Abstract
Diabetic peripheral neuropathy (DPN) is the most common complication in both type 1 and type 2 diabetes. Here we studied some phenotypic features of a well-established animal model of type 2 diabetes, the leptin receptor-deficient db−/db− mouse, and also the effect of long-term (6 mo) treatment with coenzyme Q10 (CoQ10), an endogenous antioxidant. Diabetic mice at 8 mo of age exhibited loss of sensation, hypoalgesia (an increase in mechanical threshold), and decreases in mechanical hyperalgesia, cold allodynia, and sciatic nerve conduction velocity. All these changes were virtually completely absent after the 6-mo, daily CoQ10 treatment in db−/db− mice when started at 7 wk of age. There was a 33% neuronal loss in the lumbar 5 dorsal root ganglia (DRGs) of the db−/db− mouse versus controls at 8 mo of age, which was significantly attenuated by CoQ10. There was no difference in neuron number in 5/6-wk-old mice between diabetic and control mice. We observed a strong down-regulation of phospholipase C (PLC) β3 in the DRGs of diabetic mice at 8 mo of age, a key molecule in pain signaling, and this effect was also blocked by the 6-mo CoQ10 treatment. Many of the phenotypic, neurochemical regulations encountered in lumbar DRGs in standard models of peripheral nerve injury were not observed in diabetic mice at 8 mo of age. These results suggest that reactive oxygen species and reduced PLCβ3 expression may contribute to the sensory deficits in the late-stage diabetic db−/db− mouse, and that early long-term administration of the antioxidant CoQ10 may represent a promising therapeutic strategy for type 2 diabetes neuropathy.
Keywords: neurodegeneration, neuropeptide, electrophysiology
Diabetic peripheral neuropathy (DPN) is a debilitating complication of both type 1 and type 2 diabetes, affecting up to 50% of diabetes patients (1–5). DPN can be observed early in human diabetes (6, 7). Early neurophysiological studies indicated that at initial stages of the illness, the severity of the polyneuropathy was similar in type 1 and type 2 diabetes patients (8). However, more recent studies showed distinct differences both in humans and rodent models. Thus, progressive axonal atrophy and loss are more serious in type 1 diabetes, which in contrast to type 2 shows nodal and paranodal degenerative changes, as well as more severe downstream effects on neuroskeletal and adhesive proteins (9).
In type 1 and type 2 diabetes, many patients initially experience painful diabetic neuropathy, allodynia, which subsides and then is replaced by loss of sensation, hypoalgesia (5, 10, 11). A similar course of disease has been noted in the db−/db− mouse (12), whose diabetic phenotype was originally discovered by Hummel et al. (13) (SI Text). The db−/db− mouse, just one of many animal models of diabetes (SI Text), has since then been extensively studied as a particularly robust model for type 2 DPN (14).
Previous studies of mammalian DPN models have reported a decrease in impulse conduction velocity (CV) in several peripheral nerves, interpreted as being caused by a reduction in number of large or medium-sized axons (15–20), demyelination (18, 19, 21–23), or axon shrinkage/atrophy (24, 25). Unmyelinated and myelinated fibers in the skin are also affected (26). Zochodne et al. (27) raised the question as to whether diabetes also targets sensory neuronal cell bodies. However, the authors only observed few changes in markers in dorsal root ganglia (DRGs) in 12-mo-old streptotozin (STZ)-treated rats, later confirmed (28, 29). Thus, the mechanisms behind DPN seem different from the dramatic phenotypic changes seen in neuronal somata in DRGs after various types of peripheral nerve injury (30–32).
DPN develops as a result of hyperglycemia, hypoxia, and inflammation, all conditions that cause increased production of reactive oxygen species. The pathogenic mechanism is attributed mainly to oxidative stress and also impaired insulin and insulin growth factor (IGF) activity (33–35). In agreement, the db−/db− mouse has severe hyperglycemia, inflammation secondary to hyperlipidemia and obesity, and express markers of oxidative stress with lower IGF-I activity, reduced IGF-I levels, and increased IGF binding protein-I (2, 33–35).
Coenzyme Q10 (CoQ10) is a well-known antioxidant and has bioenergetic and anti-inflammatory effects, and protects against apoptosis of neurons (36–40) and should in principle represent a relevant approach to treat DPN (36, 41). In fact, beneficial effects of CoQ10 on DPN in an animal model have been reported (15), but in clinical trials with short-term treatment antioxidants lacked therapeutic effects in DPN (41) and in diabetes in general (42).
The present study was undertaken to analyze, at different time intervals up to 8 mo of age, the sensory phenotype of the diabetic db−/db− mouse with focus on DRGs, using behavioral, electrophysiological, biochemical, and histochemical methods. Moreover, we monitored the total number of neurons in lumbar (L) 5 DRGs using a stereological counting method. The effect of long-term treatment with the antioxidant CoQ10 was studied on diverse phenotypic changes.
Results
Metabolic Parameters.
The design of the experiments is shown in Fig. S1. The male db−/db− mice were obese and hyperglycemic already at 5/6 wk of age (WoA), and body weight and glucose increased further with age (Fig. S2 A and B). Six months of CoQ10 treatment did not affect body weight or blood glucose levels (Fig. S2 C and D). Female db−/db− mice showed similar changes as the male mice (Fig. S2 E and F). None of the changes were observed in nondiabetic control mice (Fig. S2 C–F).
CoQ10 Prevents Hypoalgesia.
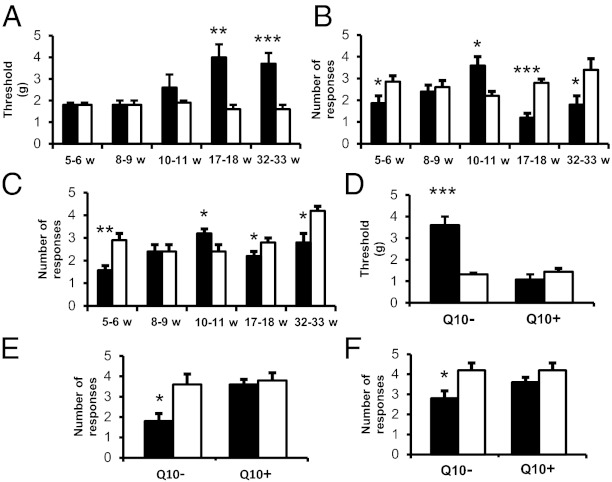
db−/db− and control mice were tested with regard to sensory parameters at regular intervals (5/6, 8/9, 10/11, 17/18, 32/33 WoA). In the von Frey hair test, the male db−/db− mice showed a significantly higher withdrawal threshold (tactile hypoalgesia) than controls at 17/18 and 32/33 WoA (Fig. 1A). In the pin-prick and acetone tests these mice showed mechanical hyperalgesia and cold allodynia at 10/11 WoA, but this was reversed and followed by hypoalgesia at the two later time points (Fig. 1 B and C). At 32/33 WoA, similar results were seen in female db−/db− mice both after the von Frey hair test and cold stimulation (Fig. S2 G and H), even though the results with noxious stimulation did not reach statistical significance (Fig. S2I). In the CoQ10-treated db−/db− mice there was no significant difference from control mice with regard to tactile, mechanical, and thermal hypoalgesia at 32/33 WoA, both in male (Fig. 1 D–F) and female mice (Fig. S2 G–I). We also observed that diabetic animals and controls demonstrated a similar degree of autotomy, a behavior considered to be pain-related (43), in the ipsilateral hind paws 1 wk after sciatic nerve transection (Fig. S3).
