Abstract
The nucleus accumbens (NAc) regulates motivated behavior by, in part, processing excitatory synaptic projections from several brain regions. Among these regions, the prefrontal cortex (PFC) and basolateral amygdala, convey executive control and affective states, respectively. Whereas glutamatergic synaptic transmission within the NAc has been recognized as a primary cellular target for cocaine and other drugs of abuse to induce addiction-related pathophysiological motivational states, the understanding has been thus far limited to drug-induced postsynaptic alterations. It remains elusive whether exposure to cocaine or other drugs of abuse influences presynaptic functions of these excitatory projections, and if so, in which projection pathways. Using optogenetic methods combined with biophysical assays, we demonstrate that the presynaptic release probability (Pr) of the PFC-to-NAc synapses was enhanced after short-term withdrawal (1 d) and long-term (45 d) withdrawal from either noncontingent (i.p. injection) or contingent (self-administration) exposure to cocaine. After long-term withdrawal of contingent drug exposure, the Pr was higher compared with i.p. injected rats. In contrast, within the basolateral amygdala afferents, presynaptic Pr was not significantly altered in any of these experimental conditions. Thus, cocaine-induced procedure- and pathway-specific presynaptic enhancement of excitatory synaptic transmission in the NAc. These results, together with previous findings of cocaine-induced postsynaptic enhancement, suggest an increased PFC-to-NAc shell glutamatergic synaptic transmission after withdrawal from exposure to cocaine. This presynaptic alteration may interact with other cocaine-induced cellular adaptations to shift the functional output of NAc neurons, contributing to the addictive emotional and motivational state.
Keywords: addiction, multiple probability fluctuation analysis, channelrhodopsin
Medium spiny neurons (MSNs) within the nucleus accumbens (NAc) shell function to gate the emotional and motivational arousals for behavioral output (1). Glutamatergic synaptic input to the NAc provides the major driving force for MSNs and is targeted by drugs of abuse to produce adaptive changes (2). These drug-induced synaptic adaptations may substantially reshape the functional output of MSNs, leading to a preferential prioritization of addiction-related behavioral output. As such, elucidating cocaine-induced adaptive changes at NAc glutamatergic synapses has been a major task in understanding the neural basis underlying cocaine addiction. Whereas up-regulation of postsynaptic responsiveness of the NAc glutamatergic synapses after cocaine withdrawal are evident (3–5), it is not well understood whether the presynaptic input is also concurrently altered. Thus, the effect of cocaine on NAc glutamatergic synapses as a whole remains largely incomplete.
A number of limbic and paralimbic regions project glutamatergic inputs to NAc shell neurons, each presumably carrying different aspects of emotional and motivational information (6, 7). Among these afferents, we focused on the medial prefrontal cortex (mPFC)- and basolateral amygdala (BLA)-to-NAc (BLA-NAc) pathways given their important roles in several defining characteristics of cocaine addiction. The prefrontal cortex (PFC)-to-NAc (PFC-NAc) pathway is particularly important for cocaine-induced increase in impulsivity (8, 9) and reinstatement of drug seeking after extinction (10). The BLA-NAc pathway is essential for cue-induced motivated behaviors (11–13) and also mediates cue-induced cocaine relapse (14). In the present study, we used a combination of optogenetic and biophysical tools to detect cocaine-induced presynaptic alterations in these two critical pathways by following two different cocaine procedures [i.p. injection vs. self-administration (SA)]. Our results, together with previous findings of cocaine-induced postsynaptic enhancement (4, 5), suggest that the PFC-NAc synaptic transmission becomes exceedingly strengthened during long-term withdrawal from cocaine SA.
Results
Combination of Optogenetics and Multiple-Probability Fluctuation Analysis Allows Input-Specific Quantification of Quantal Parameters of Synaptic Transmission.
We used a combination of optogenetic tools and multiple-probability fluctuation analysis (MPFA) to investigate pathway-specific synaptic properties, including probability of presynaptic glutamate release (Pr), the quantal size of the glutamatergic release (Q), and the number of active release sites (N) (15, 16). To establish and verify this procedure, we injected an adeno-associated viral vector (AAV) that expressed channelrhodopsin-2 H134R venus fusion protein (ChR2Y) into the mPFC of newborn mice or rats. Three to four weeks later, we prepared acute coronal PFC slices, which contained yellow fluorescent neurons. We first characterized the properties of ChR2Y, specifically in respect to its desensitization characteristics. With whole-cell voltage-clamp recording, we measured light (λ = 473 nm) evoked currents with varying pulse durations (Fig. 1B). During a five-pulse train (1 ms duration, 20 Hz), the amplitude of the current progressively decreased to ∼70% of the initial amplitude, consistent with a desensitization of the ChR2Y (normalized photocurrent amplitudes: 100%, 82% ± 4, 74% ± 5, 71% ± 5, 68% ± 5, for first through fifth pulse, respectively, n = 6; Fig. 1C) (17). Similar desensitization patterns were observed by optical trains with different stimulation duration (normalized photocurrent amplitudes: 0.1-ms pulse, 100%, 91% ± 3, 81% ± 5, 71% ± 5, 76% ± 5, n = 5; 0.5-ms pulse, 100%, 82% ± 2, 75% ± 3, 70% ± 3, 67% ± 3, n = 6; 2-ms pulse, 100%, 77% ± 6, 69% ± 6, 64% ± 6, 61% ± 7, n = 3). Thus, desensitization is an intrinsic property for ChR2-mediated depolarization currents. However, this desensitization property does not appear to prevent ChR2-mediated current from generating consistent action potential firing. In current clamp recordings, action potentials (APs) were triggered reliably during 100 trials of the five-pulse trains, despite the ChR2 desensitization (AP success rate, 1-ms pulse: 100% ± 0, for all five light pulses, n = 6; Fig. 1 A and C). This result was further confirmed in three other five-train protocols with different pulse durations (AP success rate, 0.1-ms pulse: 99.7% ± 0.3, n = 7; 0.5-ms pulse: 100% ± 0, n = 7; 1-ms pulse: 100% ± 0, n = 6; 2-ms pulse: 100% ± 0, n = 6, Fig. 1D). These results suggest that desensitization does not prevent the five-pulse train to consistently elicit repetitive action potentials, which is likely due to the high level of ChR2 expression.
