The unexplained collapse of honeybee (Apis mellifera) colonies across the world continues to fascinate both the scientific and mainstream media alike. This is mainly due to the worldwide importance of honeybees in ecological and commercial sectors. We recently reported how the ectoparasitic mite, Varroa destructor, altered the viral landscape in the Hawaiian archipelago by decreasing the viral genetic diversity while increasing the prevalence of a particular virus species, deformed wing virus (DWV). Here we explain why DWV is now the most likely candidate responsible for the majority of the colony losses that have occurred across the world during the past 50 years.
Historically, extensive losses of honeybee colonies are not unusual and have occurred repeatedly over many centuries and locations (Oldroyd, PLoS Biol 2007). They all have in common the disappearance of adult worker bees, which can occur over periods of weeks or months. It is generally assumed that all honeybee collapses are considered to have the same underlying cause. Currently there are two main categories of colony collapse; those associated with the presence of the parasitic Varroa destructor (Varroa) mite (Fig. 1) and those where the mite is not directly involved, such as the recently observed “colony collapse disorder” (CCD) (e.g., Oldroyd, PLoS Biol 2007) and over-wintering colony losses (OCL) (Highfield et al., Appl Environ Microbiol 2009). Due to the economic importance of honeybees, considerable interest in honeybee toxicology and pathology have implicated various pests, pathogens, pesticides and even mobile phones in honeybee colony collapses (discussed in Martin et al., Science 2012).
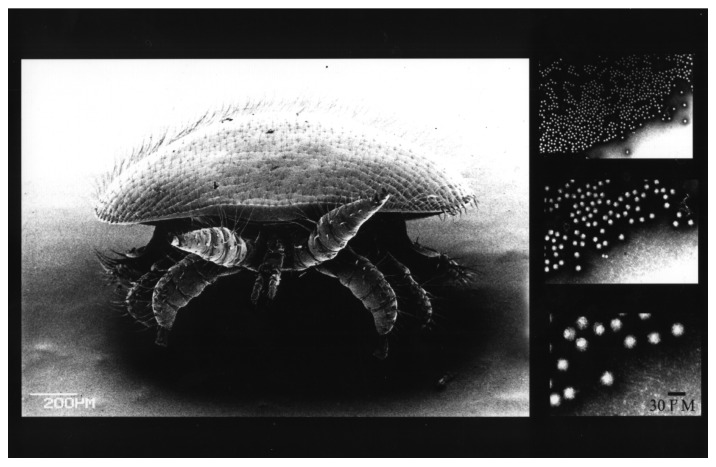
Figure 1. Electron micrographs of the ectoparasitic mite, Varroa destructor, and picorna-like virion particles of deformed wing virus (magnified series of three micrographs).
The collapse of millions of honeybee colonies associated with the global spread of Varroa has resulted in this pest being attributed to many of the overt colony collapses during the past 50 years. Numerous studies have revealed a complex association between Varroa, its honeybee host and a range of viral pathogens (e.g., Hung et al., Amer Bee J 1995; Martin J Appl Ecol 2001). However, Varroa has been ruled out as the cause of unexplained losses such as those attributed to CCD (Cox-Foster et al., Science 2007) and OCL (Highfield et al., Appl Environ Microbiol 2009). This is mainly due to the mite numbers remaining low in the collapsing colonies because of the various Varroa control measures applied by the beekeeper.
In honeybees, 18 viruses have been identified and physically characterized to date. Most resemble picorna-like viruses; 30 nm isometric particles containing single stranded positive sense RNA (Fig. 1). Five “species” have been routinely linked with honeybee colony collapses, in particular, acute bee paralysis virus (ABPV) and deformed wing virus (DWV) in Europe, and Kashmir bee virus (KBV), Israel acute paralysis virus (IAPV) and DWV in the US. It is now generally accepted that ABPV, IAPV and KBV all belong to the same cloud of ABPV variants (Baker and Schroeder, Virology J 2008; de Miranda et al., J Invert Pathol 2010) assigned to the family Dicistroviridae, order Picornavirales. ABPVs follow a classic acute-type infection strategy since relatively low loads (hundreds of viral particles per honeybee) can rapidly translate into overt symptoms of paralysis and ultimately death for the honeybee. Virulence is highly dependent on the mode of transmission and type of the variant present. Generally speaking, less than 100 APBV particles injected into a honeybee causes death within 4–8 days but 10,000,000,000 viral particles are required to kill a bee when introduced via an oral route i.e., feeding (Bailey and Gibbs, J Ins Pathol 1964). Modeling work (Sumpter and Martin, J Animal Ecol 2004) revealed that in the honeybee-Varroa-virus system, pathogens such as APBV with a high virulence can only kill a colony when very large mite populations (10,000–20,000) are present, since acutely virulent pathogens transmitted by the mites kill the adult bees on which they live (Fig. 2A) and the honeybee brood (Fig. 2B) on which they reproduce. Therefore, the death of the developing brood prevents any subsequent mite reproduction, as the mites die, entombed within the brood cells. This along with adults dying outside the colony helps break the viral transmission cycle (Fig. 3). It is therefore our contention that despite the acute virulent nature of the ABPV variants, they have never been consistently linked with colony collapse even in the presence of the Varroa mite.