Fig. 1.
CoQ10 treatment prevents hypoalgesia in male db−/db− mice. (A–C) Response to innoxious (von Frey filaments; A), noxious (pin prick; C), or cold (acetone; B) stimulations in db−/db− and control groups at different weeks of age. (D–F) After CoQ10 treatment, response to mechanical (von Frey filaments; D), noxious (pin prick; F), or cold (acetone; E) stimulations in db−/db− and control groups at 32/33 WoA. Q10− and Q10+, without and with CoQ10 treatment, respectively. db−/db−, filled columns; control, open columns. *P < 0.05, **P < 0.01, ***P < 0.001 vs. control. All results are means ± SEM n = 5–20 mice.
CoQ10 Prevents the Decrease in Impulse Conduction.
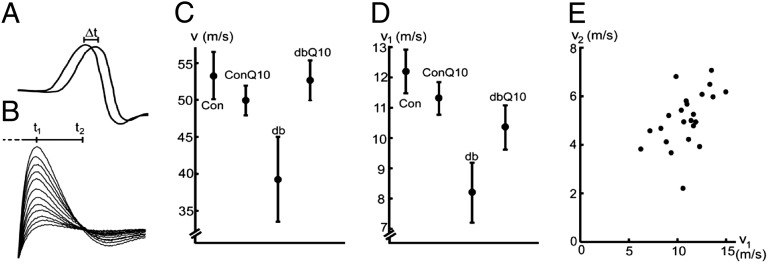
The CVs of the sciatic nerve of the db−/db− mice were reduced at 32/33 WoA in the in vivo measurements of motor responses from the tibial branch (26%, calculated from the time shift of muscle responses at 34 °C) and the in vitro measurements of whole nerve (33%, at 22 °C) (Fig. 2 and Table S1) compared with control mice. No difference between the results for female and male mice was noted. The CoQ10 treatment of the db−/db− mice reduced the CV decrease: from 26% to 1% in the in vivo motor response measurements and from 33% to 16% in the in vitro measurements (Fig. 2 and Table S1), assessed by a two-way ANOVA (Table S2) (P = 0.033 and P = 0.066, for in vivo and in vitro, respectively). In the db−/db− mice, the compound action potential, reflecting the composition of axon-size classes, appeared stretched along the time axis (anisotropic scaling) without specific dips or peaks, compared with that of controls. This result is reflected in Fig. 2E, where CVs calculated from two different time points of the action potential (t1 and t2) (Fig. 2B) were compared, suggesting an equal CV reduction in the motor and sensory fibers (correlation coeficient = 0.51). The coefficient of variation (Cv) varied between 11.4% and 32.6% (Table S1).
Fig. 2.
CoQ10 treatment increases CV of the diabetic sciatic nerve at 32/33 WoA. (A) In vivo measurements. Superponated muscle responses of the gastrocnemius muscle to stimulation at the tibial (distal) and the sciatic (proximal) nerve sites, respectively (Materials and Methods). (B) In vitro measurements. Superponated compound action potentials from the sciatic nerve at increasing stimulation. Measurements at 24 °C. (C) CVs under in vivo conditions. (D) CVs under in vitro conditions. Con, control; ConQ10, CoQ10-treated control; db, diabetic; dbQ10, CoQ10-treated db−/db− mice. Error bars indicate the SEM. Each data point in C and D is based on measurements from three to seven nerves (n = 21 in vivo and n = 23 in vitro) (Table S1). An ANOVA (Materials and Methods and SI Text) showed an interaction effect; CoQ10 treatment is beneficial for nerve CV in db−/db− mice (P = 0.033 and P = 0.066 for in vivo and in vitro, respectively). (E) Relationship between CVs of fast (v1) and slower (v2) axons. Data from in vitro experiments.
CoQ10 Counteracts Neuronal Cell Loss in L5 DRG.
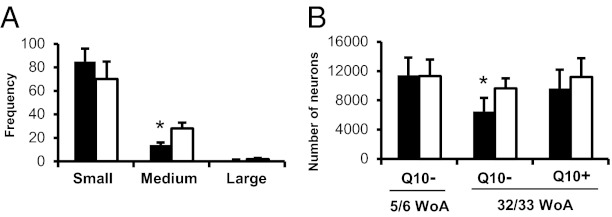
The histochemical study showed a significant reduction in the proportion of medium-sized neuron profiles (NPs) (Fig. 3A) (P < 0.05). The stereological analysis displayed a 32.6% neuronal cell loss in diabetic male mice at 32/33 WoA compared with controls [6,495 neurons (SD: 1,868; Cv: 0.29) vs. 9,643 neurons (SD: 1,377; Cv: 0.14)] (Fig. 3B and Table S3) (P < 0.05), and this reduction was only 13.9% after CoQ10 treatment [9,628 neurons (SD: 2,567; Cv: 0.27) vs. 11,180 neurons (SD: 2,582; Cv: 0.24)] (Fig. 3B and Table 3) (P > 0.05). At 5/6 WoA there was no difference in cell numbers between db−/db− and control mice [11,447 neurons (SD: 2,400; Cv: 0.20) vs. 11,315 neurons (SD: 2,266; Cv: 0.20)] (Fig. 3B and Table S3) (P > 0.05). Seven days after axotomy there was no further neuronal cell loss seen in the axotomized DRGs in 32/33 WoA db−/db− mice compared with control mice [6,697 neurons (SD: 1,028; Cv: 0.15) vs. 6,329 neurons (SD: 1,257; Cv: 0.20); P > 0.05].
Fig. 3.
CoQ10 attenuates neuron loss of DRG neurons in male db−/db− mice. (A) Size distribution of DRG NPs of db−/db− and control mice at 32/33 WoA. (B) Number of L5 DRG neurons in db−/db− compared with control mice at 32/33 or 5/6 WoA. Q10− and Q10+, without and with CoQ10 treatment, respectively. db−/db−, filled columns; control, open columns. *P < 0.05 vs. control. The results are means ± SEM (for NP) or SD (for number of neurons). n = 5–6 mice.
Biomarker Expression in DRG.
Western blot analysis revealed a decrease in the prosurvival factor p-Akt and an increase in the proapoptotic protein Bax in male db−/db− compared with control mice at 32/33 WoA, whereas p-Erk1/2 and Bcl-2 did not show an obvious difference (Fig. S4A).
The immunohistochemical analysis at 32/33 WoA showed that after peripheral axotomy calcitonin gene-related peptide (CGRP)-immunoreactive (IR) NPs were significantly decreased to the same extent (by ∼30%; P < 0.05) in axotomized DRGs of both diabetic and control male animals (Fig. S4B). Galanin- (Fig. S4C) and neuropeptide tyrosine (NPY)- (Fig. S4D) like immunoreactivities (LIs) were strongly up-regulated ipsilaterally in both diabetic and control mice. For galanin this was mainly related to small-sized NPs, and for NPY mainly to medium- and large-sized NPs (Fig. S4 G and H) both in diabetic and control mice. For galanin no significant difference was found between the two groups (Fig. S4C), whereas NPY-LI was increased in fewer NPs in diabetic compared with control mice (Fig. S4D) (P < 0.05). With regard to some further axotomy-sensitive proteins, down-regulation of substance P (Fig. S5 A and B) was observed in neurons, and up-regulation, in neurons or glial cells, was observed for superoxide dismutase 2 (Fig. S5 C and D), p-p38 (Fig. S5 E and F), Iba1(Fig. S5 G and H), and GFAP (Fig. S5 I and J), but without any certain difference between diabetic and control mice.
