Abstract
The application of advanced imaging techniques to fundamental questions in immunology has provided insight into dendritic cell function and has challenged dogma created using static imaging of lymphoid tissue. The history of dendritic cell biology has a storied past and is tightly linked to imaging. The development of imaging techniques that emphasize live cell imaging in situ has provided not only breath-taking movies, but also novel insights into the importance of spatiotemporal relationships between antigen presenting cells and T cells. This review serves to provide a primer on two-photon microscopy, TIRF microscopy, spinning disk confocal microscopy and optical trapping and provides selective examples of insights gained from these tools on dendritic cell biology.
Keywords: microscopy, TIRF, spinning disk, two photon microscopy, optical traps, optical tweezer
Introduction
“You know what your problem is, it’s that you haven’t seen enough movies—all of life’s riddles are answered in the movies.” —Steve Martin, actor
Infection is a result of the failure of the immune system to prevent the offending microorganism from causing disease. On the pathogen side, the most successful microbes have sophisticated machinery to subvert the immune system. On the host side, the immune system uses redundant system of cells and soluble factors to thwart infection. Much work has been done to understand the rules that govern the immune response.1,2 Recently, immunologists have divided the mammalian immune response into two arms: the innate and adaptive immune system.3-5 Innate immunity is composed of phagocytic cells including neutrophils, macrophages and dendritic cells (DCs) as well as soluble factors including complement and antimicrobial peptides. Their principle task is to identify invading pathogens using in part Toll-like receptors (TLRs) that recognize pathogen-associated molecular patterns on microorganisms.6-8 In most cases, these cells serve to neutralize quickly these pathogens. In contrast, constituents of the adaptive immune system provide long-term antigen-specific immunity. While this division of the immune system provides a conceptual framework, there are notable exceptions including NK cells and DCs that limit the usefulness of this characterization of the immune system. DCs bridge the gap between innate immunity and adaptive immunity.9 Using surface disposed and endosomal TLRs, immature DCs lie in the periphery waiting for pathogens. Upon activation, microorganisms are taken up into membrane-delimited compartments termed phagosomes. Instead of rapid degradation of the content, the goal of DCs is to extract antigens from this compartment to load class II MHC molecules and present these molecules on the cell surface for inspection by antigen-specific T cells. DCs are the only antigen presenting cell known that has the capacity to stimulate naïve T cells. By discharging these duties, DCs bridge the innate and adaptive immune system and thus play a critical role in the host defense against infections.
The role of microscopy in the study of DC biology is inexorably linked. In fact, Ralph Steinman, a Nobel laureate physician scientist at the Rockefeller University, first described the presence of DCs in cell cultures from mouse spleens using phase microscopy.10 Both phase microscopy and electron microscopy provided our first glimpse into this important cell type. Its peculiar shape and behavior in live cell imaging captured Steinman’s attention for the bulk of his career that led to many seminal findings11,12 that provided enough insight to justify the use of DCs in clinical trials for many types of cancer.13,14
Microscopy encompasses many modalities of imaging, so I have chosen to focus on those systems that permit time-lapse imaging of live cells. Recent advances in cellular imaging techniques permit direct observation of these events in situ with good spatiotemporal resolution.15,16 The recent application of in vivo imaging to characterize the dynamics of T-cell activation by DCs has reshaped long-held beliefs of how adaptive immune responses are initiated. However, to improve our fundamental understanding, these new observations must be evaluated against diverse paradigms within the field of immunology, most of which were established before the advent of these cutting-edge techniques. To determine the rules that govern the ability of antigen-bearing DCs to activate naïve T cells, numerous factors have been elucidated including the density and spatial orientation of class II MHC molecules as well as costimulatory molecules found on the surface of DCs, and the signal transducing pathways associated with surface T cell receptors.17,18 The opportunity to view T-cell activation by DCs as a sum of independent factors allows for the integration of disparate observations into testable predictions for further experimentation.
