Abstract
Recently, we reported microchimerism to be oppositely associated with maternal breast and colon cancer. In women with a blood test positive for male microchimerism the risk of breast cancer development was reduced to one third, whereas the risk of colon cancer was elevated 4-fold. In this article addendum, I report the survival of cases in the original study after being diagnosed with cancer. Despite small numbers, the analysis suggests that microchimerism may be positively associated with survival after breast and maybe colon cancer diagnosis. Despite the findings on colon cancer in our original report, I speculate whether microchimerism could have a general beneficial role in cancer, which in some sites may not be evident because an allogeneic maternal immune reaction hastens cancer development.
Keywords: breast cancer, colon cancer, microchimerism, survival, epidemiology, Denmark
Recently, we reported microchimerism to be oppositely associated with maternal breast and colon cancer. In women with a blood test positive for male microchimerism the risk of breast cancer development was reduced to one third, whereas the risk of colon cancer was elevated 4-fold.1 This study is the most recent in only a handful of investigations of the association between microchimerism and cancer,2-5 which generally point toward a possible beneficial role of microchimerism in cancer. I believe that this poorly characterized and seemingly important link deserves further attention. Therefore, I used data from our original study1 to investigate whether male microchimerism was associated with survival among women diagnosed with breast and colon cancer, respectively. I followed women from the time of cancer diagnosis to time of death, emigration or end of follow-up, whichever occurred first. Cause of death was classified according to the tenth revision of the International Classification of Diseases (ICD-10). Deaths from breast (ICD-10 code C50) and colon (ICD-10 code C18) cancer were considered events, whereas deaths from other causes entailed censoring at the time of death.
Women were followed for up to 13.5 y after their first cancer diagnosis. Of the 89 women diagnosed with breast cancer, eight (9.0%) died from breast cancer and three (3.4%) died from other causes. Two (25.0%) of the eight breast cancer deaths occurred in microchimerism positive, and six (75.0%) occurred in microchimerism negative women. Similarly, of the 67 women diagnosed with colon cancer, 22 (32.8%) died from colon cancer and 9 (13.4%) died from other causes. Twenty (90.9%) of the 22 colon cancer deaths occurred in microchimerism positive, and 2 (9.1%) occurred in microchimerism negative women. The survival of women diagnosed with breast and colon cancer, respectively, according to microchimerism status and time since cancer diagnosis, is depicted in Figure 1 below. To increase comparability between breast and colon cancer, Figure 1 depicts the survival of women aged 64 y at time of diagnosis (approximate mean in both groups). In microchimerism-positive women diagnosed with breast cancer, the 10-y survival was 95.8%, whereas the corresponding survival was 88.6% in microchimerism-negative women with breast cancer. No deaths from breast cancer occurred after approximately 4 y from the time of diagnosis. Overall comparison of the survival curves under adjustment for age at cancer diagnosis yielded a hazard ratio of 0.34 (95% confidence interval 0.06–1.78) for death due to breast cancer in microchimerism-positive compared with negative women diagnosed with breast cancer. In women diagnosed with colon cancer, the 10-y survival was 62.2% in microchimerism positive women and 68.4% in microchimerism negative women. The last death from colon cancer occurred approximately 8 y after time of diagnosis. The corresponding age-adjusted hazard ratio of death from colon cancer was 0.89 (95% confidence interval 0.20–3.92).
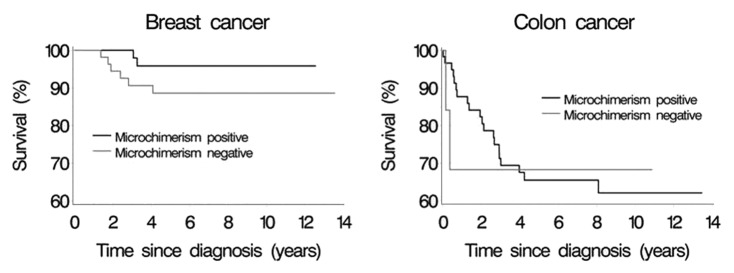
Figure 1. Survival among women with breast and colon cancer, respectively, according to microchimerism status and time since cancer diagnosis.
