Abstract
Within cloud water, microorganisms are metabolically active and, thus, are expected to contribute to the atmospheric chemistry. This article investigates the interactions between microorganisms and the reactive oxygenated species that are present in cloud water because these chemical compounds drive the oxidant capacity of the cloud system. Real cloud water samples with contrasting features (marine, continental, and urban) were taken from the puy de Dôme mountain (France). The samples exhibited a high microbial biodiversity and complex chemical composition. The media were incubated in the dark and subjected to UV radiation in specifically designed photo-bioreactors. The concentrations of H2O2, organic compounds, and the ATP/ADP ratio were monitored during the incubation period. The microorganisms remained metabolically active in the presence of ●OH radicals that were photo-produced from H2O2. This oxidant and major carbon compounds (formaldehyde and carboxylic acids) were biodegraded by the endogenous microflora. This work suggests that microorganisms could play a double role in atmospheric chemistry; first, they could directly metabolize organic carbon species, and second, they could reduce the available source of radicals through their oxidative metabolism. Consequently, molecules such as H2O2 would no longer be available for photochemical or other chemical reactions, which would decrease the cloud oxidant capacity.
Keywords: biodegradation, cloud chemistry
The cloud system is an ideal medium for the development of complex multiphase chemistry, in which chemical species from the gas, solid, and aqueous phases are transformed. This process perturbs the homogeneous gas phase chemistry through the dissolution of various chemical compounds that undergo efficient photochemical processing. During a cloud’s lifetime, cloud chemistry can lead to the formation of new, low volatile compounds, such as organic and inorganic acids, that modify the physical and chemical properties of aerosols after cloud evaporation and can also contribute to the formation of secondary aerosols (1, 2). The formation of clouds is, consequently, modified, and this process remains one of the major uncertainties in climate models that assess the earth’s radiative balance (3).
Within this framework, the presence of free radicals and oxidants in the cloud system leads to aqueous phase oxidations, transforming both inorganic and organic compounds. Cloud chemistry models predict that the ●OH radicals represent the most important oxidant in the cloud aqueous phase (4). This oxidant can either be transferred from the gas phase or produced in situ in the aqueous phase through photochemical processes or related reactions with hydrogen peroxide and transition metal ions, such as iron (5). Multiple other oxidants that are produced in clouds can also oxidize chemicals, and these oxidation processes must be better understood because they impact atmospheric chemical cycles and radiation. Indeed, the resulting aerosols increase or decrease the scattering albedo, thus modifying the radiative forcing by clouds.
Many volatile organic compounds from secondary formations are associated with moderately high Henry’s law constants and are, consequently, dissolved into the tropospheric aqueous phase (6). Additionally, organic compounds constitute a significant mass fraction of tropospheric aerosol particles, which can also be transferred into cloud water. Hence, the dissolved organic matter is able to interact directly or indirectly with the aqueous chemistry of radicals, radical anions, nonradical oxidants, and transition metal ions. A large proportion of the dissolved organic matter is still not characterized, but carboxylic acids could represent a significant proportion of this soluble matter. Among these acids, the formic and acetic acids are the most abundant (mainly produced in the gaseous phase), oxalic acid is commonly the third dominant species and the main di-carboxylic acid, followed by succinic, malonic, and maleic acids (predominantly dissolved from organic particles) (7–10).
Cloud water also hosts microbial populations that are primary biological aerosols and the dominant living aerosols that are present in the atmosphere (11–14). These aerosols can be integrated into clouds because they can serve as cloud condensation nuclei for droplet formation (13–15). This environment is stressful for airborne microorganisms (low temperature, desiccation, oxidation, UV radiation, acidic pH in the aqueous phase, and so forth) (13, 16). Low temperatures appear to represent one of the major obstacles for cellular activity because they are directly linked to decreased molecular motion and reaction rates. However, bacteria can sustain growth in cloud water at temperatures at or below 0 °C (17), and they are capable of maintaining metabolic activity at subzero temperatures down to −20 °C (18–20). Cultivable microorganisms (fungal spores, yeasts, and bacteria) have been found in fog and cloud water (21–23). Bauer et al. demonstrated that the majority of bacteria that are present in cloud water are viable (up to 95% in two samples) (21). The ATP concentrations in cloud water that were measured by Amato et al. (24) suggest that a significant fraction of the microorganisms that are present in these environments are metabolically active.
The discovery of microbial activity in this environment has indicated that there are biologically mediated processes in the chemistry of clouds. In the recent past, researchers investigated the microbial activity in cloud water containing organic compounds, such as carboxylic acids, formaldehyde, and methanol (25, 26). Inferred estimates indicated that the activity of microorganisms was likely to affect the chemistry of these compounds in warm clouds and could even drive their reactivity during the night (27–29).
All of these studies are pertinent, but they were conducted under conditions that were different from those in real clouds. In particular, the presence of reactive oxygenated species, such as hydrogen peroxide (H2O2) and free radicals, was ignored. Although these compounds are toxic to cell life, the survival of microorganisms in clouds strongly suggests that cloud-borne microorganisms can resist the high concentrations that are found in the atmosphere. This result is likely to be because of the efficient, antioxidative stress metabolism that involves specialized enzymes (such as catalases, peroxidases, and superoxide dismutase) and nonenzymatic compounds (30, 31).
