Abstract
Tuberous sclerosis complex 1 (Tsc1) is a tumor suppressor negatively regulating mammalian target of rapamycin complex 1 (mTORC1). It is reported that mice lacking Tsc1 gene in oocytes show depletion of primordial follicles, resulting in premature ovarian failure and subsequent infertility. A recent study indicated that deletion of Tsc1 in somatic cells of the reproductive tract caused infertility of female mice. However, it is not known whether specific disruption of Tsc1 in granulosa cells influences the reproductive activity of female mice. To clarify this problem, we mated Tsc1flox/flox mice with transgenic mice strain expressing cyp19-cre which exclusively expresses in granulosa cells of the ovary. Our results demonstrated that Tsc1flox/flox; cyp19-cre mutant mice were fertile, ovulating more oocytes and giving birth to more pups than control Tsc1flox/flox mice. Progressive accumulation of corpora lutea occurred in the Tsc1flox/flox; cyp19-cre mutant mice with advanced age. These phenotypes could be explained by the elevated activity of mTORC1, as indicated by increased phosphorylation of rpS6, a substrate of S6 in the Tsc1flox/flox; cyp19-cre mutant granulosa cells. In addition, rapamycin, a specific mTORC1 inhibitor, effectively rescued the phenotype caused by increased mTORC1 activity in the Tsc1cko ovaries. Our data suggest that conditional knockout of Tsc1 in granulosa cells promotes reproductive activity in mice.
Introduction
In mammals, folliculogenesis is strictly controlled by FSH and LH. FSH supports follicles to develop to the preovulatory stage, and the LH surge causes ovulation and rapidly initiates terminal differentiation of ovulated follicles into the corpora lutea [1], [2]. Numerous signaling pathways participate in these processes, such as phosphoinositide-3 kinase (PI3K), ERK1/2 and cAMP/protein kinase A pathways. These pathways coordinate expression of a huge number of genes in granulosa cells stimulated by FSH and LH [3].
Phosphoinositide-3 kinase (PI3K) signaling is a well known pathway, playing a vital role in many biological processes related to cancer, immunity, metabolism, and others [4]. FSH rapidly activates the PI3K pathway, initiating AKT phosphorylation. Activated AKT then phosphorylates its target proteins, FOXO1 (Forkhead winged helix box O1) and FOXO3 (Forkhead winged helix box O3) to control granulosa cell function and differentiation [3], [5], [6], [7]. In vitro experiments demonstrate that PI3K pathway mediated by FSH in granulosa cells is essential for differentiation and expansion of granulosa cells [6], [7], [8], [9]. In addition, oocyte-specific deletion of PI3K pathway members causes premature ovarian failure because of global primordial follicle activation [10], [11], [12], [13]. Moreover, conditional knockout of Pten in ovary granulosa cells promotes ovulation and causes progressive accumulation of corpora lutea [14]. These results indicate that the PI3K pathway is very important for ovarian functions.
In oocytes, the activation of S6-RPS6 by Pten deletion largely depends on mammalian target of rapamycin complex I (mTORC1) [12]. This indicates that mTORC1 is one critical downstream effector of the PI3K pathway [15]. As a serine/threonine kinase that regulates cell growth and proliferation by modulating processes such as ribosome biogenesis, protein synthesis and cell autophagy, the activity of mTORC1 is negatively regulated by a heterodimeric complex consisting of two proteins: TSC1 (hamartin) and TSC2 (tuberin) [15], [16]. Tsc1 and Tsc2 are two tumor suppressor genes, inactivating mutations in either of which explains genetically why patients suffer multiple tumors in various tissues and organs [17]. In cells, TSC1 and TSC2 form a heterodimeric complex, and TSC1 stabilizes TSC2 by protecting it from ubiquitination and degradation. The TSC1/2 complex controls cell growth, metabolism and proliferation by suppressing mTORC1 activation through a GTPase mechanisms [18], [19].