Phospholipase C β3 Expression in DRG.
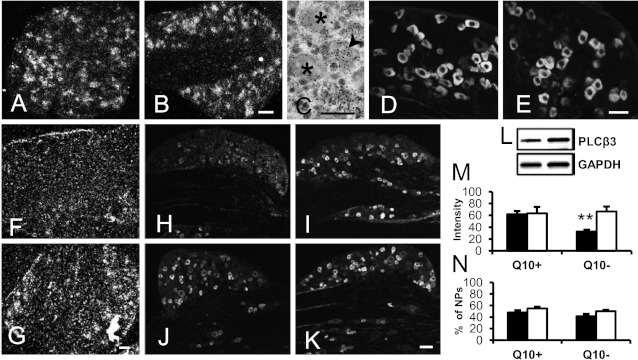
The level of phospholipase C β3 (PLCβ3) mRNA (Fig. 4 A vs. B) and protein (Fig. 4 D vs. E) in DRG neurons was similar in db−/db− and control mice at 5/6 WoA. At 32/33 WoA, a reduction of PLCβ3 transcript was seen in db−/db− but not in control mice (Fig. 4 F vs. G). With immunohistochemistry, a similar percentage of PLCβ3-IR NPs was found in diabetic and control mice (Fig. 4N), but the intensity of PLCβ3-LI (fluorescence levels) was significantly reduced in the diabetic mice (Fig. 4 H vs. I, M) (P < 0.01). Six months after CoQ10 treatment, the intensity of the PLCβ3-LI was as strong in DRGs of db−/db− mice as in controls at the protein level (Fig. 4 J vs. K, M) (P > 0.05). Western blot also confirmed the recovery of the expression of PLCβ3 after CoQ10 treatment (Fig. 4L).
Fig. 4.
CoQ10 treatment prevents a reduction of PLCβ3 expression in diabetic DRGs of male mice. (A–E) Studies on DRGs of db−/db− and control mice showing PLCβ3 mRNA (A–C) or protein expression (D and E) in db−/db− (A and D) and control (B, C, and E) mice at 5/6 WoA, respectively. (F and G) PLCβ3 mRNA in db−/db− (F) and control (G) mice at 32/33 WoA. (H–K) PLCβ3-LI in db−/db− (H and J) and control (I and K) mice at 32/33 WoA without (H and I) or with CoQ10 (J and K) treatment. (L) Western blot shows PLCβ3 in db−/db− mice (Left bands) and db−/db− mice treated with CoQ10 (Right bands). (M and N) Intensity (M) or percentage (N) of PLCβ3-IR NPs in db−/db− and control mice with or without CoQ10 treatment. **P < 0.01 vs. control. The results are means ± SEM db−/db−, filled columns; control, open columns. n = 5–10 mice. [Scale bars: 50 µm (A = B and C; D = E; F = G and H–K).]
Discussion
The present study in db−/db− mice, a type 2 diabetes model, shows that early, long-term treatment of these mice with the antioxidant CoQ10 prevents nerve conduction impairment, several sensory symptoms of DPN, and maintains DRG neuron levels of PLCβ3, a key molecule in pain processing. Moreover, the number of L5 DRG neurons was reduced by 33% at 32/33 WoA in db−/db− mice, a reduction that was significantly attenuated by CoQ10 treatment. In contrast, no neuronal cell loss was observed at 5/6 WoA. We also confirm that the dramatic neurochemical phenotypic changes seen in DRGs of rodents after various types of peripheral nerve injury (e.g., axotomy or spared nerve injury) are essentially absent in this particular animal model of type 2 diabetes.
CV Decrease.
Earlier studies on DPN have provided strong evidence for peripheral sensory and motor deficits (15–22), the mechanisms being differential with respect to experimental type 1 and type 2 models. Internodal structures seem primarily affected in type 2 models, but in type 1 models mainly nodal structures are affected (44–46). Furthermore, modeling studies show that the CV is surprisingly insensitive to modified nodal parameters (47). The present results from the sciatic nerve, both the in vivo (motor axons) and the in vitro (motor and sensory axons) measurements, confirm the velocity decrease in the db−/db− mouse. The results are robust; the in vivo measurements are supported by the in vitro measurements, where the observational error is 10-fold smaller than in the in vivo measurements (see SI Text). The decrease in velocity seems equal for large- and medium-sized axons, but velocities of smaller-sized axons are difficult to estimate with reasonable resolution. The higher CV in the in vivo motor response measurements compared with the in vitro whole-nerve measurements (approximately fivefold) could most likely be explained by the different temperatures used in the measurements and the different ways to estimate the CV used (Materials and Methods).
Time Course.
Many type 2 diabetes patients (5, 10, 48–51) and animals with diabetes (12, 52) show an initial painful DPN/tactile allodynia, which subsides and is then replaced by hypoalgesia. This replacement probably reflects a progressive damage/loss of nociceptive fibers, and as shown here in the db−/db− mouse, a substantial loss of DRG neuron cell bodies. We can in part confirm this biphasic curve herein. Thus, both cold and pain sensitivities are increased at 10/11 wk and are then replaced by hyposensitivity.
Neuron Loss.
Many studies on diabetes animal models, using electrophysiological and morphometric methods, have shown decreased motor nerve CV and degenerative changes in myelinated and unmyelinated fibers (19, 27). In contrast, a possible involvement of the neuronal cell bodies in the DRGs has been less clear. A reduced perikaryal volume of primary sensory neurons was noted early (53) and confirmed by Shimosige et al. (54), but when unbiased stereological methods (55) were used, no significant change in DRG neuron numbers was observed in 12-mo-old STZ rats (17, 27). However, a 27% loss of small-sized DRG neurons has been reported in type 1 diabetic BB/Wor rats by Kamiya et al. (56). Apoptotic changes in DRGs have been reported in other experimental diabetic animal models (SI Text).
Here we show that diabetic db−/db− mice at 32/33 but not at 5/6 WoA, have around 33% fewer L5 DRG neurons than the heterozygotes. CoQ10 caused a partial protection against this loss. To what extent the loss has influenced our own and others’ pain tests in this mouse remains to be analyzed. We observed a transient increase in cold and pain sensitivity but no mechanical allodynia in the db−/db− mouse. A transient, mechanical allodynia has been described during the early stage (6–12 WoA) of diabetes (12). Moreover, we observed a statistical trend for the reduction of CV in the in vitro study (P = 0.066) and the significant reduction of CV in the in vivo study (P = 0.033) (see the legend to Fig. 2 and Table S2). Therefore, we think that the significant loss of DRG neurons, including medium-sized ones, may also contribute the reduction of neuronal CV, especially under in vivo conditions.
Our results show that proapoptotic Bax is strongly elevated versus a strong decrease in antiapoptotic p-Akt, indicating involvement of the classic apoptotic pathway in the DRG neuron loss seen in db−/db− mice. Other groups have reported, in DRGs, low p-Akt levels in STZ mice (57) and low Bcl-2 levels in STZ rats (58, 59). However, no change in Bax or Bcl-2 was found in rat DRGs after STZ treatment for 8 wk (28).
