Abstract
During pregnancy, fetal cells cross into the maternal organs where they reside postpartum. Evidence from multiple laboratories suggests that these microchimeric fetal cells contribute to maternal tissue repair after injury. In mouse models, most injury experiments are performed during pregnancy; however, in a clinical setting most injuries or diseases occur postpartum. Therefore, experiments using animal models should be designed to address questions in the time period following delivery. In order to provide a baseline for such experiments, we analyzed the natural history of fetal cells in the postpartum maternal organs. Female C57BL/6J mice were mated to males homozygous for the enhanced green fluorescent protein gene. Fetal cells in the maternal lungs and bone marrow were identified by their green fluorescence using in a high-speed flow cytometer and their counts were compared between the lung and bone marrow. Spearman correlation analysis was used to identify relationships between the duration of time postpartum and the cell counts and ratio of live and dead cells. Our results show that fetal cells persist in these organs until at least three months postpartum in healthy female mice. We show a two-stage decline, with an initial two and a half-week rapid clearance followed by a trend of gradual decrease. Additionally, an increase in the ratio of live to dead cells within the lung over time suggests that these cells may replicate in vivo. The results presented here will inform the design of future experiments and may have implications for women’s health.
Keywords: microchimerism, fetal cell trafficking, pregnancy, postpartum, green fluorescent protein, lungs, bone marrow, flow cytometry, mouse
Introduction
During pregnancy, in both humans and mice a bi-directional exchange of cells occurs between the pregnant female and her fetus. Immediately following delivery, the number of fetal cells in the maternal circulation and organs decreases quickly.1-3 Despite rapid clearance, a microchimeric fetal cell population survives for several decades in the postpartum woman.4,5
Evidence from mice and humans suggests that persistent fetal cells can contribute to maternal tissue repair during injury. In humans, fetal cells have been found in thyroid follicles in a woman with an adenomatous goiter,6 the liver parenchyma in a woman with hepatitis C7 and the appendix in pregnant women with appendicitis.8 In all of these reports, the fetal cells were entirely integrated within the maternal tissue, distinguishable only by the presence of a Y chromosome. In mice, fetal cells have been shown to contribute to induced injuries. For example, Kara et al.9 reported that in pregnant females fetal cells differentiated into cardiomyocytes in infarcted heart tissue. Other investigators have demonstrated that fetal cells respond to maternal liver chemical injury10 and contact dermatitis.11
In mouse models, most injury experiments are performed during pregnancy since the number of fetal cells is highest immediately prior to delivery.3,12 In a clinical setting, however, most injuries or diseases occur postpartum. Therefore, experiments using animal models should be designed to address questions in the time period following delivery. In order to provide a baseline for such experiments, a comprehensive analysis of the natural history of fetal cells in the postpartum maternal organs is needed.
Our laboratory previously described the quantitative pattern of fetomaternal trafficking in mice during pregnancy3 and suggested that the number of fetal cells would likely increase in the postpartum period. These cells form the basis of the population of microchimeric cells that persist long-term. Other laboratories have examined fetal cell presence at specific time-points postpartum.12-15 In this paper we present a detailed analysis of the natural history of fetal cells in the murine maternal lungs and bone marrow in the postpartum period. We use a model system in which a wild type virgin female is mated to a transgenic male that is homozygous for the enhanced green fluorescent protein gene, Egfp. The maternal lungs contain the largest number of fetal cells during pregnancy.3 For this reason, we chose the lungs for this experiment. Furthermore, since fetal cells have some similar properties to bone marrow stem cells, such as the ability to respond to injury and inflammation,16,17 we also examined bone marrow. Finally, we used flow cytometry since it is the most sensitive and specific method for detection of rare microchimeric fetal cells.3,18 We previously validated expression of Egfp in GFP+ sorted cells by qRT-PCR.19
Results
Forty-eight lung and 40 bone marrow samples were analyzed between postpartum days 0.5 to 90. For most samples 10–30 million events were analyzed per lung, and 8–20 million per bone marrow. Propidium iodide (PI) staining was used to identify live (PI-) and dead (PI+) GFP+ fetal cells (Fig. 1).