In order to determine the role of Tsc1/2 in development in vivo, mouse models for studying function of Tsc1 and Tsc2 have been developed. Because of embryonic lethality caused by conventional deletion of Tsc1 or Tsc2, conditional knockout of Tsc1 and Tsc2 in specific organs have been introduced via the Cre-loxP system [20], [21]. Specific deletion of Tsc1 or Tsc2 caused abnormalities in brain, heart, and kidney [22], [23], [24], [25]. In the ovary, oocyte specific disruption of either Tsc1 or Tsc2 leads to global activation of primordial follicles at the time of puberty, resulting in early follicle depletion and premature ovarian failure (POF) [26], [27]. So far, there are also data about deletion of Tsc1 in somatic cells of the mouse reproductive tract. Disruption of Tsc1 introduced by Amhr2-cre caused defects in ovarian folliculogenesis, compromised oocyte/embryo integrity, obstruction of oviduct and failure of implantation, resulting in female infertility [28]. As described above, it is not clear whether specific depletion of Tsc1 in granulosa cells contributes to the fertility/infertility in the Tsc1flox/flox; Amhr2-cre female mice, because of the wide expression of Amhr2-cre in some somatic cells of the reproductive tract [28], [29].
In the current study, in order to investigate the role of Tsc1 in granulosa cells in female reproductive activity, we used cyp19-cre to specifically delete Tsc1 expression in granulosa cells [30]. Our results show that increased activity of mTORC1 in granulosa cells caused by Tsc1 deletion does not cause sterility. On the contrary, Tsc1 depletion improves reproductive capacity to some extent, stimulates folliculogenesis, and leads to progressive accumulation of corpora lutea.
Materials and Methods
Mice
Tsc1flox/flox mice were maintained with a mixed genomic background of 129S4/SvJae and C57/BL6 [20], and cyp19-cre mice were maintained with C57/BL6 genomic background [30]. Tsc1flox/flox mice were crossed with cyp19-cre mice to generate Tsc1flox/flox; cyp19-cre (Tsc1cko) mutant mice which are homozygous for the Tsc1 floxed allele and heterozygous for cyp19-cre. Animals that are homozygous for Tsc1 floxed allele and cyp19-cre negative were used as control mice. Mice were housed in 12-hour alternating light/dark cycles, with free access to water and food. All experiments were conducted with the approval of the Animal Research Committee of the Institute of Zoology, Chinese Academy of Sciences, China.
Fertility Superovulation and Natural Ovulation Analysis
To evaluate the reproductive activity, six individually housed Tsc1flox/flox and Tsc1cko female mice at the age of 6 weeks were crossed to Tsc1flox/flox male mice with known fertility. The numbers of pups and litters were recorded up to 6 months. At the age of 23d, female mice of both genotype were injected with 5 IU of PMSG (Sansheng, Ningbo China) followed 48 h later with 5IU of hCG (Sansheng, Ningbo China) for superovulation analysis. For natural ovulation, female mice in estrus were mated with male mice. The next morning, female mice with plugs were euthanized, and fertilized eggs were separated from the oviduct and counted.
Western Blot Analysis
Granulosa cells were collected from COC of six Tsc1flox/flox or Tsc1cko mice at the age of 23d after superovulation. Proteins extracted from cell lysis were quantified for western blot analysis. The primary antibodies used were: Tsc1, Tsc2, Akt, phospho-Akt (ser473), and phospho-rpS6 (ser240/4) from Cell Signaling Technology (USA), rpS6 from Bioworld (USA). β-Tubulin from Abmart (USA) was used as a loading control. Western blot were carried out according to the instructions by suppliers of the respective antibodies and viewed using molecular imager™ (Bio-Rad).
Histological Analysis of Ovaries
Ovaries were fixed in 4% paraformaldehyde, dehydrated in a graded ethanol series, cleared in xylene, and embedded in paraffin wax. The paraffin-embedded ovaries were sectioned serially at 8 µm and stained with hematoxylin and eosin for histological analysis.
Rapamycin Treatment
Rapamycin (LC Laboratories, Worburn, MA) was dissolved to 50 mg/ml in ethanol and diluted in a vehicle containing 0.25% Tween-20 and 0.25% polyethylene glycol in PBS. Mice were given intraperitoneal injection with either rapamycin (a daily dosage of 5 mg/kg body weight) or vehicle alone. Tsc1cko mice were injected daily from PD21 to PD42 and euthanized at PD43, then one ovary of each mouse was weighed, fixed, dehydrated and embedded for morphological analysis; the other one was lysed for western blot analysis. For superovulation analysis, Tsc1cko mice were intraperitoneally injected with rapamycin (a daily dosage of 3 mg/kg body weight) or vehicle from PD21 to PD42, followed by PMSG (Sansheng, Ningbo China) and hCG (Sansheng, Ningbo China) treatment.