Phenotypic Changes in DPN.
It is well known that peripheral nerve injury causes dramatic changes in DRG neurons (30–32). However, only very few alterations were observed in STZ-treated rats (28, 29), as well as in the present study. These results strongly suggest that DPN and the commonly used models of (mechanical) peripheral nerve injury are distinctly different (SI Text).
Possible Role of PLCβ3.
Excitatory neuropeptides and neurotrophins have been implicated in allodynia during the early phase of DPN in db−/db− mice (12, 60–64). PLCβ3, expressed in a subpopulation of DRG neurons, is another molecule involved in pain processing (65–68). PLCβ3 is down-regulated after peripheral nerve injury (66) and, as shown here, also in DPN. Activation of PLCβ3 causes an increase in intracellular Ca2+ and presumably release of excitatory transmitters, such as glutamate, and excitatory neuropeptides; that is, the enzyme is pronociceptive. In fact, inhibition of PLCβ3 causes a long-lasting (∼48 h) increase in the pain threshold in mice after nerve injury (66). We hypothesize that the down-regulation of pronociceptive PLCβ3 in db−/db− mice at 32/33 WoA, but not 5/6 WoA, is one factor contributing to the subsiding of the initial allodynia (ref. 12 and present study). A possible contribution of CoQ10, and thus of antioxidant effects, to the involvement of PLCβ3 in the elevation of the pain threshold needs further analysis. In addition, the loss of the DRG neurons may be associated with hypoalgesia in late-stage diabetes. The down-regulation of PLCβ3 may also be involved in the reduction of the CV, possibly via an associated reduction of axoplasmic Ca2+ concentration, affecting axoskeletal structures critical for the impulse propagation (69).
DPN Treatment Strategies.
Several strategies have been outlined for treatment of early, painful DPN (70–74), and for reversal of neuropathic deficits (75). However, there are fewer opportunities with regard to the hypoalgesia, which can lead to significant morbidity by predisposing, especially in the lower extremities, to injury, ulceration, and ultimately amputation (76, 77).
Intensive metabolic control of blood glucose reduces the incidence of new clinically detected neuropathy, but diabetes patients can still develop DPN (78). Intensified glucose-lowering therapy increases the risk of hypoglycemic episodes and can even be dangerous (79, 80). There is evidence that NGF can improve hypoalgesia (81) and restore myelinated nerve fiber morphology (82, 83). The insulin-sensitizer Rosiglitazone prevents development of thermal hypoalgesia in DBA/2J mice (84). Moreover, C-peptide partially prevents DPN in type 1 diabetic BB/Wor rats (85) and in humans (86, 87). Morphological changes in DRG neurons were significantly reduced in STZ rats after treatment (>1 y) with an aldose reductase inhibitor (zenarestat) (54). Recently, methylglyoxal has been identified as a new target for treatment of DPN (88).
Despite evidence that antioxidant processes play an important role in the pathogenesis of diabetes, a recent review concluded that “there is not any established benefit for short term treatment (3 months) with CoQ10 antioxidants use in the (clinical) management of diabetic complications” (42). However, Ayaz et al. (15) have shown that treatment of STZ rats with CoQ10 for 5 wk stops the shift toward slower CVs. CoQ10 treatment improves endothelial function and blood flow; thus, long-term treatment may be effective by improving oxygenation of the peripheral nerves (89). Finally, Hernandez-Ojeda et al. (90) reported that a 12-wk treatment with ubiquinone significantly improves diabetic polyneuropathy in patients with type 2 diabetes.
Our results indicate that early, long-term (∼6 mo), peroral treatment with CoQ10 can prevent the late hypoalgesia (decreases in mechanical hyperalgesia and cold allodynia) associated with DPN in 8-mo-old db−/db− mice. This finding includes a significant effect of CoQ10 on the CV impairment in the sciatic nerve of db−/db− mice, shown both in vivo and in vitro experiments, probably with a similar effect on all axon classes (SI Text). In addition, CoQ10 prevents the late sensory deficits associated with DPN, including the reduction of PLCβ3 expression in DRGs. Finally, CoQ10 significantly counteracts the marked loss of DRG neurons (SI Text). The reason for the profound, preventive effects with CoQ10 in our studies may be the early start of the treatment, in fact essentially initiated parallel to elevation of glucose levels.
The mechanisms underlying the results after CoQ10 treatment remains to be elucidated. In a parallel study on db−/db− mice with a similar design involving CoQ10 treatment, but focusing on kidney function, evidence is provided that oxidative stress activates uncoupling protein 2 (91). CoQ10 treatment reduces oxidative stress and uncoupling protein 2 protein levels, as well as normalizes oxygen consumption, and prevents mitochondrial fragmentation (91). Possibly, similar mechanisms operate in DRGs, leading to preserved sensibility and reduced loss of DRG neurons.
In conclusion, by using db−/db− mice we demonstrate marked effects of CoQ10 on preventing development of DPN, counteracting nerve conduction impairment and neuronal phenotypic changes, as well as protecting against neuron loss. These data identify CoQ10 as a potential candidate for future treatment of DPN in type 2 diabetes.
Materials and Methods
Animals.
C57BL/KsJm/Leptdb (db−/db−) mice (Stock 000662) and their normoglycemic heterozygous littermates were obtained from Charles River Laboratories. The mice were housed, fed, and treated with CoQ10. The commercial basal diet contained 18.5% protein, 55.7% carbohydrates, and 4% fat but not cholesterol. CoQ10 was dissolved in acetone followed by impregnation of the pellets. CoQ10 was added to the food pellets (1 g/kg), starting at 7 WoA until 32/33 WoA. The experimental procedures were approved by the Northern Stockholm Ethical Committee for Care and Use of Laboratory Animals.
Blood-glucose levels, body weight, and sensory thresholds were analyzed at regular intervals between ages 5/6 and 32/33 wk. Expression of selected markers was studied in L4 and L5 DRGs at the transcript and protein levels at 32/33 WoA, and some parameters in 5/6-WoA mice (SI Text). The design of the experiments is shown in Fig. S1.
Nerve Injury Model.
The left sciatic nerve was transected (axotomy) at midthigh level under isoflurane anesthesia, and the animals were allowed to survive for 7 d after surgery (SI Text).
Behavioral Tests.
Mechanical allodynia threshold was assessed using von Frey monofilaments, mechanical hyperalgesia using the pin-prick test, and cold allodynia by applying a drop of acetone solution (SI Text). After axotomy, the onset, extent, and incidence of autotomy were recorded by using modified, previously published methods (SI Text).
Electrophysiology.
For the in vivo experiments, the sciatic nerve was exposed, and the CV was calculated from the time-shift (Δt) of the responses of the gastrocnemius muscle to electrical stimulation at two locations of the nerve (Fig. 2A). For the in vitro experiments, a section of the nerve was excised and transferred into a temperature-controlled recording chamber. The distance between stimulating and recording electrodes was variable and measured with a caliper, and action currents were sampled at 100 kHz. The CV was calculated from the time from stimulation artifact to peak of the compound action potential (t1) (Fig. 2B). The in vivo CV experiments were carried out at 34 °C, whereas the in vitro experiments were mainly done at room temperature, but also in the range from 5 to 35 °C.