We investigated the metabolic activity of microorganisms in microcosms that were more similar to the real cloud environment. We used cloud water samples that were collected at the puy de Dôme mountain, which is a reference site in France for cloud observations and is part of the European ACTRIS (Aerosols, Clouds, and Trace gases Research InfraStructure Network) project. The samples contained complex mixtures of organic and inorganic compounds, oxidants, such as iron complexes and H2O2, and the endogenous microflora. The cloud water samples were either kept intact or were sterilized by filtration and were then incubated in the dark or subjected to UV-light radiation in specially designed photo-bioreactors (Fig. S1). This procedure resulted in the separation of biologically driven processes and other chemical phenomena (including photochemistry) that occurred in the cloud water under controlled conditions. The biological and chemical characterizations were conducted throughout the incubation. Under these conditions, the microorganisms were exposed to H2O2 and potential ●OH radical photoproduction. This study had four specific objectives: (i) to study the possible biodegradation processes of H2O2; (ii) to determine the energetic state (ADP/ATP ratios) of cells under these stressful conditions; (iii) to investigate the impact of the presence of reactive oxygenated species on the biodegradation rates of organic acids (acetate, formate, oxalate, malonate, and succinate) and formaldehyde; and (iv) to compare abiotic and biotic processes.
The major result of this work helps to answer two questions: (i) Do microorganisms interact with reactive oxygenated species? and (ii) Consequently, does microbial activity control the oxidant capacity and the organic carbon budget in natural clouds?
Results and Discussion
Three cloud events were sampled in June 2010 at puy de Dôme mountain (1,465 m above sea level). The backward trajectories from the National Oceanic and Atmospheric Administration Hysplit model were plotted for the various sampled air-masses and are displayed in Fig. S2. Three cloud types were selected for their contrasting features: cloud 1 had a northwestern marine origin, cloud 2 was from the continental southwest, and cloud 3 was from the continental northeastern flux but was influenced by anthropogenic emissions. The average temperatures during sampling were 10 °C for clouds 1 and 3 and 13.5 °C for cloud 2 (see Table 1 for additional physico-chemical parameters).
Table 1.
Initial bio-physico-chemical characteristics for the three cloud events, sampled at the puy de Dôme station
| Characteristic | Cloud 1 | Cloud 2 | Cloud 3 |
| Air-mass origin | Northwestern | Southwestern | Northeastern |
| Air-mass type | Marine | Continental | Urban |
| Date of sampling | 6/1/10 8:20 PM | 6/8/10 12:05 PM | 6/18/10 11:15 AM |
| Duration of sampling | 6:30 | 11:20 | 19:45 |
| Temperature | 10 °C | 13.5 °C | 10 °C |
| pH | 6.1 | 5.2 | 3.9 |
| Conductivity (µS⋅cm−1) | 3.5 | 37.6 | 78.6 |
| TOC (DOC) (mg⋅L−1) | 1.1 (1.1) | 6.8 (6.7) | 6.9 (6.8) |
| Compound | Concentration (µM) | ||
| Acetate | 4.5 | 25.4 | 23.2 |
| Formate | 4.9 | 42.7 | 33.2 |
| Succinate | — | 3.1 | 3.8 |
| Oxalate | 1.0 | 9.7 | 9.3 |
| Malonate | — | 3.1 | 3.5 |
| Cl− | 3.0 | 7.7 | 11.3 |
| NO3− | 4.5 | 70.6 | 228.7 |
| SO42- | 1.8 | 46.1 | 64.0 |
| Na+ | 2.2 | 10.1 | 8.8 |
| NH4+ | 8.5 | 100.3 | 122.3 |
| K+ | — | 1.5 | 2.2 |
| Mg2+ | 1.0 | 2.1 | 2.7 |
| Ca2+ | 1.7 | 3.8 | 3.8 |
| Fe (total) | 0.9 | 1.1 | 1.3 |
| Fe (II) | 0.3 | 0.5 | 0.5 |
| Formaldehyde | 1.5 | 2.7 | 6.1 |
| H2O2 | 3.6 | 33.4 | 57.7 |
| ATP (pmol⋅mL−1) | 0.8 | 2.3 | 2.1 |
| ADP (pmol⋅mL−1) | 1.1 | 0.7 | 1.1 |
| ADP/ATP ratio | 1.4 | 0.3 | 0.5 |
| Total fungal spores and yeasts (cells/mL−1) | 9 × 103 | 3 × 103 | 3 × 103 |
| Total bacteria (cells/mL−1) | 3 × 104 | 8 × 104 | 9 × 104 |
DOC, dissolved organic carbon; TOC, total organic carbon.
Chemical and Biological Content of Cloud Water Samples.