Application of live-cell imaging tools to the interface of immunology and infectious disease is in its infancy though preliminary observations challenge our view of long-held beliefs about immune responses to microorganisms. To control Mycobacterium tuberculosis, the host forms granulomas, histologically-distinct structures regularly seen in clinical tissue of infected persons. Conventional wisdom suggested that macrophages, T cells and occasionally DCs formed this static barrier enveloping organisms to control spread. The use of intravital two-photon microscopy into the liver of mice infected with Mycobacterium bovis demonstrated that immune cells showed remarkable dynamic movements to initiate and maintain these granulomas.19 TNF-α derived signals were required to recruit uninfected macrophages to granulomas and preserve the involvement of T cells. These observations challenge the dogma that granulomas are static warehouses of organisms locked in by dormant immune cells. Instead, granulomas require constant signaling to maintain this architecture, providing new insight into the pathogenesis of reactivation of mycobacterial disease and possible pathways to prevent reactivation.
To provide a framework for this review, I have divided the sections into different microscopy platforms with a primer on the technique followed by some of the key findings of DC biology elucidated using these systems. This review is not meant to be comprehensive as other papers have reviewed these platforms in great detail.20 I have chosen to focus the attention on two-photon microscopy, total internal reflection microscopy, spinning disk confocal microscopy and optical traps.
Two Photon Microscopy
Technique
The generation of the immune response requires precise choreography of cells in space and time within the lymph node and other immunologic tissue. Standard immunology assays to provide limited insight into these processes include immunohistochemistry of fixed tissues or flow cytometry of cells after disruption of normal tissue architecture. In a sense, we had used these still shots to understand a motion picture. Earlier attempts to visualize immune cells in vivo were hampered by three main problems that limit optical resolution.21 First, high-numerical-aperture objective lenses used in fluorescence microscopy have a narrow depth of field. Second, emitted light from biological tissues becomes scattered, so that image is limited to close to the surface. Third, the intense excitation light bleaches the fluorophore over time and photodamages cells.21 In order to overcome these technical obstacles, intravital two photon microscopy was adapted to real-time imaging of the immune system to permit direct visualization of cells in a spatiotemporal manner, at a level of resolution that previously had not been seen.
The term “two-photon” refers to the fact that excitation of the fluorophore occurs when there is near-simultaneous absorption of energy from two separate photons.15,21-24 This approach permits the three aforementioned technical obstacles to be overcome. Longer wavelengths of light penetrate tissue deeper than shorter wavelengths of light permitting visualizing of tissues deeper than typical fluorescence microscopy. The use of a light source emitting longer wavelengths of light also minimizes photodamage and bleaching because excitation only occurs at the focal plane. Finally, stromal elements can be seen using second-harmonic generation because of the regularly repeating structure of polymers including collagen without the need of a second fluorophore. Coupled with mice that express fluorescent fusion proteins within immune cells including DCs and surgical techniques that allow explants of lymphoid tissue in living mice, two photon microscopy quickly became an important modality in the imaging toolkit for immunologists.
Insights
With the opportunity to view DCs in their native environment, many observations obtained through a reductionist approach toward the immune response could be integrated into a cohesive model. Mempel et al. showed that T-cell priming by DCs occurs in three successive stages:25 (1) transient serial encounters, (2) stable contact between DC and T cell culminating in cytokine production and (3) high motility and rapid proliferation seen in activated T cells.25 In the absence of antigen, DCs scan at least 500 different T cells per hour, a number that far exceeded the estimations from static-based images.26 DCs tightly entrap the T cells within a complex net of membrane extensions requiring both Rac1 and Rac2 for the formation of dendrites in mature DCs and T cell cognate pairs.27 The timing of DC-T cell contact became an important determinant for robust T cell activation. Stable DC-T cell interactions occurred during the induction of priming, whereas brief contacts contributed to the induction of T cell tolerance.28 At least six hours of contact was required for efficient CD4+ T cell activation in vivo.29 Recently, two photon microscopy has been applied to clinically-relevant models tissue rejection.30 Tracking DCs and T cells in an ear skin graft model in mice, Celli et al. showed that donor dermal DCs migrated rapidly from the graft and were replaced by host CD11b+ macrophages. The infiltrating host cells captured donor antigen, reached the draining lymph node and cross-primed graft-reactive CD8+ T cells. These T cells disseminated throughout the graft but many became arrested in the tissue.30 This application of microscopy to graft rejection provides more questions that should be addressed and raises the possibility of new pathways to modulate this clinical challenge.