Fig. 1.
Verification of ChR2Y-based electrophysiological recordings. (A and B) Sample traces (first and 100th) of light-evoked APs (A) and photocurrents (B) with five flashes (1 ms at 20 Hz) of MSNs in rat PFC, transduced with AAV-ChR2Y. (C) Summary showing that although the whole-cell currents elicited by the optical stimulation train (five-pulse) decreased gradually, they were sufficient to elicit reliable action potential firing (n = 6).
We next tested whether the ChR2-based five-pulse train is suitable for the MPFA. We obtained coronal NAc slices from mice with intra-PFC injection of ChR2. The NAc slices contained yellow fluorescent fibers, indicating that PFC-NAc axonal projections expressed ChR2. With whole-cell voltage-clamp recording, we evoked PFC-NAc pathway-specific synaptic transmission with short light (1 ms) flashes (Fig. 2). We then applied a five-pulse train (at 20 Hz) to the NAc slice for stimulation of PFC-NAc presynaptic terminals. This five-pulse train has been used to set excitatory synapses to five repetitive and consistent releasing states (with different Prs) for the MPFA (18). Note that the Pr is a function of release site occupancy and intrinsic release probability, both of which are influenced during short-term plasticity. It is assumed that along the stimulus train, the Pr changes uniformly and consistently across all release sites (18). To test whether the light-evoked repetitive stimuli followed the same rule, we plotted the coefficients of variation squared of the amplitudes of the first and second stimulus against the ratio of their mean amplitude (Fig. S1A) (19). The data points are in the quadrant of the horizontal and vertical dashed line at 1 and on or below the identity line, suggesting a decrease of the Pr in the second compared with the first pulse. We then plotted the variance of the EPSC amplitude of each of the five stimuli of 30–100 sweeps against the mean amplitude (Fig. 2 A and C) (18). We derived the Q and N by fitting with a parabola and calculated the Pr for the first stimulus (16) (see Materials and Methods for details). Data from individual cells typically fit a symmetrical parabola curve, consistent with a binomial model of synaptic transmission. To test whether this assay accurately measured changes in the Pr, we applied 10 µM 4-aminopyridine (4-AP) to block presynaptic potassium channels, which can enhance transmitter release (20, 21). To confirm the presynaptic action of 4-AP, we fitted the decay phase of the first EPSC with a single exponential function. The decay time constant increased after 4-AP significantly, consistent with a prolonged glutamate release by a broadened presynaptic action potential (τ = 3.9 ± 0.3 ms; 4-AP: 4-AP τ = 5.5 ± 0.03 ms; P < 0.05, paired t test). Application of 4-AP increased the mean EPSC amplitude of the first pulse, whereas its variance decreased, suggesting an increase in the Pr (Fig. 2 A–C). The Pr increased significantly, whereas the Q and N remained unchanged, suggesting that application of 4-AP did not recruit additional synapses nor altered the postsynaptic AMPA receptor (R) function (Pr = 0.53 ± 0.05; 4-AP: Pr = 0.75 ± 0.04, n = 6, P < 0.01, paired t test; Q = 9.63 ± 2.31; 4-AP Q = 9.39 ± 2.35, n = 6, P = 0.91, paired t test; n = 22.38 ± 4.14; 4-AP n = 21.50 ± 5.69, n = 6, P = 0.89, paired t test; Fig. 2 D–F). When we plotted the ratio of the second to the first EPSC, the ratio decreased after application of 4-AP, suggesting a 4-AP–mediated increase in Pr [paired-pulse ratio (PPR) = 0.44 ± 0.06; 4-AP PPR = 0.32 ± 0.05, n = 8, P < 0.05, paired t test; Fig. 2G]. An additional analysis of the coefficient of variation squared of the first and second pulses in the presence of 4-AP and the coefficient of variation squared of the first pulse in control and in 4-AP against the ratio of the mean amplitudes confirmed a presynaptic mechanism for both the changes in amplitude during the stimulation train and the pharmacological procedure (Fig. S1 B and C). The lack of change in Q suggested that AMPAR desensitization did not significantly influence the five-pulse train-based assay, even when glutamate release is extended with 4-AP. These results show that the combination of pathway-specifically expressed ChR2Y and MPFA allowed the quantification of quantal parameters of synaptic transmission.
Fig. 2.
A combination of in vivo ChR2Y expression and MPFA allows the measurement of pathway-specific synaptic properties. (A and B) AMPAR EPSCs were triggered by optical stimulation of the NAc slice from animals with intra-PFC injection of ChR2Y. Example traces of 30 overlaid synaptic responses (gray) and the average response (black) in normal ACSF and after addition of 10 µM 4-AP in MSNs of the mouse NAc shell. (C) MPFA of the example recording in A (open circles) and after addition of 10 µM 4-AP (filled circles). (D–F) Quantal parameters Q and N were derived from the parabolic fit of the vaviance-mean analysis (n = 6) before and after 10 µM 4-AP addition. The Pr was calculated according to Eq. 1 in Materials and Methods. (G) Changes of the PPR (second to first) of EPSCs before and after addition of 4-AP on n = 8 cells. *P < 0.05;.**P < 0.01.
