Abstract
It is well known that helminth parasites have immunomodulatory effects on their hosts. They characteristically cause a skew toward TH2 immunity, stimulate Treg cells while simultaneously inhibiting TH1 and TH17 responses. Additionally, they induce eosinophilia and extensive IgE release. The exact mechanism of how the worms achieve this effect have yet to be fully elucidated; however, parasite-derived secretions and their interaction with antigen presenting cells have been centrally implicated. Herein, we will review the effects of helminth excretory-secretory fractions on dendritic cells and discuss how this interaction is crucial in shaping the host response.
Keywords: ES, helminth, dendritic cell, Th2, TLR, parasite
Introduction
Parasitic worms have co-evolved with vertebrate hosts over millions of years. As a result, they have developed numerous survival strategies including potent factors for immunomodulation. Infections can be long-lived and comprise complex and morphologically distinct life cycle stages. Worms display multiple mechanisms not only to evade host immunity for maturation and transmission, but also to preserve the host long enough for them to do so. Their success depends not only on their specialized ways of colonizing their host but also on host-specific genetic and immunological factors. Differences in worm burden among communities with equivalent levels of exposure has been repeatedly noted for a variety of helminth infections in both animal models and humans1-3 with familial patterns pointing to a genetic link to susceptibility. For example, linkage analysis has highlighted three genes with strong associations to Ascaris worm burden in humans,4 TNSF13B and ABF1, a TNF family member and transcription factor, respectively, that play central roles in B cell regulation, as well as IL-7, a cytokine influencing mucosal lymphocytes whose production is associated with protection against intestinal worms.5 These gene products have perceptible links to adaptive immunity pointing to both genetic and immunological factors as determinants of the course and severity of infection.
Helminths affect the immune system differently to other pathogen classes. They present as large multicellular entities that cannot be readily phagocytosed yet they elicit a strong adaptive humoral response.6 This point begs the question of how their components enter the antigen presentation pathway. The answer likely lies within worm-derived secretions, namely the excretory-secretory fraction (ES), components of which are released into the host throughout the life cycles of most parasitic helminths.
The use of “excretory-secretory” to describe the mixture of material released by helminths into their host organism fails to distinguish between components that are actively secreted and those that are released as a consequence of physiological processes such as egg laying and digestion. Individual characterization of components that are purposefully host-targeted may be more useful in understanding a given worm’s adaptation to its developmental milieu, but the immunological affront inflicted on the host during infection derives from both passively and actively secreted antigens. Although ES composition of parasitic worms of different taxonomies varies significantly, secretions have been found to contain a mixture of glycoproteins, proteins, glycolipids, and polysaccharides.7 Effects of ES on cellular physiology are vast, but here we will focus on what is known about ES influence on dendritic cells and how these elements are able to direct adaptive immunity.
Helminth ES
Parasitic worms can progress through intricate life cycles stages, occupying distinct niches in the host and taking vastly different physical forms. Echinococcus granulosus, a cestode tapeworm, exists in the canine intestine as a segmented adult worm but once transferred to an intermediate host like a human, its larval stage develops into fluid filled cysts.8 Likewise, adult worms of the nematode Trichinella spiralis infect the intestinal tract whereas its larval stage encysts in striated muscle and can survive in this form for years.9 Each parasitic form and corresponding niche undoubtedly involve different types of interactions with the host immune system and distinct DC subtypes, a point that is reflected in the dynamic reshaping of surface molecules and secreted elements at each stage. The interactions of adult T. spiralis in the intestine vs. those of its intracellular larval stage with the host immune system are inevitably quite different. Therefore, the composition of its excretory-secretory fraction is dependent on how it needs to interface with its host to ensure progression to the next life-cycle stage.
Characterizing the ES and defining these interactions is an enormous challenge when dealing with organisms that cannot be modeled in vitro. In the example of Trichinella, adult worms can be extracted from the intestine and cultured for a few days as can larvae enzymatically digested out of muscle tissue. Neither of these stages truly reflects what is being secreted by the encysted larvae within the muscle tissue, which is arguably most biologically relevant. Many helminth parasites cannot be cultured at all, relying on mouse models and ex vivo studies to infer what is being released by the worms during natural infection. Furthermore, barring certain pioneering studies done in schistosomes,10,11 these worms are genetically intractable, posing an added challenge.
The viability of the worms in culture varies between organisms, for example T. spiralis L1 stage larvae will only survive 4–5 d in culture medium whereas adult Heligmosomoides polygrus may be cultured for up to 20 d. In all examples discussed, the environmental cues that would be present in the native system are absent and this will undoubtedly have an effect on the secretion and metabolic pattern of the parasite. Methods for collecting ES depend on the parasite, its life cycle and the form in which ES components are released, a recently discovered route being through exosomes.12 In some cases many stages of the parasite are accessible, whereas others prove extremely restrictive. Nippostrongylus brasiliensis is a rodent gastrointestinal nematode closely related to the human and sheep/goat hookworms. N. brasiliensis adults lay eggs in the gut that are excreted by the host. Eggs can be collected from feces and hatched. The larvae then develop from L1 to L3 (infective stage larvae) that can be cultured in liquid media and ES is collected from the supernatant. N. brasiliensis ES is therefore representative of the free-living stage that in nature enters the host via the skin and transits to the lungs. Adults may also be isolated from the host intestines and cultured for ES, representing parasitic components that encounter the intestinal microenvironment.
Ascaris lumbricoides, Ascaris suum and Toxocara canis are large intestinal roundworms that may be collected from the intestines or the feces of infected hosts. Adult (gut) L2 (circulating) and L3/4 (lung) stage parasites can also be extracted and cultured for ES.
Brugia malayi and Onchocerca volvulus are examples of filarial nematodes that are transmitted to humans via mosquito or blackfly vectors respectively. In the laboratory, adult parasites are collected from nodules in the lymphatic system of jird rodents and cultured for ES. Microfilariae and L3 larvae may also be isolated from the vector. Studies have demonstrated how filarial ES proteins vary between stages and are gender-specific.13,14
Various stages of the human blood fluke Schistosoma mansoni can be isolated for study. Eggs can be collected from the liver or feces of hosts and schistosomula can be recovered from lung tissue for ES collection. Adult schistosomes may also be collected by dissection or perfusion, but adult yields are low and the site of their residence varies between animals.
Often used in laboratory studies of cestodes are the tapeworms Echinococcus granulosus and Taenia solium, which also parasitize humans. Adult cestodes usually reside in the intestine of a definitive host, from which eggs are shed in the feces and ingested by an intermediate host. Parasites can be dissected out of the hydatid cysts of intermediate hosts and cultured, as can eggs isolated from feces that hatch, releasing oncospheres from which ES can be collected. Occasionally adults may also be extracted from the gut of infected animals.
Since it is a challenge to purify ES that is representative of the life cycle stage of interest, it is reasonable that helminth extracts, which contain a high concentration of helminth antigens, are routinely prepared for immunological analyses. These extracts can be prepared after immediate isolation of the parasite, without culturing or manipulation, and have proven useful for learning more about important parasite antigens and their effect on the immune response. For example, soluble S. mansoni egg antigen (SEA) and soluble schistosomule antigen (SSA) are often used, as are whole extracts prepared from nematode and cestode parasites. Although a crude extract, this will contain products from the parasite secretory organs and therefore the ES products themselves, pre-secretion. They are also rich in parasite-specific modifications that decorate ES components. Lewisx, an abundant parasitic glycan, is found in SEA and SSA but also on omega-1 and α-1, glycoproteins that are amply secreted by schistosome eggs.15-17 Lex is also expressed by nematodes such as Dictyocaulus viviparus18 and antisera against structures containing Lex are raised during infection with Taenia crassiceps and T. spiralis.19,20 In addition, many helminths have been shown to express an enzyme responsible for the production of Lex.21-24
Therefore, many of the studies that have truly implicated ES in immune interactions, such as those with DCs, have either been done in vitro, or in vivo using elements shown to contain both secreted and non-secreted helminth components. For the purposes of this review, we include these studies since our current understanding of helminth-provoked effects on DCs are in large part owing to this work.