Total Internal Reflection Fluorescence (TIRF) Microscopy
Technique
In some experiments, it is important to restrict the zone of observation to the plasma membrane or just beneath it. The adoption of TIRF microscopy is perfectly suited for this application. Using laser light at an angle to generate an evanescent wave, fluorophores are excited only if they are located near the glass/cell membrane interface. The evanescent wave is created only when the light is totally reflected internally at the coverslip-cell surface interface. As the composition and organization of cell surface molecules are critical to the production of an effective immune response, this imaging modality is helpful to illustrate surface characteristics of immune cells.
Insights
DCs undergo a remarkable transformation upon exposure to microorganisms or stimulation with TLR agonists. In addition to increased mobility and decreased phagocytic capacity, DCs translocate class II MHC molecules from the endolysosomal compartment to the cell surface. Indeed, high level of class II MHC on the cell surface of DCs is a veritable marker of a mature cell. The mechanism of trafficking of class II MHC to the cell surface has been studied using TIRF microscopy31 as well as spinning disk confocal microscopy32 (see below). These studies convincingly showed that class II MHC molecules are rapidly deployed to the cell surface using long tubular structures containing other lysosomal membrane proteins.33-35 Migration of DCs from the tissue to the regional lymph node requires remarkable reorganization of the cytoskeleton. Using TIRF microscopy, Lämmermann et al. showed that CDC42, a small GTPase, in DCs is required for in vivo motility, but dispensable for cell polarization and formation of protusions.36
Spinning Disk Confocal Microscopy
Technique
Epifluorescence microscopy provides detailed subcellular localization of fluorescently-labeled cellular constituents. However, out-of-focus light obscures detail when imposing the image of a three-dimensional object into two dimensions. The similar problem exists in radiographic images in clinical medicine. Chest roentograms suffer from precise localization of pathology. To mitigate this, images taken at 90° are taken to augment localization of findings. The development of confocal microscopy for biological samples addressed this issue in microscopy. Multiple pinhole apertures placed in the emission light path allow light to pass that originates from the point of focus, but block out of focus light. Spinning disk confocal microscopes use a rotating disk with thousands of lenslets aligned with pinholes such that thousands of points of light scan the specimen simultaneously. To continue the analogy, spinning disk confocal microscopy is similar to CT radiographic imaging (CT scan), where images are taken a focal plane and all out-of-focus light is discarded. Multiple images can be obtained by moving the focal plane in small increments. Three-dimensional rendering of the image can be done by reformatting this Z stack of images. For spinning disc confocal microscopes to provide illustrative insight into biology, high-intensity laser light sources including water-cooled lasers and diodes and sensitive digital cameras including EM-CCD cameras were necessary to add to the system. These systems permitted video-rate imaging with minimal photobleaching and led to several insights into DC biology.