To compare the CV of the in vivo and in vitro measurements, we determined the temperature dependence between 5 and 35 °C (SI Text). To compare the CV of different fiber size classes, we used measurements of t1 and t2 (time from stimulation artifact to the trajectory-baseline crossing) (Fig. 2B).
Western Blot Analysis.
Total protein of L4 and L5 DRGs from db−/db− or heterozygous mice was extracted and processed for the analysis. Antibodies for markers used are listed in SI Text. The membranes were incubated with HRP-conjugated secondary antibodies, developed (Amersham Biosciences) and exposed to X-ray film (SI Text).
Immunohistochemistry.
Animals were deeply anesthetized and fixed by vascular perfusion with formalin. The L5 DRGs were dissected, frozen, and cut in a cryostat. The sections were incubated with various antibodies/antisera and reagents (listed in Table S4) and processed using a commercial kit (TSA Plus; NEN Life Science Products). The sections were analyzed in a Bio-Rad Radiance Plus confocal scanning microscope. For quantitative evaluation of NPs, the percentage and intensity of IR DRG NPs was counted (SI Text). The size distribution of NPs was measured using the Nikon Eclipse E 600 fluorescence microscope with Wasabi Image Software, as described in earlier studies (SI Text).
Stereology.
The counting was performed using a Leica TCS SPE Systems (D-35578) confocal laser-scanning system. The total number of L5 DRG neurons was monitored as described previously (92), based on the Cavalieri method for volume estimation of the DRG (55) using L5 DRGs from db–/db– and heterozygous control mice. The number of neurons was calculated as the product of the DRG volume and the numerical density (SI Text).
In Situ Hybridization.
Db–/db– and heterozygous control mice were decapitated, the L5 DRGs dissected and cut in a cryostat. The sequences of the synthetic oligonucleotides complementary to mRNA encoding PLCβ3 are listed in SI Text. Probes were labeled at the 3′- end with [33P] ATP using terminal deoxynucleotidyl transferase. Hybridization procedure and control experiments were carried out as previously described (93) (SI Text).
Statistical Analysis.
Regarding the percentage, intensity, and size distribution of positive NPs or the number of neurons in DRG neurons, data were expressed as mean ± SEM (for NP) or SD (for neuron number). Differences between groups were compared using Student t test (two groups). Some data (n = 5 samples) were also tested by the nonparamettric Kruskal–Wallis test and Mann–Whitney test, and the same statistical results were obtained. A P value less than 0.05 was regarded as being significant.
For the electrophysiological study, a factorial ANOVA was used to assess the statistical significance of the outcome of the experiments, with the two independent variables being the presence of diabetic neuropathy and CoQ10 treatment, and the dependent variable the CV. The significance levels were set to α = 0.05. The Shapiro–Wilk test was used to test if data were normally distributed (94) (SI Text).
Supplementary Material
Acknowledgments
We thank Drs. L. Terenius (Karolinska Institutet) and I. Nylander (Uppsala University) (calcitonin gene-related peptide), E. Theodorsson (Linköping University) (galanin), R. Elde (University of Minnesota) (P2X3), and the late J. Walsh and H. Wong (University of California Los Angeles) (NPY and Y1R) for generous supply of antisera. This study was supported by the Swedish Research Council (04X-2887, 33X-10815, 04224, 55X-0422437-3), the Marianne and Marcus Wallenberg Foundation, the Knut and Alice Wallenberg Foundation, Funds from Karolinska Institutet, the Swedish Brain Foundation, The Peter and Patricia Gruber Foundation, the Family Erling Persson Foundation, the European Commission project FUNCFOOD (FP7-KBBE-2009-245030), and Natural Scientific Research Innovation Foundation from Harbin Institute of Technology (HIT.NSRIF 2006).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1220794110/-/DCSupplemental.
References
- 1.Boulton AJ, et al. American Diabetes Association Diabetic neuropathies: A statement by the American Diabetes Association. Diabetes Care. 2005;28(4):956–962. doi: 10.2337/diacare.28.4.956. [DOI] [PubMed] [Google Scholar]
- 2.Sima AA. New insights into the metabolic and molecular basis for diabetic neuropathy. Cell Mol Life Sci. 2003;60(11):2445–2464. doi: 10.1007/s00018-003-3084-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tomlinson DR, Gardiner NJ. Diabetic neuropathies: components of etiology. J Peripher Nerv Syst. 2008;13(2):112–121. doi: 10.1111/j.1529-8027.2008.00167.x. [DOI] [PubMed] [Google Scholar]
- 4.Zochodne DW. Diabetic polyneuropathy: An update. Curr Opin Neurol. 2008;21(5):527–533. doi: 10.1097/WCO.0b013e32830b84cb. [DOI] [PubMed] [Google Scholar]
- 5.Feldman EL, et al. In: The Diabetes Mellitus Manual. Inzucchi SE, editor. New York: McGraw-Hill; 2005. pp. 366–384. [Google Scholar]
- 6.Pirart J. Diabetic neuropathy: A metabolic or a vascular disease? Diabetes. 1965;14:1–9. doi: 10.2337/diab.14.1.1. [DOI] [PubMed] [Google Scholar]
- 7.Thomas PK, Eliasson SG. In: Peripheral Neuropathy. Dyck PJ, Thomas PK, Lampert EH, editors. Philadelphia: Saunders; 1975. pp. 956–981. [Google Scholar]
- 8.Hendriksen PH, Oey PL, Wieneke GH, Bravenboer B, Banga JD. Subclinical diabetic neuropathy: Similarities between electrophysiological results of patients with type 1 (insulin-dependent) and type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1992;35(7):690–695. doi: 10.1007/BF00400264. [DOI] [PubMed] [Google Scholar]
- 9.Sima AA, Kamiya H. Diabetic neuropathy differs in type 1 and type 2 diabetes. Ann N Y Acad Sci. 2006;1084:235–249. doi: 10.1196/annals.1372.004. [DOI] [PubMed] [Google Scholar]
- 10.Feldman EL, et al. Diabetic Peripheral and Autonomic Neuropathy. Contemporary Endocrinology. Totowa, NJ: Humana; 2002. [Google Scholar]
- 11.Said G, Goulon-Goeau C, Slama G, Tchobroutsky G. Severe early-onset polyneuropathy in insulin-dependent diabetes mellitus. A clinical and pathological study. N Engl J Med. 1992;326(19):1257–1263. doi: 10.1056/NEJM199205073261905. [DOI] [PubMed] [Google Scholar]