Cocaine Induces Pathway-Specific Increases in the Pr After Short-Term Drug Withdrawal.
We then tested whether exposure to cocaine-induced presynaptic alterations of PFC-NAc and BLA-NAc excitatory synapses. We used two experimental procedures: (i) noncontingent (15 mg/kg per d for 5 d i.p.), and (ii) contingent (i.v. SA, 0.75 mg/kg per infusion, 2 h/d for 5 d). Both cocaine procedures produce very different cellular and behavioral effects; typically the contingent procedure offers, arguably, a higher face value of drug addiction (22). For the cocaine SA procedure, we adjusted the duration of the training session such that animals received approximately the same amount of cocaine each day for 5 d as their i.p. injection counterparts (Fig. S2). To selectively target the PFC or BLA afferents to the NAc shell, we injected the AAV-ChR2Y into either the infralimbic PFC or BLA of 3- to 4-wk-old rats. Three weeks later, a large number of neurons of the injection regions and the projecting fibers in the NAc shell exhibited yellow fluorescent signals (Fig. 3 A, B, G, and H), indicating the expression of ChR2Y in neurons and their projection fibers to the NAc shell. We first tested the synaptic parameters in animals on withdrawal day 1 from the cocaine procedures. In the NAc slices, AP trains of the projection fibers were triggered by repetitive activation of ChR2Y, resulting in EPSCs in NAc shell neurons (Fig. 3 C–E and I–K). We tested the stability of EPSC responses by plotting the EPSC amplitudes over time and tested whether there are significant differences between the first 10 EPSCs and last 10 EPSCs (Fig. S3). One hundred ten neurons exhibited no significant difference for at least 40 sweeps. In 93 of these 110 neurons within the PFC-NAc pathway, the variance of the EPSCs against the mean amplitudes could be fitted to calculate the quantal parameters (Fig. 3 C–E). The MPFA revealed that the basal presynaptic release probability at PFC-NAc synapses in saline-exposed rats was approximately 0.5 [Pr = 0.48 ± 0.03, number of cells (n)/number of animals (m) = 13/4; Fig. 3F], which is similar to the value obtained in mice with optogenetic triggering (Fig. 2) and electrical stimulation (23). Exposure to cocaine via either i.p. injection or SA increased the release probability by approximately 30% (F2,7 = 11.75, P < 0.01, ANOVA; i.p. cocaine: Pr = 0.65 ± 0.02, n/m = 14/3, P < 0.01 vs. saline; cocaine SA: Pr = 0.62 ± 0.03, n/m = 14/3, P < 0.01 vs. saline; Fig. 3F). In contrast, no significant alterations were observed in either the number of release sites (F2,7 = 0.78, P = 0.49, ANOVA; saline: n = 20 ± 7, n/m = 13/4; i.p. cocaine: n = 11 ± 4, n/m = 14/3, cocaine SA: n = 15 ± 5, n/m = 14/3) or quantal size (F2,7 = 1.01, P = 0.41; saline: Q = 17.14 ± 4.18, n/m = 13/4, i.p. cocaine: Q = 24.44 ± 9.63, n/m = 14/3, cocaine SA: Q = 31.08 ± 9.72, n/m = 14/3). Note that the number of release sites highly depended on the number of synapses activated in each recording, which may exhibit a high variability that is not related to the effect of cocaine. Additionally, we estimated the quantal size of nonspecific synaptic transmission onto MSNs by following different procedures with an independent approach (Fig. S4). We measured the amplitude of spontaneously released quanta in our recorded traces. These averaged between 20 and 25 pA with no significant difference between the procedures and similar magnitudes as the pathway-specific quantal sizes, confirming no gross alteration in Q after cocaine procedures. In an independent set of MPFA experiments, we varied the light pulse duration to test, whether the Pr is influenced by different levels of ChR2Y activation, which might result from differences in ChR2Y expression levels. The Pr was not significantly different using light pulses with different duration (0.5, 1, or 2 ms), suggesting that activation of different amounts of ChR2Y in our experimental conditions did not influence the measurement of Pr [F2,10 = 0.43, P = 0.66, ANOVA; Pr (0.5 ms) = 0.51 ± 0.06, n = 5; Pr (1 ms) = 0.46 ± 0.02, n = 5; Pr (2 ms) = 0.50 ± 0.04, n = 3; Fig. S5].
Fig. 3.

The Pr of excitatory synapses within the PFC-NAc pathway, but not the BLA-NAc pathway, is increased during short-term drug withdrawal (1 d). (A, B, G, and H) Images showing the injection site of viral vectors and expression of ChR2Y in the rat infralimbic PFC (A), the BLA (G) and projecting ChR2Y-expressing axons in NAc shell (B and H). (Scale bars: 100 µm). (C–E) Five-pulse trains (at 20 Hz) of AMPAR EPSCs elicited within the PFC-NAc pathway in an example NAc shell MSN and the corresponding variance-mean plot of EPSCs from a rat with repeated i.p. injections of saline (C), i.p. injections of cocaine (D), or SA of cocaine (E). The solid line was generated from the parabolic fitting. (F) Summarized data showing that the Pr of PFC-NAc pathway is increased during short-term withdrawal from cocaine, both i.p. and SA. (I–K) Light-evoked AMPAR EPSCs and analysis (as in C–E) in the BLA-NAc pathway for the indicated experimental procedures. (L) Summarized data showing that the Pr of BLA-NAc pathways is not affected during short-term withdrawal from cocaine. **P < 0.01.