The chemical and biological data from the three cloud water samples are summarized in Table 1. The chemical properties of these three samples are consistent with their respective origins. For example, cloud 3, collected from an “urban” air-mass, was more acidic and oxidant (pH = 3.9 and [H2O2] = 57.7 µM) than cloud 1, which had a “marine” origin (pH = 6.1 and [H2O2] = 3.6 µM). Cloud 2, from the “continental” air-mass, represented an intermediate condition.
The five most abundant carboxylic acids that are usually found in cloud water [i.e., formic, acetic, oxalic, succinic, and malonic acids (7–9)] and the most abundant aldehyde (i.e., formaldehyde) were present in clouds 2 and 3, but succinic and malonic acids were not detected in cloud 1. The contribution of carboxylic acids and formaldehyde to dissolved organic carbon was ∼19%, 25%, and 23% in clouds 1, 2, and 3, respectively. The total number of microbial cells was of the same order of magnitude as the typical previous measurements at the puy de Dôme site (23, 32) and was very similar to samples from Mt. Rax in Austria (1,644 m above sea level) (21).
Hydrogen Peroxide Biotransformation in Real Cloud Water Microcosms.
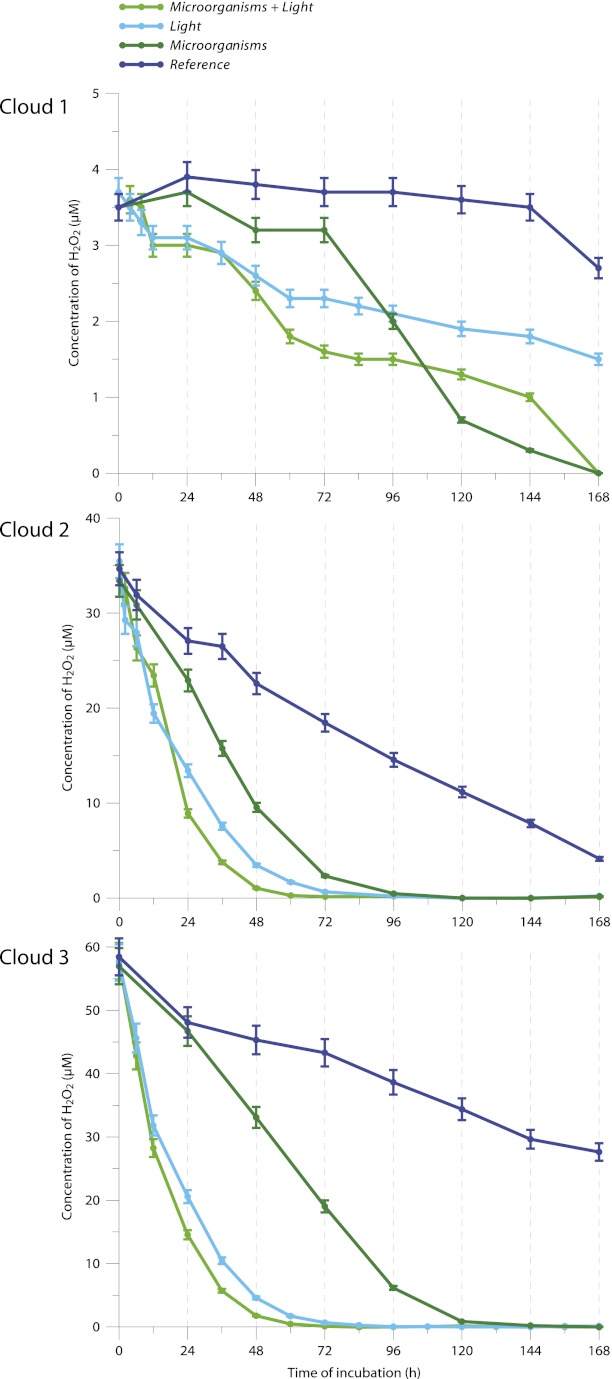
The three cloud water samples were incubated at 17 °C under four incubation regimes: unfiltered and in the presence or absence of UV radiation (“Microorganisms + Light” and “Microorganisms,” respectively) and filtered and in the presence or absence of UV radiation (“Light” and “Reference,” respectively). H2O2 concentrations were measured periodically over the incubation period and are plotted in Fig. 1; the corresponding degradation rates are reported in Table 2.
Fig. 1.
Temporal evolution of H2O2 concentrations (µM) in the presence or absence of UV light or microorganisms during incubation of cloud water (clouds 1, 2, 3). Cloud water samples were incubated at 17 °C under four incubation regimes for 7 d: unfiltered and in the presence or absence of UV radiation (Microorganisms + Light and Microorganisms, respectively), filtered and in the presence or absence of UV radiation (Light and Reference, respectively). Error bars represent the SEs of the enzymatic assay (5%).
Table 2.
Initial degradation rates of H2O2 in the presence and absence of UV light or microorganisms during the incubation of natural cloud waters
| Incubation regime | Cloud 1 |
Cloud 2 |
Cloud 3 |
| Rate of H2O2 transformation (× 10−11 M⋅s−1) | |||
| Reference* | 0 | −9.8 | −7.2 |
| Light | −0.6 | −48.6 | −105.1 |
| Microorganisms | −2.9 (72 h to end) | −28.5 | −30.0 |
| Microorganisms + Light | −0.9 | −68.4 | −126.9 |
A negative value indicates the disappearance of H2O2 from the medium. In the case where a noncontinuous transformation occurred, the time period used for the linear regression is indicated in brackets.