DC and Helminths: Th2 Polarizing Response
Many subtypes of DCs have been implicated in the host response to parasitic worms.25-29 Each is characterized by a basic combination of surface markers, however new subtypes and slight variants of known subtypes are constantly being discovered. The specific phenotypes differ based on host organism (mouse or human), whether a study was done in vitro or in vivo, what location or organ they are extracted from and how they were matured. The particular adaptations of DC subtypes to their distinct microenvironments makes studying them in situ of paramount importance to understanding their behavior. This point is particularly pertinent for helminth infections given their diverse tissue specificity. In light of the experimental obstacles outlined above, localized analysis of DC response to worms is often impossible. As a result, the majority of published work on this topic heavily relies on either ex vivo observations or monocyte and bone-marrow derived DCs and their characteristic plasticity, wherein their behavior can be largely driven by microbial cues.30
Parasitic worms have long been known to promote host immune responses heavily skewed toward the TH2 phenotype.31 Unlike viruses and bacteria, worms do not elicit release of pro-inflammatory cytokines and responses targeted toward killing the pathogen itself. Perhaps this phenomenon is partly due to the worms being large, multicellular entities difficult to damage via direct inflammatory mechanisms.7
Type 2 immunity to helminths revolves around the CD4+ T helper 2 cell, whereby depletion of these cells is associated with an inability to control32 or clear33 infection. Through cytokine secretions, this cell type is able to elicit a range of downstream responses, including B cell-derived antibody production and granulocyte-derived release of inflammatory effectors. In the context of a helminth infection, these events, in turn, result in both direct and indirect challenges against the pathogen. Immunoglobulins can directly opsonize antigens,6 while effector-induced muscle contractions and mucus release can work toward mechanically expelling the parasite.34,35
Why do helminths specifically drive TH2 responses? The answer to this question is still largely unknown. One hypothesis that has been suggested is that this happens as a default, a result of no TH1 polarizing stimuli on the part of worms.36 Another is that TH2 responses promote wound repair, important during worm infections where skin and gut epithelia are often damaged.37 The mechanisms behind TH2 polarization likely combine elements from both these hypotheses. In an effort to distill the underlying processes involved, much work has been done toward characterizing the host receptors and parasitic ligands responsible for initiating the immune response during helminth infections. These studies have collectively implicated a range of pattern recognition receptors (PRR) and identified the dendritic cell as an essential regulator of TH2 polarization. For instance, in vivo removal of DCs via CD11c-selected depletion results in a remarkable decrease in both TH2 cytokine release and expansion of T helper cells upon parasitic challenge.38 Conversely, adoptively transferred-DCs pulsed with ES antigens from the rodent nematode N. brasiliensis can efficiently drive TH2 responses in naïve recipient mice.28 A synergistic role for basophils in the cooperative generation of TH2 immunity to worms with DCs has also recently been described. However, despite their ability to antigen present, to expand T cell populations and to elicit production of regulatory cytokines, their presence seems to be dispensable to these processes, unlike that of DCs.39
How Do Dendritic Cells Interface with Parasite ES?
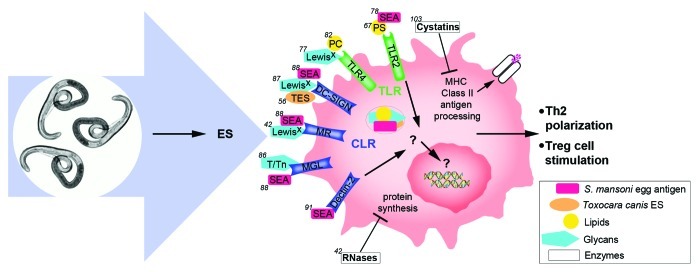
The main function of dendritic cells is to capture, process and present antigen to T cells, serving as mediators between innate and adaptive immunity. The mechanisms by which they detect antigen are manifold; however pattern recognition receptors (PRR) are particularly important to this process. These receptors comprise several molecular families including cytoplasmic DNA sensors, Toll-like (TLR), C-type lectin (CLR), NOD-like (NLR) and RIG-1-like receptors and have evolved to recognize pathogen-associated molecular patterns (PAMPs) in order to allow DCs the ability to rapidly detect and respond to foreign antigens. In the context of helminth infections, ES-derived and parasite surface molecules are the agents responsible for the contact and interaction with host immune cells (Fig. 1).
Figure 1. The molecular interactions involved in DC conditioning by components found in helminth excretory-secretory products (ES) and in S. mansoni soluble egg antigen (SEA) are illustrated. ES and SEA contain a mixture of immunogenic proteins and lipids, many of which are glycosylated. Many of the most potent antigens in SEA are also found in ES. Interactions are C-type lectin (CLR) or Toll-like receptor (TLR) dependent or pattern recognition receptor (PRR) independent. DCs are polarized toward a TH2 phenotype and Treg cells are stimulated. Each interaction is numbered based on the corresponding reference in which it was reported.
Parasite-derived signals
As previously mentioned, ES contains a complex mixture of proteins, lipids and metabolic by-products. Many of these proteins and lipids are highly glycosylated. It is widely understood that it is these carbohydrate moieties, or glycans, that are most important for the interface with host DCs.40 Evidence shows that the functional protein portion of the ES is not necessarily required for DC recognition but does play a role in the overall response. For example, heat treated ES from the nematodes Heligmosomoides polygyrus and Nippostrongylus brasiliensis is still able to elicit a response representative of Th2 polarization in mouse BMDCs.41 However, Everts et al. recently showed that although the carbohydrate domain alone of omega-1, a glycoprotein secreted from Schistosoma mansoni eggs, is sufficient for binding to DCs, the protein domain is still required for conditioning the Th2 response.42
Immunodominant helminth glycans were first identified, and have largely been characterized via the analysis of protective antibodies from the serum of immune animals.21 Lewisx or Lex was one of the first helminth glycan antigens to be described in the immune sera of mice infected with S. mansoni.43-45 Later, it was found that LacdiNAc (GalNAcβ1–4GlcNAc-R, LDN) and fucosylated LacdiNAc (LDNF) are also immunodominant glycoproteins and glycolipids from soluble extracts of S. mansoni and in immune sera to Trichinella spiralis and Hemonchus contortus.46-50 Lacto-N-fucopentaose III (LNFPIII) is found in the urine of S. mansoni infected animals and in those vaccinated with irradiated larvae51 but also in human milk and the urine of pregnant women. LNFPIII contains the terminal trisaccharride Lex. LDN and LDN-F share similar motifs to mammalian glycoconjugates and, along with LNFPIII, may be described as host-like helminth glycans. Other host-like helminth antigens include the T and Tn antigens which are GalNAc-O-Ser/Thr motifs often found on surface glycoproteins of mammalian cancer cells.52 T and Tn antigens are common to cestodes, nematodes and trematodes.53 There is some speculation that the expression of host-like antigens may aid protection of the parasite from immune attack and clearance.54,55 There are however helminth glycans that are parasite or even genus specific such as the O-methylated glycans from the nematode Toxocara canis56 and the glycan terminal tyvelose (3,6-dideoxy-D-arabino-hexose) from Trichinella spiralis.57-59 Alternatively, parasite glycans may be helminth-specific but common among different groups, such as the α(1–3)-linked core fucosylation of LacdiNAc60 and chitin, a polysaccharide that is found in the cuticle of parasitic and non-parasitic worms.61,62 Phosphorylcholine {PC, [(CH3)3N+CH2CH2 PO4−]} is a small zwitterionic molecule.63 It is found in the LPS of various pathogenic bacteria as well as helminth ES products such as ES-62, an abundant ES glycoprotein antigen from Acanthocheilonema viteae, a rodent filarial nematode. It is also found on the ES-62 homolog from Brugia pahangi and the N-acetylglucosaminyl-transferase from Brugia malayi and seems to be nematode specific.64-66 Other lipid antigens include phosphatidylserine (PS), which is found in S. mansoni and the dog heartworm Dirofilaria imitis.67,68
Host DC receptors: TLR
Although many helminth antigens have been identified, our knowledge of the host DC receptors responsible for their recognition, binding and internalization is more limited. There are 11 known mammalian TLRs that recognize bacterial, viral and fungal pathogen-derived antigens. TLR2, TLR4 and TLR9 are all expressed on DCs and have been well characterized for their interaction with potent TH1-inducing agents such as bacterial LPS. DC TLR2 has also been linked to TH2 immunity in response to certain pathogenic stimuli, and more recent evidence suggests that TLR4 may also be important for cross-talk between TH1 and TH2 immunity.69,70 Pre-treatment with helminth ES has been shown to inhibit classical LPS signaling through DC TLR receptors.71-74
In vitro activation of DCs by SEA and SSA has been shown to be TLR4 independent.75,76 In contrast, pulsing DCs with synthetic forms of the Lex containing carbohydrates LNFPIII and LNFPIII-Dex (both found in SEA and SSA) was shown to drive DC activation in a TLR4-dependent manner.77 Additionally, Gao et al. showed that TLR2 deficient mouse BMDCs were unable to produce IL-12p70 and IL-10 in response to SSA and SEA.78 This result was not observed in TLR4-deficient BMDCs, suggesting that TRL2 but not TLR4 is essential for the Th2-specific DC response to SSA and SEA but that LNFPIII alone may signal via TLR4.
