αβ T cells suppresses development of other cell lineages in thymus.
Keywords: differentiation, survival
Interkeukin (IL)-7 is a cytokine essential for lymphocyte development and survival. The IL-7 receptor (IL-7R) consists of a common cytokine receptor γ-chain (γc) and a unique IL-7R α-chain (IL-7Rα). IL-7Rα also forms the receptor for thymic stromal lymphopoietin (TSLP) by interacting with the TSLP receptor. Mice deficient in either IL-7 or IL-7Rα show markedly reduced numbers of T and B cells. The IL-7R transmits signals for proliferation and survival and for V(D)J recombination of Ig heavy chain and T-cell receptor (TCR) γ-chain loci in early lymphocytes (1, 2). In the periphery, IL-7 regulates T-cell homeostasis by enhancing survival and proliferation of naive and memory T cells (3). Upon IL-7 binding, the IL-7R activates JAK1 and JAK3 tyrosine kinases, which then activates STAT5 and phosphatidylinositol 3-kinase. IL-7 signaling promotes cell survival by inducing expression of antiapoptotic factors such as Bcl-2, Bcl-xL, and Mcl-1 in T cells (4). Thus, introduction of Bcl-2 transgenes significantly rescues T-cell development in IL-7Rα–deficient mice (5, 6).
The γc cytokine signal controls differentiation of CD8 single positive (SP) thymocytes. The IL-7R is expressed on CD4−8− double negative (DN) thymocytes but then down-regulated in CD4+8+ double positive (DP) thymocytes. After positive selection, transient cessation of TCR signaling induces IL-7Rα reexpression on postselection thymocytes (7). The IL-7R permits thymocytes to differentiate into the CD8 lineage (8). Both Runx3 and STAT transcription factors function in CD8 T-cell differentiation by interacting with CD8 enhancer, which is responsive to IL-7 (7). In contrast, another group reported that CD8 lineage specification occurs normally in the absence of IL-7R signaling by anti–IL-7R antibody (9). In addition, IL-15 also functions in CD8 T-cell differentiation (10). However, little is known about precise roles of the IL-7R in CD8 T-cell development, because IL-7Rα–deficient mice show severely impaired expansion of DN thymocytes.
The roles of the IL-7R in development of regulatory T cells (Tregs) and natural killer T (NKT) cells remain controversial. In γc-deficient mice, Tregs are severely reduced in the thymus (11–13). Although the proportion of Tregs to non-Treg CD4 T cells is not altered in the IL-7Rα–deficient thymus, IL-7Rα/IL-2Rβ doubly deficient mice show more severe reduction of Tregs than do IL-2Rβ–deficient mice, and the absolute number of Tregs is reduced in IL-7Rα–deficient mice (11–14). As for NKT cell development, it is reported that γc-deficient mice lack thymic NKT cells (15). In contrast, the proportion of NKT cells is not reduced in the IL-7– or IL-7Rα–deficient thymus, although absolute numbers of thymic NKT cells are drastically reduced (15, 16). Matsuda et al. observed that IL-7/IL-15 doubly deficient mice show more severe reduction in NKT cells than do IL-15–deficient mice (16). Nonetheless, the roles of the IL-7R in development of Tregs and NKT cells in the thymus remain unclear.
Early thymocyte development is severely impaired in IL-7Rα–deficient mice, making it difficult to precisely determine IL-7Rα function in late stages of T-cell development. To address this question, we used a conditional knockout approach and show that IL-7Rα regulates development and maturation of multiple thymocyte subpopulations in late developmental stages.
Results
Generation of IL-7RcKO Mice.
To investigate the roles of the IL-7R in the late stages of T-cell development, we generated IL-7Rα–floxed (IL-7Rαf/f) mice (Fig. S1A and B) and bred them with CD4-Cre transgenic (Tg) mice (IL-7RcKO). IL-7Rα expression on DN thymocytes of IL-7RcKO mice was comparable to that seen in control mice (Fig. S1C). Although IL-7Rα is down-regulated on DP thymocytes, reexpression of IL-7Rα on CD4SP, CD8SP, Treg, and NKT cells did not occur and IL-7R on γδ T cells was not changed in IL-7RcKO mice (Fig. S1C). Therefore, we conclude that CD4-Cre transgene-mediated deletion of the IL-7Rα gene was successfully achieved.
IL-7R Is Required for Development of CD8 T Cells in Thymus.
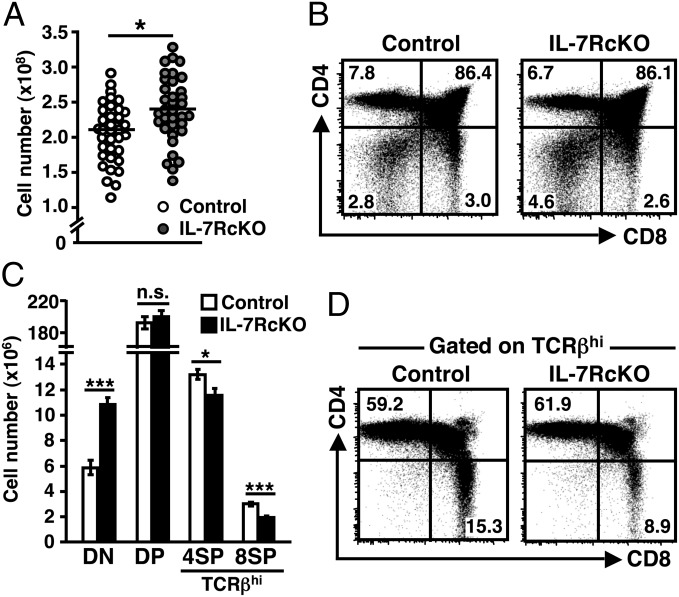
First, we compared the total cell number of thymocytes between IL-7RcKO and littermate control mice. Unexpectedly, thymocyte number was slightly increased in IL-7RcKO mice (control: 212 ± 7.1 × 106 versus IL-7RcKO: 241 ± 8.4 × 106) (Fig. 1A). IL-7RcKO mice exhibited an almost normal proportion of DP, CD4SP, and CD8SP thymocytes, whereas the proportion and absolute number of DN thymocytes increased by twofold (Fig. 1 B and C). The proportion and absolute number of TCRβhigh (TCRβhi) thymocytes was reduced by 30% in IL-7RcKO mice (Fig. S2A). Gating on TCRβhi thymocytes revealed that the frequency of CD8SP thymocytes was reduced (Fig. 1D). Similar results were obtained by analyzing IL-7RcKO mice crossed with OT-I and H-Y TCR Tg mice, whereas the frequency of CD4SP thymocytes was unchanged in IL-7RcKO mice crossed with OT-II TCR Tg mice (Fig. S2B). The absolute number of TCRβhi CD8SP and CD4SP thymocytes decreased by 40% and 10%, respectively, in IL-7RcKO mice (Fig. 1C). Consistent with previous reports that γc cytokines influence CD8 T-cell commitment (8), our results confirm that IL-7 is not the only factor that regulates development of CD8SP thymocytes. Our data also suggest that the IL-7R is required for CD4SP thymocytes, although to a lesser extent.