*Reference: sterilized sample (filtration 0.22 µm) incubated in darkness.
In the absence of UV radiation in filtered cloud water (Reference), a slow degradation of H2O2 was observed in clouds 2 and 3. This phenomenon can be explained by the reactivity of H2O2 with chemical species, such as transition metal ions (a.k.a. “Fenton reactions”) or sulphite (33, 34). The zero-order kinetic constants were the same for the two clouds, most likely because of the very similar iron concentrations. In cloud 1, the degradation of H2O2 was even slower, most likely because the species responsible for the radical reactions (S and Fe) were present at lower concentrations than in clouds 2 and 3 (Table 1). Under UV light, the first-order kinetics had similar constants, indicating that H2O2 was photolyzed, producing ●OH radicals. Interestingly, in the presence of microorganisms without UV light, H2O2 was also efficiently degraded in cloud 2 (vc = 28.5 × 10−11 M⋅s−1) and cloud 3 (vc = 30.0 × 10−11 M⋅s−1). In cloud 1, the microbial degradation of H2O2 was very slow until 72 h but then increased (vc = 2.9 × 10−11 M⋅s−1). In darkness, the presence of microorganisms enhanced the rate of H2O2 degradation by a factor of 2.9 and 4.2 for clouds 2 and 3, respectively. Combining UV light and microorganisms resulted in higher degradation rates for H2O2 than either individual condition alone. For clouds 2 and 3, the degradation rates for H2O2 appeared to be additive: Light and Microorganisms ∼ Microorganisms + Light (Table 2). These results demonstrate that cloud microorganisms can metabolize H2O2 into O2 and H2O using catalases, which are ubiquitous oxidoreductase enzymes.
Furthermore, it was possible to quantify the relative impact of biotic activity vs. abiotic H2O2 transformations (Fig. S3 and Tables S1 and S2). During the day, three types of H2O2 degradation mechanisms were active in clouds 2 and 3. Photodegradation was the major process (54% and 76%, respectively), followed by biodegradation (30% and 18%, respectively). Other abiotic reactions accounted for 16% in cloud 2 and 6% in cloud 3. In cloud 1, only light was involved initially. During the night, photodegradation was no longer involved, and microbial activity was the major process, accounting for 66% (cloud 2) and 76% (cloud 3) of H2O2 degradation; nonphotochemical abiotic reactions only accounted for 34% (cloud 2) and 24% (cloud 3). These reactions were too slow to be measured in cloud 1 during the first few hours. Clearly, the impact of microbial activity controlling the oxidant capacity in warm clouds may be important, particularly at night, when it may be dominant.
In addition, the results obtained during the combined photo-biodegradation processes indicate that photoproduced radicals are not toxic to cloud microflora; this statement is consistent with the oxidative stress metabolism of microorganisms. This type of metabolism involves not only catalases but also peroxidases and superoxide dismutase, as well as other nonenzymatic antioxidants (31).
Microbial Energetic States in Real Cloud Water Microcosms.
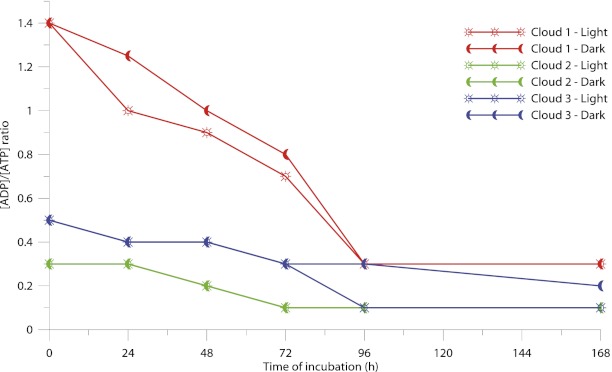
In the previous section, we noted that microorganisms were exposed to photoproduced radicals, which are known to be potentially toxic to microbial cells. Therefore, we investigated the ability of cloud microorganisms to resist these oxidative stresses by quantifying the ADP/ATP ratios during the same incubations. Essentially, growing bacteria present a ratio of ∼0.25, whereas dead cells have a ratio >6 (35). The plots presented in Fig. 2 reflect the evolution of the energetic states of these microorganisms over time.
Fig. 2.
[ADP]/[ATP] ratios of microbial cells in the presence and absence of UV light during the incubation of unfiltered cloud water samples (clouds 1, 2, 3).