As expected, survival after diagnosis of breast cancer was found to be considerably better than that after colon cancer. Although not statistically significant, there was some indication that male microchimerism positivity was associated with better survival in women with breast cancer. Among women diagnosed with colon cancer, there was less indication that microchimerism status affected survival. If anything, survival was best in microchimerism-positive women. Few deaths occurred, especially among microchimerism-positive women with breast cancer and microchimerism-negative women with colon cancer. Therefore the contrast between microchimerism-positive and -negative women was poorly estimated after both breast and colon cancer. If truly associated, the improved survival among microchimerism-positive women presented here cannot be ascribed to more aggressive breast cancers in the microchimerism-negative compared with -positive women. This is true because adjustment for breast cancer stage did not change the estimated association. I did not have access to colon cancer stage at diagnosis, which is why I cannot rule out that a possible association between microchimerism and survival after colon cancer was masked by different distribution of aggressive cancers in microchimerism-positive and -negative women.
In the original report, we speculate about biologic mechanisms underlying the observed associations between microchimerism presence and development of maternal cancer. One such mechanism could be increased maternal immune reactivity. Breast cancer risk may be reduced in microchimerism-positive women because malignant cells are detected and destroyed at increased rates, or because chimeric cells participate in maternal tissue repair.6 Colon cancer risk, on the other hand, may be increased because of an allogeneic immune reaction against chimeric cells fused with the maternal colon, leading to chronic inflammation. This, in turn increases the risk of colon cancer.7 If so, however, the detrimental effect of the allogeneic immune reaction against the colon must exceed the beneficial effect of increased surveillance of malignant cells and/or tissue repair. Although numbers are small, the analyses presented here suggest that microchimerism may have beneficial effects on breast and maybe colon cancer survival. Despite the findings on colon cancer in our original report, I speculate whether microchimerism could have a general beneficial role in cancer, which in some sites may not be evident because an allogeneic maternal immune reaction hastens cancer development. It is my hope that future studies associating microchimerism and cancer at various sites will help clarify this.
Acknowledgments
This work was supported by the Danish Council for Independent Research, Medical Sciences (grant no. 10-093896).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/chimerism/article/22770
References
- 1.Kamper-Jørgensen M, Biggar RJ, Tjønneland A, Hjalgrim H, Kroman N, Rostgaard K, et al. Opposite effects of microchimerism on breast and colon cancer. Eur J Cancer. 2012;48:2227–35. doi: 10.1016/j.ejca.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 2.Gadi VK, Nelson JL. Fetal microchimerism in women with breast cancer. Cancer Res. 2007;67:9035–8. doi: 10.1158/0008-5472.CAN-06-4209. [DOI] [PubMed] [Google Scholar]
- 3.Gadi VK, Malone KE, Guthrie KA, Porter PL, Nelson JL. Case-control study of fetal microchimerism and breast cancer. PLoS One. 2008;3:e1706. doi: 10.1371/journal.pone.0001706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cirello V, Perrino M, Colombo C, Muzza M, Filopanti M, Vicentini L, et al. Fetal cell microchimerism in papillary thyroid cancer: studies in peripheral blood and tissues. Int J Cancer. 2010;126:2874–8. doi: 10.1002/ijc.24993. [DOI] [PubMed] [Google Scholar]
- 5.Gilmore GL, Haq B, Shadduck RK, Jasthy SL, Lister J. Fetal-maternal microchimerism in normal parous females and parous female cancer patients. Exp Hematol. 2008;36:1073–7. doi: 10.1016/j.exphem.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 6.Fugazzola L, Cirello V, Beck-Peccoz P. Fetal cell microchimerism in human cancers. Cancer Lett. 2009;287:136–41. doi: 10.1016/j.canlet.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 7.Ekbom A, Helmick C, Zack M, Adami HO. Increased risk of large-bowel cancer in Crohn’s disease with colonic involvement. Lancet. 1990;336:357–9. doi: 10.1016/0140-6736(90)91889-I. [DOI] [PubMed] [Google Scholar]


