Abstract
Retinoic acid (RA), an active vitamin A derivative, is essential for mammalian spermatogenesis. Genetic studies have revealed that oxidation of vitamin A to retinal by retinol dehydrogenase 10 (RDH10) is critical for embryonic RA biosynthesis. However, physiological roles of RDH10 in postnatal RA synthesis remain unclear, given that Rdh10 loss-of-function mutations lead to early embryonic lethality. We conducted in vivo genetic studies of Rdh10 in postnatal mouse testes and found that an RDH10 deficiency in Sertoli cells, but not in germ cells, results in a mild germ cell depletion phenotype. A deficiency of RDH10 in both Sertoli and germ cells in juvenile mice results in a blockage of spermatogonial differentiation, similar to that seen in vitamin A-deficient animals. This defect in spermatogenesis arises from a complete deficiency in juvenile testicular RA synthesis and can be rescued by retinoid administration. Thus, in juvenile mice, the primary, but not exclusive, source of RA in the testes is Sertoli cells. In contrast, adult Rdh10-deficient mice exhibit phenotypically normal spermatogenesis, indicating that during development a change occurs in either the cellular source of RA or the retinaldehyde dehydrogenase involved in RA synthesis.
Keywords: synchronous spermatogenesis, seminiferous epithelium, synchrony factor
Mammalian spermatogenesis begins shortly after birth, and continues to complete the first wave of spermatogenesis until puberty. This provides the framework for subsequent spermatogenic waves that occur in a continuous, well-coordinated manner in the adult (1–3). Spermatogenesis relies on the proper function of spermatogonial stem cells, which are among the undifferentiated spermatogonia that include A single (As), A paired (Ap), and A aligned (Aal) spermatogonia (4). The undifferentiated spermatogonia transform into A1 spermatogonia without a mitotic division. The A1 spermatogonia further generate, in succession, A2, A3, A4, In (intermediate), and B spermatogonia via a series of proliferative divisions. The differentiated spermatogonia then continue to differentiate into spermatocytes, haploid spermatids, and spermatozoa (4).
Retinoic acid (RA), an active derivative of vitamin A [retinol (Rol)], is essential for mammalian spermatogenesis (5–9). Vitamin A-deficient (VAD) rodents display a block at the transition of the undifferentiated spermatogonia into A1 spermatogonia, resulting in seminiferous epithelium that contains only spermatogonia and Sertoli cells. Administration of bioactive retinoids to VAD animals reinitiates spermatogenesis in a synchronous manner by releasing the block on spermatogonial differentiation (10–13). Recent studies have shown that Rol can induce spermatogonial differentiation in cryptorchid testes as well (14). Normally, testicular RA is synthesized in situ rather than originating from the circulation (15, 16). To date, however, which molecules are involved in RA biosynthesis in the testes and which cells are the primary sources of RA remain unknown.
RA functions as a ligand for nuclear receptors [RA receptors (RARs)], which bind to RA-response elements (RAREs) in regulatory regions of target genes and control the expression of target genes (17). The RARs include three isotypes—Rara, Rarb, and Rarg—that are widely expressed in the testes (18). RA is synthesized from an inactive precursor through a two-step enzymatic oxidation reaction in which Rol is first converted to retinaldehyde (Ral) then to RA. The first step is mediated by two families of enzymes: the cytosolic alcohol dehydrogenase (ADH) family (e.g., ADH1, ADH3, ADH4), which are medium-chain dehydrogenases/reductases, and Rol dehydrogenases (e.g., RDH1, RDH10, RDH11, RDH13, RDH14, RDH15), which are short-chain dehydrogenases/reductases (19, 20). Enzymes belonging to the Ral dehydrogenase (RALDH) family are responsible for the second step of RA synthesis. The RALDH family includes RALDH1, RALDH2, and RALDH3, which are encoded by Aldh1a1, Aldh1a2, and Aldh1a3, respectively (21, 22). Studies with gene loss-of-function and RA reporter mice have suggested that RDH10 and RALDH2 are critical for RA biosynthesis in the embryo (22–26). In contrast to its well-established importance in the embryo, the physiological roles of RDH10 in postnatal RA synthesis remain elusive, given that Rdh10 loss of function results in early embryonic lethality.
To address this question, we generated testicular cell-specific conditional knockout (cKO) alleles of Rdh10, which permitted an evaluation of RDH10 on postnatal testicular RA synthesis. We report here that Rdh10 loss of function in both Sertoli and germ cells or only in Sertoli cells leads to a defect in spermatogonial differentiation in juvenile mice. Through RA reporter mouse analyses, we found that Rdh10 loss of function in both Sertoli and germ cells completely impairs testicular RA signaling in juvenile animals. Interestingly, spermatogenesis was progressively recovered in adult Rdh10 cKO mice. These findings demonstrate that RDH10 is essential for juvenile spermatogenesis, but not for adult spermatogenesis.
Results
Male Rdh10 Mutants Display Impaired Spermatogenesis.
To determine the role of Rdh10 in postnatal mouse development and disease, we used a cKO strategy that generated an Rdh10 floxed line (Rdh10fl/fl), in which exon 2 of the Rdh10 allele is flanked by loxP sites (Fig. S1). Global inactivation of Rdh10 causes embryonic lethality (24–26). Affymetrix array data and in situ hybridization findings revealed Rdh10 expression in postnatal mouse testes, including Sertoli cells and germ cells (Tables S1 and S2 and Fig. S2). To explore the role of RDH10 in postnatal testes, we deleted Rdh10 in Sertoli cells using the Amh-Cre transgenic line (Rdh10fl/fl, Amh-Cre+) (27), in germ cells using the Stra8-Cre transgenic line (Rdh10fl/fl, Stra8-Cre+) (28), or in both Sertoli and germ cells (Rdh10fl/fl, Amh-Cre+, Stra8-Cre+). Amh-Cre transgenic mice exhibit high recombinase activity as early as embryonic day 14.5 (27). Stra8-Cre male mice start to express Cre in spermatogonia at postnatal day 3 (3 dpp), whereas female germ cells do not express Cre (28). To confirm the Cre expression in mutants, we generated compound mutant mice by crossing the Cre mice with a double-fluorescent reporter line, ROSA26mTmG (29). Membrane-targeted EGFP allowed visualization of cellular patterns of testicular Cre activity, demonstrating Sertoli cell- or germ cell-specific Cre expression, consistent with previous reports (27, 28). To avoid global deletion of the Rdh10 floxed allele, we used only Rdh10fl, Stra8-Cre+ females for breeding. For this study, we designated the four different genotypes of mice as control (Rdh10fl/fl), Rdh10 gKO (germ cell-specific KO; Rdh10fl/fl, Stra8-Cre+), Rdh10 sKO (Sertoli cell-specific KO; Rdh10fl/fl, Amh-Cre+), and Rdh10 sgKO (Sertoli and germ cell-specific KO; Rdh10fl/fl, Amh-Cre+, Stra8-Cre+).