In all cases, the microbial energetic state improved over time as the ADP/ATP ratios decreased. The microorganisms in clouds 2 and 3 had similar initial energetic states (ADP/ATP ratios of 0.3 and 0.5, respectively) that decreased slightly over the incubation period and reached 0.1 and 0.2, respectively, after 72 h. The initial energetic state of bacteria from cloud 1 was lower (ADP/ATP ratio 1.4) than the other cloud samples. This difference could be because of some specific, unfavorable stress that was encountered by the microorganisms during the air-mass history. The ADP/ATP ratio increased over time and attained an energetic state that was similar to clouds 2 and 3 after 72 h. This recovery of a higher energetic state after 72 h reflects an activation of the microbial metabolism. Consequently, the biodegradation rate of H2O2 increased, as displayed in Fig. 1 (cloud 1), when microorganisms were present in the incubation medium.
The ADP/ATP ratios during the microbial incubations under dark and UV light conditions were similar. Clearly, photodegradation in the presence of H2O2, notably the production of ●OH radicals under light conditions, did not affect the cells’ energy metabolism. This result is in agreement with the additive nature of the photochemical and biological processes that are involved in H2O2 degradation (Table 2).
Impact of the Presence of Reactive Oxygen Species on the Biotransformation of Carboxylic Acids and Formaldehyde in Real Cloud Water Microcosms.
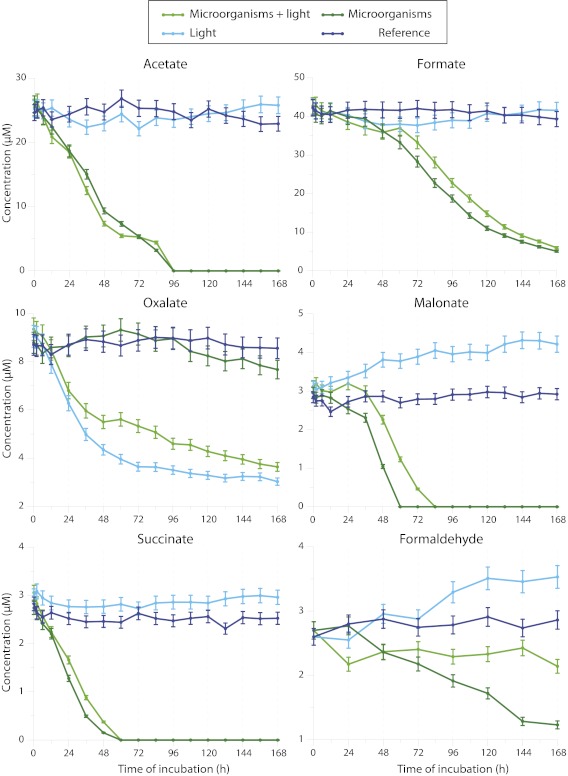
Formate, acetate, succinate, oxalate, malonate, and formaldehyde were also measured in the four incubation regimes that were used for H2O2. Focusing on cloud 2, the evolution of the concentrations of carboxylic acids and formaldehyde are plotted in Fig. 3, and the production and degradation rates of carboxylic acids and formaldehyde are presented in Table 3. Because they are very similar to cloud 2, the results for the other clouds are presented in Figs. S4 and S5. Acetate, formate, and succinate were only degraded in the presence of microorganisms, and oxalate was only degraded in the presence of UV light. Malonate and formaldehyde were photo-produced during the experiment and degraded in the presence of microorganisms. The biotransformation was not delayed for acetate, succinate, malonate, and formaldehyde, but in the case of formate we observed a lag time (up to 48 h) before the biodegradation began. The degradation of oxalate by UV light in the absence of microorganisms indicated that photochemical reactions were involved. This conclusion is consistent with the concomitant H2O2 photolysis under the same conditions. It is also important to note that the degradation rates in the Reference sample were close to zero, demonstrating that the major processes were photodegradation and biodegradation.
Fig. 3.
Temporal evolution of carboxylic acids and formaldehyde concentrations during the incubation of cloud 2. The cloud 2 water sample was incubated at 17 °C under four incubation regimes for 7 d: unfiltered and in the presence or absence of UV radiation (Microorganisms + Light and Microorganisms, respectively), filtered and in the presence or absence of UV radiation (Light and Reference, respectively). Error bars represent the SEs of the chemical analysis (5%).
Table 3.
Initial transformation rates of carboxylic acids and formaldehyde in the presence and absence of UV light and/or microorganisms during the incubation of cloud 2
| Acetate |
Formate |
Succinate |
Oxalate |
Malonate |
Formaldehyde |
|
| Incubation regime | Rate of transformation (× 10−11 M⋅s−1) | |||||
| Reference* | 0 | 0 | 0 | 0 | 0 | 0 |
| Light | 0 | 0 | 0 | −4.0 (0 h to 60 h) | 0.3 (0 h to 60 h) | 0.2 |
| Microorganisms | −15.5 | −17.5 (48 h to end) | −4.5 | 0 | −4.2 (36 h to end) | −0.3 |
| Microorganisms + Light | −15.6 | −16.1 (48 h to end) | −3.5 | −2.7 (0 h to 60 h) | −4.3 (36 h to end) | 0 |
A negative value indicates the disappearance of the organic compounds from the medium. Values in bold represent production of the compounds in question. In the case where a noncontinuous transformation occurred, the time period used for the linear regression is indicated in parenthesis.