Fig. 1.
IL-7R is required for development of CD8 T cells in thymus. (A) Absolute numbers of thymocytes from control (IL-7R+/+ CD4-Cre or IL-7Rfl/fl) and IL-7RcKO (IL-7Rfl/fl CD4-Cre) mice of 4–5 wk old. Cell numbers from individual mice (circles) and means (horizontal bars) are shown (n = 35). (B) CD4 and CD8 expression in total thymocytes. (C) Absolute numbers of indicated populations (mean ± SEM, n = 35). (D) Expression of CD4 and CD8 in thymocytes gated on TCRβhi cells. Representative data are shown (n > 10) (B and D).
IL-7R Controls Proliferation of Mature CD4SP and CD8SP Thymocytes.
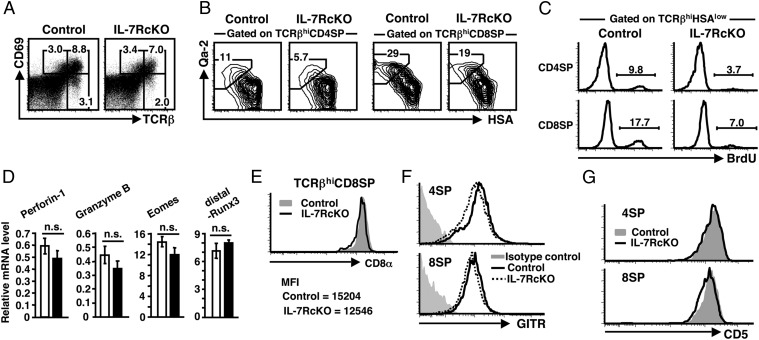
During positive selection, thymocytes undergo progressive stages of development from TCRβintermediate (TCRβint)CD69+ to TCRβhiCD69+. After positive selection, SP thymocytes first acquire a semimature phenotype (CD69+ heat stable antigen (HSA)hiQa-2lowCD62Llow) and then differentiate into mature phenotype (CD69−HSAlowQa-2hiCD62Lhi). Whereas the IL-7R is down-regulated in most DP thymocytes, its reexpression begins in TCRβintCD69+ DP cells (17). Although the frequency of TCRβintCD69+ cells was unchanged in IL-7RcKO mice, the frequency of TCRβhiCD69− and TCRβhiHSAlow cells was more severely reduced than the frequency of TCRβhiCD69+ and TCRβhiHSAhi cells in IL-7RcKO mice (Fig. 2A and Fig. S3A). Compared with control mice, thymocytes with HSAlowQa-2hi and HSAlowCD62Lhi mature phenotypes were decreased in both CD4SP and CD8SP thymocytes in IL-7RcKO mice (Fig. 2B and Fig. S3B). These results demonstrate that generation of both mature CD4SP and CD8SP thymocytes is impaired in IL-7RcKO mice.
Fig. 2.
IL-7R controls proliferation of mature CD4SP and CD8SP thymocytes. (A) Expression of TCRβ and CD69 in thymocytes. (B) Expression of HSA and Qa-2 in TCRβhiCD4SP excluding CD4+CD8low and TCRβhiCD8SP thymocytes. (C) BrdU uptake by TCRβhiHSAlow CD4SP and CD8SP thymocytes. Data represent four independent experiments with similar results. (D) Real-time RT-PCR analysis showing expression of indicated genes in TCRβhiCD8SP thymocytes from control (open bars) and IL-7RcKO (filled bars) mice. Transcripts levels were normalized to hypoxanthine-guanine phosphoribosyltransferase (HPRT) mRNA. Values are the mean ± SEM of triplicate data points from three to six independent experiments. (E) CD8α expression on TCRβhiCD8SP thymocytes. Representative data and mean fluorescence intensity (MFI) are shown (n > 10). (F and G), GITR (F) and CD5 (G) expression on TCRβhiCD4SP and TCRβhiCD8SP thymocytes. Data represent five independent experiments with similar results.
Because mature thymocytes reportedly proliferate in the thymus (18), we performed BrdU incorporation assay. Twenty-four hours after injection, the frequency of BrdU+ cells was reduced by two- to threefold in TCRβhiHSAlow CD4SP and CD8SP thymocytes of IL-7RcKO compared with control mice, although some proliferation still occurred without IL-7R signaling (Fig. 2C). We obtained similar results even 4 h after a BrdU pulse (Fig. S3C). Consistent with a previous observation (19), these results demonstrate that the IL-7R drives mature SP thymocytes to proliferate in the thymus but that other signals also regulate CD4SP and CD8SP thymocytes.
To determine the role of the IL-7R in acquiring functional competence by SP thymocytes, we analyzed expression of genes characteristic for each population. Transcripts of Runx1 and GATA3, CD4SP-enriched transcription factors, in TCRβhiCD4SP thymocytes were comparable between control and IL-7RcKO mice (Fig. S3D). Next we measured CD8SP-enriched mRNAs encoding perforin-1, granzyme B, and Eomes. There was no statistically significant difference between control and IL-7RcKO mice (Fig. 2D). Although it was reported that IL-7 signaling induces Runx3 transcription in postselected DP thymocytes in vitro (8), Runx3 was not changed in TCRβhiCD8SP thymocytes of IL-7RcKO mice (Fig. 2D).