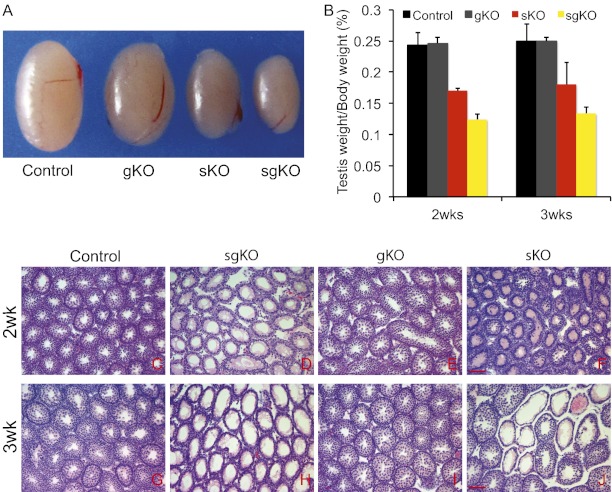
To explore the role of Rdh10 in the testes of juvenile males, we collected testes from mice at age 2-3 wk. There were no significant differences in body weight among the four genotypes. The relative weights of testes were significantly lower in Rdh10 sKO and sgKO mice compared with controls at both 2 wk and 3 wk [at age 2 wk: control, 0.243 ± 0.021 (mean ± SD testes weight/body weight × 100); Rdh10 sKO, 0.170 ± 0.003, P < 0.05; Rdh10 sgKO, 0.124 ± 0.009, P < 0.01; at age 3 wk: control, 0.249 ± 0.028; Rdh10 sKO, 0.180 ± 0.035, P < 0.05; Rdh10 sgKO, 0.133 ± 0.011, P < 0.01]. In contrast, there were no significant differences between controls and Rdh10 gKO (at age 2 wk: Rdh10 gKO, 0.246 ± 0.010, P = 0.51; at age 3 wk: Rdh10 gKO, 0.250 ± 0.007, P = 0.49) (Fig. 1 A and B). In addition, the testes weight index was dramtically lower at both 2 and 3 wk in Rdh10 sgKO mutants compared with Rdh10 sKO mutants (P < 0.05). Histological analyses showed that the control testes (Fig. 1 C and G) and Rdh10 gKO mutant testes (Fig. 1 E and I) were indistinguishable; at 2 wk, both genotypes had spermatogonia and preleptene, leptene, zygotene, and pachytene spermatocytes, and by 3 wk, round spermatids had formed in both genotypes. In contrast, in the Rdh10 sgKO testes, most seminiferous tubules (>85%) were devoid of meiotic cells and retained only undifferentiated spermatogonia-like cells and Sertoli cells (Fig. 1 D and H). These defects were first apparent in the 7-d-old juveniles. An Rdh10 deficiency exclusively in Sertoli cells (Rdh10 sKO) had milder phenotypes with ∼45% degenerated tubules (Fig. 1 F and J). Numerous degenerated germ cells were present in the Rdh10 sgKO and sKO testes (Fig. S3 A–D). A TUNEL assay detected more TUNEL-positive germ cells in Rdh10 sKO and sgKO mutant testes than in control and gKO testes (Fig. S3 E–H).
Fig. 1.
Impaired spermatogenesis occurs in Rdh10 sgKO and sKO juvenile mice. (A) Gross morphology of representative testes from a 2-wk-old control and age-matched Rdh10 gKO, sKO, and sgKO mutants. (B) Comparisons of testis weight from 2- or 3-wk-old controls and mutants (n = 4–8 for each genotype per data point). (C–J) H&E staining of control (C and G), Rdh10 sgKO (D and H), gKO (E and I), and sgKO (F and J) testes at age 2 or 3 wk. (Scale bars: 20 μm.)
Rdh10 Deficiency in both Sertoli and Germ Cells Causes Blockage of Differentiation of Aal Spermatogonia into A1 Spermatogonia.
The remaining germ cells in Rdh10 sgKO testes were immunostained with the molecular markers of differentiated spermatogonia and spermatocytes. A marker for the formation of DNA double-strand breaks in early meiosis, γ-H2AX (30), was absent within most seminiferous tubules of Rdh10 sgKO testes of 2-wk-old (Fig. 2 D and F) and 3-wk-old mutants (Fig. S4 D and F) but was present in control testes (Fig. 2 A and C and Fig. S4 A and C), suggesting noninitiation of meiosis in the germ cells of Rdh10 sgKO testes. Immunostaining for STRA8, a marker for differentiated spermatogonia (also a RA-responsive gene), was done to determine whether spermatogonia in Rdh10 sgKO testes differentiate. As expected, control testes contained many seminiferous tubules with STRA8-positive germ cells (Fig. 2 G and I), but STRA8-positive cells were rarely observed in Rdh10 sgKO testes from 2-wk-old (Fig. 2 J and L) and 3-wk-old mutants (Fig. S4 J and L), indicating nondifferentiation of spermatogonia. We performed concomitant immunostaining for GCNA, a germ cell-specific maker (31), to differentiate between somatic cells and germ cells in the testes. Consistent with the foregoing histological study, the GCNA staining showed that only one layer of germ cells was located along the basement membrane in Rdh10 sgKO testes (Fig. 2 E and K and Fig. S4 E and K), in contrast to the multiple layers of germ cells within the seminiferous epithelium of control testes (Fig. 2 B and H and Fig. S4 B and H). Control testes contained seminiferous tubules with KIT-positive germ cells, whereas KIT-positive cells were rarely observed in Rdh10 sgKO testes from 2-wk-old mice (Fig. S5). Collectively, the observed defects in Rdh10 sgKO testes phenocopy the abnormalities observed in VAD animals.
Fig. 2.