* Reference: sterilized sample (filtration 0.22 µm) incubated in darkness.
The most interesting results clearly show that the endogenous microflora were not inhibited by the presence of reactive oxygen species (H2O2 and photoproduced ●OH radicals). In the case of acetate, formate, and succinate, the degradation rates that were measured for Microorganisms (15.5, 17.5, and 4.5 × 10−11 M⋅s−1, respectively) and Microorganisms + Light (15.6, 16.1, and 3.5 × 10−11 M⋅s−1, respectively) were very similar. Therefore, the presence of light, and thus of ●OH radicals, had no influence on microbial carbon metabolism. In the case of formaldehyde, the photoproduction rate was 0.2 × 10−11 M⋅s−1 (Light), and the biodegradation rate (Microorganisms) was 0.3 × 10−11 M⋅s−1. When the two processes were combined (Microorganisms + Light), the resulting rate of transformation was null, indicating that the addition of the processes occurred without any inhibition. The case of malonate is rather similar to that of formaldehyde; the rate observed during the combined photo-biodegradation processes after 36 h (4.3 × 10−11 M⋅s−1) corresponded to the addition of the photoproduction rate (0.3 × 10−11 M⋅s−1) and the biodegradation rate (4.2 × 10−11 M⋅s−1). The conclusions related to cloud 2 can be extended to clouds 1 and 3 (see comments in the SI Text); they are straightforward in the case of cloud 3, but the case of cloud 1 is more complex to interpret. This difficulty in interpretation could be because of the unusually low concentration of organic and inorganic compounds in cloud 1 (Table 1) and to the lower initial energetic state of the microorganisms (Fig. 2), which explains why biodegradation only began after 72 h. Global comments about the three clouds are provided in the SI Text and are related to Figs. S4 and S5 and Table S3, which lists all of the transformation rates.
The principle of the noninhibition of reactive oxygen species to the biodegradation process was observed in all of the study clouds, and the addition of the various photo- and biotransformation rates remains valid (SI Text). This result has major consequences when considering the impact of microbial activity on carbon budgets; it suggests that microorganisms could play a role not only in cloud chemistry at night, as previously indicated (27–29), but also during the daytime, when ●OH radicals are photoproduced. The implication of microorganisms in carbon flux in the atmosphere at the global scale was estimated (SI Text and Table S4). This rough calculation results in a global release of 51–215 million tons of CO2 per year through microbial respiration.
Conclusions
We studied microbial activity in real cloud samples that represent the three major categories of air-masses on the puy de Dôme mountain (marine, continental, and urban origins). Their physical and chemical compositions thus represented varied experimental scenarios, and the endogenous microflora in each sample were likely to be different. Indeed, the microbial composition of cloud waters greatly depends on the sources of microbial aerosolization (vegetation, oceans, urban areas, and so forth) that are present on the air-mass trajectories and also on the chemical composition of clouds, which can favor the survival of some species over others (11). During their transport over long distances, these microorganisms have been subjected to numerous stresses, including evaporation-condensation cycles; nevertheless, they remained active, as shown by their good energetic states (ADP/ATP ratios range to 0.3–1.4) and their efficiency at biodegradation. Microorganisms can adapt very easily to changing conditions and stresses in the atmosphere, as observed in other extreme environments (36).
Our experiments were conducted in innovative microcosms that were designed to mimic more realistic environmental cloud conditions. Using unfiltered samples in a homemade photo-bioreactor, we investigated the activities of microorganisms that were exposed to UV light and H2O2, which is a major source of ●OH radicals in cloud waters. The experiments that combined both the photo- and biodegradation processes were compared with experiments with biodegradation (absence of UV light) or photodegradation alone (filtered sample).
First, our results indicate that the microorganisms that were present in the cloud samples and exposed to UV light remained metabolically active in the presence of ●OH radicals that were photoproduced from H2O2 because of the oxidative stress metabolism of the cells. This phenomenon is clearly demonstrated by the similar ADP/ATP ratios that were measured when the microorganisms were exposed or not exposed to UV light, indicating that the microbial energetic state was unchanged. This result was also clearly demonstrated by the degradation rates under combined conditions (Microorganisms + Light), where photodegradation and biodegradation are additive processes. There was no inhibition of microbial activities toward the organic, biodegradable compounds (acetate, formate, succinate, malonate, and formaldehyde) that were tested in the presence of reactive oxygen species. This information is particularly important when considering the potential role of microorganisms in cloud chemistry and the resulting carbon balance. Previously, studies were conducted in the absence of such reactive oxygen species, and the resulting biodegradation rates were subject to debate. The results obtained here reinforce the hypothesis that the actual activity of microorganisms in clouds is an alternative route in photochemistry. These two transformation processes could coexist in cloud droplets and be modulated by the bio-physico-chemical conditions that are encountered in natural warm clouds.