- 12.Cheng HT, Dauch JR, Hayes JM, Hong Y, Feldman EL. Nerve growth factor mediates mechanical allodynia in a mouse model of type 2 diabetes. J Neuropathol Exp Neurol. 2009;68(11):1229–1243. doi: 10.1097/NEN.0b013e3181bef710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hummel KP, Dickie MM, Coleman DL. Diabetes, a new mutation in the mouse. Science. 1966;153(3740):1127–1128. doi: 10.1126/science.153.3740.1127. [DOI] [PubMed] [Google Scholar]
- 14.Sullivan KA, et al. Mouse models of diabetic neuropathy. Neurobiol Dis. 2007;28(3):276–285. doi: 10.1016/j.nbd.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ayaz M, Tuncer S, Okudan N, Gokbel H. Coenzyme Q(10) and alpha-lipoic acid supplementation in diabetic rats: Conduction velocity distributions. Methods Find Exp Clin Pharmacol. 2008;30(5):367–374. doi: 10.1358/mf.2008.30.5.1186072. [DOI] [PubMed] [Google Scholar]
- 16.Behse F, Buchthal F, Carlsen F. Nerve biopsy and conduction studies in diabetic neuropathy. J Neurol Neurosurg Psychiatry. 1977;40(11):1072–1082. doi: 10.1136/jnnp.40.11.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kishi M, Tanabe J, Schmelzer JD, Low PA. Morphometry of dorsal root ganglion in chronic experimental diabetic neuropathy. Diabetes. 2002;51(3):819–824. doi: 10.2337/diabetes.51.3.819. [DOI] [PubMed] [Google Scholar]
- 18.Lennertz RC, Medler KA, Bain JL, Wright DE, Stucky CL. Impaired sensory nerve function and axon morphology in mice with diabetic neuropathy. J Neurophysiol. 2011;106(2):905–914. doi: 10.1152/jn.01123.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robertson DM, Sima AA. Diabetic neuropathy in the mutant mouse [C57BL/ks(db/db)]: A morphometric study. Diabetes. 1980;29(1):60–67. doi: 10.2337/diab.29.1.60. [DOI] [PubMed] [Google Scholar]
- 20.Sima AA, Robertson DM. Peripheral neuropathy in mutant diabetic mouse [C57BL/Ks (db/db)] Acta Neuropathol. 1978;41(2):85–89. doi: 10.1007/BF00689757. [DOI] [PubMed] [Google Scholar]
- 21.Hildebrand J, Joffroy A, Graff G, Coërs C. Neuromuscular changes with alloxan hyperglycemia. Electrophysiological, biochemical, and histological study in rats. Arch Neurol. 1968;18(6):633–641. doi: 10.1001/archneur.1968.00470360055005. [DOI] [PubMed] [Google Scholar]
- 22.Preston GM. Peripheral neuropathy in the alloxan-diabetic rat. J Physiol. 1967;189(2):49P–50P. [PMC free article] [PubMed] [Google Scholar]
- 23.Valls-Canals J, Povedano M, Montero J, Pradas J. Diabetic polyneuropathy. Axonal or demyelinating? Electromyogr Clin Neurophysiol. 2002;42(1):3–6. [PubMed] [Google Scholar]
- 24.Dyck PJ, et al. Acute hyperosmolar hyperglycemia causes axonal shrinkage and reduced nerve conduction velocity. Exp Neurol. 1981;71(3):507–514. doi: 10.1016/0014-4886(81)90028-5. [DOI] [PubMed] [Google Scholar]
- 25.Sima AA, Bouchier M, Christensen H. Axonal atrophy in sensory nerves of the diabetic BB-Wistar rat: A possible early correlate of human diabetic neuropathy. Ann Neurol. 1983;13(3):264–272. doi: 10.1002/ana.410130307. [DOI] [PubMed] [Google Scholar]
- 26.McCarthy BG, et al. Cutaneous innervation in sensory neuropathies: Evaluation by skin biopsy. Neurology. 1995;45(10):1848–1855. doi: 10.1212/wnl.45.10.1848. [DOI] [PubMed] [Google Scholar]
- 27.Zochodne DW, Verge VM, Cheng C, Sun H, Johnston J. Does diabetes target ganglion neurones? Progressive sensory neurone involvement in long-term experimental diabetes. Brain. 2001;124(Pt 11):2319–2334. doi: 10.1093/brain/124.11.2319. [DOI] [PubMed] [Google Scholar]
- 28.Burnand RC, Price SA, McElhaney M, Barker D, Tomlinson DR. Expression of axotomy-inducible and apoptosis-related genes in sensory nerves of rats with experimental diabetes. Brain Res Mol Brain Res. 2004;132(2):235–240. doi: 10.1016/j.molbrainres.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Brussee V, et al. Distal degenerative sensory neuropathy in a long-term type 2 diabetes rat model. Diabetes. 2008;57(6):1664–1673. doi: 10.2337/db07-1737. [DOI] [PubMed] [Google Scholar]
- 30.Hökfelt T, Zhang X, Wiesenfeld-Hallin Z. Messenger plasticity in primary sensory neurons following axotomy and its functional implications. Trends Neurosci. 1994;17(1):22–30. doi: 10.1016/0166-2236(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 31.Costigan M, et al. Replicate high-density rat genome oligonucleotide microarrays reveal hundreds of regulated genes in the dorsal root ganglion after peripheral nerve injury. BMC Neurosci. 2002;3:16. doi: 10.1186/1471-2202-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiao HS, et al. Identification of gene expression profile of dorsal root ganglion in the rat peripheral axotomy model of neuropathic pain. Proc Natl Acad Sci USA. 2002;99(12):8360–8365. doi: 10.1073/pnas.122231899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boulton AJ, Malik RA, Arezzo JC, Sosenko JM. Diabetic somatic neuropathies. Diabetes Care. 2004;27(6):1458–1486. doi: 10.2337/diacare.27.6.1458. [DOI] [PubMed] [Google Scholar]
- 34.Pop-Busui R, Sima A, Stevens M. Diabetic neuropathy and oxidative stress. Diabetes Metab Res Rev. 2006;22(4):257–273. doi: 10.1002/dmrr.625. [DOI] [PubMed] [Google Scholar]
- 35.Vincent AM, Callaghan BC, Smith AL, Feldman EL. Diabetic neuropathy: Cellular mechanisms as therapeutic targets. Nat Rev Neurol. 2011;7(10):573–583. doi: 10.1038/nrneurol.2011.137. [DOI] [PubMed] [Google Scholar]
- 36.Villalba JM, Parrado C, Santos-Gonzalez M, Alcain FJ. Therapeutic use of coenzyme Q10 and coenzyme Q10-related compounds and formulations. Expert Opin Investig Drugs. 2010;19(4):535–554. doi: 10.1517/13543781003727495. [DOI] [PubMed] [Google Scholar]
- 37.Littarru GP, Tiano L, Belardinelli R, Watts GF. Coenzyme Q(10), endothelial function, and cardiovascular disease. Biofactors. 2011;37(5):366–373. doi: 10.1002/biof.154. [DOI] [PubMed] [Google Scholar]
- 38.Quinzii CM, Hirano M. Primary and secondary CoQ(10) deficiencies in humans. Biofactors. 2011;37(5):361–365. doi: 10.1002/biof.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bentinger M, Brismar K, Dallner G. The antioxidant role of coenzyme Q. Mitochondrion. 2007;7(Suppl):S41–S50. doi: 10.1016/j.mito.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Y, Turunen M, Appelkvist EL. Restricted uptake of dietary coenzyme Q is in contrast to the unrestricted uptake of alpha-tocopherol into rat organs and cells. J Nutr. 1996;126(9):2089–2097. doi: 10.1093/jn/126.9.2089. [DOI] [PubMed] [Google Scholar]
- 41.Vincent AM, Edwards JL, Sadidi M, Feldman EL. The antioxidant response as a drug target in diabetic neuropathy. Curr Drug Targets. 2008;9(1):94–100. doi: 10.2174/138945008783431754. [DOI] [PubMed] [Google Scholar]