IL-7 signaling reportedly regulates CD8α coreceptor expression (20). In IL-7RcKO mice, CD8α expression on TCRβhiCD8SP thymocytes was marginally decreased and was not changed in peripheral CD8 T cells (Fig. 2E and Fig. S3E). Gating on TCRβhiHSAhi, but not TCRβhiHSAlow, thymocytes (including 4SP, DP, and 8SP) revealed that CD8α expression on the CD8α+ population was significantly reduced in IL-7RcKO mice (Fig. S3F). We also found that a costimulatory molecule, the glucocorticoid-induced TNF receptor (GITR), was slightly reduced especially in TCRβhiCD4SP thymocytes and peripheral CD4T cells of IL-7RcKO mice (Fig. 2F and Fig. S3G). Furthermore, CD5 expression on CD8SP thymocytes was lower in IL-7RcKO compared with control mice, whereas that on CD4SP thymocytes was unchanged (Fig. 2G). These results suggest that the IL-7R plays some role in quality control of both CD4SP and CD8SP thymocytes.
Bcl-2 Expression Rescues Development and CD5 Expression of CD8SP Thymocyte.
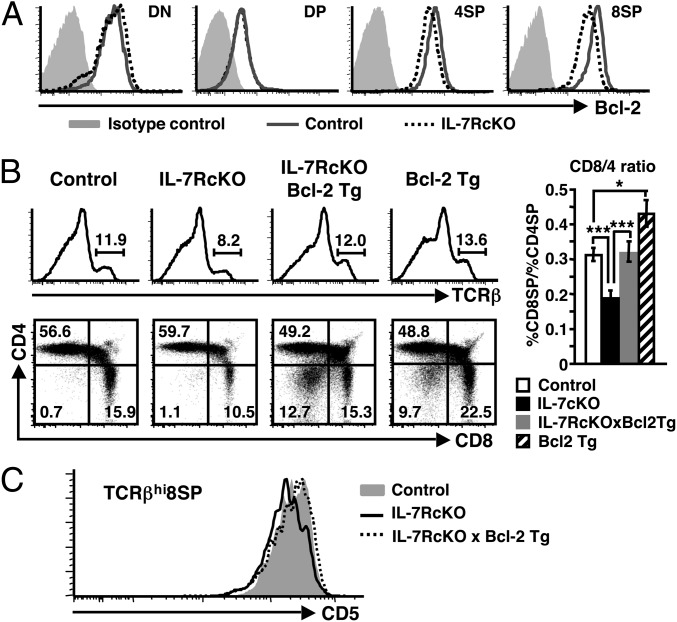
Enforced expression of Bcl-2 reportedly promotes significant recovery of thymic T cells in IL-7Rα–deficient mice (5, 6), indicating that the IL-7R primarily serves a survival signal. Therefore, we compared Bcl-2 expression in thymocyte subpopulations between control and IL-7RcKO mice. Like IL-7Rα expression, Bcl-2 levels were almost comparable in DN and DP thymocytes but were significantly reduced in CD4SP and CD8SP thymocytes in IL-7RcKO mice (Fig. 3A). Furthermore, IL-7RcKO thymocytes showed higher caspase-3 activity than control mice with or without IL-7 in vitro (Fig. S4A). In contrast, Bcl-xL and Mcl-1 were unchanged (Fig. S4 B and C).
Fig. 3.
IL-7R transmits survival signals in CD8SP thymocytes through Bcl-2. (A) Expression of Bcl-2 in thymocyte fractions. 4SP and 8SP indicate TCRβhiCD25−Foxp3−CD4SP and TCRβhiCD8SP, respectively. Data represent five independent experiments with similar results. (B) Histograms (Upper) indicate expression of TCRβ in total thymocytes from mice of each genotype. Dot plots (Lower) show CD4 and CD8 expression in TCRβhi thymocytes. Bar graph (Right) shows the ratio of CD8SP to CD4SP cells in TCRβhi thymocytes (mean ± SEM, n = 8–11). (C) CD5 expression in TCRβhiCD8SP thymocytes from indicated mice. Data represent four independent experiments with similar results.
To test whether Bcl-2 expression rescues SP thymocyte development in IL-7RcKO mice, we bred IL-7RcKO mice with Bcl-2 Tg mice. Introduction of the Bcl-2 transgene completely restored the frequency of TCRβhi thymocytes in IL-7RcKO mice (Fig. 3B). Furthermore, the ratio of CD8SP to CD4SP thymocytes and absolute cell number of CD8SP thymocytes were also recovered in IL-7RcKO mice harboring the Bcl-2 transgene, although they did not reach those of Bcl-2 Tg mice (Fig. 3B and Fig. S4D). In addition, introduction of the Bcl-2 transgene also restored CD5 expression on CD8SP thymocytes in IL-7RcKO mice (Fig. 3C), although Bcl-2 misexpression in control mice did not affect CD5 level on TCRβhiCD8SP thymocytes (Fig. S4E). These results suggest that the IL-7R transmits a survival signal for CD8SP thymocytes primarily through Bcl-2 and that reduced CD5 expression in IL-7RcKO mice may be attributable to reduced survival.
IL-7R Promotes Treg Proliferation in the Thymus.
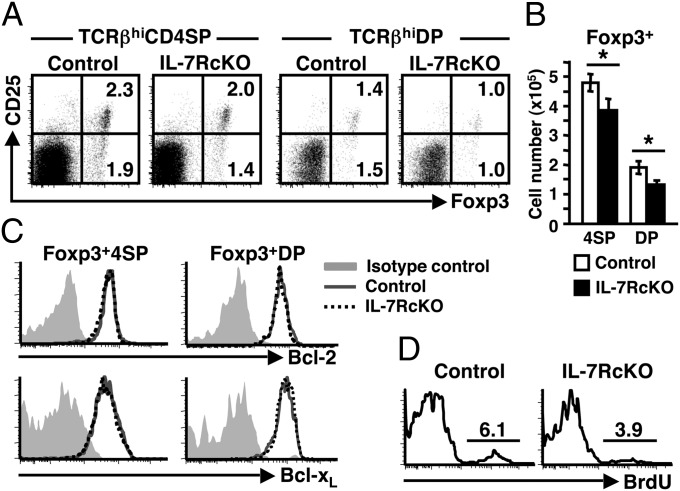
Although γc signaling is critical for thymic Treg development, it is unclear whether IL-7Rα functions in this process. To address this question, we analyzed development of thymic Tregs in IL-7RcKO mice. The frequency and absolute number of both CD25− and CD25+ Foxp3+ cells were decreased by 20% in TCRβhiCD4SP thymocytes of IL-7RcKO mice (Fig. 4 A and B). Because some Tregs develop from DP thymocytes in the medulla (21), we also examined Tregs in the TCRβhiDP fraction. The frequency and absolute number of Foxp3+ cells were also reduced by 30% in TCRβhiDP cells (Fig. 4 A and B). These results suggest that the IL-7R participates in thymic Treg development.