Decreased expression of markers for spermatogonial differentiation in Rdh10 sgKO mutant testes. (A–F) Immunofluorescence staining for γH2AX (red) in sections of 2-wk-old control (A–C) and Rdh10 sgKO (D–F) testes, with costaining for germ cell marker GCNA (green). (G–L) Immunohistochemical staining for STRA8 (red) in sections of 2-wk-old control (G–I) and Rdh10 sgKO (J–L) testes, with costaining for germ cell marker GCNA (green). (M) Quantitative RT-PCR analysis of mRNA levels of markers for spermatogonial differentiation in control, Rdh10 sgKO, sKO, and gKO mutant testes at age 2 wk. Data are expressed as mean ± SD fold differences compared with controls, normalized to Rps2. n = 4–6; Student t test. (Scale bars: 20 μm.)
We used levels of mRNA for key markers for spermatogonial differentiation and meiotic initiation to examine the differentiation of spermatogonia in Rdh10 sgKO testes. Markers of differentiated (A1) spermatogonia included Stra8 and c-kit. Markers of early meiotic prophase included Spo11, which encodes a topoisomerase (32); Dmc1, which encodes a meiosis-specific recombinase (33); and Sycp3, which encodes a component of the synaptonemal complex (34). mRNA levels of spermatogonial differentiation markers (Stra8 and c-kit) and meiosis markers (Spo11, Dmc1, and Sycp3) were significantly reduced in Rdh10 sgKO mutant testes and even in Rdh10 sKO testes, as measured by quantitative RT-PCR (Fig. 2M). Taken together, these data clearly indicate that spermatogonia in Rdh10 sgKO testes do not enter differentiation and thus do not initiate meiosis.
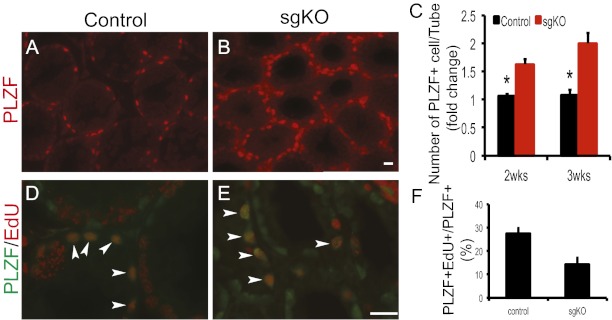
To further characterize the state of the remaining spermatogonia, we used staining with an antibody to an undifferentiated spermatogonial marker, PLZF. The number of PLZF-positive spermatogonia was significantly elevated in Rdh10 sgKO testes (Fig. 3 A–C). We then performed immunostaining for SOX9, a marker for Sertoli cells (35), to determine the state of Sertoli cells in Rdh10 sgKO. The numbers of Sertoli cells in control and Rdh10 sgKO testes did not differ significantly [control, 32.58 ± 1.94 (mean ± SD SOX9+/tubule); sgKO, 33.05 ± 1.95; n = 3; P > 0.05] (Fig. S6). We administered a short-duration (2 h) 5-ethynyl-2′-deoxyuridine (EdU) pulse to control and Rdh10 sgKO mutant mice, and found a lower ratio of both EdU-positive and PLZF-positive cells to PLZF-positive cells (EdU+PLZF+/PLZF+) in Rdh10 sgKO testes compared with controls (Fig. 3 D–F), indicating lower proliferation rates in these PLZF-positive spermatogonia. A TUNEL assay on control and Rdh10 sgKO testes revealed little if any difference in apoptosis of PLZF-positive spermatogonia [control, 1.6 ± 0.51 (mean ± SD TUNEL+PLZF+/PLZF+ × 100); sgKO, 1.9 ± 0.46; n = 3–5; P = 0.21]. Taken together, these findings provide strong evidence that the accumulation of undifferentiated (PLZF-positive) spermatogonia in the Rdh10 sgKO testes results from blockage of the differentiation of Aal into A1 spermatogonia, rather than from hyperproliferation and/or decreased apoptosis.
Fig. 3.
Accumulation of PLZF-positive spermatogonia in Rdh10 sgKO mutant testes. (A and B) Immunofluorescence staining for PLZF (red) in sections of 2-wk-old control (A) and Rdh10 sgKO (B) testes. (Scale bar: 20 μm.) (C) Quantification of PLZF-positive cells per seminiferous tubule in 2-wk-old testes. (D and E) Immunostaining for PLZF (green) and EdU (red) in sections of 2-wk-old control (D) and Rdh10 sgKO (E) testes. Arrowheads indicate both PLZF- and EdU-positive spermatogonia (orange). (Scale bar: 20 μm.) (F) Quantification of proliferative spermatogonia in control and Rdh10 sgKO testes at age 2 wk. The number of EdU-positive cells per number of PLZF-positive cells was recorded. All seminiferous tubules at each section were counted (n = 3–5). Error bars represent SD. *P < 0.05, Student t test.
RDH10 Deficiency Affects RA Signaling in Testis.
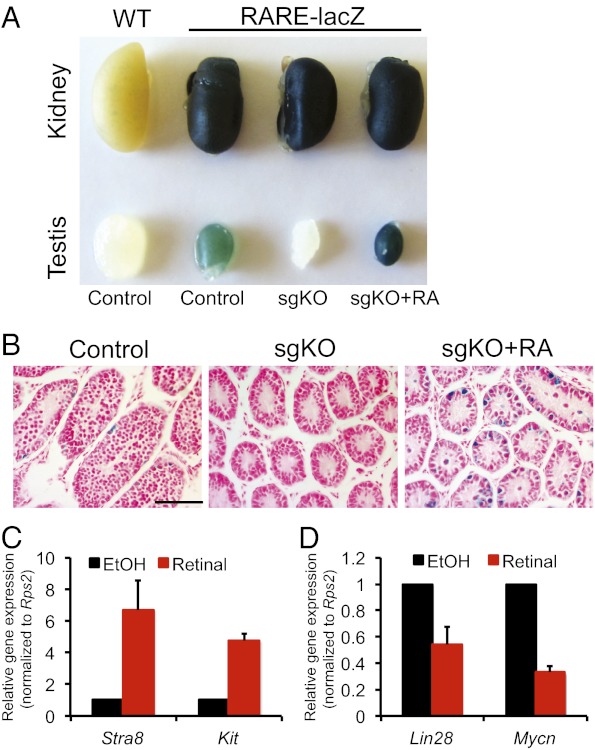
RDH10 is known to be essential for the synthesis of embryonic Ral, the intermediate metabolite in RA biosynthesis (24–26). We used a RARElacZ reporter line harboring a RA-responsive transgene, which allows visualization of the distribution of RA signaling by X-Gal staining, to examine RA levels in the testes of the mutant mice (36). We found no lacZ activity in testes from Rdh10 sgKO mutants, but strong lacZ staining in the control testes (Fig. 4A). We then verified impaired RA signaling in the Rdh10 sgKO mutant testes by treating 2-wk-old Rdh10 sgKO mice with RA for 24 h. The Rdh10 sgKO testes demonstrated rescue of lacZ expression after RA treatment (Fig. 4A), indicating that these testes lacked RA. Histologically, seminiferous tubes in control testes contained lacZ-positive spermatogonia and spermatocytes (Fig. 4B and Fig. S7A). Significantly, virtually no lacZ-positive cells were found in Rdh10 sgKO testes (Fig. 4B and Fig. S7B), but >80% of the seminiferous tubes in these testes contained lacZ-positive spermatogonia after exposure to RA (Fig. 4B and Fig. S7C).