ES-62 carries the phospholipid PC and is expressed by A. viteae in a stage-specific manner. A number of studies have evaluated the molecular interaction of ES-62 with DCs.64,79 ES-62 alone is able to stimulate DCs and induce a Th2 phenoype.80,81 This glycoprotein was found to signal via DC TLR4 and its adaptor MyD88 but not via TLR2.82 The mechanism of this interaction however was shown to be unconventional, i.e., mutated TLR4 that can no longer respond to LPS, still responds to ES-62. Further studies by the same group showed that the PC portion alone (as PC conjugated to ovalbumin) was still able to redirect DC maturation, and this effect was also found to be TLR4 and MyD88 dependent. The phospholipid PS from S. mansoni was also shown to activate DCs toward a Th2 phenotype, but unlike PC, it is most likely this occurs via TLR2 signaling.67 There is therefore a role for both TLR2 and TLR4 in the recognition of helminth antigens by DCs and there may be some interplay between the two receptors.
CLR
CLRs contain an extracellular carbohydrate recognition domain (CRD) that binds antigenic glycans, a process that requires calcium. DC CLRs that are involved in pathogen recognition include DC-specific ICAM-3 grabbing nonintegrin (DC-SIGN), which binds high mannose glycans.83 DC-SIGN plays an important role in viral recognition and binding to the HIV antigen gp 120 has been well characterized.84 DC-SIGN activation can in turn activate TLR signaling.85 Macrophage galactose binding lectin receptor (MGL) recognizes, among others, the helminth and tumor Tn antigen motif.86 Mannose receptor (MR) also recognizes mannose-containing glycans.54
Van Die et al. and others have shown that DC-SIGN is also a receptor for Lex and that antibodies against Lex inhibit the binding of SEA to DC-SIGN.83,87 The mechanism of binding, however, is not the same as that observed in the interaction between DC-SIGN and HIV gp120. The carbohydrate recognition domain (CRD) of DC-SIGN is still responsible for binding Lex, but site-directed mutagenesis of the receptor showed that the regions within the CRD that mediate interaction are not conserved. Furthermore, the internalization of SEA is not only mediated by DC-SIGN, but also by MGL and MR.88 DC-SIGN is also able to bind total ES products from Toxocara canis but not synthetic versions of the most abundant T. canis glycans.56 DC-SIGN, MGL and MR receptors appear to provide some form of redundancy whereby the inhibition of any one of these receptors at any one time still allows the helminth-associated activation of the DC.88 Everts et al. recently demonstrated that the MR, but not DC-SIGN, is responsible for the binding and internalization of the S. mansoni secreted glycoprotein omega-1 by DCs.42 Omega-1 is a T2 RNase enzyme decorated with a Lex-containing glycan.89 Although the carbohydrate domain alone is sufficient for binding DCs, the RNase activity of the glycoprotein is required to induce Th2 polarization in vitro. Another DC CLR receptor that has been shown to bind SEA is Dectin-2. Dectin-2 is a PRR whose role in fungal antigen recognition and innate immunity has been well characterized.90 Ritter et al. showed that the Dectin-2/FcRγ complex on BMDCs is required for SEA-induced production of the inflammatory cytokine IL-1β.91 This was not the case when Dectin-1, CD36 or indeed MR were tested. Interestingly, IL-1β production was not observed after heat or proteinase K inactivation of the SEA, again implicating an important role for the functional proteins in SEA.
What Happens to Dendritic Cells upon ES Exposure?
Of all antigen-presenting cells, DCs express the highest levels of MHC class II and are the most efficient processors of exogenous antigens. Once they have received the necessary stimuli via PRRs, DCs undergo a process of maturation to become efficient antigen presenters via a series of subcellular and morphological changes. They translocate class II MHC molecules loaded with antigenic peptides to the cell surface, they upregulate CD40, CD80 and CD86 costimulatory molecules on the cell surface, and they release pro-inflammatory cytokines and chemokines including IL-12, IL-6, IL-23 and TNF.92 These events enable the DC to communicate with other cells types and to determine the course of the immune response. Classically TLR-activated DCs will release IL-12 which signals effector T cells to expand and also recruits macrophages to the site of infection. In addition, IL-12 acts as a signal to neighboring cells to release other pro-inflammatory cytokines (IFN-γ and TNF-α) that drive a TH1 response.
In the context of helminth infections, DCs exhibit a very different phenotype. As a general rule, they fail to classically mature in that they do not upregulate coreceptors nor do they release pro-inflammatory cytokines.93 This phenomenon seems to be common to most helminth infections, irrespective of their taxonomic classification (nematode, cestode, trematode). DCs exposed to ES derived from Taenia crassiceps, a cestode tapeworm, fail to upregulate CD83, HLA-DR, CD80 or CD86.73 This immature phenotype persists even when DC are subsequently stimulated with LPS. DC stimulated with schistosome SEA exhibit the same behavior, with low expression of CD80, CD86, class II MHC and CD4094 and no IL-12 production. Like their cestode-exposed counterparts, they too fail to classically respond to LPS stimulation. Nematode ES also inhibits DC maturation, with ES from the filarial worm Brugia malayi blocking IL-12 production,95 and ES from Trichinella spiralis inhibiting both the upregulation of costimulatory molecules as well as LPS-responsiveness.96 By stimulating ES-exposed DC to various TLR agonists, the authors of this study show that the inhibitory effects of the T. spiralis secretions are specific to TLR4. However, TL4-mediated non-responsiveness in unlikely to be caused by differences in absolute levels of TLR4 surface expression since SEA-exposed DC, that also present with the same phenotype, have equivalent levels of this receptor on their surface as compared with unexposed controls.70 More likely, the downstream signaling pathways that are stimulated following helminth ES exposure are liable for the observed differences. TLR signaling is known to proceed via mitogen activated protein kinases (MAPK) ERK and p38. Induction of TH1 responses, namely the release of IL-12 by DCs, results from p38 phosphorylation, whereas the ERK pathway is associated with TH2 polarization via stabilization of the c-fos transcription factor that suppresses IL-12 release.97,98 Consistent with these signaling associations, SEA favors ERK phosphorylation.70,99 Therefore, the anti-TH1 bias may partially arise from the subcellular signaling cascades that are triggered upon ES exposure.
In addition to their rapid, PPR-induced activities, DCs ingest, process and present antigen to CD4+ helper T cells. Protein peptides are presented in association with class II MHC molecules, but glycolipids—which helminth ES is rich in—can also be presented via cell-surface CD1d.100 This protein seems to play a critical role in DC-mediated TH2 priming, since DCs from CD1d−/− mice were unable to drive expansion of SEA-specific TH2 lymphocytes.101 The fact that proper antigen processing and presentation are necessary components to an effective host response is another aspect of DC functionality with which parasitic worms can interfere. As mentioned above, helminth infections are associated with decreased MHC class II expression. Low levels of expression may either result from interference with protein synthesis and molecular assembly102 or from worm-induced inhibition of class II MHC-peptide complex translocation to the cell surface. In line with the latter idea, the rodent intestinal nematode Nippostrongylus brasiliensis secretes nippocystatin, a cysteine protease inhibitor that effectively decreases antigen processing. Furthermore, mice with anti-cystatin circulating antibodies are able to better control their infection103 suggesting that foreign peptides are not efficiently presented and downstream effector mechanisms that control worm burden are disrupted. Onchocerca volvulus and Brugia malayi are also known to secrete similar protease inhibitors which interfere with host endolysosomal proteases and potentially inhibit proper processing and loading of worm proteins onto class II MHC.104,105
How Do ES-Exposed Dendritic Cells Affect the Downstream Immune Response?
Despite the demonstrated necessity for DCs in the development of TH2-polarization to helminth infections, the specific signals they send and the mechanisms through which they interact with other cells are still quite poorly characterized. The host response to parasitic worms characteristically begins with an active TH2 effector phase that is then downregulated.106 The presence of certain ligands on the surface of DCs has been found to be essential for proper anti-helminth TH2 responses to develop. One such molecule is OX40L, where an OX40L−/− model demonstrated that expression of this ligand on DCs is central to the development of a TH2 effector response.107 Similarly, DCs derived from a CD40−/− mouse were also found to be deficient in their ability to induce TH2 responses.108 Both these studies were done in the context of S. mansoni infections, but given the consistency of the host response to parasitic worms irrespective of taxonomy, it is likely that the importance of CD40 and OX40L will carry over to other helminth infections as well. Another mechanism by which DC may polarize a TH2 response is through mechanical means.109 DC exposed to SEA fail to display the ruffled appearance and adherence of classically activated DC. This altered cytoskeletal morphology correlates with decreased ability to form stable conjugates with CD4 T cells. The authors of this study hypothesize that fewer interactions between DC and T cells sends a low-dose antigenic signal to DCs which is known to favor TH2 responses.110,111
As previously discussed, DCs are central to the initial TH2 polarization, but they are apparently also essential for subsequently moderating its strength.