Fig. 4.
IL-7R promotes Treg proliferation in thymus. (A) Expression of Foxp3 and CD25 in TCRβhiCD4SP and TCRβhiDP thymocytes. Data represent eight independent experiments with similar results. (B) Absolute numbers of Foxp3+ cells in CD4SP and DP thymocytes (mean ± SEM, n = 8). (C) Bcl-2 and Bcl-xL expression in Foxp3+TCRβhi CD4SP (Left) and Foxp3+TCRβhi DP (Right) thymocytes. Data represent five (Bcl-2) and two (Bcl-xL) independent experiments with similar results. (D) BrdU incorporation in Foxp3+TCRβhi CD4SP Tregs. BrdU was injected 24 h before analysis. Data represent three independent experiments with similar results.
Next we analyzed the basis of Treg reduction in IL-7RcKO mice. Bcl-2 expression in Foxp3+ 4SP and DP thymocytes was comparable between control and IL-7RcKO mice (Fig. 4C, Upper). Bcl-xL expression was also unchanged (Fig. 4C, Lower). Introduction of the Bcl-2 transgene appeared to rescue the frequency of Foxp3+ CD4SP thymocytes in IL-7RcKO mice (Fig. S5A). However, CD4SP thymocytes of Bcl-2 Tg mice contain more Foxp3+ cells compared with control mice, and the frequency of Foxp3+ cells in IL-7RcKO × Bcl-2 Tg mice did not reach the levels seen in Bcl-2 Tg mice (Fig. S5A). These results suggest that factors downstream of the IL-7R other than survival signals regulate Treg development. On the other hand, the frequency of BrdU+ cells was reduced in Tregs of IL-7RcKO mice (Fig. 4D). The costimulatory molecules cytotoxic T-lymphocyte antigen (CTLA)-4 and GITR are reportedly constitutively expressed on Tregs and regulate their activity (22). However, expression of intracellular CTLA-4 and surface GITR was unchanged in Tregs of IL-7RcKO mice (Fig. S5B). Overall, these results suggest that the IL-7R is required for proliferation but not essential for functional maturation of thymic Tregs.
IL-7R Promotes Proliferation and Maturation of Invariant NKT Cells in Thymus.
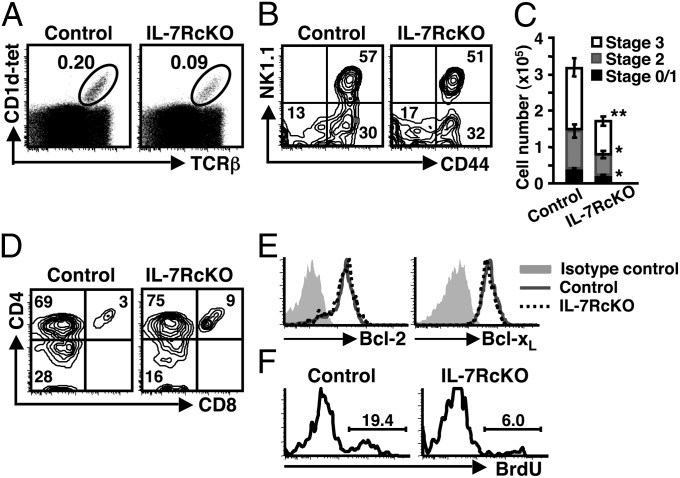
Invariant NKT (iNKT) cells are derived from DP thymocytes through interaction of invariant Vα14-Jα18 TCRα chain with CD1d expressed on DP thymocytes. Although the proportion of iNKT cells was not reduced in IL-7– or IL-7Rα–deficient thymus (15, 16), it is unclear whether the IL-7R participates in NKT cell development. To address this question, we analyzed IL-7RcKO thymocytes by using CD1d-tetramer loaded with α-GalCer. The frequency of CD1d-tetramer+ iNKT cells was reduced twofold in IL-7RcKO compared with control mice (Fig. 5A). After selection, iNKT cells begin to mature from stage 0 (HSA+) and then proceed to stage 1 (CD44−NK1.1−), stage 2 (CD44+NK1.1−), and stage 3 (CD44+NK1.1+) (23). We found that frequency of stage 3 iNKT cells was reduced in IL-7RcKO mice (Fig. 5B). Absolute numbers of iNKT cells were decreased in IL-7RcKO mice by 30%, 45%, and 50% in stages 0/1, 2, and 3, respectively, suggesting that iNKT cell maturation is partially blocked (Fig. 5C). iNKT cells reportedly differentiate primarily into CD4+ NKT cells and to a lesser extent into DN NKT cells. DN iNKT arise from CD4+ precursors before stage 3 and DN and CD4+ iNKT cells are suggested to be functionally different (24, 25). In IL-7RcKO mice, frequency of DN iNKT cells was reduced compared with control mice (Fig. 5D), suggesting that differentiation or survival of DN iNKT cells depends on IL-7R signaling more than CD4+ NKT cells. Similar results were also observed in peripheral tissue (Fig. S6A). These results demonstrate that IL-7R signaling is required for iNKT cell maturation after the DP stage.
Fig. 5.
IL-7R promotes proliferation and maturation of thymic iNKT cells. (A) TCRβ and CD1d tetramer (CD1d-tet) expression in total thymocytes. Representative data are shown (n = 12). (B) Expression of CD44 and NK1.1 in TCRβ+CD1d-tet+ thymocytes. Representative data are shown (n = 11). (C) Absolute numbers of TCRβ+CD1d-tet+ thymocytes in stage 0/1, stage 2, and stage 3. Cell numbers were calculated based on flow cytometry data in A and B (mean ± SEM, n = 11). (D) Expression of CD4 and CD8 in TCRβ+CD1d-tet+ thymocytes. Data represent eight independent experiments with similar results. (E) Bcl-2 and Bcl-xL expression in TCRβhiCD1d-tet+ thymocytes. Data represent three (Bcl-2) and two (Bcl-xL) independent experiments with similar results. (F) BrdU incorporation by TCRβ+CD1d-tet+ thymocytes 24 h after the pulse. Data represent three independent experiments with similar results.