Insights
DCs had long been implicated in the pathogenesis of HIV-1 infection. Using spinning disk confocal microscopy, McDonald et al. showed that DCs can bind and transfer HIV without becoming infected themselves.37 This process was facilitated by DC-T cell interactions. To communicate with antigen specific T cells, DCs must display peptide-loaded class II MHC molecules on the cell surface. The molecular analysis of vesicular transport of peptide-loaded class II MHC molecules from the endolysosomal compartment to the cell surface has been greatly aided by spinning disk confocal microscopy. Boes et al. showed that DCs for long class II MHC-positive tubules upon DC stimulation.32 These tubules containing multiple endolysosomal proteins and require microtubules for transport (Fig. 1). In addition to these intracellular tubules, DCs are capable of forming intercellular connections called tunneling nanotubules.38 When DCs and monocytes are triggered to flux calcium, the signal can be propagated within seconds to other cells at distances hundreds of microns away through these nanotubules.38
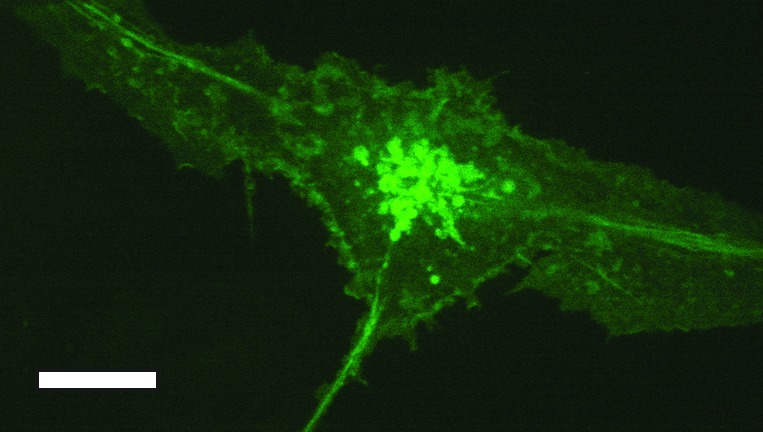
Figure 1. Spinning disk confocal image of class II MHC-GFP expressing primary bone marrow derived DC. Note that most of the GFP signal is found intracellularly. Endolysosomal tubes are present. Scale bar represents 5 µm.
The other cellular process that it is important to DC function is phagocytosis. Ingestion of microorganisms generates a novel subcellular compartment termed a phagosome.39,40 This compartment is modified over time in an effort to neutralize the offending pathogen and extract antigens from this compartment to load onto MHC molecules. The study of remodeling of the phagosome has been greatly aided by spinning disk confocal microscopy. One common theme that emerged from these collective studies is that the content of phagosomes dictates the community of mammalian membrane proteins recruited.41-44 Specifically, CD63 and CD82, members of the tetraspanin family of proteins, are specifically recruited to phagosomes containing fungal pathogens (Vids. S1 and S2) as compared with size-matched polystyrene beads devoid of any TLR or carbohydrate lectin receptor ligands.41,42
Optical Trap (Tweezers)
Technique
Research in biophysics and mechanics has developed novel technologies, in particular optical trapping, to probe non-destructively and study the mechanical properties of biological systems. By utilizing optical trapping, one can study the role of force and mechanics in biology to evaluate quantitatively and interpret the specific contribution of molecular and cellular machinery. Optical trapping is a technique used for non-contact, non-destructive manipulation of a microparticle, such as a cell or pathogen, and it is based on radiation pressure induced by a laser beam tightly focused by a high numerical aperture lens. The radiation pressure exerted on a particle or organism by the optical trap results when a focused laser beam illuminates the particle and is refracted twice, once when it enters, and once when it exits the particle.45 The difference in the photon momentum before and after refraction is transferred to the particle such that the radiation pressure is exerted in the direction opposite to the momentum change. The sum of the forces at each point in the particle is directed to the highest-intensity region at the focal point. Thus, the particle is attracted to the focused beam and is trapped in the vicinity of the focal point. The force of the radiation pressure is on the order of piconewtons and can remotely control and manipulate cells and other microparticles on any imaging plane.