DCs exposed to a wide array of helminth ES products have been shown to promote the expansion of CD4+CD25+Foxp3+ regulatory T cells.74,95,96,112 In turn, regulatory T cells are necessary for averting the pathology and tissue damage arising from an unchecked TH2 response in addition to contributing to the suppression of a TH1 response.113 DC matured in the presence of S. mansoni phosphatidylserine seem to acquire the capacity to potently drive naïve T-cells to become regulatory in nature67 and T. spiralis and H. polygrus stimulate expansion of existing Treg cell populations in vitro and ex vivo, respectively.96,114 DC conditioning by ES also results in release of regulatory cytokines such as IL-4 and IL-10. These cytokines are most commonly produced either by DC-stimulated T cells or by DCs themselves.99
Although helminths elicit Type 2 immunity, prolonged infection necessitates a subsequent dampening of these responses in order to preserve host integrity.115 Whether this downregulation is a direct effect of the parasite or, more likely, a defense mechanism against tissue damage on the part of the host, is not entirely clear.
Parasite ES contains host glycan “mimics” and their interaction with DCs are thought to play a central role in the downregulation of TH2 responses. These “self glycans” engage C-type lectins on the surface of DCs and stimulate expansion of T regulatory cell populations which, in turn, promote tolerance and downregulate inflammatory responses.54 Therefore, worm infections can help balance Th1-mediated pathologies given their Th2-promoting bias, and they can also control Th2-mediated conditions due to their stimulation of T regulatory populations.
Multiple sclerosis (MS) and murine experimental autoimmune encephalomyelitis (EAE), a mouse model system for MS, are both characterized by high Th1 and Th17 cell responses.116 EAE can be significantly suppressed by treatment with T. spiralis, S. mansoni and T. suis ES.117 Inhibition of DC-derived TNF-α and IL-12, coupled with upregulation of OX40L suggests that DCs may play a central role in the observed immune modulation. Likewise, DC-driven TH2 responses also seem to protect against development of Type 1 diabetes in non-obese diabetic (NOD) mice. If exposure to S. mansoni antigens is established at a young age, DCs stimulate IL-10 release and Treg cell expansion which protects against the otherwise spontaneous development of the disease.118,119
Several studies have shown an association between helminth infection and the suppression of Th2-driven allergic responses.120,121 Perhaps the most convincing evidence of a link comes from the central principles underlying the “hygiene hypothesis” which stipulate that industrialization—which brings a reduction in infections—is associated with corresponding increases in allergies and autoimmune diseases.122 For instance, it is known that infection with Ascaris suum suppresses allergic immune responses in mice123 in an IL-10 independent fashion. Reduced activation of DCs was deemed responsible for this difference, since cytokine production and receptor expression was suppressed in DCs exposed to parasite-derived products.
Conclusion
Although much remains to be discovered about the signals DCs receive and send in order to modulate the immune response during helminth infection, their behavior is incrementally being defined. Herein, we have attempted to comprehensively review these efforts, focusing on (1) the recognition elements between the parasites and the DC, (2) the subcellular changes these interactions induce within the DC and finally (3) how these changes translate into signals that drive TH2 polarization and subsequent control. Like all pathogens, worms have evolved skillful and innovative ways by which to modulate host immunity to their own benefit. They secrete ES whose glycosylated components regulate fundamental processes of antigen recognition, processing and presentation. By expressing parasite specific as well as “host” glycans, they induce DCs to stimulate both inflammation and tolerance. The ability to induce and then downregulate TH2 responses, which allows worms to establish persistent infections, can also be exploited therapeutically for certain allergic and autoimmune diseases. Further research is necessary to isolate specific components that can be used as “parasite therapeutics,” and unpair them from the pathology that also accompanies helminth infection. It is likely that DCs—and the molecular mechanisms by which they interact with parasite components—hold many more clues of how to attain this goal.
Acknowledgments
K.A.T. is supported by a Wellcome Trust Career Development Fellowship and R.R.W. by an MRC PhD studentship.
Footnotes
Previously published online: www.landesbioscience.com/journals/virulence/article/22832
References
- 1.Williams-Blangero S, McGarvey ST, Subedi J, Wiest PM, Upadhayay RP, Rai DR, et al. Genetic component to susceptibility to Trichuris trichiura: evidence from two Asian populations. Genet Epidemiol. 2002;22:254–64. doi: 10.1002/gepi.0187. [DOI] [PubMed] [Google Scholar]
- 2.Wassom DL, Brooks BO, Cypess RH, David CS. Trichinella spiralis: role of non-H-2 genes in resistance to primary infection in mice. Exp Parasitol. 1983;55:153–8. doi: 10.1016/0014-4894(83)90009-7. [DOI] [PubMed] [Google Scholar]
- 3.Quinnell RJ. Genetics of susceptibility to human helminth infection. Int J Parasitol. 2003;33:1219–31. doi: 10.1016/S0020-7519(03)00175-9. [DOI] [PubMed] [Google Scholar]
- 4.Williams-Blangero S, VandeBerg JL, Subedi J, Aivaliotis MJ, Rai DR, Upadhayay RP, et al. Genes on chromosomes 1 and 13 have significant effects on Ascaris infection. Proc Natl Acad Sci U S A. 2002;99:5533–8. doi: 10.1073/pnas.082115999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolowczuk I, Nutten S, Roye O, Delacre M, Capron M, Murray RM, et al. Infection of mice lacking interleukin-7 (IL-7) reveals an unexpected role for IL-7 in the development of the parasite Schistosoma mansoni. Infect Immun. 1999;67:4183–90. doi: 10.1128/iai.67.8.4183-4190.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harris N, Gause WC. To B or not to B: B cells and the Th2-type immune response to helminths. Trends Immunol. 2011;32:80–8. doi: 10.1016/j.it.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lightowlers MW, Rickard MD. Excretory-secretory products of helminth parasites: effects on host immune responses. Parasitology. 1988;96(Suppl):S123–66. doi: 10.1017/S0031182000086017. [DOI] [PubMed] [Google Scholar]
- 8.McManus DP, Zhang W, Li J, Bartley PB. Echinococcosis. Lancet. 2003;362:1295–304. doi: 10.1016/S0140-6736(03)14573-4. [DOI] [PubMed] [Google Scholar]
- 9.Despommier DD. Trichinella spiralis and the concept of niche. J Parasitol. 1993;79:472–82. doi: 10.2307/3283370. [DOI] [PubMed] [Google Scholar]
- 10.Skelly PJ, Da’dara A, Harn DA. Suppression of cathepsin B expression in Schistosoma mansoni by RNA interference. Int J Parasitol. 2003;33:363–9. doi: 10.1016/S0020-7519(03)00030-4. [DOI] [PubMed] [Google Scholar]