Fig. 4.
Expression of RA-responsive genes in Rdh10 sgKO testes. (A) X-Gal staining of testes and kidneys from Rdh10 sgKO, RA-rescued Rdh10 sgKO, and control littermates harboring a RARE-Hspa1b-lacZ transgene at age 2 wk. X-Gal staining of kidneys from mutants and controls served as a positive control, and X-Gal staining of WT without a RARE-Hspa1b-lacZ transgene served as a negative control. (B) Histological analysis of X-Gal staining of testes from Rdh10 sgKO, RA-rescued Rdh10 sgKO, and control littermates harboring a RARE-Hspa1b-lacZ transgene at age 2 wk. Cells with a positive signal are shown in blue. Nuclei were counterstained with fast red. (Scale bar: 20 μm.) (C and D) Quantitative RT-PCR analysis of mRNA levels of RA-induced genes (C) and RA-repressed genes (D) in Ral-rescued Rdh10 sgKO testes at age 2 wk. Data are expressed as mean ± SD fold differences compared with controls (Rdh10 sgKO), normalized to Rps2. n = 3. *P < 0.05, Student t test.
We treated RDH10 sgKO mice with Ral in an attempt to rescue the phenotype. Exogenous Ral administration significantly induced expression of Stra8 and Kit in Rdh10 sgKO mutant testes, as determined by quantitative RT-PCR (Fig. 4C). In addition, expression of Lin28 and Mycn, which are RA-repressed genes (37, 38), was dramatically inhibited after RA treatment (Fig. 4D). Taken together, these data support the idea that testicular cell-specific Rdh10 deficiency impairs RA signaling, and thus Rdh10 sgKO spermatogenesis defects result from the lack of an RA signal.
Retinoid Induces Synchrony of Spermatogenesis in Rdh10 sgKO Mutants.
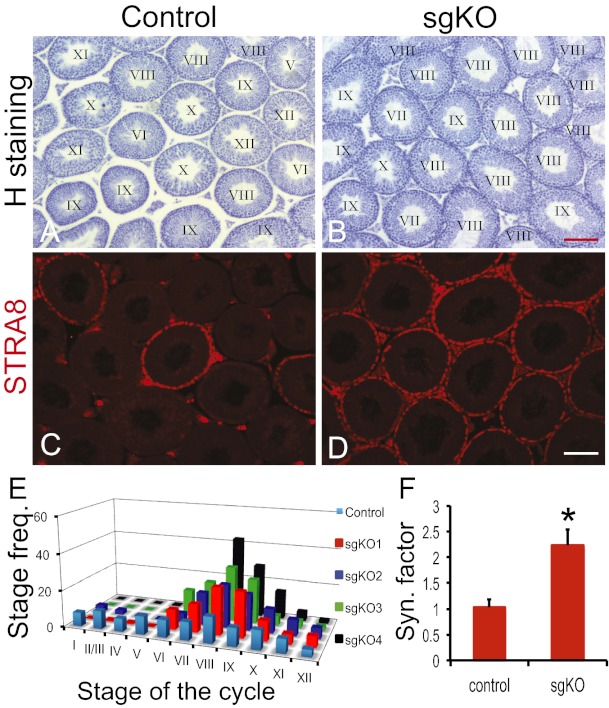
Spermatogenesis in mammals is highly coordinated process of germ cell differentiation occurring in the seminiferous tubules, where various types of germ cells form well-defined cellular associations (39). These typical cell associations are stages of the cycle of the seminiferous epithelium, 12 of which are present in the mouse (39). VAD male mice exhibit arrested spermatogonial differentiation; however, administration of vitamin A or RA to VAD animals results in the reinitiation of spermatogenesis, such that the epithelium becomes stage-synchronized. Given that a loss of RDH10 in mouse testes causes testicular defects similar to those seen in VAD mice, we examined whether RA or Ral treatment also induces synchronization of the seminiferous epithelium in Rdh10 sgKO mutant mice.
In this experiment, 3-wk-old control and Rdh10 sgKO mutant male mice received a single RA or Ral injection and were then fed a normal vitamin A-containing diet for 35 d. Most seminiferous tubules from representative RA-treated Rdh10 sgKO males were enriched in stages VII–IX of the cycle of the seminiferous epithelium, whereas retinoid treatment did not enrich the stages of the seminiferous epithelial cycle in any control individuals (Fig. 5 A and B). STRA8 immunostaining further demonstrated the enrichment of stage VIII in the RA-treated Rdh10 sgKO mutants (Fig. 5 C and D). Stage frequency analysis and synchrony factor calculations confirmed that spermatogenesis was more synchronous in RA-treated mutants than in RA-treated controls (Fig. 5 E and F). Results after Ral treatment were similar to those after RA treatment, whereas Rol treatment cannot induce synchronized spermatogenesis in mutants (mean synchrony factor, 1.08 ± 0.09; n = 3; P > 0.05).
Fig. 5.