To determine the mechanism underlying reduction of iNKT cells in IL-7RcKO mice, we first checked expression of antiapoptotic factors. Bcl-2 and Bcl-xL expression in iNKT cells was comparable between control and IL-7RcKO mice (Fig. 5E). In contrast to CD8SP thymocytes and Tregs, introduction of the Bcl-2 transgene did not rescue the frequency of iNKT cells in IL-7RcKO mice (Fig. S6B). Because iNKT cells rapidly proliferate after selection (26), we next analyzed proliferation. Twenty-four hours after BrdU injection, BrdU+ iNKT cells were greatly decreased in IL-7RcKO compared with control mice (Fig. 5F). These results suggest that the IL-7R is important for proliferation of thymic iNKT cells.
IL-7RcKO Mice Show Increased Numbers of γδ T Cells, B Cells, and Dendritic Cells in Thymus.
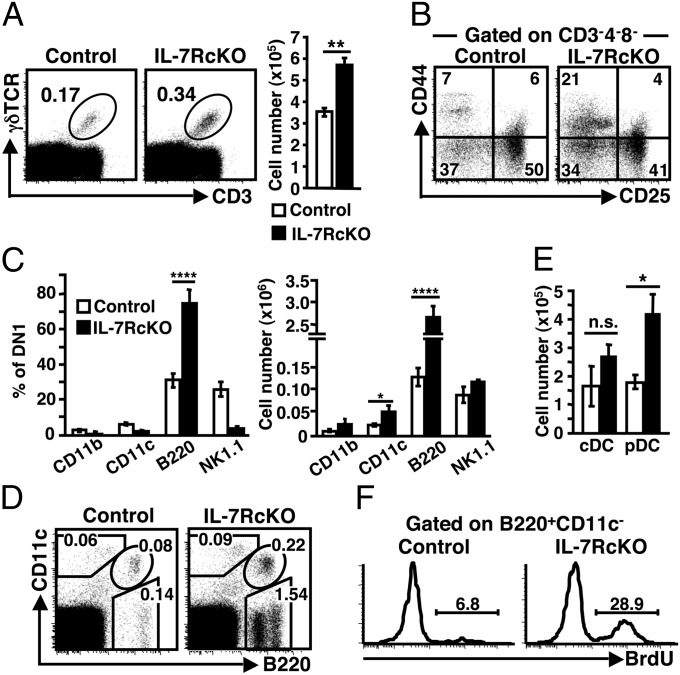
The frequency and absolute number of DN thymocytes increased twofold in IL-7RcKO mice (Fig. 1 B and C). To define what cell type had increased, we first checked γδ T cells. Both frequency and absolute number of γδ T cells were increased in IL-7RcKO thymus by 1.5-fold (Fig. 6A). In addition, we found that NK1.1+ γδ T cells were increased in IL-7RcKO thymus (Fig. S7A). By contrast, the absolute number of CD3−NK1.1+ NK cells was comparable (Fig. S7B).
Fig. 6.
IL-7RcKO mice show increased numbers of γδ T cells, B cells, and DCs in thymus. (A) Dot plots (Left) show CD3 and γδTCR expression in thymocytes. Bar graph (Right) indicates the absolute numbers of CD3+γδTCR+ cells (mean ± SEM, n = 14). (B) Expression of CD44 and CD25 in CD3−CD4−CD8− thymocytes. Representative data are shown (n = 11). (C) Expression of CD11b, CD11c, B220, and NK1.1 in CD3−CD4−CD8−CD44+CD25− DN1 cells was analyzed by flow cytometry. Percentages (Left) and absolute cell numbers (Right) of each marker positive population are shown (mean ± SEM, n = 7). (D) B220 and CD11c expression of thymocytes isolated with collagenase. Data represent six independent experiments with similar results. (E) Absolute numbers cDC (B220−CD11c+) and pDC (B220+CD11c+) (mean ± SEM, n = 6). (F) BrdU incorporation by B220+CD11c− B lineage cells 24 h after treatment. Data represent four independent experiments with similar results.
Although γδ T cells were increased, it did not account for the overall increase of DN thymocytes in IL-7RcKO mice (Fig. 1C). Therefore, we assessed CD3−CD4−CD8− triple negative thymocytes based on CD25 and CD44 expression. Interestingly, the frequency of DN1 (CD25−CD44+) cells was threefold higher in IL-7RcKO mice (Fig. 6B). Next, we analyzed the expression of several lineage markers and found that the frequency of B220+ cells increased by 2- to 3-fold (Fig. 6C). The absolute number of B220+ DN1 cells increased by 20-fold in IL-7RcKO mice. These cells were confirmed to be B lineage cells, because they expressed both MHC class II and CD19 (Fig. S7C), and about 30% of the B220+CD19+ cells were surface IgM+ (Fig. S7D). The absolute number of CD11c+ cells in the DN1 fraction also increased in IL-7RcKO mice (Fig. 6C). In total thymocytes, the frequency and absolute number of CD11c+B220+ plasmacytoid dendritic cells (pDCs) increased by 2-fold in IL-7RcKO thymus, whereas the numbers of CD11c+B220− conventional DCs (cDCs) did not show a statistically significant difference (Fig. 6 D and E).
To examine the mechanism underlying the increase in γδ T cells, B cells and DCs in IL-7RcKO thymus, we analyzed proliferation. Twenty-four hours after BrdU injection, we detected a greater number of BrdU+ B cells in IL-7RcKO than in control mice (Fig. 6F). In contrast, the frequency of BrdU+ cells in γδ T cells and plasmacytoid DCs was unchanged in IL-7RcKO mice at 24 h after injection (Fig. S7E). These results suggest that IL-7R expression on αβ T cells suppresses B-cell proliferation in thymus.
Discussion
The IL-7R may influence negative selection of CD8SP thymocytes, because the IL-7R transmits survival signal in CD8SP thymocytes. CD5 expression correlates positively with the strength of TCR signal during positive selection (27). IL-7R signaling may be required for resistance against negative selection via Bcl-2 induction. In IL-7RcKO mice, negative selection might be enhanced, deleting CD8SP thymocytes receiving a relatively strong TCR signal, which normally results in survival. Actually, introduction of the Bcl-2 transgene in IL-7RcKO mice restored CD5 expression on CD8SP thymocytes (Fig. 3C). Another possibility is that reduced CD8α expression in IL-7RcKO mice might have attenuated TCR signaling by coreceptor tuning (20), which results in lower CD5 expression. In contrast to our results, it was reported that IL-7R signaling does not play a role in postselection thymocytes (9). Although the reason for the discrepancy is not clear, it might be that the blocking of IL-7R signaling by antibody was not complete.