- 11.Krautz-Peterson G, Skelly PJ. Schistosome asparaginyl endopeptidase (legumain) is not essential for cathepsin B1 activation in vivo. Mol Biochem Parasitol. 2008;159:54–8. doi: 10.1016/j.molbiopara.2007.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marcilla A, Trelis M, Cortés A, Sotillo J, Cantalapiedra F, Minguez MT, et al. Extracellular vesicles from parasitic helminths contain specific excretory/secretory proteins and are internalized in intestinal host cells. PLoS One. 2012;7:e45974. doi: 10.1371/journal.pone.0045974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bennuru S, Semnani R, Meng Z, Ribeiro JM, Veenstra TD, Nutman TB. Brugia malayi excreted/secreted proteins at the host/parasite interface: stage- and gender-specific proteomic profiling. PLoS Negl Trop Dis. 2009;3:e410. doi: 10.1371/journal.pntd.0000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moreno Y, Geary TG. Stage- and gender-specific proteomic analysis of Brugia malayi excretory-secretory products. PLoS Negl Trop Dis. 2008;2:e326. doi: 10.1371/journal.pntd.0000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Everts B, Perona-Wright G, Smits HH, Hokke CH, van der Ham AJ, Fitzsimmons CM, et al. Omega-1, a glycoprotein secreted by Schistosoma mansoni eggs, drives Th2 responses. J Exp Med. 2009;206:1673–80. doi: 10.1084/jem.20082460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schramm G, Gronow A, Knobloch J, Wippersteg V, Grevelding CG, Galle J, et al. IPSE/alpha-1: a major immunogenic component secreted from Schistosoma mansoni eggs. Mol Biochem Parasitol. 2006;147:9–19. doi: 10.1016/j.molbiopara.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Wuhrer M, Balog CIA, Catalina MI, Jones FM, Schramm G, Haas H, et al. IPSE/alpha-1, a major secretory glycoprotein antigen from schistosome eggs, expresses the Lewis X motif on core-difucosylated N-glycans. FEBS J. 2006;273:2276–92. doi: 10.1111/j.1742-4658.2006.05242.x. [DOI] [PubMed] [Google Scholar]
- 18.Haslam SM, Coles GC, Morris HR, Dell A. Structural characterization of the N-glycans of Dictyocaulus viviparus: discovery of the Lewis(x) structure in a nematode. Glycobiology. 2000;10:223–9. doi: 10.1093/glycob/10.2.223. [DOI] [PubMed] [Google Scholar]
- 19.Dissanayake S, Amith RS, Shahin A. Taenia crassiceps carbohydrates stimulate IL-6 expression in naïve murine macrophages via Toll-like receptors (TLRs) Mol Immunol. 2004;41:391–8. doi: 10.1016/j.molimm.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 20.Zhang J, Otter A, Bundle DR. Synthesis and conformational studies of the tyvelose capped, Lewis-x like tetrasaccharide epitope of Trichinella spiralis. Bioorg Med Chem. 1996;4:1989–2001. doi: 10.1016/S0968-0896(96)00182-4. [DOI] [PubMed] [Google Scholar]
- 21.DeBose-Boyd RA, Nyame AK, Jasmer DP, Cummings RD. The ruminant parasite Haemonchus contortus expresses an alpha1,3-fucosyltransferase capable of synthesizing the Lewis x and sialyl Lewis x antigens. Glycoconj J. 1998;15:789–98. doi: 10.1023/A:1006912032273. [DOI] [PubMed] [Google Scholar]
- 22.Pöltl G, Kerner D, Paschinger K, Wilson IBH. N-glycans of the porcine nematode parasite Ascaris suum are modified with phosphorylcholine and core fucose residues. FEBS J. 2007;274:714–26. doi: 10.1111/j.1742-4658.2006.05615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marques ET, Jr., Weiss JB, Strand M. Molecular characterization of a fucosyltransferase encoded by Schistosoma mansoni. Mol Biochem Parasitol. 1998;93:237–50. doi: 10.1016/s0166-6851(98)00033-4. [DOI] [PubMed] [Google Scholar]
- 24.Hokke CH, Neeleman AP, Koeleman CA, van den Eijnden DH. Identification of an alpha3-fucosyltransferase and a novel alpha2-fucosyltransferase activity in cercariae of the schistosome Trichobilharzia ocellata: biosynthesis of the Fucalpha1-->2Fucalpha1-->3[Gal(NAc)beta1-->4]GlcNAc sequence. Glycobiology. 1998;8:393–406. doi: 10.1093/glycob/8.4.393. [DOI] [PubMed] [Google Scholar]
- 25.Silva SR, Jacysyn JF, Macedo MS, Faquim-Mauro EL. Immunosuppressive components of Ascaris suum down-regulate expression of costimulatory molecules and function of antigen-presenting cells via an IL-10-mediated mechanism. Eur J Immunol. 2006;36:3227–37. doi: 10.1002/eji.200636110. [DOI] [PubMed] [Google Scholar]
- 26.Segura M, Su Z, Piccirillo C, Stevenson MM. Impairment of dendritic cell function by excretory-secretory products: a potential mechanism for nematode-induced immunosuppression. Eur J Immunol. 2007;37:1887–904. doi: 10.1002/eji.200636553. [DOI] [PubMed] [Google Scholar]
- 27.Fujiwara RT, Cançado GGL, Freitas PA, Santiago HC, Massara CL, Dos Santos Carvalho O, et al. Necator americanus infection: a possible cause of altered dendritic cell differentiation and eosinophil profile in chronically infected individuals. PLoS Negl Trop Dis. 2009;3:e399. doi: 10.1371/journal.pntd.0000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Balic A, Harcus Y, Holland MJ, Maizels RM. Selective maturation of dendritic cells by Nippostrongylus brasiliensis-secreted proteins drives Th2 immune responses. Eur J Immunol. 2004;34:3047–59. doi: 10.1002/eji.200425167. [DOI] [PubMed] [Google Scholar]
- 29.D’Elia R, Else KJ. In vitro antigen presenting cell-derived IL-10 and IL-6 correlate with Trichuris muris isolate-specific survival. Parasite Immunol. 2009;31:123–31. doi: 10.1111/j.1365-3024.2008.01088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shortman K, Liu Y-J. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151–61. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 31.Scott P, Pearce E, Cheever AW, Coffman RL, Sher A. Role of cytokines and CD4+ T-cell subsets in the regulation of parasite immunity and disease. Immunol Rev. 1989;112:161–82. doi: 10.1111/j.1600-065X.1989.tb00557.x. [DOI] [PubMed] [Google Scholar]
- 32.Vignali DA, Crocker P, Bickle QD, Cobbold S, Waldmann H, Taylor MG. A role for CD4+ but not CD8+ T cells in immunity to Schistosoma mansoni induced by 20 krad-irradiated and Ro 11-3128-terminated infections. Immunology. 1989;67:466–72. [PMC free article] [PubMed] [Google Scholar]
- 33.Katona IM, Urban JF, Jr., Finkelman FD. The role of L3T4+ and Lyt-2+ T cells in the IgE response and immunity to Nippostrongylus brasiliensis. J Immunol. 1988;140:3206–11. [PubMed] [Google Scholar]
- 34.Hasnain SZ, Evans CM, Roy M, Gallagher AL, Kindrachuk KN, Barron L, et al. Muc5ac: a critical component mediating the rejection of enteric nematodes. J Exp Med. 2011;208:893–900. doi: 10.1084/jem.20102057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akiho H, Blennerhassett P, Deng Y, Collins SM. Role of IL-4, IL-13, and STAT6 in inflammation-induced hypercontractility of murine smooth muscle cells. Am J Physiol Gastrointest Liver Physiol. 2002;282:G226–32. doi: 10.1152/ajpgi.2002.282.2.G226. [DOI] [PubMed] [Google Scholar]
- 36.Pulendran B, Tang H, Manicassamy S. Programming dendritic cells to induce T(H)2 and tolerogenic responses. Nat Immunol. 2010;11:647–55. doi: 10.1038/ni.1894. [DOI] [PubMed] [Google Scholar]
- 37.Chen F, Liu Z, Wu W, Rozo C, Bowdridge S, Millman A, et al. An essential role for TH2-type responses in limiting acute tissue damage during experimental helminth infection. Nat Med. 2012;18:260–6. doi: 10.1038/nm.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Phythian-Adams AT, Cook PC, Lundie RJ, Jones LH, Smith KA, Barr TA, et al. CD11c depletion severely disrupts Th2 induction and development in vivo. J Exp Med. 2010;207:2089–96. doi: 10.1084/jem.20100734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siracusa MC, Comeau MR, Artis D. New insights into basophil biology: initiators, regulators, and effectors of type 2 inflammation. Ann N Y Acad Sci. 2011;1217:166–77. doi: 10.1111/j.1749-6632.2010.05918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomas PG, Harn DA., Jr Immune biasing by helminth glycans. Cell Microbiol. 2004;6:13–22. doi: 10.1046/j.1462-5822.2003.00337.x. [DOI] [PubMed] [Google Scholar]