- 42.Golbidi S, Ebadi SA, Laher I. Antioxidants in the treatment of diabetes. Curr Diabetes Rev. 2011;7(2):106–125. doi: 10.2174/157339911794940729. [DOI] [PubMed] [Google Scholar]
- 43.Koplovitch P, Minert A, Devor M. Spontaneous pain in partial nerve injury models of neuropathy and the role of nociceptive sensory cover. Exp Neurol. 2012;236(1):103–111. doi: 10.1016/j.expneurol.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 44.Brismar T, Sima AA. Changes in nodal function in nerve fibres of the spontaneously diabetic BB-Wistar rat: Potential clamp analysis. Acta Physiol Scand. 1981;113(4):499–506. doi: 10.1111/j.1748-1716.1981.tb06928.x. [DOI] [PubMed] [Google Scholar]
- 45.Sima AA, Zhang W, Li ZG, Murakawa Y, Pierson CR. Molecular alterations underlie nodal and paranodal degeneration in type 1 diabetic neuropathy and are prevented by C-peptide. Diabetes. 2004;53(6):1556–1563. doi: 10.2337/diabetes.53.6.1556. [DOI] [PubMed] [Google Scholar]
- 46.Sima AA, et al. A comparison of diabetic polyneuropathy in type II diabetic BBZDR/Wor rats and in type I diabetic BB/Wor rats. Diabetologia. 2000;43(6):786–793. doi: 10.1007/s001250051376. [DOI] [PubMed] [Google Scholar]
- 47.Moore JW, Joyner RW, Brill MH, Waxman SD, Najar-Joa M. Simulations of conduction in uniform myelinated fibers. Relative sensitivity to changes in nodal and internodal parameters. Biophys J. 1978;21(2):147–160. doi: 10.1016/S0006-3495(78)85515-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barrett AM, et al. Epidemiology, public health burden, and treatment of diabetic peripheral neuropathic pain: A review. Pain Med. 2007;8(Suppl 2):S50–S62. doi: 10.1111/j.1526-4637.2006.00179.x. [DOI] [PubMed] [Google Scholar]
- 49.Clarke P, Gray A, Holman R. Estimating utility values for health states of type 2 diabetic patients using the EQ-5D (UKPDS 62) Med Decis Making. 2002;22(4):340–349. doi: 10.1177/0272989X0202200412. [DOI] [PubMed] [Google Scholar]
- 50.Currie CJ, et al. The financial costs of healthcare treatment for people with Type 1 or Type 2 diabetes in the UK with particular reference to differing severity of peripheral neuropathy. Diabet Med. 2007;24(2):187–194. doi: 10.1111/j.1464-5491.2006.02057.x. [DOI] [PubMed] [Google Scholar]
- 51.Martin CL, et al. DCCT/EDIC Research Group Neuropathy among the diabetes control and complications trial cohort 8 years after trial completion. Diabetes Care. 2006;29(2):340–344. doi: 10.2337/diacare.29.02.06.dc05-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kamiya H, Murakawa Y, Zhang W, Sima AA. Unmyelinated fiber sensory neuropathy differs in type 1 and type 2 diabetes. Diabetes Metab Res Rev. 2005;21(5):448–458. doi: 10.1002/dmrr.541. [DOI] [PubMed] [Google Scholar]
- 53.Sidenius P, Jakobsen J. Reduced perikaryal volume of lower motor and primary sensory neurons in early experimental diabetes. Diabetes. 1980;29(3):182–186. doi: 10.2337/diab.29.3.182. [DOI] [PubMed] [Google Scholar]
- 54.Shimoshige Y, et al. Thirteen-month inhibition of aldose reductase by zenarestat prevents morphological abnormalities in the dorsal root ganglia of streptozotocin-induced diabetic rats. Brain Res. 2009;1247:182–187. doi: 10.1016/j.brainres.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 55.Tandrup T. Unbiased estimates of number and size of rat dorsal root ganglion cells in studies of structure and cell survival. J Neurocytol. 2004;33(2):173–192. doi: 10.1023/b:neur.0000030693.91881.53. [DOI] [PubMed] [Google Scholar]
- 56.Kamiya H, Zhang W, Sima AA. Degeneration of the Golgi and neuronal loss in dorsal root ganglia in diabetic BioBreeding/Worcester rats. Diabetologia. 2006;49(11):2763–2774. doi: 10.1007/s00125-006-0379-0. [DOI] [PubMed] [Google Scholar]
- 57.Chattopadhyay M, Walter C, Mata M, Fink DJ. Neuroprotective effect of herpes simplex virus-mediated gene transfer of erythropoietin in hyperglycemic dorsal root ganglion neurons. Brain. 2009;132(Pt 4):879–888. doi: 10.1093/brain/awp014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang TJ, Sayers NM, Verkhratsky A, Fernyhough P. Neurotrophin-3 prevents mitochondrial dysfunction in sensory neurons of streptozotocin-diabetic rats. Exp Neurol. 2005;194(1):279–283. doi: 10.1016/j.expneurol.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 59.Srinivasan S, Stevens M, Wiley JW. Diabetic peripheral neuropathy: Evidence for apoptosis and associated mitochondrial dysfunction. Diabetes. 2000;49(11):1932–1938. doi: 10.2337/diabetes.49.11.1932. [DOI] [PubMed] [Google Scholar]
- 60.Brewster WJ, Fernyhough P, Diemel LT, Mohiuddin L, Tomlinson DR. Diabetic neuropathy, nerve growth factor and other neurotrophic factors. Trends Neurosci. 1994;17(8):321–325. doi: 10.1016/0166-2236(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 61.Hellweg R, Raivich G, Hartung HD, Hock C, Kreutzberg GW. Axonal transport of endogenous nerve growth factor (NGF) and NGF receptor in experimental diabetic neuropathy. Exp Neurol. 1994;130(1):24–30. doi: 10.1006/exnr.1994.1181. [DOI] [PubMed] [Google Scholar]
- 62.Tomlinson DR, Fernyhough P, Diemel LT. Neurotrophins and peripheral neuropathy. Philos Trans R Soc Lond B Biol Sci. 1996;351(1338):455–462. doi: 10.1098/rstb.1996.0042. [DOI] [PubMed] [Google Scholar]
- 63.Zochodne DW. Neurotrophins and other growth factors in diabetic neuropathy. Semin Neurol. 1996;16(2):153–161. doi: 10.1055/s-2008-1040971. [DOI] [PubMed] [Google Scholar]
- 64.Yasuda H, et al. Diabetic neuropathy and nerve regeneration. Prog Neurobiol. 2003;69(4):229–285. doi: 10.1016/s0301-0082(03)00034-0. [DOI] [PubMed] [Google Scholar]
- 65.Han SK, Mancino V, Simon MI. Phospholipase Cbeta 3 mediates the scratching response activated by the histamine H1 receptor on C-fiber nociceptive neurons. Neuron. 2006;52(4):691–703. doi: 10.1016/j.neuron.2006.09.036. [DOI] [PubMed] [Google Scholar]