The IL-7R may also function in development of conventional CD4 T cells (Fig. 1C). Bcl-2 expression and BrdU incorporation were severely reduced in CD4SP thymocytes. This is mainly attributable to conventional CD4SP cells, because Bcl-2 levels were unchanged in Tregs and NKT cells, and BrdU+CD4SP excluding Foxp3+ and CD1d-tetramer+ cells were still reduced in IL-7RcKO mice (Fig. S8A). Therefore, our study suggests that the IL-7R likely plays multiple roles in proliferation, survival, and maturation during conventional CD4 T-cell development.
The molecular link between the IL-7R and thymocyte proliferation is still unclear. Degradation of p27Kip1 by its phosphorylation is important for IL-7–induced proliferation (28). The IL-7R induces the transcription of pim-1 (29), and pim-1 can phosphorylate p27Kip1 (30). However, expression of pim-1 was unchanged in SP thymocytes of IL-7RcKO mice (Fig. S8B). In another possibility, like IL-2, the IL-7R activates Akt, which phosphorylates Forkhead transcription factors and inhibits the transcription of p27Kip1 gene (31). However, p27Kip1 mRNA was not increased in IL-7RcKO mice (Fig. S8B). Because Akt can directly phosphorylates p27Kip1 (32), p27Kip1 degradation mediated directly by Akt might be responsible for IL-7–induced proliferation.
The role of the IL-7R in Treg development has been a matter of debate. Previous studies with IL-7Rα–deficient mice led to varying conclusions (11–14). In this study, we found that IL-7R is partially responsible for intrathymic Treg proliferation (Fig. 4D). Bcl-2 misexpression rescued Tregs in IL-7RcKO mice (Fig. S5A). However, alteration in cell survival might not be the major reason for reduced Treg number, because Bcl-2 and Bcl-xL expression was unchanged in IL-7RcKO mice and the frequency of Tregs in IL-7RcKO mice harboring a Bcl-2 transgene did not reached the levels seen in Bcl-2 Tg mice (Fig. 4C and Fig. S5A). We hypothesize that the increased number of Foxp3+ cells in Bcl-2 Tg mice are progeny that escape negative selection and that Tregs are not in fact rescued in IL-7RcKO mice carrying the Bcl-2 transgene. The second possibility is that Tregs with lower Bcl-2 expression did not survive in IL-7RcKO mice and that the remaining cells were those with Bcl-2 levels that had reached a certain threshold.
The IL-7R appears to play multiple roles in NKT cell development. IL-7RcKO mice showed markedly reduced numbers of thymic iNKT cells with impaired proliferation (Fig. 5 C and F). The IL-7R seems not to be essential for NKT cell survival, because Bcl-2 and Bcl-xL expression was unchanged in NKT cells and Bcl-2 misexpression in IL-7RcKO mice did not rescue iNKT cells (Fig. 5E and Fig. S6B). Because Bcl-xL, but not Bcl-2, reportedly rescues iNKT cell development in IL-15−/− mice (33), it is possible that iNKT cells with lower Bcl-xL expression did not survive in IL-7RcKO mice and that the remaining cells were those with Bcl-xL levels that had reached a certain threshold by receiving IL-15 signal.
B-cell development in the thymus is influenced by several factors. For example, TCRβ-deficient mice show a 10-fold increase in the number of thymic B cells (34), suggesting that disrupting αβ T-cell development alone is sufficient to increase the number of thymic B cells. In the IL-7RcKO thymus, B cells were increased 20-fold (Fig. 6C). Although less prominent than B cells, γδ T cells and DCs were also increased in the IL-7RcKO thymus (Fig. 6 A, C, and E). γδ T cells, some immature B cells, and pDCs express IL-7R in both control and IL-7RcKO mice (Figs. S1C and S7F). IL-7R expression in pDCs was slightly reduced in IL-7RcKO mice, as previously suggested that CD4-Cre mice partially deletes floxed allele in CD11c+ cells (35). IL-7 concentrations may be higher in and around the medulla of IL-7RcKO thymus than control thymus, because SP thymocytes do not consume IL-7 produced by thymic epithelial cells. Therefore, we suggest that suppression of B-cell development in thymus requires adequate IL-7 concentrations in addition to T-cell specification via Notch signaling. This is in agreement with a recent report that overexpression of IL-7 promotes extensive B-cell development in the thymus (36). TSLP concentrations might also contribute to this mechanism.
Although not analyzed mainly, peripheral T cells of IL-7RcKO mice showed several characteristics as expected from previous studies. First, peripheral T-cell numbers were greatly reduced in IL-7RcKO mice (Fig. S9 A–G). Lymphopenia-induced proliferation was also impaired in T cells (Fig. S9H). As previously reported in IL-7R−/− mice (37, 38), TCR-induced proliferation was also reduced in IL-7RcKO mice (Fig. S9I), although TCR expression and early activation, as monitored by CD69 expression, were normal (Fig. S9 J and K). This result suggests that IL-7R is required for expansion of effecter T cells, that is, generation of memory T-cell precursors. Suppressive activity of Treg from IL-7RcKO mice was also diminished (Fig. S9L), as previously reported in IL-7R−/− mice (13).
In summary, we characterized roles of the IL-7R in survival, proliferation, and maturation of different subpopulations of αβ thymocytes. These findings will accelerate the understanding of IL-7R function in thymus and the periphery, and IL-7Rα–floxed mice should serve as a powerful tool to determine the mechanism underlying how the IL-7R controls T-cell development and immune responses.
Materials and Methods
Mice.
Generation of IL-7Rα–floxed mice is described in SI Materials and Methods. CD4-Cre Tg mice were reported previously (39). H2K–Bcl-2 Tg mice were described previously (40). All mice were analyzed at 4–5 wk of age. Control (IL-7R+/+ CD4-Cre or IL-7Rfl/fl) and IL-7RcKO (IL-7Rfl/fl CD4-Cre) mice were compared. All mice were maintained under specific pathogen-free conditions at the Experimental Research Center for Infectious Diseases at the Institute for Virus Research, Kyoto University, Kyoto. All mouse protocols were approved by the Animal Research Committee at the Institute for Virus Research, Kyoto University.
