Effective treatment of many infections has progressively become more difficult due to the worldwide increase in antimicrobial resistance and the paucity of new antimicrobial development. Urinary tract infections (UTIs) are among the most common infections afflicting primarily women, and often resulting in recurrences or chronic infections that require frequent retreatment or long-term prophylaxis with antimicrobials. Either approach exposes patients to frequent antimicrobial use and its consequences. This is leading to a serious medical impasse requiring innovative therapeutic strategies. Uropathogenic Escherichia coli (UPEC) are the predominant UTI causative agent. This article highlights work that we have performed, distinguishing the contribution of two UPEC virulence determinants, the QseBC two-component system and type 1 pili, in pathogenesis. We discuss our findings on the impact of QseC disruption alone and in the presence of mannosides, orally bioavailable small-molecular weight inhibitors of the FimH adhesin that are highly efficacious in the treatment of UTI in a murine model. Our work provides insights toward the development of alternative therapeutics for UTIs.
Urinary tract infections (UTI) are among the most common bacterial infections, accounting for over 15 million cases annually and over 3.5 billion dollars in health care costs in the US alone, due to their prevalence and tendency toward recurrence and/or chronicity (Foxman, Nat Rev Urol 2010). More than 85% of UTI are caused by uropathogenic Escherichia coli (UPEC) (Griebling 2007, Chapter 18 in Urol. Diseases in America). During infection, UPEC utilize the FimH adhesin at the tip of type 1 pili to bind mannosylated receptors on bladder epithelial cells, mediating colonization and invasion of the host bladder (Thankavel et al., J Clin Invest 1997; Martinez et al., EMBO J 2000; Hung et al., Mol Microbiol 2002; Bouckaert et al., Mol Microbiol 2005). Internalized UPEC avoid TLR-4 mediated exocytosis by escaping into the host cell cytoplasm, where they replicate into biofilm-like intracellular bacterial communities (IBC) (Anderson et al., Science 2003; Justice et al., Proc Natl Acad Sci U S A 2004; Bishop, et al., Nat Med 2007). Upon IBC maturation, bacteria detach from the IBC biomass, flux out of the infected cell and spread to naïve epithelial cells capable of re-initiating the IBC developmental cycle (Justice et al., Proc Natl Acad Sci U S A 2004). Thus, IBC formation allows bacteria to evade extracellular host defenses, while rapidly expanding in numbers (Anderson et al., Science 2003). Exfoliated bladder epithelial cells containing IBCs have been observed in urine obtained from women with recurrent UTIs but not in healthy controls or in cases of UTI caused by gram-positive pathogens (Rosen et al., PLoS Med 2007). Mutations in fimH are postulated to drive the evolution of UPEC strains and those mutations that confer a fitness advantage within the urinary tract are selected in this body habitat (Chen et al., Proc Natl Acad Sci U S A 2009). Recent studies have also demonstrated that higher IBC numbers correlate with higher frequency of chronic UTI, indicating that IBC formation is a critical determinant for persistence (Schwartz et al., Infect Immun 2011).
Antibiotics are currently the primary course of treatment for UTI; however, given the impact on microbiota and the rise in antibiotic resistant uropathogens, finding novel treatment strategies is becoming a pressing issue. Type 1 pili mediate colonization and invasion of the bladder and thus, previous studies in our lab focused on the development of innovative compounds, mannosides ZFH-02056 and ZFH-04269, which attenuate UPEC virulence in a murine model, by specifically blocking FimH binding to bladder epithelial cells, thereby preventing bacterial adherence, invasion and IBC formation (Han et al., J Med Chem 2010; Cusumano et al., Sci Transl Med 2011). In particular, we have shown that ZFH-02056 and ZFH-04269 dramatically reduce the bacterial bladder population when dosed orally in mice, showing great potential in treating chronic cystitis and preventing acute UTI (Cusumano et al., Sci Transl Med 2011). Moreover, ZFH-02056 potentiated TMP-SMZ efficacy against PBC-1, a strain clinically resistant to TMP-SMZ (Cusumano et al., Sci Transl Med 2011). Thus, by preventing bacterial invasion of bladder epithelial cells, mannosides compartmentalize UPEC to the bladder lumen, where antibiotic concentrations are well above the minimum inhibitory concentration (MIC), resulting in bacterial death (Cusumano et al., Sci Transl Med 2011). Translated to clinical practice, mannosides could be a cost-effective treatment that lowers the antibiotic resistance rate, which is as high as 30% in some cases (van der Starre et al., Curr Infect Dis Rep 2011). Consequently, among the ongoing efforts in our laboratories is to optimize and/or potentiate mannoside efficacy.