Second, and most importantly, we have demonstrated that H2O2, a precursor to oxidant species in clouds, is biodegraded by the endogenous microflora through the actions of catalases. To our knowledge, this report of such an effect in the atmospheric environment is unique. Moreover, the biodegradation process is significant compared with the photochemical process. This finding has major consequences for atmospheric chemistry because it shows that microorganisms may have an impact on the oxidant capacity of clouds. This concept is clearly unique and should be considered in much greater detail.
However, we are aware that our microcosms are still unlike real cloud systems, which are polydisperse, with highly variable spatial and temporal parameters. However, this work suggests that microorganisms could play a double role in atmospheric chemistry and, more specifically, on the carbon budget of the atmosphere. First, they could directly metabolize organic carbon species. Second, they could destroy a portion of the source of radicals because of their oxidative metabolism, and as a result, these molecules, such as H2O2, would no longer be available for photochemical or other chemical reactions.
Materials and Methods
Three cloud water samples from three different origins were collected at the puy de Dôme station in 2010 and were analyzed chemically and biologically. The samples were incubated in photo-bioreactors at 17 °C under four incubation regimes for 7 d: unfiltered and in the presence or absence of UV radiation (Microorganisms + Light and Microorganisms, respectively), and filtered and in the presence or absence of UV radiation (Light and Reference, respectively). We recorded the concentrations of formate, acetate, oxalate, succinate, malonate, formaldehyde, H2O2, and the ADP/ATP ratio during the incubation period. Additional details about the methodology are provided in SI Text.
Supplementary Material
Acknowledgments
We thank the French Ministry of Research for PhD scholarships, Bruce Moffett for correcting the manuscript, and C. Bernard and M. Ribeiro for technical support. This research was funded by the Centre National de la Recherche Scientifique/Institut National des Sciences de l’Univers and the French Ministry of Research under the Les Enveloppes Fluides et l'Environnement – Chimie Atmosphérique (LEFE-CHAT) program.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1205743110/-/DCSupplemental.
References
- 1.Lim YB, Tan Y, Perri MJ, Seitzinger SP, Turpin BJ. Aqueous chemistry and its role in secondary organic aerosol (SOA) formation. Atmos Chem Phys. 2010;10(21):10521–10539. [Google Scholar]
- 2.Ervens B, Turpin BJ, Weber RJ. Secondary organic aerosol formation in cloud droplets and aqueous particles (aqSOA): A review of laboratory, field and model studies. Atmos Chem Phys. 2011;11(21):11069–11102. [Google Scholar]
- 3.Solomon S, et al., editors. Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK, and New York, NY: Cambridge University Press; 2007. Contribution of Working Group I. [Google Scholar]
- 4.Herrmann H, Hoffmann D, Schaefer T, Bräuer P, Tilgner A. Tropospheric aqueous-phase free-radical chemistry: Radical sources, spectra, reaction kinetics and prediction tools. ChemPhysChem. 2010;11(18):3796–3822. doi: 10.1002/cphc.201000533. [DOI] [PubMed] [Google Scholar]
- 5.Deguillaume L, et al. Transition metals in atmospheric liquid phases: Sources, reactivity, and sensitive parameters. Chem Rev. 2005;105(9):3388–3431. doi: 10.1021/cr040649c. [DOI] [PubMed] [Google Scholar]
- 6.Sander R. Henry's Law Constants. In: Linstrom PJ, Mallard WG, editors. NIST Chemistry WebBook. Gaithersburg MD: National Institute of Standards and Technology; 1999. Available at http://webbook.nist.gov. Accessed December 13, 2012. [Google Scholar]
- 7.Chebbi A, Carlier P. Carboxylic acids in the troposphere, occurrence, sources, and sinks: A review. Atmos Environ. 1996;30(24):4233–4249. [Google Scholar]
- 8.Löflund M, et al. Formic, acetic, oxalic, malonic and succinic acid concentrations and their contribution to organic carbon in cloud water. Atmos Environ. 2002;36(9):1553–1558. [Google Scholar]
- 9.Marinoni A, Laj P, Sellegri K, Mailhot G. Cloud chemistry at the Puy de Dôme: Variability and relationships with environmental factors. Atmos Chem Phys. 2004;4(3):715–728. [Google Scholar]
- 10.Legrand M, et al. Origin of C2-C5 dicarboxylic acids in the European atmosphere inferred from year-round aerosol study conducted at a west-east transect. J Geophys Res. 2007;112(D23):D23S07. [Google Scholar]
- 11.Burrows SM, Elbert W, Lawrence MG, Pöschl U. Bacteria in the global atmosphere—Part 1: Review and synthesis of literature data for different ecosystems. Atmos Chem Phys. 2009;9(3):10777–10827. [Google Scholar]