Radiation pressure was first observed46 and applied47,48 to optical tweezer systems and since then, optical tweezers has matured into a technology to probe biological phenomena. To simultaneously trap and image microparticles, optical tweezer systems are usually built in conjunction with epifluorescent microscope systems. Tweezer systems are typically implemented with laser scanning confocal microscopes,49,50 but the scan rate of the single laser beam are typically too slow to image cellular processes in real time. Recently, an optical trapping system was developed on a spinning disk confocal microscope platform.51,52
Insights
The adaptation of this technology to the field of immunology and host-pathogen interactions is at its infancy though the potential is significant to quantify mechanical forces imposed on particles destined for phagocytosis by professional antigen presenting cells including DCs. In the first proof of principle studies, Tam et al. showed that an optical trap can be used to control the spatiotemporal interaction of Aspergillus fumigatus with a macrophage (Vid. S3).51 The ability to perform force measurements permit quantification of the forces imparted on particles undergoing phagocytosis. Coupled with synthetic polystyrene beads displaying ligands for CLRs and TLRs53 and primary cells from mice lacking critical components of the innate immune response, the contribution of specific signaling pathways on the net mechanical forces of phagocytosis may be evaluated. Some studies that have used optical traps to promote formation of immune synapses to characterize the dynamic protein rearrangement for optimal signaling or track HIV-1 transfer from one T cell to another.54,55 Indeed, one consistent theme in DC biology has been that spatiotemporal interactions potently influence DC function and affect the resulting immune response. The ability to control the spatio-temporal interactions of DCs and pathogens using optical trapping will permit us to ask questions that could not be addressed using other technologies.
Conclusions
The love affair between immunology and imaging techniques shows no signs of dissolving. After years of reductionist approach which served to catalog all of the constituents of the immune system, live cell imaging has provided integration of this information coupled with new insights into the importance of spatio-temporal relationships between host cells and pathogens and antigen presenting cells and T cells. New questions arise from these observations that require the use of ever more sophisticated imaging modalities. The ability to illustrate novel critical pathways in immune activation not appreciated by static imaging justifies the continued use of these optical-based techniques.
Supplementary Material
Acknowledgments
J.M.V. is supported by NIH grant R01 AI092084. Post-doctoral fellows in the lab are supported by NIH grant T32AI007061. The author thanks all of the current and former members of the laboratory.
Footnotes
Previously published online: www.landesbioscience.com/journals/virulence/article/22981
References
- 1.Neefjes J, Jongsma ML, Paul P, Bakke O. Towards a systems understanding of MHC class I and MHC class II antigen presentation. Nat Rev Immunol. 2011;11:823–36. doi: 10.1038/nri3084. [DOI] [PubMed] [Google Scholar]
- 2.Vyas JM, Van der Veen AG, Ploegh HL. The known unknowns of antigen processing and presentation. Nat Rev Immunol. 2008;8:607–18. doi: 10.1038/nri2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janeway CA., Jr. The immune system evolved to discriminate infectious nonself from noninfectious self. Immunol Today. 1992;13:11–6. doi: 10.1016/0167-5699(92)90198-G. [DOI] [PubMed] [Google Scholar]
- 4.Janeway CA., Jr. How the immune system recognizes invaders. Sci Am. 1993;269:72–9. doi: 10.1038/scientificamerican0993-72. [DOI] [PubMed] [Google Scholar]