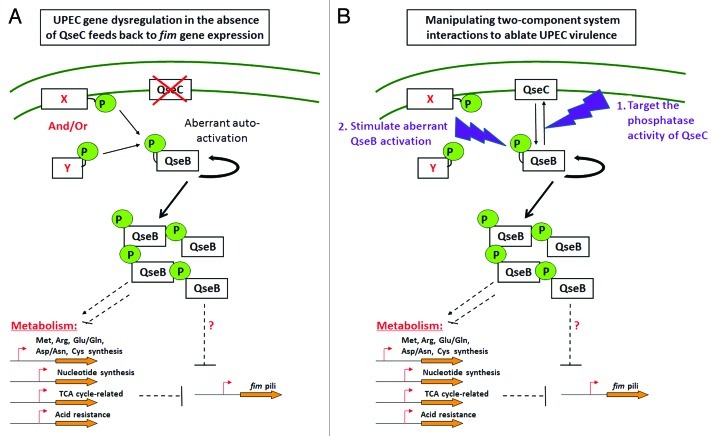
We have previously shown that the QseBC two-component system (TCS) is implicated in UPEC virulence gene regulation (Kostakioti et al., Mol Microbiol 2009; Hadjifrangiskou et al., Mol Microbiol 2011). More specifically, deletion of the QseC sensor kinase leads to aberrant activation of the QseB response regulator, which in turn interferes with the canonical progression of central metabolic processes, thereby impacting virulence gene expression and causing UPEC attenuation (Kostakioti et al., Mol Microbiol 2009; Hadjifrangiskou et al., Mol Microbiol 2011) (Fig. 1A). We have shown that QseC is a bi-functional sensor kinase/phosphatase and that its phosphatase activity is imperative for maintaining the equilibrium between QseB phosphorylated and dephosphorylated states (Kostakioti et al., Mol Microbiol 2009). In the absence of QseC, QseB can still become phosphorylated via an alternative phosphor-donor, but cannot become dephosphorylated and deactivated. Given that active QseB can upregulate its own expression (Clarke and Sperandio, Mol Microbiol 2005), deletion of QseC triggers a positive feedback loop that operates uncontrolled, leading to accumulation of phosphorylated QseB and dysregulation of target genes (Kostakioti et al., Mol Microbiol 2009) (Fig. 1A). Thus, ablation of QseC function tips the balance toward unidirectional QseB phosphorylation and activation, causing virulence attenuation. These attributes make QseC an attractive drug target (Fig. 1).
Figure 1. Two-component system interactions as drug targets. Model depicting perturbations in gene expression upon deletion of the qseC sensor in UPEC and how these perturbations could be exploited toward ablating UPEC virulence. (A) In the absence of QseC, the QseB response regulator becomes readily phosphorylated by another phosphodonor molecule that cannot adequately dephosphorylate and deactivate QseB. As a result, phosphorylated QseB accumulates at higher than normal levels, leading to dysregulation of multiple genes (representative examples are highlighted) including type 1 pili, and causing virulence attenuation. The observed downregulation of type 1 pili stems, at least in part, from downregulation of TCA cycle genes. We have currently no evidence that QseB directly modulates expression of type 1 pili. (B) The pathway linking QseBC to fim gene expression can be exploited for the development of alternative therapeutics: (1) deletion of the QseC sensor can be recapitulated by identifying inhibitors that interfere with the QseC phosphatase activity, thereby enabling the QseB over-activation; (2) irreversible QseB activation may be chemically stimulated in the presence of QseC.