Synchrony of spermatogenesis in Rdh10 sgKO testes after RA treatment. (A and B) Hematoxylin staining of representative testes from 3-wk-old control (A) and Rdh10 sgKO (B) mice with a vitamin A-containing diet for 5 wk, after an RA injection. Roman numerals indicate the stage of the seminiferous epithelial cycle. (C and D) Immunostaining for STRA8 (red) in sections of representative testes from 3-wk-old control (C) and Rdh10 sgKO (D) mice after RA treatment. The seminiferous epithelium contains STRA8-positive cells, a characteristic marker of stage VIII. (E) Stage frequency of seminiferous epithelial cycle in control and Rdh10 sgKO individuals after RA treatment. (F) Calculated synchronization factors of control and Rdh10 sgKO mice after RA treatment. n = 5–8. Error bars represent SD. *P < 0.05, Student t test. (Scale bars: 100 μm.)
Spermatogenesis Is Recovered in Adult Rdh10 sgKO Mutant Males.
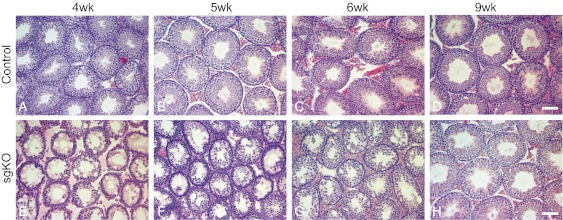
Given the importance of Rdh10 in regulating juvenile spermatogenesis, we next investigated the requirement for Rdh10 in support of adult spermatogenesis. We found that Rdh10 sgKO males aged <7 wk were infertile or subfertile, but those aged >9 wk were fertile. Moreover, litters produced from controls and mutants did not differ in size. Histologically, spermatogenesis in most seminiferous tubules from Rdh10 sgKO mutants was reinitiated at age 4 wk, because spermatocytes were present (Fig. 6). Spermatids were rarely observed in 5- and 6-wk-old Rdh10 sgKO testes (Fig. 6). By age 9 wk, spermatogenesis was completed in Rdh10 sgKO testes, with all seminiferous tubules containing the expected spermatogonia, spermatocytes, and spermatids (Fig. 6). Examination of recovery of spermatogenesis in adult Rdh10 sgKO males showed that only 1 out of 48 exhibited spermatogenesis with an altered stage frequency.
Fig. 6.
Histological analysis of testes from peripubertal and adult control (A–D) and Rdh10 sgKO (E–H) mice. (Scale bars: 20 μm.)
Discussion
In this study, we investigated the biological function of Rdh10 in postnatal mouse testes using a conditional targeted deletion, and found that Rdh10 is required for spermatogenesis in juveniles. Specifically, we found that Rdh10 is essential for juvenile testicular RA biosynthesis (i.e., generation of Ral for use as a substrate for RALDH synthesis of RA), and thus that the testicular defects in Rdh10 mutants result from lack of RA. Interestingly, we found that Rdh10 in Sertoli and/or germ cells is dispensable for spermatogenesis in the adult.
In mammals, under physiological conditions, testicular RA derives from local synthesis within the seminiferous tubule rather than from the circulation, because of the presence of a metabolic barrier (15, 18). Testicular RA is synthesized in a two-step process from Rol provided either by the circulation or by the local stores of retinyl esters. The initial step, conversion of Rol into Ral, is the rate-limiting and reversible step in RA synthesis (40). We found that Rdh10 is expressed in both mouse Sertoli cells and spermatogonia. This finding, together with a wealth of evidence that RDH10-mediated Ral production is a critical step in embryonic RA synthesis, leads us to postulate that RDH10 may be responsible for the postnatal testicular RA synthesis. The data presented here support this hypothesis in several respects. First, loss of the RDH10 enzyme either in both Sertoli cells and germ cells or only in Sertoli cells results in defects in spermatogonial differentiation characterized by the presence of only Sertoli cells and undifferentiated spermatogonia within the seminiferous tubules. This phenotype is identical to the testicular abnormalities observed in VAD mice. Second, a lack of RDH10 activity in RARElacZ reporter mice causes complete loss of lacZ activity in testes; however, RA administration rescues expression of the reporter in mutant testes. Finally, retinoid supplementation not only reinitiates spermatogonial differentiation, but also induces synchrony of spermatogenesis in Rdh10 sgKO mutants, similar to what occurs in RA-rescued VAD rodents. Thus, although many enzymes displaying all-trans- and/or cis-Rol dehydrogenase, such as RDH11, RDH13, RDH14, ADH1, ADH3, and ADH4 (41), are expressed in juvenile mouse testes, it is clear that the RDH10 enzyme contributes significantly to physiological synthesis of RA during juvenile spermatogenesis.
It is interesting to note that Sertoli cell-specific Rdh10 deficiency could result in defects in spermatogonial differentiation, although the phenotype is not as severe as that seen with a deficiency in both Sertoli cells and germ cells. In contrast, germ cell-specific Rdh10-deficient juvenile mice demonstrated normal spermatogenesis. These findings suggest that both Sertoli cells and germ cells produce Ral by RDH10, but Sertoli cells may be the major source of Ral in juvenile mouse testes. Ral is subsequently converted into RA in Sertoli cells, which express Aldh1a1 and Aldh1a2 in juvenile mouse testes (18). The fact that Rdh10 sgKO mutants can be rescued by Ral treatment, which exposes all cells of the body to Ral, suggests that spatially restricted availability of Ral is not critical for spermatogenesis. This suggestion is consistent with the observation that embryo development is not dependent on localized Ral production (24, 25).
A notable finding is that spermatogenesis in Rdh10 sgKO mutants was reinitiated starting at age 4 wk and was completed by age 9 wk. In one study, Ral-rescued Rdh10 global KO mice survived to adulthood and were fertile (24). Although the status of spermatogenesis was not reported in that study, it is possible—as we have demonstrated in Rdh10 sgKO mice—that spermatogenesis was also impaired in the juvenile Rdh10 global KO mice and then recovered in adult mice. The ability of the Rdh10 sgKO adult mice to recover from early impaired spermatogenesis suggests that other Rol-oxidizing enzymes must be involved. For example, ADH KO studies have demonstrated a postnatal role for ADHs in Rol metabolism, although in these KOs, even Adh-del compound KOs lacking all six ADHs (Adh1, 2, 3, 4, 5a, and 5b), were fertile (42). ADHs and/or other RDHs are expressed along with RDH10 in the testes. In the absence of RDH10, they could be the source of Ral in the testes. In addition, β-carotene 15,15′-monoxygenase, which can produce Ral from provitamin A, is expressed in >20-d-old mouse testes, suggesting that it could play a role in the recovery seen in Rdh10 sgKO adult testes (18, 43). Alternatively, the testes could obtain Ral for RA synthesis from the circulation. Nonetheless, it is clear that these enzymes do not compensate for the absence of RDH10-mediated Ral production in juvenile mouse testes.