Using the same approach, we next examined the presynaptic properties of BLA-NAc excitatory synapses after exposure to cocaine (Fig. 3 I–L). The basal presynaptic release probability within this pathway is significantly higher than that in the PFC-NAc pathway (Pr = 0.65 ± 0.01, n/m = 10/4; P < 0.01 vs. PFC-NAc, t test). Furthermore, neither i.p. injection nor SA of cocaine significantly affected the release probability of BLA-NAc synapses (F2,12 = 0.32, P = 0.72, ANOVA; i.p. cocaine: Pr = 0.65 ± 0.04, n/m = 12/4, cocaine SA: Pr = 0.68 ± 0.02, n/m = 17/7; Fig. 3R). In addition, neither the number of release sites (F2,12 = 0.35, P = 0.71, ANOVA; i.p. saline, n = 7 ± 3, n/m = 10/4; i.p. cocaine, n = 8 ± 1, n/m = 12/4; cocaine SA, n = 12 ± 5, n/m = 17/7) nor the quantal size (F2,12 = 0.46, P = 0.64, ANOVA; i.p. saline, Q = 24.75 ± 5.11, n/m = 10/4; i.p. cocaine, Q = 21.94 ± 3.71, n/m = 12/4; cocaine SA: Q = 21.16 ± 3.84, n/m = 17/7) was significantly changed by following either cocaine procedure.
The high Pr is often associated with multivesicular release from a single active zone, and if this release saturates postsynaptic receptors, the parabola of the variance–mean relationship bends (24). To test for receptor saturation, we perfused the NAc slice with 0.5 mM kynurenic acid in some experiments and repeated the stimulation to measure the variance–mean relationship with an apparent lower synaptic glutamate concentration (24, 25). The degrees of blockades of the first, second, and third stimuli before and after kynurenic acid were similar, all approximately 50%, suggesting that potential multivesicular release, if any, did not saturate the AMPARs under our conditions (Fig. S6).
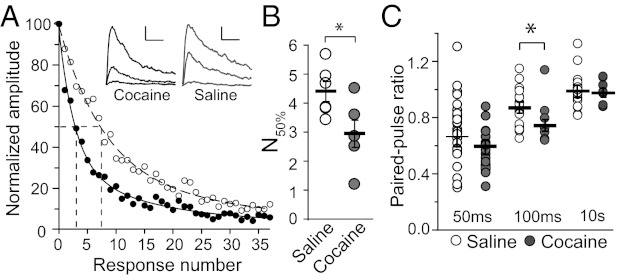
We next performed two independent assays to verify our MPFA-based finding that the Pr at PFC-NAc synapses was increased by cocaine exposure. The first approach involves measuring the time course of MK-801–mediated inhibition of NMDAR EPSCs. Typically, NMDARs at synapses with a high Pr would be activated more frequently and, thus, more rapidly inhibited by the use-dependent antagonist MK-801 (26). After a stable baseline of NMDAR EPSCs from the PFC-NAc synapses, we started a continuous perfusion of 20 µM MK-801 and observed that the inhibition of NMDAR EPSCs was achieved significantly faster in slices from cocaine-exposed animals than saline controls (Fig. 4A). The blocking rate was measured as the number of activations via which the inhibition of NMDAR EPSCs reached 50% (N50%) (inhibition course fit with two exponentials; i.p. saline, N50% = 4.41 ± 0.35, n/m = 19/6; i.p. cocaine, N50% = 2.94 ± 0.55, n/m = 15/5, P < 0.05, t test; Fig. 4B). The second approach involves measuring PPR of AMPAR EPSCs, which, to some extent, reflect the property of the Pr. In cocaine-exposed rats, whereas the change in the PPR with an interstimulus interval (ISI) of 50 ms was not significant, a significant decrease was observed in the PPR with the ISI of 100 ms (PPR 100 ms ISI: saline, 0.87 ± 0.02, n/m = 22/5; cocaine, 0.75 ± 0.02, n/m = 10/3, P = 0.01; PPR 50 ms ISI: saline, 0.63 ± 0.07, n/m = 33/6; cocaine, 0.60 ± 0.05, n/m = 22/5, P = 0.6, t test, Fig. 4C). With the ISI of 10 s, the PPR returned to ∼1 as the second EPSCs recovered from the influence of the first EPSCs (saline, 1.02 ± 0.05, n/m = 22/5; cocaine, 0.98 ± 0.02, n/m = 10/3, P = 0.5, t test; Fig. 4C). Thus, results from both the MK-801 and PPR assays confirmed the conclusion from the MPFA that cocaine exposure increased the Pr at PFC-NAc synapses.
Fig. 4.
Use-dependent blocking time course of NMDARs and PPRs within the PFC-NAc pathway. (A) Blocking rate of NMDAR EPSC amplitudes from the PFC-NAc synapses in i.p. cocaine- and saline-treated animals with sample traces of the first, eighth, and 35th NMDAR EPSC in Inset. (Scale bars: 100 pA, 25 ms.) (B) Summary of N50% values of analysis of individual traces as in A. (C) PPRs of two AMPAR EPSC amplitudes with interstimulus intervals of 50 ms, 100 ms, and 10 s. Each dot represents one recorded MSN (n). Average values (black bars) are based on the number of animals (m). *P < 0.05.
Cocaine Induces Procedure-Dependent Long-Term Presynaptic Changes in PFC-NAc.