- 5.Medzhitov R, Janeway CA., Jr. Innate immunity: impact on the adaptive immune response. Curr Opin Immunol. 1997;9:4–9. doi: 10.1016/S0952-7915(97)80152-5. [DOI] [PubMed] [Google Scholar]
- 6.Akira S. Toll-like receptors and innate immunity. Adv Immunol. 2001;78:1–56. doi: 10.1016/S0065-2776(01)78001-7. [DOI] [PubMed] [Google Scholar]
- 7.Takeuchi O, Akira S. Toll-like receptors; their physiological role and signal transduction system. Int Immunopharmacol. 2001;1:625–35. doi: 10.1016/S1567-5769(01)00010-8. [DOI] [PubMed] [Google Scholar]
- 8.Kaisho T, Akira S. Dendritic-cell function in Toll-like receptor- and MyD88-knockout mice. Trends Immunol. 2001;22:78–83. doi: 10.1016/S1471-4906(00)01811-1. [DOI] [PubMed] [Google Scholar]
- 9.Wan H, Dupasquier M. Dendritic cells in vivo and in vitro. Cell Mol Immunol. 2005;2:28–35. [PubMed] [Google Scholar]
- 10.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973;137:1142–62. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inaba K, Young JW, Steinman RM. Direct activation of CD8+ cytotoxic T lymphocytes by dendritic cells. J Exp Med. 1987;166:182–94. doi: 10.1084/jem.166.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheong C, Matos I, Choi JH, Dandamudi DB, Shrestha E, Longhi MP, et al. Microbial stimulation fully differentiates monocytes to DC-SIGN/CD209(+) dendritic cells for immune T cell areas. Cell. 2010;143:416–29. doi: 10.1016/j.cell.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valone FH, Small E, MacKenzie M, Burch P, Lacy M, Peshwa MV, et al. Dendritic cell-based treatment of cancer: closing in on a cellular therapy. Cancer J. 2001;7(Suppl 2):S53–61. [PubMed] [Google Scholar]
- 14.Oshita C, Takikawa M, Kume A, Miyata H, Ashizawa T, Iizuka A, et al. Dendritic cell-based vaccination in metastatic melanoma patients: phase II clinical trial. Oncol Rep. 2012;28:1131–8. doi: 10.3892/or.2012.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Germain RN, Bajénoff M, Castellino F, Chieppa M, Egen JG, Huang AY, et al. Making friends in out-of-the-way places: how cells of the immune system get together and how they conduct their business as revealed by intravital imaging. Immunol Rev. 2008;221:163–81. doi: 10.1111/j.1600-065X.2008.00591.x. [DOI] [PubMed] [Google Scholar]
- 16.Germain RN, Robey EA, Cahalan MD. A decade of imaging cellular motility and interaction dynamics in the immune system. Science. 2012;336:1676–81. doi: 10.1126/science.1221063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sumen C, Mempel TR, Mazo IB, von Andrian UH. Intravital microscopy: visualizing immunity in context. Immunity. 2004;21:315–29. doi: 10.1016/S1074-7613(04)00237-7. [DOI] [PubMed] [Google Scholar]
- 18.Henrickson SE, von Andrian UH. Single-cell dynamics of T-cell priming. Curr Opin Immunol. 2007;19:249–58. doi: 10.1016/j.coi.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 19.Egen JG, Rothfuchs AG, Feng CG, Horwitz MA, Sher A, Germain RN. Intravital imaging reveals limited antigen presentation and T cell effector function in mycobacterial granulomas. Immunity. 2011;34:807–19. doi: 10.1016/j.immuni.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frigault MM, Lacoste J, Swift JL, Brown CM. Live-cell microscopy - tips and tools. J Cell Sci. 2009;122:753–67. doi: 10.1242/jcs.033837. [DOI] [PubMed] [Google Scholar]
- 21.Cahalan MD, Parker I, Wei SH, Miller MJ. Two-photon tissue imaging: seeing the immune system in a fresh light. Nat Rev Immunol. 2002;2:872–80. doi: 10.1038/nri935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang AY, Qi H, Germain RN. Illuminating the landscape of in vivo immunity: insights from dynamic in situ imaging of secondary lymphoid tissues. Immunity. 2004;21:331–9. doi: 10.1016/S1074-7613(04)00235-3. [DOI] [PubMed] [Google Scholar]