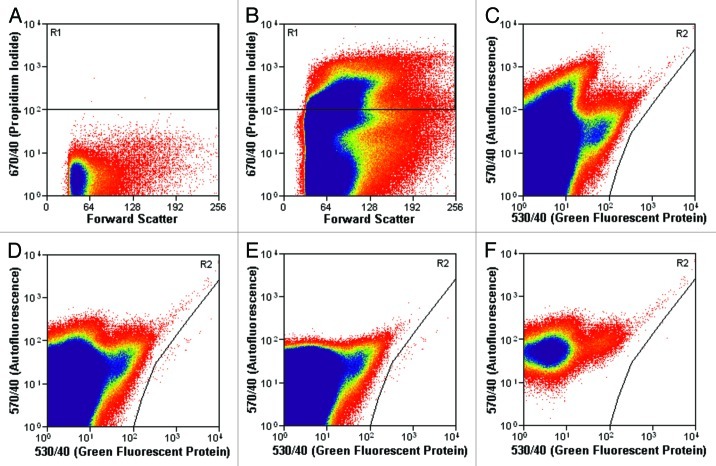
Figure 1. Gates used during the flow cytometry experiments to quantify fetal cells. (A) Unstained virgin female lung sample showing gate R1 used for PI. (B) Virgin female lung sample stained with PI showing gate R1 used for PI. The cells in the upper region (gate R1) are PI positive, and therefore dead. Cells not contained within the R1 gate (at the bottom of the image) are PI negative, and therefore alive. (C) Virgin female lung sample stained with PI showing gate R2 used for GFP+ cell counting. (D−F) Postpartum female lung sample stained with PI showing gate R2 used for GFP+ cell counting. Cells within the R2 gate (at the right of the image) are GFP+ and therefore fetal. (D) Ungated window displaying the total number of fetal cells in gate R2. (E) The same sample gated for PI (gate R1 shown in AandB), showing GFP+ PI+ dead fetal cells in area R2. (F) The same sample gated to exclude PI, showing GFP+ PI- live fetal cells in area R2.
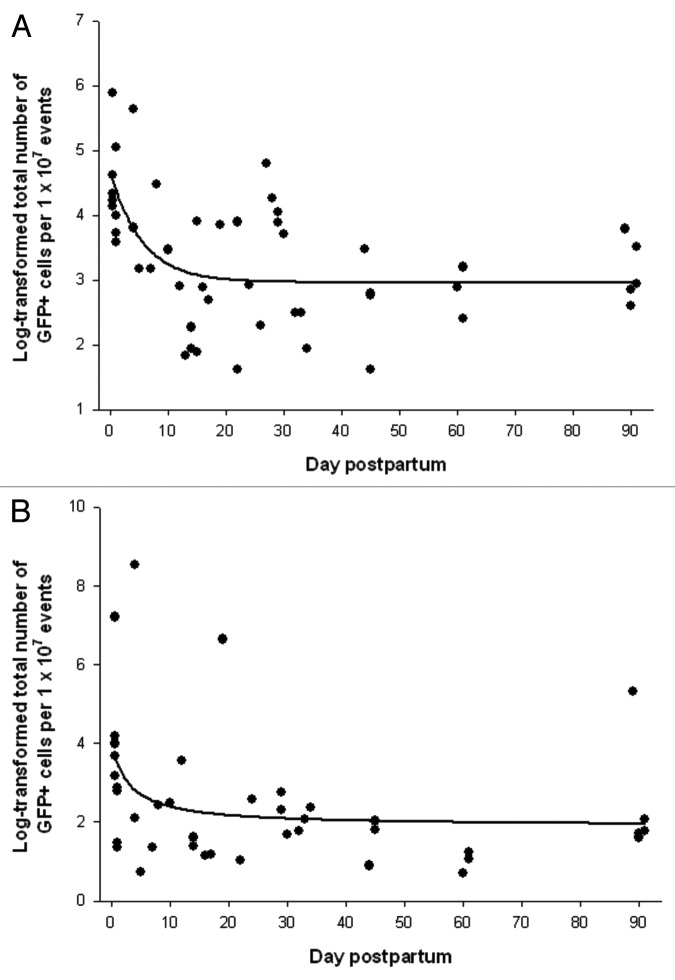
Fetal cells were detectable in the pregnant mice for the duration of the experiment, up to 90 d postpartum in both lungs and bone marrow (Fig. 2). In the lungs (Fig. 2A), there was a steep decline in the number of fetal cells immediately following delivery (r = -0.76, p < 0.0001). This was followed by a slower decrease that started around days 17–18 but did not reach statistical significance (r = -0.21 and p = 0.32). In the bone marrow (Fig. 2B), the pattern was similar (r = -0.65, p = 0.0025 in early postpartum and r = -0.30, p = 0.20 after days 17–18).