We next examined the presynaptic properties of excitatory synapses within both the PFC-NAc and BLA-NAc pathways after 45 d withdrawal from repeated i.p. injections or SA of cocaine. At PFC-NAc synapses, the basal Pr from long-term saline withdrawal rats was similar to that in rats with short-term saline withdrawal (0.53 ± 0.03, n/m = 15/3; P = 0.31 vs. short-term withdrawal; t test; Fig. 5 A and D). The Pr remained significantly up-regulated after long-term withdrawal from repeated i.p. injection of cocaine (F2,8 = 28.01, P < 0.01, ANOVA; i.p. cocaine, Pr = 0.64 ± 0.02, n/m = 19/4; P < 0.05 vs. saline; cocaine SA, Pr = 0.73 ± 0.01, n = 19/4, P < 0.01 vs. saline, P < 0.01 vs. i.p. cocaine; Fig. 5 B–D). Moreover, in cocaine self-administering rats, the Pr was further increased after long-term withdrawal from the already up-regulated level after short-term withdrawal (P < 0.05 vs. short-term withdrawal, t test), whereas this withdrawal-dependent additional enhancement was not observed in rats with i.p. cocaine injections (P = 0.81 vs. short-term withdrawal, t test). Again, no change was detected in the number of release sites (F2,8 = 0.05, P = 0.95, ANOVA; i.p. saline, n = 23 ± 4, n/m = 15/3; i.p. cocaine: n = 26 ± 9, n/m = 19/4; cocaine SA: n = 23 ± 10, n/m = 19/4) or the quantal size (F2,8 = 2.00, P = 0.82, ANOVA; i.p. saline: Q = 15.77 ± 2.47, n/m = 13/2; i.p. cocaine, Q = 13.65 ± 3.02, n/m = 14/4; cocaine SA, Q = 22.24 ± 4.79, n/m = 14/5) within this pathway after long-term withdrawal.
Fig. 5.
Selective increase in release probability in the PFC-NAc synapses during prolonged (45 d) withdrawal in cocaine self-administering rats. (A–C) Trains of AMPAR EPSCs elicited within the PFC-NAc pathway and the corresponding variance-mean plot of EPSCs in an example NAc shell MSN from a rat with repeated i.p. injection of saline (A), i.p. injection of cocaine (B), or cocaine SA (C). Solid lines were generated from parabolic fitting with Eq. 2. (D) Summarized data showing that the Pr of synapses in the PFC-NAc pathway is increased after long-term withdrawal from both i.p. injections and SA of cocaine with the magnitude of increase higher in cocaine self-administering rats. (E–G) Sample recordings and variance-mean analysis for the BLA-NAc pathway for the indicated experimental procedures. (H) Summarized data showing that the Pr of synapses in the BLA-NAc pathway is not affected during long-term withdrawal from both i.p. injection and SA of cocaine. *P < 0.05; **P < 0.01; ***P < 0.001.
Within BLA-NAc excitatory synapses, the basal presynaptic Pr (in saline-pretreated rats) remained stable throughout the 45 d withdrawal period (P = 0.25 vs. short-term withdrawal, t test; Pr = 0.62 ± 0.02, n/m = 13/2; Fig. 5 E and H). Furthermore, the release probability did not change significantly by either i.p. injections or SA of cocaine SA (F2,8 = 1.01, P = 0.40; i.p. cocaine, Pr = 0.66 ± 0.03, n/m = 14/4, P = 0.82 vs. saline; cocaine SA, Pr = 0.70 ± 0.04, n/m = 14/5, P = 0.34 vs. saline; P = 0.81 i.p. cocaine vs. cocaine SA; Fig. 5 E–H). Similarly, no changes were observed in either the number of release sites (F2,8 = 0.04, P = 0.92, ANOVA; i.p. saline, n = 12 ± 2, n/m = 13/2; i.p. cocaine, n = 12 ± 2, n/m = 14/4; cocaine SA, n = 13 ± 3, n/m = 14/5) or the quantal size (F2,8 = 0.04, P = 0.96, ANOVA; i.p. saline, Q = 19.66 ± 2.39, n/m = 13/2; i.p. cocaine, Q = 17.68 ± 5.93, n/m = 14/4; cocaine SA, Q = 19.96 ± 5.85, n/m = 14/5) during long-term withdrawal.
To compare the relative increase in Pr between the two different pathways, we normalized the single Prs of the cocaine-treated groups to the averaged Pr of their saline control groups. The magnitude of cocaine-induced increase in Pr was significantly higher in the PFC-NAc pathways than in BLA-NAc pathways after 1-d withdrawal from both the noncontingent and contingent exposure and after 45 d withdrawal from contingent cocaine exposure (F3,13 = 12.02, P < 0.001, ANOVA; i.p. cocaine: PFC-NAc: 1.34 ± 0.03, n/m = 14/3, BLA-NAc: 1.00 ± 0.06, n/m = 12/4 P < 0.05; cocaine SA: PFC-NAc: 1.28 ± 0.06, n/m = 14/3, BLA-NAc: 1.05 ± 0.03, n/m = 17/7 P < 0.05; Fig. S7A). Furthermore, after 45 d withdrawal, the magnitude of cocaine-induced increase in Pr within the PFC-NAC pathway was significantly higher in rats receiving cocaine by SA than passively exposed rats (F3,12 = 6.43, P < 0.01, ANOVA; i.p. cocaine: PFC-NAc: 1.21 ± 0.03, n/m = 19/4, P < 0.05 vs. PFC-NAc i.p. cocaine, BLA-NAc: 1.07 ± 0.04, n/m = 14/4; cocaine SA: PFC-NAc: 1.38 ± 0.03, n/m = 19/4, BLA-NAc: 1.17 ± 0.08, n/m = 14/4 P < 0.05; Fig. S7B), suggesting the procedure- and withdrawal-dependent features of this cocaine-induced presynaptic adaptation.
Discussion
By selectively activating different glutamatergic afferents to NAc MSNs, we demonstrated that after exposure to cocaine, the Pr was increased in the PFC, but not the BLA, afferents to the NAc in a procedure-dependent manner. These results establish long-term, pathway-specific presynaptic alterations as a consequence of cocaine exposure on excitatory synaptic transmission to the NAc shell and imply a shift in the driving force of different brain areas onto MSNs in regulating the functional output of NAc neurons.
Cocaine Increases Selectively the Pr of PFC-NAc Synapses.