- 23.Germain RN. Imaging dynamic interactions in immune responses. Semin Immunol. 2005;17:385–6. doi: 10.1016/j.smim.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Bajénoff M, Germain RN. Seeing is believing: a focus on the contribution of microscopic imaging to our understanding of immune system function. Eur J Immunol. 2007;37(Suppl 1):S18–33. doi: 10.1002/eji.200737663. [DOI] [PubMed] [Google Scholar]
- 25.Mempel TR, Henrickson SE, Von Andrian UH. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427:154–9. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- 26.Bousso P, Robey E. Dynamics of CD8+ T cell priming by dendritic cells in intact lymph nodes. Nat Immunol. 2003;4:579–85. doi: 10.1038/ni928. [DOI] [PubMed] [Google Scholar]
- 27.Benvenuti F, Hugues S, Walmsley M, Ruf S, Fetler L, Popoff M, et al. Requirement of Rac1 and Rac2 expression by mature dendritic cells for T cell priming. Science. 2004;305:1150–3. doi: 10.1126/science.1099159. [DOI] [PubMed] [Google Scholar]
- 28.Hugues S, Fetler L, Bonifaz L, Helft J, Amblard F, Amigorena S. Distinct T cell dynamics in lymph nodes during the induction of tolerance and immunity. Nat Immunol. 2004;5:1235–42. doi: 10.1038/ni1134. [DOI] [PubMed] [Google Scholar]
- 29.Celli S, Lemaître F, Bousso P. Real-time manipulation of T cell-dendritic cell interactions in vivo reveals the importance of prolonged contacts for CD4+ T cell activation. Immunity. 2007;27:625–34. doi: 10.1016/j.immuni.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 30.Celli S, Albert ML, Bousso P. Visualizing the innate and adaptive immune responses underlying allograft rejection by two-photon microscopy. Nat Med. 2011;17:744–9. doi: 10.1038/nm.2376. [DOI] [PubMed] [Google Scholar]
- 31.Chow A, Toomre D, Garrett W, Mellman I. Dendritic cell maturation triggers retrograde MHC class II transport from lysosomes to the plasma membrane. Nature. 2002;418:988–94. doi: 10.1038/nature01006. [DOI] [PubMed] [Google Scholar]
- 32.Boes M, Cerny J, Massol R, Op den Brouw M, Kirchhausen T, Chen J, et al. T-cell engagement of dendritic cells rapidly rearranges MHC class II transport. Nature. 2002;418:983–8. doi: 10.1038/nature01004. [DOI] [PubMed] [Google Scholar]
- 33.Bertho N, Cerny J, Kim YM, Fiebiger E, Ploegh H, Boes M. Requirements for T cell-polarized tubulation of class II+ compartments in dendritic cells. J Immunol. 2003;171:5689–96. doi: 10.4049/jimmunol.171.11.5689. [DOI] [PubMed] [Google Scholar]
- 34.Boes M, Bertho N, Cerny J, Op den Brouw M, Kirchhausen T, Ploegh H. T cells induce extended class II MHC compartments in dendritic cells in a Toll-like receptor-dependent manner. J Immunol. 2003;171:4081–8. doi: 10.4049/jimmunol.171.8.4081. [DOI] [PubMed] [Google Scholar]
- 35.Vyas JM, Kim YM, Artavanis-Tsakonas K, Love JC, Van der Veen AG, Ploegh HL. Tubulation of class II MHC compartments is microtubule dependent and involves multiple endolysosomal membrane proteins in primary dendritic cells. J Immunol. 2007;178:7199–210. doi: 10.4049/jimmunol.178.11.7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lämmermann T, Renkawitz J, Wu X, Hirsch K, Brakebusch C, Sixt M. Cdc42-dependent leading edge coordination is essential for interstitial dendritic cell migration. Blood. 2009;113:5703–10. doi: 10.1182/blood-2008-11-191882. [DOI] [PubMed] [Google Scholar]
- 37.McDonald D, Wu L, Bohks SM, KewalRamani VN, Unutmaz D, Hope TJ. Recruitment of HIV and its receptors to dendritic cell-T cell junctions. Science. 2003;300:1295–7. doi: 10.1126/science.1084238. [DOI] [PubMed] [Google Scholar]
- 38.Watkins SC, Salter RD. Functional connectivity between immune cells mediated by tunneling nanotubules. Immunity. 2005;23:309–18. doi: 10.1016/j.immuni.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 39.Stuart LM, Ezekowitz RA. Phagocytosis: elegant complexity. Immunity. 2005;22:539–50. doi: 10.1016/j.immuni.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 40.Stuart LM, Ezekowitz RA. Phagocytosis and comparative innate immunity: learning on the fly. Nat Rev Immunol. 2008;8:131–41. doi: 10.1038/nri2240. [DOI] [PubMed] [Google Scholar]