Figure 2. Log-transformed normalized total number of GFP+ fetal cells in the maternal organs during the postpartum period. (A) Lung, regression function: f(y) = 3.0 + 1.7 × exp(-0.2x), R2 = 0.34. (B) Bone marrow, regression function: f(y) = 2.2 + 53.8 × exp(-6.5x), R2 = 0.18.
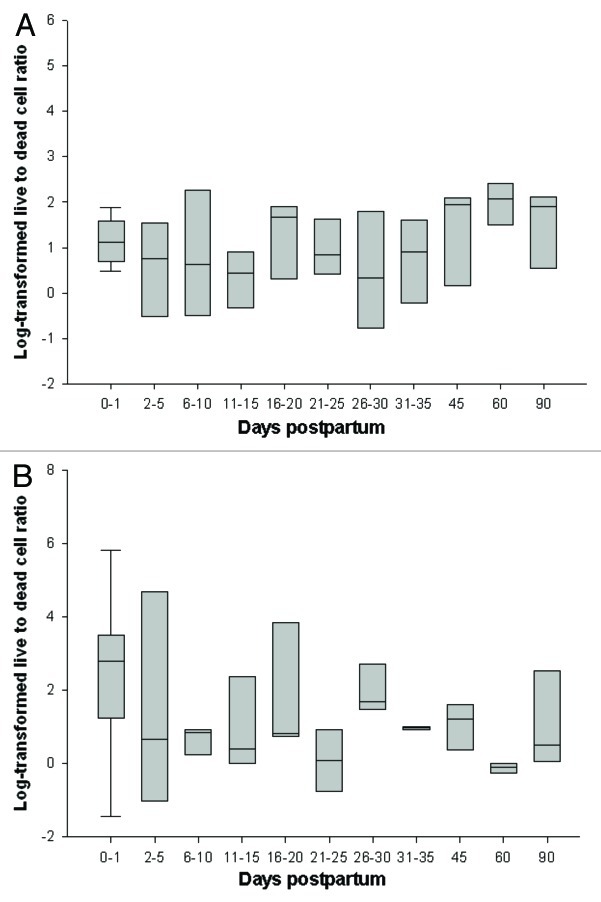
The number of fetal cells detected was consistently higher in the lungs [median (interquartile): 27 (13–51)] compared with bone marrow [7 (3–16); p < 0.0001], with a strong positive correlation between the total number of fetal cells in each organ for a given animal (r = 0.58, p = 0.0001). Over time, the ratio of live to dead cells in bone marrow significantly decreased (Fig. 3), particularly during early postpartum (r = -0.58, p = 0.0075) with a similar trend detected thereafter (r = -0.33, p = 0.16). An opposite pattern was observed for the lung with no change in the ratio of live to dead cells during early postpartum (r = -0.07, p = 0.74) followed by a significant increase between 18 and 90 d (r = 0.42, p = 0.035).
Figure 3. Ratio of log-transformed normalized live to dead cells in the maternal (A) lung and (B) bone marrow during the postpartum period. Horizontal lines represent median, box represents 25th to 75th percentile and whiskers represent 10th to 90th percentile.
After adjusting for the number of days postpartum, the number of pups in the litter negatively correlated with the total number of fetal cells in the bone marrow (r = -0.36, p = 0.028). There was no statistically significant relationship between litter size and cell number in the lungs (r = -0.19, p = 0.24).
Discussion
Here we report a two-stage pattern in the number of fetal cells in postpartum murine organs. The first stage is characterized by a rapid clearance of fetal cells during the two and a half weeks immediately following delivery. This is followed by a gradual trend through at least day 90 postpartum, and likely longer. Although the quick decline immediately postpartum has been previously reported in both humans and mice,1-3 the two-stage pattern is a novel finding. These results inform study design, and we recommend that murine injury experiments investigating the potential postpartum effects of fetal cells on maternal tissue repair should start at a minimum of 17–18 d after delivery to be sure that the initial clearance stage has been completed.
We recently showed that during pregnancy, there are at least three main types of fetal cells present in the maternal lungs: trophoblasts, mesenchymal stem cells (MSCs) and cells of the immune system.19 In humans, trophoblasts are found in the maternal lung during pregnancy but are quickly cleared postpartum.20,21 It is therefore possible that the rapid postpartum decline may represent the clearance of trophoblasts, while the longer-term persistent cells observed after days 17–18 may be MSCs and/or immune cells. Previous work showed that fetal cells have characteristics similar to both MSCs and hematopoietic stem cells.17 Future work should establish what cell types comprise the postpartum fetal cell population to test if this may be true.