- 41.Massacand JC, Stettler RC, Meier R, Humphreys NE, Grencis RK, Marsland BJ, et al. Helminth products bypass the need for TSLP in Th2 immune responses by directly modulating dendritic cell function. Proc Natl Acad Sci U S A. 2009;106:13968–73. doi: 10.1073/pnas.0906367106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Everts B, Hussaarts L, Driessen NN, Meevissen MH, Schramm G, van der Ham AJ, et al. Schistosome-derived omega-1 drives Th2 polarization by suppressing protein synthesis following internalization by the mannose receptor. J Exp Med. 2012;209:1753–67. doi: 10.1084/jem.20111381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harrison J, Ridley DS. Heterologous reactions involving parasites, blood group antibodies and tissue components. Trans R Soc Trop Med Hyg. 1975;69:312–7. doi: 10.1016/0035-9203(75)90125-X. [DOI] [PubMed] [Google Scholar]
- 44.Nash TE, Ottesen EA, Cheever AW. Antibody response to a polysaccharide antigen present in the schistosome gut. II. Modulation of antibody response. Am J Trop Med Hyg. 1978;27:944–50. doi: 10.4269/ajtmh.1978.27.944. [DOI] [PubMed] [Google Scholar]
- 45.Nash TE. Antibody response to a polysaccharide antigen present in the schistosome gut. I. Sensitivity and specificity. Am J Trop Med Hyg. 1978;27:939–43. doi: 10.4269/ajtmh.1978.27.939. [DOI] [PubMed] [Google Scholar]
- 46.Wuhrer M, Koeleman CAM, Deelder AM, Hokke CH. Repeats of LacdiNAc and fucosylated LacdiNAc on N-glycans of the human parasite Schistosoma mansoni. FEBS J. 2006;273:347–61. doi: 10.1111/j.1742-4658.2005.05068.x. [DOI] [PubMed] [Google Scholar]
- 47.Nyame AK, Leppänen AM, Bogitsh BJ, Cummings RD. Antibody responses to the fucosylated LacdiNAc glycan antigen in Schistosoma mansoni-infected mice and expression of the glycan among schistosomes. Exp Parasitol. 2000;96:202–12. doi: 10.1006/expr.2000.4573. [DOI] [PubMed] [Google Scholar]
- 48.Van der Kleij D, Van Remoortere A, Schuitemaker JHN, Kapsenberg ML, Deelder AM, Tielens AG, et al. Triggering of innate immune responses by schistosome egg glycolipids and their carbohydrate epitope GalNAc beta 1-4(Fuc alpha 1-2Fuc alpha 1-3)GlcNAc. J Infect Dis. 2002;185:531–9. doi: 10.1086/338574. [DOI] [PubMed] [Google Scholar]
- 49.Aranzamendi C, Tefsen B, Jansen M, Chiumiento L, Bruschi F, Kortbeek T, et al. Glycan microarray profiling of parasite infection sera identifies the LDNF glycan as a potential antigen for serodiagnosis of trichinellosis. Exp Parasitol. 2011;129:221–6. doi: 10.1016/j.exppara.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Geldhof P, Newlands GFJ, Nyame K, Cummings R, Smith WD, Knox DP. Presence of the LDNF glycan on the host-protective H-gal-GP fraction from Haemonchus contortus. Parasite Immunol. 2005;27:55–60. doi: 10.1111/j.1365-3024.2005.00744.x. [DOI] [PubMed] [Google Scholar]
- 51.Richter D, Incani RN, Harn DA. Lacto-N-fucopentaose III (Lewis x), a target of the antibody response in mice vaccinated with irradiated cercariae of Schistosoma mansoni. Infect Immun. 1996;64:1826–31. doi: 10.1128/iai.64.5.1826-1831.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Springer GF. Immunoreactive T and Tn epitopes in cancer diagnosis, prognosis, and immunotherapy. J Mol Med (Berl) 1997;75:594–602. doi: 10.1007/s001090050144. [DOI] [PubMed] [Google Scholar]
- 53.Casaravilla C, Freire T, Malgor R, Medeiros A, Osinaga E, Carmona C. Mucin-type O-glycosylation in helminth parasites from major taxonomic groups: evidence for widespread distribution of the Tn antigen (GalNAc-Ser/Thr) and identification of UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase activity. J Parasitol. 2003;89:709–14. doi: 10.1645/GE-2970. [DOI] [PubMed] [Google Scholar]
- 54.van Die I, Cummings RD. Glycan gimmickry by parasitic helminths: a strategy for modulating the host immune response? Glycobiology. 2010;20:2–12. doi: 10.1093/glycob/cwp140. [DOI] [PubMed] [Google Scholar]
- 55.Maizels RM, Hewitson JP, Smith KA. Susceptibility and immunity to helminth parasites. Curr Opin Immunol. 2012;24:459–66. doi: 10.1016/j.coi.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schabussova I, Amer H, van Die I, Kosma P, Maizels RM. O-methylated glycans from Toxocara are specific targets for antibody binding in human and animal infections. Int J Parasitol. 2007;37:97–109. doi: 10.1016/j.ijpara.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 57.Wisnewski N, McNeil M, Grieve RB, Wassom DL. Characterization of novel fucosyl- and tyvelosyl-containing glycoconjugates from Trichinella spiralis muscle stage larvae. Mol Biochem Parasitol. 1993;61:25–35. doi: 10.1016/0166-6851(93)90155-Q. [DOI] [PubMed] [Google Scholar]
- 58.Reason AJ, Ellis LA, Appleton JA, Wisnewski N, Grieve RB, McNeil M, et al. Novel tyvelose-containing tri- and tetra-antennary N-glycans in the immunodominant antigens of the intracellular parasite Trichinella spiralis. Glycobiology. 1994;4:593–603. doi: 10.1093/glycob/4.5.593. [DOI] [PubMed] [Google Scholar]
- 59.Ellis LA, McVay CS, Probert MA, Zhang J, Bundle DR, Appleton JA. Terminal beta-linked tyvelose creates unique epitopes in Trichinella spiralis glycan antigens. Glycobiology. 1997;7:383–90. doi: 10.1093/glycob/7.3.383. [DOI] [PubMed] [Google Scholar]
- 60.Haslam SM, Coles GC, Munn EA, Smith TS, Smith HF, Morris HR, et al. Haemonchus contortus glycoproteins contain N-linked oligosaccharides with novel highly fucosylated core structures. J Biol Chem. 1996;271:30561–70. doi: 10.1074/jbc.271.48.30561. [DOI] [PubMed] [Google Scholar]
- 61.Johnston WL, Krizus A, Dennis JW. Eggshell chitin and chitin-interacting proteins prevent polyspermy in C. elegans. Curr Biol. 2010;20:1932–7. doi: 10.1016/j.cub.2010.09.059. [DOI] [PubMed] [Google Scholar]
- 62.Foster JM, Zhang Y, Kumar S, Carlow CKS. Parasitic nematodes have two distinct chitin synthases. Mol Biochem Parasitol. 2005;142:126–32. doi: 10.1016/j.molbiopara.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 63.Weiser JN, Goldberg JB, Pan N, Wilson L, Virji M. The phosphorylcholine epitope undergoes phase variation on a 43-kilodalton protein in Pseudomonas aeruginosa and on pili of Neisseria meningitidis and Neisseria gonorrhoeae. Infect Immun. 1998;66:4263–7. doi: 10.1128/iai.66.9.4263-4267.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Haslam SM, Khoo KH, Houston KM, Harnett W, Morris HR, Dell A. Characterisation of the phosphorylcholine-containing N-linked oligosaccharides in the excretory-secretory 62 kDa glycoprotein of Acanthocheilonema viteae. Mol Biochem Parasitol. 1997;85:53–66. doi: 10.1016/S0166-6851(96)02807-1. [DOI] [PubMed] [Google Scholar]
- 65.Stepek G, Auchie M, Tate R, Watson K, Russell DG, Devaney E, et al. Expression of the filarial nematode phosphorylcholine-containing glycoprotein, ES62, is stage specific. Parasitology. 2002;125:155–64. doi: 10.1017/S0031182002001920. [DOI] [PubMed] [Google Scholar]
- 66.Hewitson JP, Harcus YM, Curwen RS, Dowle AA, Atmadja AK, Ashton PD, et al. The secretome of the filarial parasite, Brugia malayi: proteomic profile of adult excretory-secretory products. Mol Biochem Parasitol. 2008;160:8–21. doi: 10.1016/j.molbiopara.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 67.van der Kleij D, Latz E, Brouwers JFHM, Kruize YC, Schmitz M, Kurt-Jones EA, et al. A novel host-parasite lipid cross-talk. Schistosomal lyso-phosphatidylserine activates toll-like receptor 2 and affects immune polarization. J Biol Chem. 2002;277:48122–9. doi: 10.1074/jbc.M206941200. [DOI] [PubMed] [Google Scholar]
- 68.Srivastava AK, Jaffe JJ. Phosphatidylserine synthesis in adult Dirofilaria immitis females. Int J Parasitol. 1986;16:9–11. doi: 10.1016/0020-7519(86)90058-5. [DOI] [PubMed] [Google Scholar]
- 69.Dabbagh K, Dahl ME, Stepick-Biek P, Lewis DB. Toll-like receptor 4 is required for optimal development of Th2 immune responses: role of dendritic cells. J Immunol. 2002;168:4524–30. doi: 10.4049/jimmunol.168.9.4524. [DOI] [PubMed] [Google Scholar]