In control animals, the presynaptic Pr of the BLA-NAc shell pathway was higher compared with the PFC-NAc shell pathway with values of ∼0.6 and ∼0.5, respectively (Figs. 2, 3, and 5). These values of the Pr are largely consistent with those of cortical excitatory NAc projections onto MSNs estimated by using electrical stimulations (0.4–0.6) (23), suggesting our optogenetic approach to reliably quantify Pr. It is important to note, that in the binominal model, we assumed a uniform Pr for all synapses. However, the inhibition time course of NMDAR EPSCs by MK-801 was best fit by two exponentials, suggesting a nonuniform Pr at PFC-NAc synapses (27). Thus, the Pr values assessed by the MPFAs should be taken as averaged values. Furthermore, a uniform quantal size between different synapses or in one synapse was assumed in the MPFA. This assumption allowed the parabolic fit of Q and N by only sampling five data points (16). Although this simplification likely introduced a constant error during fitting, the experiment using a pharmacological manipulation demonstrated that a change in Pr could be reliably measured without changes in Q or N (Fig. 2).
In the dorsal striatum, the Pr of the corticostriatal synapses is lower (∼0.4) (28) than that in the NAc. Furthermore, these synapses exhibit paired pulse facilitation, whereas excitatory synapses onto NAc shell MSNs exhibited paired pulse inhibition (Figs. 1, 3, and 5). Importantly, in the striatum, the position of the stimulation electrode greatly influenced the measurement of the Pr and the PPR. When stimulated inside the striatum, dopamine D1 and D2 receptor-expressing MSNs exhibited different opposite PPRs and, thus, different Prs, but this difference was not detected when stimulated outside of the striatum (28–30). This stimulation position-related differentiation may be mediated by activation of additional neuromodulatory fibers when locally stimulated, and if such, it is unlikely to happen with our optical ChR2-based stimulation, which was selective to glutamatergic inputs.
After chronic cocaine procedures, the Pr in the PFC-NAc pathway increased significantly, whereas the BLA-NAc was not changed (Figs. 3–5 and Fig. S7). This increase, especially with contingent cocaine exposure, was long lasting because it remained at least for 45 d. Previous studies had identified an increase in the frequency of mEPSCs in NAc shell MSNs (5, 31). However, a presynaptic mechanism was excluded, because the PPR was unchanged and a postsynaptic mechanism favored (5), e.g., by activation of silent synapses during cocaine withdrawal (32). It is conceivable, that the random sampling of excitatory synapses might dilute the effect of the PFC-NAc pathway by other afferents such as the BLA-NAc pathway, which are not presynaptically affected by cocaine exposure. Moreover, our results show that different afferents exhibited different presynaptic Prs (Fig. 3). Thus, sampling different pathways together may further mask the selective effect of cocaine on certain afferents.
Exposure to cocaine generates AMPAR-silent synapses in the NAc shell (33). It is unlikely that these silent synapses interfere with the MPFA to measure quantal parameters. First, at the holding potential of −70 mV, only active synapses contribute to the MPFA and silent synapses are not sampled. Second, if a single axon makes multiple contacts on a MSN and some of them are silent after cocaine exposure, the connectivity would change. However, in our MPFA assay, N represents the active release sites of all stimulated fibers, which has been experimentally normalized in each experiment by adjusting the light intensity based on the potential number of ChR2Y-positive fibers. Third, in animals with i.p. injections of cocaine, the increase of silent synapses was reversed after withdrawal day 7 (33). Thus, if silent synapses influence MPFA, the quantal parameters would differ on withdrawal days 1 and 45, which was not the case.
Long-Term Neurophysiological Changes After Cocaine Withdrawal.
The propensity to relapse is a key feature of drug addiction (34). This propensity increases during abstinence/withdrawal and can be triggered by cues to drug taking, stress, or drug exposure. It has been a focus to identify neuronal changes in the NAc, which develop and progress during the withdrawal period. We demonstrated that the Pr in the PFC-NAc pathway is increased 1 d after both noncontingent and contingent cocaine exposure, whereas after long-term cocaine withdrawal, in the SA, the Pr is significantly higher (Fig. 5). In parallel to these presynaptic alterations is a postsynaptic up-regulation of AMPARs, which occur during withdrawal initially by insertion of GluA2-containing AMPARs and subsequent after long-term withdrawal by GluA2-lacking AMPARs (35). Blocking GluA2-lacking AMPARs within the NAc attenuates cocaine seeking (36). It remains unclear which specific pathways in which excitatory synapses undergo such withdrawal-dependent postsynaptic changes, but the increased Pr in the PFC-NAc suggests a shift of the relative weight of driving forces from different afferent pathways. Indeed, a recent in vivo study suggests that the PFC-NAc afferent is strengthened after cocaine withdrawal (37). In addition, the increased Pr might favor establishing new synaptic connections via generation and maturation of silent synapses (32).
Functional Implications for Pathway-Specific Changes in the Pr.
The PFC and amygdala, especially the projections from the medial PFC and BLA to NAc, have been critically implicated in a large number of cocaine-induced behavioral alterations, such as increased impulsivity, cue-induced relapse, extinction, and reinstatement/relapse to cocaine administration (7, 8, 34). Neuroimaging studies in cocaine addicts revealed a reduced PFC activity under baseline conditions, which is paralleled by findings in cocaine self-administering rats (38, 39). However, the PFC and NAc are hyperresponsive toward drug-associated cues or cocaine exposure both in humans and rats. Consistent with the elevated prefrontal responsiveness, the basal intrinsic membrane excitability of medial PFC neurons is increased during withdrawal (40). Together with the increased Pr in the PFC-NAc synapses (Figs. 3 and 5), these effects can be conveyed to increased activation of the NAc. Furthermore, the potential shift in responsiveness of the different NAc inputs may produce exceeding activation of the PFC-NAc transmission upon reexposure to cocaine or cocaine-related cues during withdrawal and alter behavioral responses toward addiction-related behaviors, such as craving, relapse, and other emotional and motivational alterations during cocaine withdrawal.