We further showed a positive correlation between the number of fetal cells in the lung and bone marrow for an individual animal. There was substantial variability between individual animals, similar to other reports.3,22,23 The reason for this variability is not known at this time and raises important biological questions about fetal cell trafficking. The strong positive correlation between the number of fetal cells in each organ for an animal suggests that an animal with high levels of fetal cells in one organ will also have high levels in other organs. This may simplify study design by eliminating the need to test multiple organs in healthy animals when assessing levels of microchimerism.
In addition, we found the ratio of live to dead cells changes over time with different patterns observed for the lung and bone marrow cells. The increased presence of live fetal cells in the maternal lung suggests that the fetal cells may be actively dividing after days 17–18. Such a finding would have positive implications for the potential use of fetal cells to treat maternal disease17 or negative implications when females serve as organ donors due to the risk of graft vs. host disease.5 Future work could use a marker of DNA replication such as bromodeoxyuridine to determine what percent of the fetal cells are actively proliferating.
Finally, our results showed a negative correlation between litter size and microchimeric fetal cells in bone marrow, which did not reach statistical significance in lung. Previous work in our laboratory used a hemizygous GFP male so that approximately half of the pups inherited Egfp. A prior report showed a statistically significant positive association between the number of GFP+ fetuses and fetal cells in the lung and kidney.3 Other papers have found no significant relationship between litter size and fetomaternal cell trafficking.23 These conflicting results suggest that overall there may not be an effect, but that individual studies show an association due to small sample sizes. However, it is possible that an inverse relationship may reflect different efficiencies of fetal cell clearance. One limitation of determining the true effect of litter size on the microchimeric fetal cell population is that most studies count the number of pups that are delivered, not the number of pups present at the beginning of pregnancy. In order to thoroughly address this question, sonographic assessment of the number of pups, combined with measurement of the number of fetal cells in maternal blood, would need to be performed repeatedly during gestation.
In conclusion, in this paper we report on postpartum fetal cell trafficking in mouse lungs and bone marrow. We show that there is a rapid decline immediately after delivery and a slower decline after the second week. Fetal cells were present in both organs for the 90-d duration of the experiment, and they likely persist for an even longer amount of time in mice. Our results have implications for the design of postpartum injury experiments studying potential effects of fetal cells on maternal tissue repair in mouse models. Such experiments should start at least 17 to 18 d after delivery to be sure that the initial postpartum clearance is complete. Lastly, the increased ratio of live to dead cells in the maternal lung is suggestive of fetal cell proliferation, which, if proven, may impact women’s health following pregnancy.
Materials and Methods
Mice
The Institutional Animal Care and Use Committee of the Tufts University School of Medicine Division of Laboratory Animal Medicine approved all protocols. All institutional guidelines regarding the ethical use of experimental animals were followed. Male mice homozygous for the green fluorescent protein (Egfp) transgene [C57BL/6-Tg(CAG-EGFP)C14-Y01-FM131Osb (stock number 267, Riken BioResource Center, provided by Dr Masaru Okabe, mated in-house)] were mated to 10–12 week-old wildtype C57BL/6J females (stock no. 664, Jackson Labs). With the homozygous male, all pups inherit one copy of the Egfp transgene. Egfp expression was used as a marker for all fetal cells and is independent of gender. Female mice delivered Egfp+/− litters. The pups were counted and euthanized during the first week of life. A total of 52 postpartum females were housed for 0.5–91 d postpartum in standard conditions with food and water provided ad libitum.
Flow sorting of fetal cells in maternal lung and bone marrow
During the postpartum period, female mice were euthafnized. Bone marrow cells were isolated from bilateral femoral and tibial bones. The marrow cavities were flushed with flow cytometry buffer (Dulbecco PBS with 2% bovine serum albumin and 0.1% sodium azide). The lungs were collected as previously described.19 Briefly, the thoracic cavity was opened and the pulmonary vasculature was perfused with ice-cold Dulbecco PBS. The lungs were harvested. A single cell suspension was created from each lung pair using a GentleMACS dissociator (Miltenyi Biotech) following the manufacturer’s protocol. Cell suspensions from both organs were filtered, spun at 300 × g and resuspended in flow cytometry buffer with 1.5 µg/ml PI to assess dead cells. On a MoFlo high-speed flow cytometer (DAKO), a 488 nm laser was used to excite the fluorophores. Green fluorescence was collected with a 530/40 filter, and PI with a 670/40 filter.