- 70.Agrawal S, Agrawal A, Doughty B, Gerwitz A, Blenis J, Van Dyke T, et al. Cutting edge: different Toll-like receptor agonists instruct dendritic cells to induce distinct Th responses via differential modulation of extracellular signal-regulated kinase-mitogen-activated protein kinase and c-Fos. J Immunol. 2003;171:4984–9. doi: 10.4049/jimmunol.171.10.4984. [DOI] [PubMed] [Google Scholar]
- 71.Langelaar M, Aranzamendi C, Franssen F, Van Der Giessen J, Rutten V, van der Ley P, et al. Suppression of dendritic cell maturation by Trichinella spiralis excretory/secretory products. Parasite Immunol. 2009;31:641–5. doi: 10.1111/j.1365-3024.2009.01136.x. [DOI] [PubMed] [Google Scholar]
- 72.Falcón C, Carranza F, Martínez FF, Knubel CP, Masih DT, Motrán CC, et al. Excretory-secretory products (ESP) from Fasciola hepatica induce tolerogenic properties in myeloid dendritic cells. Vet Immunol Immunopathol. 2010;137:36–46. doi: 10.1016/j.vetimm.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 73.Terrazas CA, Sánchez-Muñoz F, Mejía-Domínguez AM, Amezcua-Guerra LM, Terrazas LI, Bojalil R, et al. Cestode antigens induce a tolerogenic-like phenotype and inhibit LPS inflammatory responses in human dendritic cells. Int J Biol Sci. 2011;7:1391–400. doi: 10.7150/ijbs.7.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nono JK, Pletinckx K, Lutz MB, Brehm K. Excretory/secretory-products of Echinococcus multilocularis larvae induce apoptosis and tolerogenic properties in dendritic cells in vitro. PLoS Negl Trop Dis. 2012;6:e1516. doi: 10.1371/journal.pntd.0001516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kane CM, Jung E, Pearce EJ. Schistosoma mansoni egg antigen-mediated modulation of Toll-like receptor (TLR)-induced activation occurs independently of TLR2, TLR4, and MyD88. Infect Immun. 2008;76:5754–9. doi: 10.1128/IAI.00497-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Paveley RA, Aynsley SA, Cook PC, Turner JD, Mountford AP. Fluorescent imaging of antigen released by a skin-invading helminth reveals differential uptake and activation profiles by antigen presenting cells. PLoS Negl Trop Dis. 2009;3:e528. doi: 10.1371/journal.pntd.0000528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thomas PG, Carter MR, Da’dara AA, DeSimone TM, Harn DA. A helminth glycan induces APC maturation via alternative NF-kappa B activation independent of I kappa B alpha degradation. J Immunol. 2005;175:2082–90. doi: 10.4049/jimmunol.175.4.2082. [DOI] [PubMed] [Google Scholar]
- 78.Gao Y, Zhang M, Chen L, Hou M, Ji M, Wu G. Deficiency in TLR2 but not in TLR4 impairs dendritic cells derived IL-10 responses to schistosome antigens. Cell Immunol. 2012;272:242–50. doi: 10.1016/j.cellimm.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 79.Stepek G, Houston KM, Goodridge HS, Devaney E, Harnett W. Stage-specific and species-specific differences in the production of the mRNA and protein for the filarial nematode secreted product, ES-62. Parasitology. 2004;128:91–8. doi: 10.1017/S0031182003004220. [DOI] [PubMed] [Google Scholar]
- 80.Whelan M, Harnett MM, Houston KM, Patel V, Harnett W, Rigley KP. A filarial nematode-secreted product signals dendritic cells to acquire a phenotype that drives development of Th2 cells. J Immunol. 2000;164:6453–60. doi: 10.4049/jimmunol.164.12.6453. [DOI] [PubMed] [Google Scholar]
- 81.Goodridge HS, Wilson EH, Harnett W, Campbell CC, Harnett MM, Liew FY. Modulation of macrophage cytokine production by ES-62, a secreted product of the filarial nematode Acanthocheilonema viteae. J Immunol. 2001;167:940–5. doi: 10.4049/jimmunol.167.2.940. [DOI] [PubMed] [Google Scholar]
- 82.Goodridge HS, Marshall FA, Else KJ, Houston KM, Egan C, Al-Riyami L, et al. Immunomodulation via novel use of TLR4 by the filarial nematode phosphorylcholine-containing secreted product, ES-62. J Immunol. 2005;174:284–93. doi: 10.4049/jimmunol.174.1.284. [DOI] [PubMed] [Google Scholar]
- 83.van Liempt E, Bank CMC, Mehta P, Garcí-Vallejo JJ, Kawar ZS, Geyer R, et al. Specificity of DC-SIGN for mannose- and fucose-containing glycans. FEBS Lett. 2006;580:6123–31. doi: 10.1016/j.febslet.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 84.Geijtenbeek TB, Kwon DS, Torensma R, van Vliet SJ, van Duijnhoven GC, Middel J, et al. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100:587–97. doi: 10.1016/S0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- 85.van Stijn CMW, Meyer S, van den Broek M, Bruijns SC, van Kooyk Y, Geyer R, et al. Schistosoma mansoni worm glycolipids induce an inflammatory phenotype in human dendritic cells by cooperation of TLR4 and DC-SIGN. Mol Immunol. 2010;47:1544–52. doi: 10.1016/j.molimm.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 86.van Vliet SJ, van Liempt E, Saeland E, Aarnoudse CA, Appelmelk B, Irimura T, et al. Carbohydrate profiling reveals a distinctive role for the C-type lectin MGL in the recognition of helminth parasites and tumor antigens by dendritic cells. Int Immunol. 2005;17:661–9. doi: 10.1093/intimm/dxh246. [DOI] [PubMed] [Google Scholar]
- 87.van Die I, van Vliet SJ, Nyame AK, Cummings RD, Bank CM, Appelmelk B, et al. The dendritic cell-specific C-type lectin DC-SIGN is a receptor for Schistosoma mansoni egg antigens and recognizes the glycan antigen Lewis x. Glycobiology. 2003;13:471–8. doi: 10.1093/glycob/cwg052. [DOI] [PubMed] [Google Scholar]
- 88.van Liempt E, van Vliet SJ, Engering A, García Vallejo JJ, Bank CM, Sanchez-Hernandez M, et al. Schistosoma mansoni soluble egg antigens are internalized by human dendritic cells through multiple C-type lectins and suppress TLR-induced dendritic cell activation. Mol Immunol. 2007;44:2605–15. doi: 10.1016/j.molimm.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 89.Meevissen MHJ, Wuhrer M, Doenhoff MJ, Schramm G, Haas H, Deelder AM, et al. Structural characterization of glycans on omega-1, a major Schistosoma mansoni egg glycoprotein that drives Th2 responses. J Proteome Res. 2010;9:2630–42. doi: 10.1021/pr100081c. [DOI] [PubMed] [Google Scholar]
- 90.Saijo S, Iwakura Y. Dectin-1 and Dectin-2 in innate immunity against fungi. Int Immunol. 2011;23:467–72. doi: 10.1093/intimm/dxr046. [DOI] [PubMed] [Google Scholar]
- 91.Ritter M, Gross O, Kays S, Ruland J, Nimmerjahn F, Saijo S, et al. Schistosoma mansoni triggers Dectin-2, which activates the Nlrp3 inflammasome and alters adaptive immune responses. Proc Natl Acad Sci U S A. 2010;107:20459–64. doi: 10.1073/pnas.1010337107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol. 2003;3:984–93. doi: 10.1038/nri1246. [DOI] [PubMed] [Google Scholar]
- 93.MacDonald AS, Maizels RM. Alarming dendritic cells for Th2 induction. J Exp Med. 2008;205:13–7. doi: 10.1084/jem.20072665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.MacDonald AS, Straw AD, Bauman B, Pearce EJ. CD8- dendritic cell activation status plays an integral role in influencing Th2 response development. J Immunol. 2001;167:1982–8. doi: 10.4049/jimmunol.167.4.1982. [DOI] [PubMed] [Google Scholar]
- 95.Semnani RT, Liu AY, Sabzevari H, Kubofcik J, Zhou J, Gilden JK, et al. Brugia malayi microfilariae induce cell death in human dendritic cells, inhibit their ability to make IL-12 and IL-10, and reduce their capacity to activate CD4+ T cells. J Immunol. 2003;171:1950–60. doi: 10.4049/jimmunol.171.4.1950. [DOI] [PubMed] [Google Scholar]
- 96.Aranzamendi C, Fransen F, Langelaar M, Franssen F, van der Ley P, van Putten JP, et al. Trichinella spiralis-secreted products modulate DC functionality and expand regulatory T cells in vitro. Parasite Immunol. 2012;34:210–23. doi: 10.1111/j.1365-3024.2012.01353.x. [DOI] [PubMed] [Google Scholar]