- 12.Womack AM, Bohannan BJM, Green JL. Biodiversity and biogeography of the atmosphere. Philos Trans R Soc Lond B Biol Sci. 2010;365(1558):3645–3653. doi: 10.1098/rstb.2010.0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delort A-M, et al. A short overview of the microbial population in clouds: Potential roles in atmospheric chemistry and nucleation processes. Atmos Res. 2010;98(2–4):249–260. [Google Scholar]
- 14.Després VR, et al. Primary biological particles in the atmosphere: A review. Tellus B Chem Phys Meterol. 2012;64:1–58. [Google Scholar]
- 15.Pöschl U, et al. Rainforest aerosols as biogenic nuclei of clouds and precipitation in the Amazon. Science. 2010;329(5998):1513–1516. doi: 10.1126/science.1191056. [DOI] [PubMed] [Google Scholar]
- 16.Jones AM, Harrison RM. The effects of meteorological factors on atmospheric bioaerosol concentrations—A review. Sci Total Environ. 2004;326(1–3):151–180. doi: 10.1016/j.scitotenv.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 17.Sattler B, Puxbaum H, Psenner R. Bacterial growth in supercooled cloud droplets. Geophys Res Lett. 2001;28(2):239–242. [Google Scholar]
- 18.Christner BC. Incorporation of DNA and protein precursors into macromolecules by bacteria at −15 ° C. Appl Environ Microbiol. 2002;68(12):6435–6438. doi: 10.1128/AEM.68.12.6435-6438.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Junge K, Eicken H, Swanson BD, Deming JW. Bacterial incorporation of leucine into protein down to −20 ° C with evidence for potential activity in sub-eutectic saline ice formations. Cryobiology. 2006;52(3):417–429. doi: 10.1016/j.cryobiol.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Rivkina EM, Friedmann EI, McKay CP, Gilichinsky DA. Metabolic activity of permafrost bacteria below the freezing point. Appl Environ Microbiol. 2000;66(8):3230–3233. doi: 10.1128/aem.66.8.3230-3233.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bauer H, et al. The contribution of bacteria and fungal spores to the organic carbon content of cloud water, precipitation and aerosols. Atmos Res. 2002;64(1–4):109–119. [Google Scholar]
- 22.Ahern HE, Walsh KA, Hill TCJ, Moffett BF. Fluorescent pseudomonads isolated from Hebridean cloud and rain water produce biosurfactants but do not cause ice nucleation. Biogeosciences. 2007;4(1):115–124. [Google Scholar]
- 23.Vaïtilingom M, et al. Long-term features of cloud microbiology at the puy de Dôme (France) Atmos Environ. 2012;56:88–100. [Google Scholar]
- 24.Amato P, et al. An important oceanic source of micro-organisms for cloud water at the Puy de Dôme (France) Atmos Environ. 2007;41(37):8253–8263. [Google Scholar]
- 25.Amato P, et al. A fate for organic acids, formaldehyde and methanol in cloud water: Their biotransformation by microorganisms. Atmos Chem Phys. 2007;7(15):4159–4169. [Google Scholar]
- 26.Ariya PA, Nepotchatykh O, Ignatova O, Amyot M. Microbiological degradation of atmospheric organic compounds. Geophys Res Lett. 2002;29(22):2077–2081. [Google Scholar]
- 27.Husárová S, et al. Biotransformation of methanol and formaldehyde by bacteria isolated from clouds. Comparison with radical chemistry. Atmos Environ. 2011;45(33):6093–6102. [Google Scholar]
- 28.Vaïtilingom M, et al. Contribution of microbial activity to carbon chemistry in clouds. Appl Environ Microbiol. 2010;76(1):23–29. doi: 10.1128/AEM.01127-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vaïtilingom M, et al. Atmospheric chemistry of carboxylic acids: Microbial implication versus photochemistry. Atmos Chem Phys. 2011;11(16):8721–8733. [Google Scholar]
- 30.Kreiner M, Harvey LM, McNeil B. Oxidative stress response of a recombinant Aspergillus niger to exogenous menadione and H2O2 addition. Enzyme Microb Technol. 2002;30(3):346–353. [Google Scholar]
- 31.Sigler K, Chaloupka J, Brozmanová J, Stadler N, Höfer M. Oxidative stress in microorganisms—I. Microbial vs. higher cells—Damage and defenses in relation to cell aging and death. Folia Microbiol (Praha) 1999;44(6):587–624. doi: 10.1007/BF02825650. [DOI] [PubMed] [Google Scholar]
- 32.Amato P, et al. Microbial population in cloud water at the Puy de Dôme: Implications for the chemistry of clouds. Atmos Environ. 2005;39(22):4143–4153. [Google Scholar]
- 33.Gunz DW, Hoffmann MR. Atmospheric chemistry of peroxides: A review. Atmos Environ, A Gen Topics. 1990;24(7):1601–1633. [Google Scholar]
- 34.Deguillaume L, Leriche M, Monod A, Chaumerliac N. The role of transition metal ions on HOx radicals in clouds: A numerical evaluation of its impact on multiphase chemistry. Atmos Chem Phys. 2004;4(1):95–110. [Google Scholar]
- 35.Koutny M, et al. Acquired biodegradability of polyethylenes containing pro-oxidant additives. Polym Degrad Stabil. 2006;91(7):1495–1503. [Google Scholar]
- 36.Roszak DB, Colwell RR. Survival strategies of bacteria in the natural environment. Microbiol Rev. 1987;51(3):365–379. doi: 10.1128/mr.51.3.365-379.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.