Quantification of fetal cells
GFP and PI gates (Fig. 1) were drawn after data collection using Summit V4.3 Build 2445 software (DAKO). Bone marrow and lungs from homozygous GFP+ mice were used as GFP+ controls. C57BL/6J virgin female lungs and bone marrow were used as a negative control and the GFP gate was drawn to exclude all cells in the virgin female samples (Fig. 1C). The PI gate was drawn by comparing stained and unstained lung and bone marrow control samples from both GFP+/+ and wildtype animals (Fig. 1A and B). To account for differences in the total number of cells between organs and different animals, the total number of GFP+ fetal cells (Fig. 1D) in each sample is given as the number of fetal cells per 1 × 107 total cells. Dead fetal cells were defined as cells that were both GFP+ and PI+ (Fig. 1E). Living fetal cells were GFP+ and PI- (Fig. 1F).
Statistical analysis
Fetal cell counts were compared between the lung and bone marrow using the Wilcoxon rank-sum test to address a skewed distribution of the data. Partial Spearman correlation analysis was used to detect the association of fetal cell counts with duration of postpartum and litter size. All statistical analyses were performed using SAS/STAT software (SAS Institute, Inc.). Statistical significance was assigned at p < 0.05. A regression model was fit to the log-transformed fetal cell counts for each organ in order to describe the relationship with duration of postpartum.
Acknowledgments
The authors would like to thank Steven Kwok and Allen Parmalee for assistance with flow cytometry. This work was funded by R01 HD-049469-05 to D.W.B., T32 HD-049341-05 to D.W.B., The Provost Fund at Tufts University to D.W.B., Sackler Dean’s Fellowship Award to S.P. and UL1TR000067 to I.P.
Glossary
Abbreviations:
- Egfp
enhanced green fluorescent protein
- GFP
green fluorescent protein
- PI
propidium iodide
- MSC
mesenchymal stem cells
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/chimerism/article/22769
References
- 1.Ariga H, Ohto H, Busch MP, Imamura S, Watson R, Reed W, et al. Kinetics of fetal cellular and cell-free DNA in the maternal circulation during and after pregnancy: implications for noninvasive prenatal diagnosis. Transfusion. 2001;41:1524–30. doi: 10.1046/j.1537-2995.2001.41121524.x. [DOI] [PubMed] [Google Scholar]
- 2.Kolialexi A, Tsangaris GT, Antsaklis A, Mavroua A. Rapid clearance of fetal cells from maternal circulation after delivery. Ann N Y Acad Sci. 2004;1022:113–8. doi: 10.1196/annals.1318.018. [DOI] [PubMed] [Google Scholar]
- 3.Fujiki Y, Johnson KL, Tighiouart H, Peter I, Bianchi DW. Fetomaternal trafficking in the mouse increases as delivery approaches and is highest in the maternal lung. Biol Reprod. 2008;79:841–8. doi: 10.1095/biolreprod.108.068973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bianchi DW, Zickwolf GK, Weil GJ, Sylvester S, DeMaria MA. Male fetal progenitor cells persist in maternal blood for as long as 27 years postpartum. Proc Natl Acad Sci U S A. 1996;93:705–8. doi: 10.1073/pnas.93.2.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bianchi DW, Fisk NM. Fetomaternal cell trafficking and the stem cell debate: gender matters. JAMA. 2007;297:1489–91. doi: 10.1001/jama.297.13.1489. [DOI] [PubMed] [Google Scholar]
- 6.Srivatsa B, Srivatsa S, Johnson KL, Samura O, Lee SL, Bianchi DW. Microchimerism of presumed fetal origin in thyroid specimens from women: a case-control study. Lancet. 2001;358:2034–8. doi: 10.1016/S0140-6736(01)07099-4. [DOI] [PubMed] [Google Scholar]
- 7.Johnson KL, Samura O, Nelson JL, McDonnell M d WM, Bianchi DW. Significant fetal cell microchimerism in a nontransfused woman with hepatitis C: Evidence of long-term survival and expansion. Hepatology. 2002;36:1295–7. doi: 10.1053/jhep.2002.35622. [DOI] [PubMed] [Google Scholar]
- 8.Santos MA, O’Donoghue K, Wyatt-Ashmead J, Fisk NM. Fetal cells in the maternal appendix: a marker of inflammation or fetal tissue repair? Hum Reprod. 2008;23:2319–25. doi: 10.1093/humrep/den261. [DOI] [PubMed] [Google Scholar]