Materials and Methods
Animal Use and Cocaine Administration.
Male Sprague–Dawley rats were either i.p. injected with cocaine (15 mg/kg per d) for 5 d or self-administered (0.75 mg/kg in 0.1 mL over 6 s) for 2 h/d on a fixed ratio 1 schedule in an operant-conditioning chamber (41). More details are described in SI Materials and Methods.
Virus Preparation and in Vivo Delivery.
ChR2Y H134R (Addgene plasmid 20071) was under the control of a CAG promoter in an AAV with AAV2 ITRs: AAV-ChR2Y (15) and stereotactically injected. More details are described in SI Materials and Methods.
Electrophysiology.
Slices were prepared, essentially as described and specified in SI Materials and Methods (32). Standard whole-cell voltage-clamp recordings were done with published intra- and extracellular solutions (33). The ACSF was supplemented with 0.1 mM picrotoxin to record EPSCs and 10 µM NBQX and 50 µM APV for photocurrents/APs. MPFA was performed with 30–100 AMPAR EPSCs from each cell at five Pr conditions (16, 18). The peak amplitude of each EPSC was subtracted for the baseline and averaged. Variance of peak EPSCs was calculated and plotted against the mean amplitude of EPSCs for each eliciting condition. Quantal parameters were estimated from the parabolic fit to the variance-mean relationship.
Statistics.
Results are shown as mean ± SEM. Number of cells and animals was presented as “n” and “m,” respectively. Results of n were averaged to account for m = 1 for animal based statistics, using one-factor ANOVA, with Tukey post hoc tests or two-tailed t tests.
Supplementary Material
Acknowledgments
We thank S. Ott-Gebauer for excellent technical support, F. Kötting for custom-made devices, and Dr. C.-H. Huang for comments and discussions on MPFA. This study was supported by a European Molecular Biology Organization short-term fellowship and Göttingen Graduate School for Neurosciences, Biophysics, and Molecular Biosciences [Deutsche Forschungsgemeinschaft (DFG) Grant GSC226/1] (to A.S.), National Institutes of Health National Institute on Drug Abuse funds (to Y.D., Y.H.H., and B.R.L.), the Alexander von Humboldt Foundation, the Hope Foundation for Depression (Y.D.), and the DFG through the Cluster of Excellence “Nanoscale Microscopy and Molecular Physiology of the Brain” and SCHL592/4 (to O.M.S.). The European Neuroscience Institute-Göttingen is jointly funded by the Max Planck Society and University Medicine Göttingen.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1206287110/-/DCSupplemental.
References
- 1.Kelley AE, Berridge KC. The neuroscience of natural rewards: Relevance to addictive drugs. J Neurosci. 2002;22(9):3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolf ME. The Bermuda Triangle of cocaine-induced neuroadaptations. Trends Neurosci. 2010;33(9):391–398. doi: 10.1016/j.tins.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Churchill L, Swanson CJ, Urbina M, Kalivas PW. Repeated cocaine alters glutamate receptor subunit levels in the nucleus accumbens and ventral tegmental area of rats that develop behavioral sensitization. J Neurochem. 1999;72(6):2397–2403. doi: 10.1046/j.1471-4159.1999.0722397.x. [DOI] [PubMed] [Google Scholar]
- 4.Boudreau AC, Wolf ME. Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. J Neurosci. 2005;25(40):9144–9151. doi: 10.1523/JNEUROSCI.2252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kourrich S, Rothwell PE, Klug JR, Thomas MJ. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J Neurosci. 2007;27(30):7921–7928. doi: 10.1523/JNEUROSCI.1859-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelley AE. Memory and addiction: Shared neural circuitry and molecular mechanisms. Neuron. 2004;44(1):161–179. doi: 10.1016/j.neuron.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt HD, Anderson SM, Famous KR, Kumaresan V, Pierce RC. Anatomy and pharmacology of cocaine priming-induced reinstatement of drug seeking. Eur J Pharmacol. 2005;526(1-3):65–76. doi: 10.1016/j.ejphar.2005.09.068. [DOI] [PubMed] [Google Scholar]
- 8.Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011;69(4):680–694. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 9.Winstanley CA, et al. Increased impulsivity during withdrawal from cocaine self-administration: Role for DeltaFosB in the orbitofrontal cortex. Cereb Cortex. 2009;19(2):435–444. doi: 10.1093/cercor/bhn094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23(8):3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Ciano P, Everitt BJ. Direct interactions between the basolateral amygdala and nucleus accumbens core underlie cocaine-seeking behavior by rats. J Neurosci. 2004;24(32):7167–7173. doi: 10.1523/JNEUROSCI.1581-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ambroggi F, Ishikawa A, Fields HL, Nicola SM. Basolateral amygdala neurons facilitate reward-seeking behavior by exciting nucleus accumbens neurons. Neuron. 2008;59(4):648–661. doi: 10.1016/j.neuron.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stuber GD, et al. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011;475(7356):377–380. doi: 10.1038/nature10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.See RE, Kruzich PJ, Grimm JW. Dopamine, but not glutamate, receptor blockade in the basolateral amygdala attenuates conditioned reward in a rat model of relapse to cocaine-seeking behavior. Psychopharmacology (Berl) 2001;154(3):301–310. doi: 10.1007/s002130000636. [DOI] [PubMed] [Google Scholar]