- 66.Shi TJ, et al. Phospholipase Cbeta3 in mouse and human dorsal root ganglia and spinal cord is a possible target for treatment of neuropathic pain. Proc Natl Acad Sci USA. 2008;105(50):20004–20008. doi: 10.1073/pnas.0810899105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xie W, et al. Genetic alteration of phospholipase C beta3 expression modulates behavioral and cellular responses to mu opioids. Proc Natl Acad Sci USA. 1999;96(18):10385–10390. doi: 10.1073/pnas.96.18.10385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Joseph EK, Bogen O, Alessandri-Haber N, Levine JD. PLC-beta 3 signals upstream of PKC epsilon in acute and chronic inflammatory hyperalgesia. Pain. 2007;132(1–2):67–73. doi: 10.1016/j.pain.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 69.Bennett J, Weeds A. Calcium and the cytoskeleton. Br Med Bull. 1986;42(4):385–390. doi: 10.1093/oxfordjournals.bmb.a072156. [DOI] [PubMed] [Google Scholar]
- 70.Veves A, Backonja M, Malik RA. Painful diabetic neuropathy: Epidemiology, natural history, early diagnosis, and treatment options. Pain Med. 2008;9(6):660–674. doi: 10.1111/j.1526-4637.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- 71.Petit WA, Jr, Upender RP. Medical evaluation and treatment of diabetic peripheral neuropathy. Clin Podiatr Med Surg. 2003;20(4):671–688. doi: 10.1016/S0891-8422(03)00068-5. [DOI] [PubMed] [Google Scholar]
- 72.Hadj Tahar A. Pregabalin for peripheral neuropathic pain. Issues Emerg Health Technol. 2005;(67):1–4. [PubMed] [Google Scholar]
- 73.Bril V, et al. American Academy of Neurology American Asociation of Neuromuscular and Electrodiagnostic Medicine American Academy of Physical Medicine and Rehabilitation Evidence-based guideline: Treatment of painful diabetic neuropathy—Report of the American Association of Neuromuscular and Electrodiagnostic Medicine, the American Academy of Neurology, and the American Academy of Physical Medicine & Rehabilitation. Muscle Nerve. 2011;43(6):910–917. doi: 10.1002/mus.22092. [DOI] [PubMed] [Google Scholar]
- 74.Israili ZH. Advances in the treatment of type 2 diabetes mellitus. Am J Ther. 2011;18(2):117–152. doi: 10.1097/MJT.0b013e3181afbf51. [DOI] [PubMed] [Google Scholar]
- 75.Zochodne DW. Reversing neuropathic deficits. J Peripher Nerv Syst. 2012;17(Suppl 2):4–9. doi: 10.1111/j.1529-8027.2012.00388.x. [DOI] [PubMed] [Google Scholar]
- 76.Rathur HM, Boulton AJ. The neuropathic diabetic foot. Nat Clin Pract Endocrinol Metab. 2007;3(1):14–25. doi: 10.1038/ncpendmet0347. [DOI] [PubMed] [Google Scholar]
- 77.Gordois A, Scuffham P, Shearer A, Oglesby A, Tobian JA. The health care costs of diabetic peripheral neuropathy in the US. Diabetes Care. 2003;26(6):1790–1795. doi: 10.2337/diacare.26.6.1790. [DOI] [PubMed] [Google Scholar]
- 78.Habib AA, Brannagan TH., 3rd Therapeutic strategies for diabetic neuropathy. Curr Neurol Neurosci Rep. 2010;10(2):92–100. doi: 10.1007/s11910-010-0093-7. [DOI] [PubMed] [Google Scholar]
- 79.Frier BM. Morbidity of hypoglycemia in type 1 diabetes. Diabetes Res Clin Pract. 2004;65(Suppl 1):S47–S52. doi: 10.1016/j.diabres.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 80.Inzucchi SE, et al. Management of hyperglycemia in type 2 diabetes: A patient-centered approach: Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2012;35(6):1364–1379. doi: 10.2337/dc12-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Christianson JA, Ryals JM, Johnson MS, Dobrowsky RT, Wright DE. Neurotrophic modulation of myelinated cutaneous innervation and mechanical sensory loss in diabetic mice. Neuroscience. 2007;145(1):303–313. doi: 10.1016/j.neuroscience.2006.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Unger JW, Klitzsch T, Pera S, Reiter R. Nerve growth factor (NGF) and diabetic neuropathy in the rat: Morphological investigations of the sural nerve, dorsal root ganglion, and spinal cord. Exp Neurol. 1998;153(1):23–34. doi: 10.1006/exnr.1998.6856. [DOI] [PubMed] [Google Scholar]
- 83.Walwyn WM, et al. HSV-1-mediated NGF delivery delays nociceptive deficits in a genetic model of diabetic neuropathy. Exp Neurol. 2006;198(1):260–270. doi: 10.1016/j.expneurol.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 84.Wiggin TD, et al. Rosiglitazone treatment reduces diabetic neuropathy in streptozotocin-treated DBA/2J mice. Endocrinology. 2008;149(10):4928–4937. doi: 10.1210/en.2008-0869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sima AA, et al. C-peptide prevents and improves chronic type I diabetic polyneuropathy in the BB/Wor rat. Diabetologia. 2001;44(7):889–897. doi: 10.1007/s001250100570. [DOI] [PubMed] [Google Scholar]
- 86.Ekberg K, et al. C-Peptide replacement therapy and sensory nerve function in type 1 diabetic neuropathy. Diabetes Care. 2007;30(1):71–76. doi: 10.2337/dc06-1274. [DOI] [PubMed] [Google Scholar]
- 87.Wahren J, Kallas A, Sima AA. The clinical potential of C-peptide replacement in type 1 diabetes. Diabetes. 2012;61(4):761–772. doi: 10.2337/db11-1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Eberhardt MJ, et al. Methylglyoxal activates nociceptors through transient receptor potential channel A1 (TRPA1): A possible mechanism of metabolic neuropathies. J Biol Chem. 2012;287(34):28291–28306. doi: 10.1074/jbc.M111.328674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Watts GF, et al. Coenzyme Q(10) improves endothelial dysfunction of the brachial artery in type II diabetes mellitus. Diabetologia. 2002;45(3):420–426. doi: 10.1007/s00125-001-0760-y. [DOI] [PubMed] [Google Scholar]
- 90.Hernández-Ojeda J, et al. The effect of ubiquinone in diabetic polyneuropathy: A randomized double-blind placebo-controlled study. J Diabetes Complications. 2012;26(4):352–358. doi: 10.1016/j.jdiacomp.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 91.Persson MF, et al. Coenzyme Q10 prevents GDP-sensitive mitochondrial uncoupling, glomerular hyperfiltration and proteinuria in kidneys from db/db mice as a model of type 2 diabetes. Diabetologia. 2012;55(5):1535–1543. doi: 10.1007/s00125-012-2469-5. [DOI] [PubMed] [Google Scholar]
- 92.Shi TJ, et al. Effect of peripheral nerve injury on dorsal root ganglion neurons in the C57 BL/6J mouse: Marked changes both in cell numbers and neuropeptide expression. Neuroscience. 2001;105(1):249–263. doi: 10.1016/s0306-4522(01)00148-8. [DOI] [PubMed] [Google Scholar]
- 93.Dagerlind Å, Friberg K, Bean AJ, Hökfelt T. Sensitive mRNA detection using unfixed tissue: Combined radioactive and non-radioactive in situ hybridization histochemistry. Histochemistry. 1992;98(1):39–49. doi: 10.1007/BF00716936. [DOI] [PubMed] [Google Scholar]
- 94.Shapiro S, Wilk M. An analysis of variance test for normality (complete samples) Biometrika. 1965;52(3/4):591–611. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.