Antibodies and Flow Cytometry.
The fluorescent dye- or biotin-conjugated antibodies used in this study were described in SI Materials and Methods. Intracellular staining was performed using a Foxp3 staining buffer set (eBioscience) or Cytofix/Cytoperm (BD Biosciences) according to the manufacturer’s instructions. Stained cells were analyzed with a FACSCalibur or FACSCanto II flow cytometer (BD Biosciences). Data were analyzed using FlowJo software (Tree Star). Values in quadrants, gated area, and interval gates indicate the percentages in each population in all figures. Cell sorting was performed using a FACSAria II cell sorter (BD Biosciences).
Real-Time RT-PCR.
Total RNA was isolated and treated with RNase-free DNase to remove genomic DNA. After DNase inactivation, total RNA was reverse transcribed with random primer. cDNA was amplified by using an ABI 7500 Sequence Detector (Applied Biosystems) with QuantiTect SYBR Green PCR kit (Qiagen) with ROX reference dye (Invitrogen). PCR was carried out at 95 °C for 15 min, followed by 40 cycles consisting of 95 °C for 20 s, 55 °C for 30 s, and 72 °C for 60 s. PCR efficiency was normalized using cDNA of whole thymocytes from wild-type mice. Primer sequences are listed in SI Materials and Methods.
BrdU Incorporation Assay.
Mice were injected intraperitoneally with 1 mg of BrdU (Sigma-Aldrich). Four or 24 hr after injection, thymocytes were stained with surface markers, fixed and permeabilized with Cytofix/Cytoperm, and treated with DNase at 37 °C for 1 h. BrdU was stained with a FITC-conjugated anti-BrdU antibody (BD Biosciences). Values in histogram indicate the percentages of BrdU+ cells defined by interval gates.
Flow Cytometry for DCs.
The thymus was cut into small pieces and digested with 1.25 mg/mL collagenase D (Roche) and 0.01% DNase I (Roche) in RPMI medium 1640 for 30 min at 37 °C. The cell suspension was gently passed through 24-gauge needles twice, washed twice, and used for staining.
Statistics.
An unpaired two-tailed Student t test was used for all of the statistical analysis. Asterisks in all figures are as follows: *P < 0.05; **P < 0.01; ***P < 0.005; ****P < 0.001. NS, not significant.
Supplementary Material
Acknowledgments
We thank Drs. J. Takeda, G. Kondoh, and K. Yusa for the KY1.1 ES line and targeting system; Dr. N. G. Copeland for the Red recombination system; Drs. I. Saito, Y. Shinkai, M. Tachibana, and Y. Matsumura for AxCAFLP adenovirus; Drs. C. B. Wilson, T. Honjo, and D. Yabe for the CD4-Cre Tg mouse; Dr. J. Domen for the H2K–Bcl-2 Tg mouse; Drs. N. Minato and Y. Agata for OT-I, OT-II, and H-Y TCR Tg mice; Dr. M. Satake for Runx primer information; Mr. H. Hayashi and Ms. S. Kamioka for excellent technical assistance; and Dr. A. Singer and members of the K. Ikuta laboratory for discussion. This work was supported by Grants-in-Aid for Young Scientists (B) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan 22790460 and 24790469, by a grant from the Fujiwara Memorial Foundation, and by the BioLegend/Tomy Digital Biology Young Scientist Research Award for 2011.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. W.J.L. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1219242110/-/DCSupplemental.
References
- 1.Maki K, Sunaga S, Ikuta K. The V-J recombination of T cell receptor-γ genes is blocked in interleukin-7 receptor-deficient mice. J Exp Med. 1996;184(6):2423–2427. doi: 10.1084/jem.184.6.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corcoran AE, Riddell A, Krooshoop D, Venkitaraman AR. Impaired immunoglobulin gene rearrangement in mice lacking the IL-7 receptor. Nature. 1998;391(6670):904–907. doi: 10.1038/36122. [DOI] [PubMed] [Google Scholar]
- 3.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29(6):848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Jiang Q, et al. Cell biology of IL-7, a key lymphotrophin. Cytokine Growth Factor Rev. 2005;16(4-5):513–533. doi: 10.1016/j.cytogfr.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Akashi K, Kondo M, von Freeden-Jeffry U, Murray R, Weissman IL. Bcl-2 rescues T lymphopoiesis in interleukin-7 receptor-deficient mice. Cell. 1997;89(7):1033–1041. doi: 10.1016/s0092-8674(00)80291-3. [DOI] [PubMed] [Google Scholar]
- 6.Maraskovsky E, et al. Bcl-2 can rescue T lymphocyte development in interleukin-7 receptor-deficient mice but not in mutant rag-1-/- mice. Cell. 1997;89(7):1011–1019. doi: 10.1016/s0092-8674(00)80289-5. [DOI] [PubMed] [Google Scholar]
- 7.Singer A, Adoro S, Park JH. Lineage fate and intense debate: Myths, models and mechanisms of CD4- versus CD8-lineage choice. Nat Rev Immunol. 2008;8(10):788–801. doi: 10.1038/nri2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park JH, et al. Signaling by intrathymic cytokines, not T cell antigen receptors, specifies CD8 lineage choice and promotes the differentiation of cytotoxic-lineage T cells. Nat Immunol. 2010;11(3):257–264. doi: 10.1038/ni.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weinreich MA, Jameson SC, Hogquist KA. Postselection thymocyte maturation and emigration are independent of IL-7 and ERK5. J Immunol. 2011;186(3):1343–1347. doi: 10.4049/jimmunol.1002238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lodolce JP, et al. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9(5):669–676. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 11.Burchill MA, Yang J, Vogtenhuber C, Blazar BR, Farrar MA. IL-2 receptor β-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J Immunol. 2007;178(1):280–290. doi: 10.4049/jimmunol.178.1.280. [DOI] [PubMed] [Google Scholar]