- 97.Dunand-Sauthier I, Santiago-Raber M-L, Capponi L, Vejnar CE, Schaad O, Irla M, et al. Silencing of c-Fos expression by microRNA-155 is critical for dendritic cell maturation and function. Blood. 2011;117:4490–500. doi: 10.1182/blood-2010-09-308064. [DOI] [PubMed] [Google Scholar]
- 98.Nakahara T, Moroi Y, Uchi H, Furue M. Differential role of MAPK signaling in human dendritic cell maturation and Th1/Th2 engagement. J Dermatol Sci. 2006;42:1–11. doi: 10.1016/j.jdermsci.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 99.Correale J, Farez M. Helminth antigens modulate immune responses in cells from multiple sclerosis patients through TLR2-dependent mechanisms. J Immunol. 2009;183:5999–6012. doi: 10.4049/jimmunol.0900897. [DOI] [PubMed] [Google Scholar]
- 100.Moody DB, Besra GS, Wilson IA, Porcelli SA. The molecular basis of CD1-mediated presentation of lipid antigens. Immunol Rev. 1999;172:285–96. doi: 10.1111/j.1600-065X.1999.tb01373.x. [DOI] [PubMed] [Google Scholar]
- 101.Faveeuw C, Angeli V, Fontaine J, Maliszewski C, Capron A, Van Kaer L, et al. Antigen presentation by CD1d contributes to the amplification of Th2 responses to Schistosoma mansoni glycoconjugates in mice. J Immunol. 2002;169:906–12. doi: 10.4049/jimmunol.169.2.906. [DOI] [PubMed] [Google Scholar]
- 102.Mejri N, Müller J, Gottstein B. Intraperitoneal murine Echinococcus multilocularis infection induces differentiation of TGF-β-expressing DCs that remain immature. Parasite Immunol. 2011;33:471–82. doi: 10.1111/j.1365-3024.2011.01303.x. [DOI] [PubMed] [Google Scholar]
- 103.Dainichi T, Maekawa Y, Ishii K, Zhang T, Nashed BF, Sakai T, et al. Nippocystatin, a cysteine protease inhibitor from Nippostrongylus brasiliensis, inhibits antigen processing and modulates antigen-specific immune response. Infect Immun. 2001;69:7380–6. doi: 10.1128/IAI.69.12.7380-7386.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Manoury B, Gregory WF, Maizels RM, Watts C. Bm-CPI-2, a cystatin homolog secreted by the filarial parasite Brugia malayi, inhibits class II MHC-restricted antigen processing. Curr Biol. 2001;11:447–51. doi: 10.1016/S0960-9822(01)00118-X. [DOI] [PubMed] [Google Scholar]
- 105.Schönemeyer A, Lucius R, Sonnenburg B, Brattig N, Sabat R, Schilling K, et al. Modulation of human T cell responses and macrophage functions by onchocystatin, a secreted protein of the filarial nematode Onchocerca volvulus. J Immunol. 2001;167:3207–15. doi: 10.4049/jimmunol.167.6.3207. [DOI] [PubMed] [Google Scholar]
- 106.Everts B, Smits HH, Hokke CH, Yazdanbakhsh M. Helminths and dendritic cells: sensing and regulating via pattern recognition receptors, Th2 and Treg responses. Eur J Immunol. 2010;40:1525–37. doi: 10.1002/eji.200940109. [DOI] [PubMed] [Google Scholar]
- 107.Jenkins SJ, Perona-Wright G, Worsley AGF, Ishii N, MacDonald AS. Dendritic cell expression of OX40 ligand acts as a costimulatory, not polarizing, signal for optimal Th2 priming and memory induction in vivo. J Immunol. 2007;179:3515–23. doi: 10.4049/jimmunol.179.6.3515. [DOI] [PubMed] [Google Scholar]
- 108.MacDonald AS, Patton EA, La Flamme AC, Araujo MI, Huxtable CR, Bauman B, et al. Impaired Th2 development and increased mortality during Schistosoma mansoni infection in the absence of CD40/CD154 interaction. J Immunol. 2002;168:4643–9. doi: 10.4049/jimmunol.168.9.4643. [DOI] [PubMed] [Google Scholar]
- 109.Steinfelder S, Andersen JF, Cannons JL, Feng CG, Joshi M, Dwyer D, et al. The major component in schistosome eggs responsible for conditioning dendritic cells for Th2 polarization is a T2 ribonuclease (omega-1) J Exp Med. 2009;206:1681–90. doi: 10.1084/jem.20082462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Constant S, Pfeiffer C, Woodard A, Pasqualini T, Bottomly K. Extent of T cell receptor ligation can determine the functional differentiation of naive CD4+ T cells. J Exp Med. 1995;182:1591–6. doi: 10.1084/jem.182.5.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hosken NA, Shibuya K, Heath AW, Murphy KM, O’Garra A. The effect of antigen dose on CD4+ T helper cell phenotype development in a T cell receptor-alpha beta-transgenic model. J Exp Med. 1995;182:1579–84. doi: 10.1084/jem.182.5.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gruden-Movsesijan A, Ilic N, Colic M, Majstorovic I, Vasilev S, Radovic I, et al. The impact of Trichinella spiralis excretory-secretory products on dendritic cells. Comp Immunol Microbiol Infect Dis. 2011;34:429–39. doi: 10.1016/j.cimid.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 113.Taylor JJ, Mohrs M, Pearce EJ. Regulatory T cell responses develop in parallel to Th responses and control the magnitude and phenotype of the Th effector population. J Immunol. 2006;176:5839–47. doi: 10.4049/jimmunol.176.10.5839. [DOI] [PubMed] [Google Scholar]
- 114.Li Z, Liu G, Chen Y, Liu Y, Liu B, Su Z. The phenotype and function of naturally existing regulatory dendritic cells in nematode-infected mice. Int J Parasitol. 2011;41:1129–37. doi: 10.1016/j.ijpara.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 115.Taylor MD, van der Werf N, Maizels RM. T cells in helminth infection: the regulators and the regulated. Trends Immunol. 2012;33:181–9. doi: 10.1016/j.it.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 116.Goverman J. Autoimmune T cell responses in the central nervous system. Nat Rev Immunol. 2009;9:393–407. doi: 10.1038/nri2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kuijk LM, Klaver EJ, Kooij G, van der Pol SM, Heijnen P, Bruijns SC, et al. Soluble helminth products suppress clinical signs in murine experimental autoimmune encephalomyelitis and differentially modulate human dendritic cell activation. Mol Immunol. 2012;51:210–8. doi: 10.1016/j.molimm.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 118.Zaccone P, Burton OT, Gibbs SE, Miller N, Jones FM, Schramm G, et al. The S. mansoni glycoprotein ω-1 induces Foxp3 expression in NOD mouse CD4⁺ T cells. Eur J Immunol. 2011;41:2709–18. doi: 10.1002/eji.201141429. [DOI] [PubMed] [Google Scholar]
- 119.Zaccone P, Fehérvári Z, Jones FM, Sidobre S, Kronenberg M, Dunne DW, et al. Schistosoma mansoni antigens modulate the activity of the innate immune response and prevent onset of type 1 diabetes. Eur J Immunol. 2003;33:1439–49. doi: 10.1002/eji.200323910. [DOI] [PubMed] [Google Scholar]
- 120.Smits HH, Everts B, Hartgers FC, Yazdanbakhsh M. Chronic helminth infections protect against allergic diseases by active regulatory processes. Curr Allergy Asthma Rep. 2010;10:3–12. doi: 10.1007/s11882-009-0085-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rodrigues LC, Newcombe PJ, Cunha SS, Alcantara-Neves NM, Genser B, Cruz AA, et al. Social Change, Asthma and Allergy in Latin America Early infection with Trichuris trichiura and allergen skin test reactivity in later childhood. Clin Exp Allergy. 2008;38:1769–77. doi: 10.1111/j.1365-2222.2008.03027.x. [DOI] [PubMed] [Google Scholar]
- 122.Rook GAW. The hygiene hypothesis and the increasing prevalence of chronic inflammatory disorders. Trans R Soc Trop Med Hyg. 2007;101:1072–4. doi: 10.1016/j.trstmh.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 123.McConchie BW, Norris HH, Bundoc VG, Trivedi S, Boesen A, Urban JF, Jr., et al. Ascaris suum-derived products suppress mucosal allergic inflammation in an interleukin-10-independent manner via interference with dendritic cell function. Infect Immun. 2006;74:6632–41. doi: 10.1128/IAI.00720-06. [DOI] [PMC free article] [PubMed] [Google Scholar]