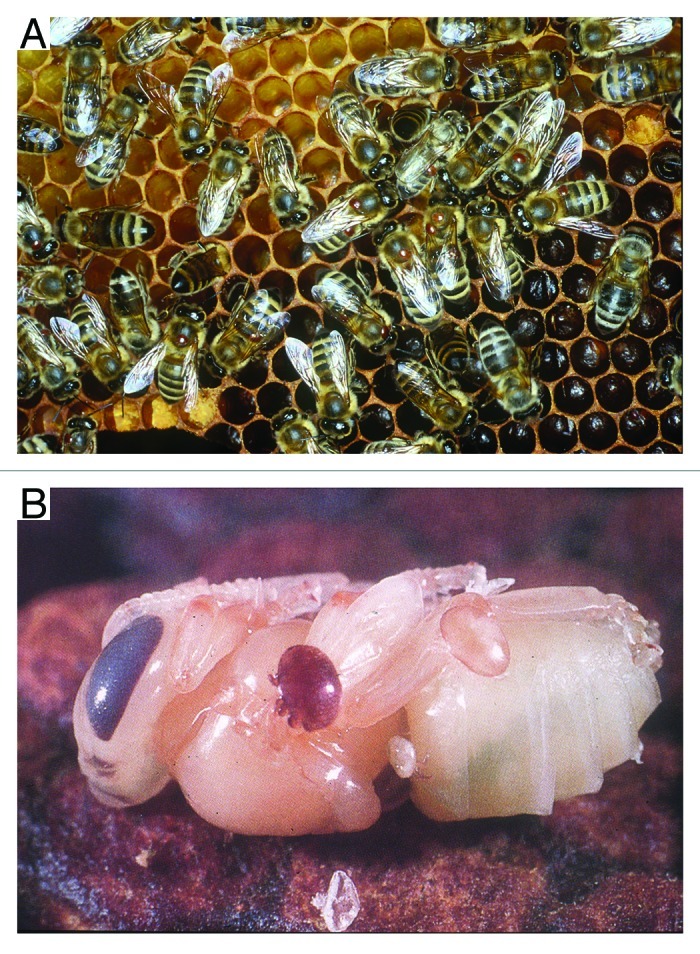
Figure 2. Photographs of Varroa destructor (brown reddish mites) feeding on (A) adult worker bees and (B) a developing pupa that was removed from its brood chamber.
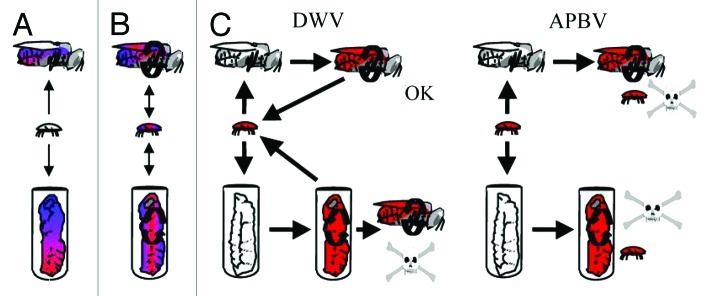
Figure 3. Role of Varroa in the transmission of deformed wing (DWV) and acute paralysis bee (APBV) viruses in the honeybee colony showing three basic phases. (A) Naturally diverse cloud of viral variants (red-blue) persisting at low levels in the honeybees. (B) Varroa feeds on an overtly infected bee introducing a new viral transmission route i.e., via injection. This over a period of years reduces the strain diversity selecting a genetically restricted cloud of highly virulent (red) strains. (C) The virulent DWV (red) strain selected by the Varroa-bee transmission cycle results in a reduced lifespan of bees infected as pupa, but not bees infected as adults, since they have a normal lifespan despite high viral loads. These adult bees become carriers, which aids the further spread of DWV to other colonies. However, in the acutely virulent ABPV, the virus quickly kills both the adult and pupa bee once the pathogen has been transmitted by the mite. This breaks the transmission cycle as the vector (mite) is also killed either by the adult bee dying away from the colony or been entombed within the brood cell.