- 12.Bayer AL, Lee JY, de la Barrera A, Surh CD, Malek TR. A function for IL-7R for CD4+CD25+Foxp3+ T regulatory cells. J Immunol. 2008;181(1):225–234. doi: 10.4049/jimmunol.181.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mazzucchelli R, et al. Development of regulatory T cells requires IL-7Ralpha stimulation by IL-7 or TSLP. Blood. 2008;112(8):3283–3292. doi: 10.1182/blood-2008-02-137414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vang KB, et al. IL-2, -7, and -15, but not thymic stromal lymphopoeitin, redundantly govern CD4+Foxp3+ regulatory T cell development. J Immunol. 2008;181(5):3285–3290. doi: 10.4049/jimmunol.181.5.3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boesteanu A, et al. Distinct roles for signals relayed through the common cytokine receptor γ chain and interleukin 7 receptor α chain in natural T cell development. J Exp Med. 1997;186(2):331–336. doi: 10.1084/jem.186.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsuda JL, et al. Homeostasis of V α 14i NKT cells. Nat Immunol. 2002;3(10):966–974. doi: 10.1038/ni837. [DOI] [PubMed] [Google Scholar]
- 17.Van De Wiele CJ, et al. Thymocytes between the β-selection and positive selection checkpoints are nonresponsive to IL-7 as assessed by STAT-5 phosphorylation. J Immunol. 2004;172(7):4235–4244. doi: 10.4049/jimmunol.172.7.4235. [DOI] [PubMed] [Google Scholar]
- 18.Pénit C, Vasseur F. Expansion of mature thymocyte subsets before emigration to the periphery. J Immunol. 1997;159(10):4848–4856. [PubMed] [Google Scholar]
- 19.Hare KJ, Jenkinson EJ, Anderson G. An essential role for the IL-7 receptor during intrathymic expansion of the positively selected neonatal T cell repertoire. J Immunol. 2000;165(5):2410–2414. doi: 10.4049/jimmunol.165.5.2410. [DOI] [PubMed] [Google Scholar]
- 20.Park JH, et al. ‘Coreceptor tuning’: Cytokine signals transcriptionally tailor CD8 coreceptor expression to the self-specificity of the TCR. Nat Immunol. 2007;8(10):1049–1059. doi: 10.1038/ni1512. [DOI] [PubMed] [Google Scholar]
- 21.Liston A, et al. Differentiation of regulatory Foxp3+ T cells in the thymic cortex. Proc Natl Acad Sci USA. 2008;105(33):11903–11908. doi: 10.1073/pnas.0801506105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilczynski JR, Radwan M, Kalinka J. The characterization and role of regulatory T cells in immune reactions. Front Biosci. 2008;13:2266–2274. doi: 10.2741/2840. [DOI] [PubMed] [Google Scholar]
- 23.Matsuda JL, Gapin L. Developmental program of mouse Valpha14i NKT cells. Curr Opin Immunol. 2005;17(2):122–130. doi: 10.1016/j.coi.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Benlagha K, Wei DG, Veiga J, Teyton L, Bendelac A. Characterization of the early stages of thymic NKT cell development. J Exp Med. 2005;202(4):485–492. doi: 10.1084/jem.20050456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Godfrey DI, Berzins SP. Control points in NKT-cell development. Nat Rev Immunol. 2007;7(7):505–518. doi: 10.1038/nri2116. [DOI] [PubMed] [Google Scholar]
- 26.Dose M, et al. Intrathymic proliferation wave essential for Valpha14+ natural killer T cell development depends on c-Myc. Proc Natl Acad Sci USA. 2009;106(21):8641–8646. doi: 10.1073/pnas.0812255106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Azzam HS, et al. CD5 expression is developmentally regulated by T cell receptor (TCR) signals and TCR avidity. J Exp Med. 1998;188(12):2301–2311. doi: 10.1084/jem.188.12.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li WQ, et al. IL-7 promotes T cell proliferation through destabilization of p27Kip1. J Exp Med. 2006;203(3):573–582. doi: 10.1084/jem.20051520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruppert SM, et al. JunD/AP-1-mediated gene expression promotes lymphocyte growth dependent on interleukin-7 signal transduction. PLoS ONE. 2012;7(2):e32262. doi: 10.1371/journal.pone.0032262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morishita D, Katayama R, Sekimizu K, Tsuruo T, Fujita N. Pim kinases promote cell cycle progression by phosphorylating and down-regulating p27Kip1 at the transcriptional and posttranscriptional levels. Cancer Res. 2008;68(13):5076–5085. doi: 10.1158/0008-5472.CAN-08-0634. [DOI] [PubMed] [Google Scholar]
- 31.Stahl M, et al. The forkhead transcription factor FoxO regulates transcription of p27Kip1 and Bim in response to IL-2. J Immunol. 2002;168(10):5024–5031. doi: 10.4049/jimmunol.168.10.5024. [DOI] [PubMed] [Google Scholar]
- 32.Fujita N, Sato S, Katayama K, Tsuruo T. Akt-dependent phosphorylation of p27Kip1 promotes binding to 14-3-3 and cytoplasmic localization. J Biol Chem. 2002;277(32):28706–28713. doi: 10.1074/jbc.M203668200. [DOI] [PubMed] [Google Scholar]
- 33.Gordy LE, et al. IL-15 regulates homeostasis and terminal maturation of NKT cells. J Immunol. 2011;187(12):6335–6345. doi: 10.4049/jimmunol.1003965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akashi K, Richie LI, Miyamoto T, Carr WH, Weissman IL. B lymphopoiesis in the thymus. J Immunol. 2000;164(10):5221–5226. doi: 10.4049/jimmunol.164.10.5221. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt-Supprian M, Rajewsky K. Vagaries of conditional gene targeting. Nat Immunol. 2007;8(7):665–668. doi: 10.1038/ni0707-665. [DOI] [PubMed] [Google Scholar]
- 36.El-Kassar N, et al. High levels of IL-7 cause dysregulation of thymocyte development. Int Immunol. 2012;24(10):661–671. doi: 10.1093/intimm/dxs067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saini M, Pearson C, Seddon B. Regulation of T cell-dendritic cell interactions by IL-7 governs T-cell activation and homeostasis. Blood. 2009;113(23):5793–5800. doi: 10.1182/blood-2008-12-192252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jacobs SR, Michalek RD, Rathmell JC. IL-7 is essential for homeostatic control of T cell metabolism in vivo. J Immunol. 2010;184(7):3461–3469. doi: 10.4049/jimmunol.0902593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee PP, et al. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity. 2001;15(5):763–774. doi: 10.1016/s1074-7613(01)00227-8. [DOI] [PubMed] [Google Scholar]
- 40.Domen J, Gandy KL, Weissman IL. Systemic overexpression of BCL-2 in the hematopoietic system protects transgenic mice from the consequences of lethal irradiation. Blood. 1998;91(7):2272–2282. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.