Finally, considering the defects in spermatogenesis in juvenile mice that were recovered in adults, our Rdh10-deficient mouse model is a unique model that can provide insight into the RA signaling pathways that control spermatogonial differentiation and meiotic initiation and regulate formation of the seminiferous epithelial cycle.
Materials and Methods
Generation of Rdh10 cKO Mice.
Rdh10-targeted ES cells were generated by the University of California Davis Knockout Mouse Project Repository. The targeting allele was designed for conditional mutagenesis using the FLP-FRT and Cre-loxP system (Fig. S1). A splice acceptor lacZ gene trap cassette flanked by an FRT site was inserted between exon 1 and exon 2 of the Rdh10 gene. The loxP sites were introduced to flank exon 2 of the Rdh10 gene. Excision of exon 2 creates a frameshift mutation in RDH10. We used a correctly targeted ES cell clone for injection into C57BL6/J (B6) blastocysts. Resulting chimeric male mice were mated to WT B6 mice, and progeny were screened by PCR for germ line transmission of the targeted allele. Mice were then bred with ACTB-FLP transgenic mice (44) to delete the gene trap cassette and obtain Rdh10 floxed mutants. The homozygous Rdh10 floxed mutants (Rdh10fl/fl) were viable and fertile. Rdh10 floxed mutants were then crossed with cell-specific expressed Cre mice for excising the loxP-flanked exon 2 to generate cell specific Rdh10 KO mice. All animal experiments were performed in accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals, and all protocols were approved by Washington State University’s Animal Care and Use Committee.
Histological, Immunohistochemical, and TUNEL Analyses.
Testes were fixed in Bouin’s solution, embedded in paraffin, and sectioned. Sections were deparaffinized, rehydrated, and stained with H&E. For immunohistochemical studies, slides were boiled in 10 mM sodium citrate buffer (pH 6.0) for 15 min, brought to room temperature, washed in PBS with 0.1% Triton X-100, and then incubated for 60 min at room temperature with blocking buffer (10% donkey serum, 1% BSA, and 0.1% Triton X-100 in PBS). The sections were then incubated with a 1:50 dilution of rat anti-GCNA IgM (kindly provided by Dr. G. Enders, University of Kansas, Kansas City, KS) and a 1:200 dilution of mouse anti-γH2AX IgG (Millipore), rabbit anti-STRA8 IgG, rabbit anti-PLZF (Santa Cruz Biotechnology), or rabbit anti-SOX9 IgG (Millipore), or a 1:50 dilution of rat anti-KIT (Millipore) overnight at 4 °C. The slides were then washed with PBS, and Alexa Fluor 488- and Alexa Fluor 594-conjugated donkey secondary antibody (Jackson ImmunoResearch Laboratories) were added at a 1:500 dilution. After 60 min at room temperature, the sections were washed in PBS, rinsed quickly in pure ethanol, mounted in Prolong Gold Antifade medium with DAPI (Molecular Probes), and then analyzed by fluorescence microscopy (Olympus). Apoptotic cells were detected using an In Situ Cell Death Detection Kit, Fluorescein (Roche Applied Science) according to the manufacturer’s instructions.
Retinoid Administration and Stage Analyses.
For short-term treatment, 2-wk-old animals were injected i.p. with all-trans RA, all-trans Ral, or all-trans Rol (Sigma-Aldrich; 400 μg per injection) with vehicle as controls twice in a 24-h period, and testes were collected at 24 h posttreatment for either RNA extraction or histological studies. For resuming spermatogenesis, 3-wk-old mice received one 500-μg i.p. injection of all-trans-RA, all-trans-Rol, or all-trans-Ral (Sigma-Aldrich) and were then fed a a normal diet until being killed. Testes were fixed in Bouin’s solution, embedded in paraffin, and sectioned at 5 μm. Sections were then stained with hematoxylin to identify the stages of the seminiferous epithelium according to previously established criteria (45). Stage frequency and synchronization factor were then determined using the methods described by Bianchi and Tiglao (46) and Siiteri et al. (47), respectively.
EdU Labeling.
Juvenile mice were injected i.p. with EdU (1 mg per injection; Invitrogen) in PBS. The mice were euthanized 2 h later, and testes were fixed in 4% paraformaldehyde/PBS solution, embedded in paraffin, and sectioned. After the sections were immunostained with PLZF or γH2AX, EdU incorporation was detected using the Click-It EdU Alexa Fluor 594 Imaging Kit (Invitrogen) according to the manufacturer’s protocol.
Quantitative RT-PCR Assays.
Total RNA was extracted using TRIzol reagent (Invitrogen) and treated with DNaseI (Ambion). Total RNA was reverse-transcribed using an iScript cDNA Synthesis Kit (Bio-Rad). Quantitative RT-PCR was performed using Fast SYBR Green PCR Master Mix (Applied Biosystems) on a 7500 Fast PCR System (Applied Biosystems). Relative gene expression was analyzed by the comparative CT method, using ribosomal protein S2 (Rps2) as a normalized control. RT-PCR primer sequences were described in Table S3.
X-Gal Staining.
Rdh10fl/fl, Amh-Cre+ (male) and Rdh10fl/fl, Stra8-Cre+ (female) mice were crossed with RARElacZ reporter line harboring RARE-Hspa1b-lacZ alleles to obtain Rdh10fl/+, Amh-Cre+, RARElacZ; Rdh10fl/+, Stra8-Cre+, RARElacZ; and Rdh10fl/+, RARElacZ mice (36). The Rdh10fl/+, Amh-Cre+, RARElacZ; Rdh10fl/+, Stra8-Cre+, RARElacZ (female); and Rdh10fl/+, RARElacZ mice were used to produce Rdh10fl/fl, Amh-Cre+, RARElacZ; Rdh10fl/fl, Stra8-Cre+, RARElacZ; and Rdh10fl/fl, RARElacZ mice. The Rdh10fl/fl, Amh-Cre+, RARElacZ males were bred to Rdh10fl/fl, Stra8-Cre+, RARElacZ females to generate Rdh10fl/fl, Stra8-Cre+, Amh-Cre+, RARElacZ and Rdh10fl/fl, RARElacZ mice. Testes, epididymides, and kidneys from mice bearing RARE-Hspa1b-lacZ alleles were fixed in 4% paraformaldehyde in PBS for 2 h at room temperature, washed, stained with X-Gal at 37 °C overnight, washed, and then photographed. The stained testes were then processed, embedded in paraffin, and sectioned. Sections were counterstained with fast red.