DWV on the other hand, is a more persistent pathogen (Highfield et al., Appl Environ Microbiol 2009) that has become synonymous with the death of mite-infested colonies across the world. This pathogen is currently assigned to the family Iflaviridae within the order Picornavirales. It is generally considered to be much less a virulent pathogen compared with the ABPV variants since adult bees can survive for weeks with very high virus loads (Fig. 3). Although in some bees, DWV can cause overt symptoms of wing deformity resulting in emerging bees that are unable to fly. The appearance of overt symptoms is far more common in Varroa infested colonies since the wing deformity is associated with the viral load being injected in the developing pupae by the mite (Gisder et al., J Gen Virol 2009). In the numerous viral surveys conducted across the world, DWV is always the most prevalent viral pathogen in honeybee colonies especially when exposed to Varroa mites. Despite prevalence levels that normally exceed 90% (e.g., Baker and Schroeder, J Invert Pathol 2008), incidence of overt DWV-associated colony collapse is now low because of the numerous husbanded practices that control mite numbers. Nonetheless, when DWV is injected into developing pupa by the feeding activities of Varroa this causes a reduction in adult lifespan of 50–75% (Martin, J Appl Ecol 2001). Therefore, when Varroa mite infestation exceeds 2,000–3,000 during the autumn period, sufficient over-wintering bees become infected with DWV causing the colony to collapse during the long winter period. This is mainly due to the fact that no new brood is produced over this period to replace the dying infected over-wintering bees (Martin, J Appl Ecol 2001). However, in Africanized bees that only inhabit tropical and sub-tropical regions of the Americas they don’t suffer a similar fate despite being infested with both Varroa and DWV. This is because new bees are produced continuously throughout the year and this rapid turnover in bees results in mite populations needing to exceed 12,000 before a colony collapses (Sumpter and Martin, J Animal Ecol 2004). In addition, behavioral adaptations of Africanized bees help maintain mite populations to between 1,000 and 6,000 per colony (Martin and Medina, Trends Parasitol 2004). So in temperate regions the control of the Varroa numbers within a colony is widely regarded as being the only preventive tool against DWV-associated colony collapse.
A key discovery from the Hawaiian study was an improved understanding of the role of Varroa in changing the viral landscape. Varroa increased both DWV prevalence and titer among the honeybee population. While this was already a well-established fact (Carreck et al., J Apicultural Res 2010), our new discovery revealed that the Varroa-honeybee transmission cycle vastly reduced a wide range of naturally occurring DWV variants to only a small subset. Moreover, these remaining DWV variants can now be found almost universally throughout the world, which raises important questions around DWV virulence. For example, the Varroa-DWV association causes wing deformities in only a small proportion of honeybees. This is despite otherwise seemingly healthy asymptomatic honeybees containing extremely high DWV loads (levels that would typically be associated with wing deformity). However, in these asymptomatic bees, DWV can affect learning behavior (Iqbal and Mueller, Proc Biol Sci 2007), aggressiveness (Fujiyuki et al., J Virol 2004) and lifespan (Martin, J Appl Ecol 2001). In the absence of Varroa, DWV have been implicated in colony death in at least five cases to date. In addition, the DWV group of variants is now known to infect a wide range of insect hosts, including beetles, ants, other bees, wasps and hoverflies (Evison et al., PLoS ONE 2012). Their true impact within asymptomatic honeybees and other insects is largely unknown. The observations from the Hawaiian study indicate that the DWV quasi-species consists of a very large cloud of variants that are capable of infecting a wide range of hosts, and potentially tissue types that may help explain the wide range of observed pathologies. This further suggests that this pathogen is evolving at different rates resulting in varying levels of virulence that is not dependent on the Varroa-honeybee transmission cycle. Consequently, the introduction of Varroa into honeybee colonies may have been selected for particular virulent forms of DWV capable of causing colony collapse in effectively Varroa free colonies. This is however yet to be proven. If this link could be made then the common denominator for unexplained colony collapses worldwide such as OCL and potentially CCD could be due to the emergence of particularity virulent form(s) of DWV.
As history has shown us, in honeybees a highly virulent virus would only be transitory in nature. This is largely due to the limited exchange of bees between colonies, although current beekeeping practices would prolong such outbreaks as colonies are often maintained in artificially high densities. It is now vital that further studies into the role of DWV in the collapse of colonies, either dependent or independent of the mite-bee transmission cycle, investigate the link between clouds of DWV variants and their virulence, to fully understand the continual evolution of an emerging pathogen whose effect may extend well beyond honeybees.
Footnotes
Previously published online: www.landesbioscience.com/journals/virulence/article/22219