- 41.Artavanis-Tsakonas K, Kasperkovitz PV, Papa E, Cardenas ML, Khan NS, Van der Veen AG, et al. The tetraspanin CD82 is specifically recruited to fungal and bacterial phagosomes prior to acidification. Infect Immun. 2011;79:1098–106. doi: 10.1128/IAI.01135-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Artavanis-Tsakonas K, Love JC, Ploegh HL, Vyas JM. Recruitment of CD63 to Cryptococcus neoformans phagosomes requires acidification. Proc Natl Acad Sci U S A. 2006;103:15945–50. doi: 10.1073/pnas.0607528103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoffmann E, Kotsias F, Visentin G, Bruhns P, Savina A, Amigorena S. Autonomous phagosomal degradation and antigen presentation in dendritic cells. Proc Natl Acad Sci U S A. 2012;109:14556–61. doi: 10.1073/pnas.1203912109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kasperkovitz PV, Cardenas ML, Vyas JM. TLR9 is actively recruited to Aspergillus fumigatus phagosomes and requires the N-terminal proteolytic cleavage domain for proper intracellular trafficking. J Immunol. 2010;185:7614–22. doi: 10.4049/jimmunol.1002760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Appleyard D, Vandermeulen K, Lee H, Lang M. Optical trapping for undergraduates. Am J Phys. 2007;75:5–14. doi: 10.1119/1.2366734. [DOI] [Google Scholar]
- 46.Ashkin A, Dziedzic JM. Optical levitation of liquid drops by radiation pressure. Science. 1975;187:1073–5. doi: 10.1126/science.187.4181.1073. [DOI] [PubMed] [Google Scholar]
- 47.Ashkin A. Optical trapping and manipulation of neutral particles using lasers. Proc Natl Acad Sci U S A. 1997;94:4853–60. doi: 10.1073/pnas.94.10.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ashkin A, Dziedzic JM, Bjorkholm JE, Chu S. Observation of a single-beam gradient force optical trap for dielectric particles. Opt Lett. 1986;11:288. doi: 10.1364/OL.11.000288. [DOI] [PubMed] [Google Scholar]
- 49.Goksör M, Enger J, Hanstorp D. Optical manipulation in combination with multiphoton microscopy for single-cell studies. Appl Opt. 2004;43:4831–7. doi: 10.1364/AO.43.004831. [DOI] [PubMed] [Google Scholar]
- 50.Hoffmann A, Horste G, Pilarczyk G, Monajembashi S, Uhl V, Greulich K. Optical tweezers for confocal microscopy. Appl Phys B. 2000;71:747–53. doi: 10.1007/s003400000454. [DOI] [Google Scholar]
- 51.Tam JM, Castro CE, Heath RJ, Cardenas ML, Xavier RJ, Lang MJ, et al. Control and manipulation of pathogens with an optical trap for live cell imaging of intercellular interactions. PLoS One. 2010;5:e15215. doi: 10.1371/journal.pone.0015215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tam JM, Castro CE, Heath RJ, Mansour MK, Cardenas ML, Xavier RJ, et al. Use of an optical trap for study of host-pathogen interactions for dynamic live cell imaging. J Vis Exp. 2011 doi: 10.3791/3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tam JM, Mansour MK, Khan NS, Yoder NC, Vyas JM. Use of fungal derived polysaccharide-conjugated particles to probe Dectin-1 responses in innate immunity. Integr Biol (Camb) 2012;4:220–7. doi: 10.1039/c2ib00089j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McNerney GP, Hübner W, Chen BK, Huser T. Manipulating CD4+ T cells by optical tweezers for the initiation of cell-cell transfer of HIV-1. J Biophotonics. 2010;3:216–23. doi: 10.1002/jbio.200900102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oddos S, Dunsby C, Purbhoo MA, Chauveau A, Owen DM, Neil MAA, et al. High-speed high-resolution imaging of intercellular immune synapses using optical tweezers. Biophys J. 2008;95:L66–8. doi: 10.1529/biophysj.108.143198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