Interestingly, type 1 pili are among the factors downregulated upon qseC deletion as a result of the aberrant QseB activity (Kostakioti et al., Mol Microbiol 2009; Hadjifrangiskou et al., Mol Microbiol 2011). More specifically, in the absence of qseC, the invertible fim promoter element switches to the OFF orientation, resulting in reduced type 1 pili expression. The prominent role of type 1 pili in UPEC pathogenesis prompted us to investigate the extent to which the QseB-mediated effects in the qseC deletion mutant are attributed to the observed type 1 pili defects. In the work published in Infection and Immunity (Kostakioti et al., Infect Immun 2012), we discern the type 1 pilus-dependent and independent effects that contribute to the virulence attenuation of a UPEC qseC deletion mutant, using a murine model of experimental UTI. We performed these studies using isogenic parent and ΔqseC strains in which the fim promoter was genetically tethered in the ON configuration. We went on to show that these strains were not over-producing type 1 pili, indicating that besides promoter inversion, additional levels of transcriptional control exist that ensure proper expression of type 1 pili. Given that deletion of qseC only affects promoter inversion, the ΔqseC “locked-on” mutant expressed type 1 pili in a manner that was similar to the isogenic wild-type strain, while it retained the remaining reported ΔqseC phenotypic defects. Using these strains we demonstrated that although restored type 1 pilus expression in the ΔqseC mutant rescued the ability of this strain to colonize the host and initiate acute infection up to 16 h post-infection, it was unable to facilitate persistence in the urinary tract, exhibiting a diminished capacity to establish chronic infection. Moreover, the ΔqseC “locked-ON” mutant was rapidly outcompeted during acute infection when co-inoculated with a wild-type strain, indicating that type 1 pili-independent effects in the absence of QseC contribute to UPEC virulence past the acute checkpoint of infection.
In order to investigate the potential of co-targeting these UPEC virulence determinants, we assessed the effects of a prophylactic oral dose of one of our most potent mannosides, ZFH-02056, on the ability of the qseC mutant to establish chronic infection. Our findings indicated that although either mannoside treatment or loss of QseC alone significantly reduced chronic colonization of the bladder by UPEC, loss of QseC in combination with mannoside treatment resulted in an additive effect thereby potentiating bacterial clearance from the bladder. Collectively, our work indicates that mannosides represent an interesting therapeutic compound for the treatment and prevention of UTIs and that compounds targeting QseC may represent an alternative that could act synergistically with mannosides for combating UTIs.
Many studies have focused on inhibiting virulence by interfering with bacterial signaling cascades, given that two-component systems constitute a central means of intercepting and translating environmental changes (Cegelski et al., Nat Rev Microbiol 2008; Rasko et al., Science 2008; Njoroge and Sperandio, EMBO Mol Med 2009). Inhibition of signal transduction systems poses an attractive means of anti-virulence therapy, by de-programming optimal gene expression and ablating virulence. Previous studies investigating the potential of inhibiting the QseC kinase activity in enterohemorrhagic E. coli identified LED209 as a promising compound (Rasko et al., Science 2008). We have shown that in UPEC, LED209 does not impact virulence-associated gene expression and pathogenesis. These data are not surprising given that the phosphatase function of QseC is critical for maintaining QseB activity under control in both UPEC and EHEC, and our analyses revealed that LED209 has no inhibitory effect on the phosphatase activity of QseC. Thus targeting QseC phosphatase activity would be an optimized strategy to decouple normal gene expression in QseC-bearing pathogens (Fig. 1B). Other possible intervention points in the QseBC pathway that could be exploited for the development of novel therapeutics may also be considered, such as stimulation of irreversible QseB activation.
Our findings identify a new area of research targeting the QseC phosphatase function, either on the enzymatic level or in its interaction with QseB. As is the case with FimH inhibitors, identifying specific QseC inhibitors would constitute a new and exciting avenue for the development of therapeutics that could be used to potentiate or replace current strategies. Such studies are currently underway in our laboratories.
Footnotes
Previously published online: www.landesbioscience.com/journals/virulence/article/22364