- 15.Petreanu L, Mao T, Sternson SM, Svoboda K. The subcellular organization of neocortical excitatory connections. Nature. 2009;457(7233):1142–1145. doi: 10.1038/nature07709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silver RA. Estimation of nonuniform quantal parameters with multiple-probability fluctuation analysis: Theory, application and limitations. J Neurosci Methods. 2003;130(2):127–141. doi: 10.1016/j.jneumeth.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 17.Mattis J, et al. Principles for applying optogenetic tools derived from direct comparative analysis of microbial opsins. Nat Methods. 2012;9(2):159–172. doi: 10.1038/nmeth.1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scheuss V, Neher E. Estimating synaptic parameters from mean, variance, and covariance in trains of synaptic responses. Biophys J. 2001;81(4):1970–1989. doi: 10.1016/S0006-3495(01)75848-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faber DS, Korn H. Applicability of the coefficient of variation method for analyzing synaptic plasticity. Biophys J. 1991;60(5):1288–1294. doi: 10.1016/S0006-3495(91)82162-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muller D, Lynch G. Evidence that changes in presynaptic calcium currents are not responsible for long-term potentiation in hippocampus. Brain Res. 1989;479(2):290–299. doi: 10.1016/0006-8993(89)91631-4. [DOI] [PubMed] [Google Scholar]
- 21.Hjelmstad GO, Nicoll RA, Malenka RC. Synaptic refractory period provides a measure of probability of release in the hippocampus. Neuron. 1997;19(6):1309–1318. doi: 10.1016/s0896-6273(00)80421-3. [DOI] [PubMed] [Google Scholar]
- 22.Jacobs EH, Smit AB, de Vries TJ, Schoffelmeer ANM. Neuroadaptive effects of active versus passive drug administration in addiction research. Trends Pharmacol Sci. 2003;24(11):566–573. doi: 10.1016/j.tips.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 23.Casassus G, Blanchet C, Mulle C. Short-term regulation of information processing at the corticoaccumbens synapse. J Neurosci. 2005;25(50):11504–11512. doi: 10.1523/JNEUROSCI.2466-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang C-H, Bao J, Sakaba T. Multivesicular release differentiates the reliability of synaptic transmission between the visual cortex and the somatosensory cortex. J Neurosci. 2010;30(36):11994–12004. doi: 10.1523/JNEUROSCI.2381-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Christie JM, Jahr CE. Multivesicular release at Schaffer collateral-CA1 hippocampal synapses. J Neurosci. 2006;26(1):210–216. doi: 10.1523/JNEUROSCI.4307-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schlüter OM, Schmitz F, Jahn R, Rosenmund C, Südhof TC. A complete genetic analysis of neuronal Rab3 function. J Neurosci. 2004;24(29):6629–6637. doi: 10.1523/JNEUROSCI.1610-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenmund C, Clements JD, Westbrook GL. Nonuniform probability of glutamate release at a hippocampal synapse. Science. 1993;262(5134):754–757. doi: 10.1126/science.7901909. [DOI] [PubMed] [Google Scholar]
- 28.Ding J, Peterson JD, Surmeier DJ. Corticostriatal and thalamostriatal synapses have distinctive properties. J Neurosci. 2008;28(25):6483–6492. doi: 10.1523/JNEUROSCI.0435-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grueter BA, Brasnjo G, Malenka RC. Postsynaptic TRPV1 triggers cell type-specific long-term depression in the nucleus accumbens. Nat Neurosci. 2010;13(12):1519–1525. doi: 10.1038/nn.2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kreitzer AC, Malenka RC. Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson’s disease models. Nature. 2007;445(7128):643–647. doi: 10.1038/nature05506. [DOI] [PubMed] [Google Scholar]
- 31.Thomas MJ, Beurrier C, Bonci A, Malenka RC. Long-term depression in the nucleus accumbens: A neural correlate of behavioral sensitization to cocaine. Nat Neurosci. 2001;4(12):1217–1223. doi: 10.1038/nn757. [DOI] [PubMed] [Google Scholar]
- 32.Brown TE, et al. A silent synapse-based mechanism for cocaine-induced locomotor sensitization. J Neurosci. 2011;31(22):8163–8174. doi: 10.1523/JNEUROSCI.0016-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang YH, et al. In vivo cocaine experience generates silent synapses. Neuron. 2009;63(1):40–47. doi: 10.1016/j.neuron.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peters J, LaLumiere RT, Kalivas PW. Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J Neurosci. 2008;28(23):6046–6053. doi: 10.1523/JNEUROSCI.1045-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCutcheon JE, Wang X, Tseng KY, Wolf ME, Marinelli M. Calcium-permeable AMPA receptors are present in nucleus accumbens synapses after prolonged withdrawal from cocaine self-administration but not experimenter-administered cocaine. J Neurosci. 2011;31(15):5737–5743. doi: 10.1523/JNEUROSCI.0350-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Conrad KL, et al. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454(7200):118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moussawi K, et al. N-Acetylcysteine reverses cocaine-induced metaplasticity. Nat Neurosci. 2009;12(2):182–189. doi: 10.1038/nn.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun W, Rebec GV. Repeated cocaine self-administration alters processing of cocaine-related information in rat prefrontal cortex. J Neurosci. 2006;26(30):8004–8008. doi: 10.1523/JNEUROSCI.1413-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: Neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12(11):652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dong Y, et al. Cocaine-induced plasticity of intrinsic membrane properties in prefrontal cortex pyramidal neurons: Adaptations in potassium currents. J Neurosci. 2005;25(4):936–940. doi: 10.1523/JNEUROSCI.4715-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mu P, et al. Exposure to cocaine dynamically regulates the intrinsic membrane excitability of nucleus accumbens neurons. J Neurosci. 2010;30(10):3689–3699. doi: 10.1523/JNEUROSCI.4063-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.