- 9.Kara RJ, Bolli P, Karakikes I, Matsunaga I, Tripodi J, Tanweer O, et al. Fetal cells traffic to injured maternal myocardium and undergo cardiac differentiation. Circ Res. 2012;110:82–93. doi: 10.1161/CIRCRESAHA.111.249037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khosrotehrani K, Reyes RR, Johnson KL, Freeman RB, Salomon RN, Peter I, et al. Fetal cells participate over time in the response to specific types of murine maternal hepatic injury. Hum Reprod. 2007;22:654–61. doi: 10.1093/humrep/del426. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen Huu S, Oster M, Uzan S, Chareyre F, Aractingi S, Khosrotehrani K. Maternal neoangiogenesis during pregnancy partly derives from fetal endothelial progenitor cells. Proc Natl Acad Sci U S A. 2007;104:1871–6. doi: 10.1073/pnas.0606490104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sunami R, Komuro M, Yuminamochi T, Hoshi K, Hirata S. Fetal cell microchimerism develops through the migration of fetus-derived cells to the maternal organs early after implantation. J Reprod Immunol. 2010;84:117–23. doi: 10.1016/j.jri.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Bonney EA, Matzinger P. The maternal immune system’s interaction with circulating fetal cells. J Immunol. 1997;158:40–7. [PubMed] [Google Scholar]
- 14.Vernochet C, Caucheteux SM, Kanellopoulos-Langevin C. Bi-directional cell trafficking between mother and fetus in mouse placenta. Placenta. 2007;28:639–49. doi: 10.1016/j.placenta.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 15.Zeng XX, Tan KH, Yeo A, Sasajala P, Tan X, Xiao ZC, et al. Pregnancy-associated progenitor cells differentiate and mature into neurons in the maternal brain. Stem Cells Dev. 2010;19:1819–30. doi: 10.1089/scd.2010.0046. [DOI] [PubMed] [Google Scholar]
- 16.Khosrotehrani K, Johnson KL, Cha DH, Salomon RN, Bianchi DW. Transfer of fetal cells with multilineage potential to maternal tissue. JAMA. 2004;292:75–80. doi: 10.1001/jama.292.1.75. [DOI] [PubMed] [Google Scholar]
- 17.Pritchard S, Hoffman AM, Johnson KL, Bianchi DW. Pregnancy-associated progenitor cells: an under-recognized potential source of stem cells in maternal lung. Placenta. 2011;32(Suppl 4):S298–303. doi: 10.1016/j.placenta.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujiki Y, Tao K, Bianchi DW, Giel-Moloney M, Leiter AB, Johnson KL. Quantification of green fluorescent protein by in vivo imaging, PCR, and flow cytometry: comparison of transgenic strains and relevance for fetal cell microchimerism. Cytometry A. 2008;73:11–118. doi: 10.1002/cyto.a.20533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pritchard S, Wick HC, Slonim DK, Johnson KL, Bianchi DW. Comprehensive analysis of genes expressed by rare microchimeric fetal cells in the maternal mouse lung. Biol Reprod. 2012;87:42. doi: 10.1095/biolreprod.112.101147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Attwood HD, Park WW. Embolism to the lungs by trophoblast. J Obstet Gynaecol Br Commonw. 1961;68:611–7. doi: 10.1111/j.1471-0528.1961.tb02778.x. [DOI] [PubMed] [Google Scholar]
- 21.Chamley LW, Chen Q, Ding J, Stone PR, Abumaree M. Trophoblast deportation: just a waste disposal system or antigen sharing? J Reprod Immunol. 2011;88:99–105. doi: 10.1016/j.jri.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 22.Khosrotehrani K, Johnson KL, Guégan S, Stroh H, Bianchi DW. Natural history of fetal cell microchimerism during and following murine pregnancy. J Reprod Immunol. 2005;66:1–12. doi: 10.1016/j.jri.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 23.Kallenbach LR, Bianchi DW, Peter I, Stroh H, Johnson KL. Maternal background strain influences fetal-maternal trafficking more than maternal immune competence in mice. J Reprod Immunol. 2011;90:188–94. doi: 10.1016/j.jri.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]