Supplementary Material
Acknowledgments
We thank Dr. G. Enders for the anti-GCNA antibody and Ms. Elizabeth B. Evans for critically reading the manuscript. This work was supported by National Institutes of Health Grants HD10808 (to M.D.G.) and HD06777 (to M.-H.T. and M.D.G.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1214883110/-/DCSupplemental.
References
- 1.Kluin PM, Kramer MF, de Rooij DG. Spermatogenesis in the immature mouse proceeds faster than in the adult. Int J Androl. 1982;5(3):282–294. doi: 10.1111/j.1365-2605.1982.tb00257.x. [DOI] [PubMed] [Google Scholar]
- 2.Bellvé AR, et al. Spermatogenic cells of the prepuberal mouse: Isolation and morphological characterization. J Cell Biol. 1977;74(1):68–85. doi: 10.1083/jcb.74.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoshida S, et al. The first round of mouse spermatogenesis is a distinctive program that lacks the self-renewing spermatogonia stage. Development. 2006;133(8):1495–1505. doi: 10.1242/dev.02316. [DOI] [PubMed] [Google Scholar]
- 4.de Rooij DG. Proliferation and differentiation of spermatogonial stem cells. Reproduction. 2001;121(3):347–354. doi: 10.1530/rep.0.1210347. [DOI] [PubMed] [Google Scholar]
- 5.Wolgemuth DJ, Chung SS. Retinoid signaling during spermatogenesis as revealed by genetic and metabolic manipulations of retinoic acid receptor alpha. Soc Reprod Fertil Suppl. 2007;63:11–23. [PMC free article] [PubMed] [Google Scholar]
- 6.Griswold MD, et al. Function of vitamin A in normal and synchronized seminiferous tubules. Ann N Y Acad Sci. 1989;564:154–172. doi: 10.1111/j.1749-6632.1989.tb25895.x. [DOI] [PubMed] [Google Scholar]
- 7.Clagett-Dame M, Knutson D. Vitamin A in reproduction and development. Nutrients. 2011;3(4):385–428. doi: 10.3390/nu3040385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson JG, Roth CB, Warkany J. An analysis of the syndrome of malformations induced by maternal vitamin A deficiency: Effects of restoration of vitamin A at various times during gestation. Am J Anat. 1953;92(2):189–217. doi: 10.1002/aja.1000920202. [DOI] [PubMed] [Google Scholar]
- 9.Wolbach SB, Howe PR. Tissue changes following deprivation of fat-soluble A vitamin. J Exp Med. 1925;42(6):753–777. doi: 10.1084/jem.42.6.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang HF, Hembree WC. Spermatogenic response to vitamin A in vitamin A-deficient rats. Biol Reprod. 1979;21(4):891–904. doi: 10.1095/biolreprod21.4.891. [DOI] [PubMed] [Google Scholar]
- 11.van Pelt AM, de Rooij DG. Synchronization of the seminiferous epithelium after vitamin A replacement in vitamin A-deficient mice. Biol Reprod. 1990;43(3):363–367. doi: 10.1095/biolreprod43.3.363. [DOI] [PubMed] [Google Scholar]
- 12.Van Pelt AM, De Rooij DG. The origin of the synchronization of the seminiferous epithelium in vitamin A-deficient rats after vitamin A replacement. Biol Reprod. 1990;42(4):677–682. doi: 10.1095/biolreprod42.4.677. [DOI] [PubMed] [Google Scholar]
- 13.Morales C, Griswold MD. Retinol-induced stage synchronization in seminiferous tubules of the rat. Endocrinology. 1987;121(1):432–434. doi: 10.1210/endo-121-1-432. [DOI] [PubMed] [Google Scholar]
- 14.Sugimoto R, Nabeshima Y, Yoshida S. Retinoic acid metabolism links the periodical differentiation of germ cells with the cycle of Sertoli cells in mouse seminiferous epithelium. Mech Dev. 2012;128(11-12):610–624. doi: 10.1016/j.mod.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 15.Kurlandsky SB, Gamble MV, Ramakrishnan R, Blaner WS. Plasma delivery of retinoic acid to tissues in the rat. J Biol Chem. 1995;270(30):17850–17857. doi: 10.1074/jbc.270.30.17850. [DOI] [PubMed] [Google Scholar]
- 16.Kane MA, Folias AE, Wang C, Napoli JL. Quantitative profiling of endogenous retinoic acid in vivo and in vitro by tandem mass spectrometry. Anal Chem. 2008;80(5):1702–1708. doi: 10.1021/ac702030f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bastien J, Rochette-Egly C. Nuclear retinoid receptors and the transcription of retinoid-target genes. Gene. 2004;328:1–16. doi: 10.1016/j.gene.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 18.Vernet N, et al. Retinoic acid metabolism and signaling pathways in the adult and developing mouse testis. Endocrinology. 2006;147(1):96–110. doi: 10.1210/en.2005-0953. [DOI] [PubMed] [Google Scholar]
- 19.Jörnvall H, et al. Short-chain dehydrogenases/reductases (SDR) Biochemistry. 1995;34(18):6003–6013. doi: 10.1021/bi00018a001. [DOI] [PubMed] [Google Scholar]
- 20.Parés X, Farrés J, Kedishvili N, Duester G. Medium- and short-chain dehydrogenase/reductase gene and protein families: Medium-chain and short-chain dehydrogenases/reductases in retinoid metabolism. Cell Mol Life Sci. 2008;65(24):3936–3949. doi: 10.1007/s00018-008-8591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rhinn M, Dollé P. Retinoic acid signalling during development. Development. 2012;139(5):843–858. doi: 10.1242/dev.065938. [DOI] [PubMed] [Google Scholar]
- 22.Duester G. Retinoic acid synthesis and signaling during early organogenesis. Cell. 2008;134(6):921–931. doi: 10.1016/j.cell.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niederreither K, Subbarayan V, Dollé P, Chambon P. Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nat Genet. 1999;21(4):444–448. doi: 10.1038/7788. [DOI] [PubMed] [Google Scholar]
- 24.Rhinn M, Schuhbaur B, Niederreither K, Dollé P. Involvement of retinol dehydrogenase 10 in embryonic patterning and rescue of its loss of function by maternal retinaldehyde treatment. Proc Natl Acad Sci USA. 2011;108(40):16687–16692. doi: 10.1073/pnas.1103877108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sandell LL, Lynn ML, Inman KE, McDowell W, Trainor PA. RDH10 oxidation of vitamin A is a critical control step in synthesis of retinoic acid during mouse embryogenesis. PLoS ONE. 2012;7(2):e30698. doi: 10.1371/journal.pone.0030698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sandell LL, et al. RDH10 is essential for synthesis of embryonic retinoic acid and is required for limb, craniofacial, and organ development. Genes Dev. 2007;21(9):1113–1124. doi: 10.1101/gad.1533407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holdcraft RW, Braun RE. Androgen receptor function is required in Sertoli cells for the terminal differentiation of haploid spermatids. Development. 2004;131(2):459–467. doi: 10.1242/dev.00957. [DOI] [PubMed] [Google Scholar]
- 28.Sadate-Ngatchou PI, Payne CJ, Dearth AT, Braun RE. Cre recombinase activity specific to postnatal, premeiotic male germ cells in transgenic mice. Genesis. 2008;46(12):738–742. doi: 10.1002/dvg.20437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45(9):593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- 30.Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 1998;273(10):5858–5868. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- 31.Enders GC, May JJ., 2nd Developmentally regulated expression of a mouse germ cell nuclear antigen examined from embryonic day 11 to adult in male and female mice. Dev Biol. 1994;163(2):331–340. doi: 10.1006/dbio.1994.1152. [DOI] [PubMed] [Google Scholar]
- 32.Romanienko PJ, Camerini-Otero RD. The mouse Spo11 gene is required for meiotic chromosome synapsis. Mol Cell. 2000;6(5):975–987. doi: 10.1016/s1097-2765(00)00097-6. [DOI] [PubMed] [Google Scholar]
- 33.Yoshida K, et al. The mouse RecA-like gene Dmc1 is required for homologous chromosome synapsis during meiosis. Mol Cell. 1998;1(5):707–718. doi: 10.1016/s1097-2765(00)80070-2. [DOI] [PubMed] [Google Scholar]
- 34.Di Carlo AD, Travia G, De Felici M. The meiotic-specific synaptonemal complex protein SCP3 is expressed by female and male primordial germ cells of the mouse embryo. Int J Dev Biol. 2000;44(2):241–244. [PubMed] [Google Scholar]
- 35.Morais da Silva S, et al. Sox9 expression during gonadal development implies a conserved role for the gene in testis differentiation in mammals and birds. Nat Genet. 1996;14(1):62–68. doi: 10.1038/ng0996-62. [DOI] [PubMed] [Google Scholar]
- 36.Rossant J, Zirngibl R, Cado D, Shago M, Giguère V. Expression of a retinoic acid response element-hsplacZ transgene defines specific domains of transcriptional activity during mouse embryogenesis. Genes Dev. 1991;5(8):1333–1344. doi: 10.1101/gad.5.8.1333. [DOI] [PubMed] [Google Scholar]
- 37.Tong MH, Mitchell DA, McGowan SD, Evanoff R, Griswold MD. Two miRNA clusters, Mir-17-92 (Mirc1) and Mir-106b-25 (Mirc3), are involved in the regulation of spermatogonial differentiation in mice. Biol Reprod. 2012;86(3):72. doi: 10.1095/biolreprod.111.096313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tong MH, Mitchell D, Evanoff R, Griswold MD. Expression of Mirlet7 family microRNAs in response to retinoic acid-induced spermatogonial differentiation in mice. Biol Reprod. 2011;85(1):189–197. doi: 10.1095/biolreprod.110.089458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clermont Y. Kinetics of spermatogenesis in mammals: Seminiferous epithelium cycle and spermatogonial renewal. Physiol Rev. 1972;52(1):198–236. doi: 10.1152/physrev.1972.52.1.198. [DOI] [PubMed] [Google Scholar]
- 40.Chen H, Namkung MJ, Juchau MR. Biotransformation of all-trans-retinol and all-trans-retinal to all-trans-retinoic acid in rat conceptal homogenates. Biochem Pharmacol. 1995;50(8):1257–1264. doi: 10.1016/0006-2952(95)02005-w. [DOI] [PubMed] [Google Scholar]
- 41.Deltour L, Haselbeck RJ, Ang HL, Duester G. Localization of class I and class IV alcohol dehydrogenases in mouse testis and epididymis: Potential retinol dehydrogenases for endogenous retinoic acid synthesis. Biol Reprod. 1997;56(1):102–109. doi: 10.1095/biolreprod56.1.102. [DOI] [PubMed] [Google Scholar]
- 42.Kumar S, Sandell LL, Trainor PA, Koentgen F, Duester G. Alcohol and aldehyde dehydrogenases: Retinoid metabolic effects in mouse knockout models. Biochim Biophys Acta. 2012;1821(1):198–205. doi: 10.1016/j.bbalip.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paik J, et al. Expression and characterization of a murine enzyme able to cleave beta-carotene: The formation of retinoids. J Biol Chem. 2001;276(34):32160–32168. doi: 10.1074/jbc.M010086200. [DOI] [PubMed] [Google Scholar]
- 44.Rodríguez CI, et al. High-efficiency deleter mice show that FLPe is an alternative to Cre-loxP. Nat Genet. 2000;25(2):139–140. doi: 10.1038/75973. [DOI] [PubMed] [Google Scholar]
- 45.Russell LD, Ettlin RA, Sinha-Hakim AP, Clegg ED, editors. Histological and Histopathological Evaluation of the Testis. Clearwater, FL: Cache River Press; 1990. [Google Scholar]
- 46.Bianchi M, Tiglao XV. Frequency of the various stages of the seminiferous epithelium in different strains of male mice. Experientia. 1976;32(4):503–504. doi: 10.1007/BF01920822. [DOI] [PubMed] [Google Scholar]
- 47.Siiteri JE, Karl AF, Linder CC, Griswold MD. Testicular synchrony: Evaluation and analysis of different protocols. Biol Reprod. 1992;46(2):284–289. doi: 10.1095/biolreprod46.2.284. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.