Statistical Analysis
All experiments were repeated at least three times for statistical analysis. For comparisons, means and standard deviations were calculated, and the difference between two groups was compared using student’s t-test. Difference was considered significant if P<0.05.
Results
Generation of Mice with Disruption of Tsc1 in Granulosa Cells
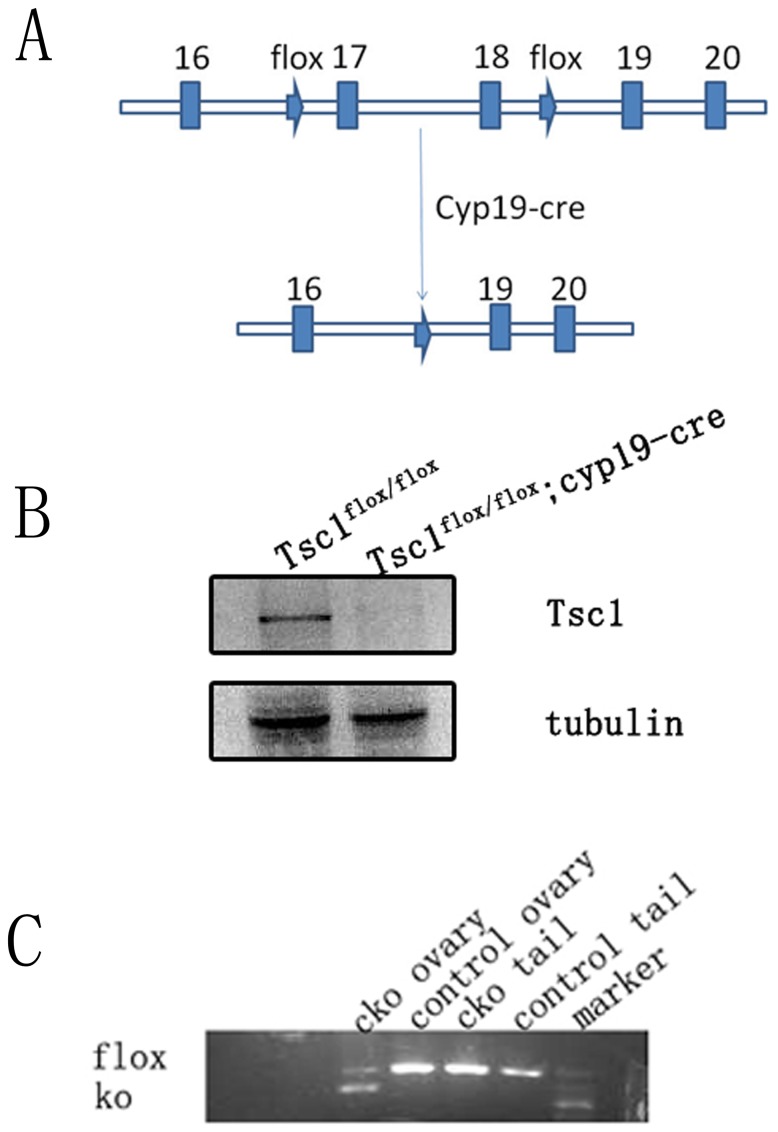
To deplete Tsc1 in granulosa cells, we crossed the Tsc1flox/flox mice [20] with transgenic mice carrying cyp19 promoter-mediated Cre recombinase(cyp19-cre) [30] (Fig. 1A). The activity of cyp19-cre was detected in granulosa cells of all antral follicles and most luteal cells, but was low or nearly undetectable in granulosa cells of primordial and primary follicles [14], [30]. First we compared the expression of TSC1 protein in the granulosa cells of control and mutant ovaries, to confirm that the expression of TSC1 was diminished in the mutant granulosa cells. Cumulus granulosa cells were isolated from cumulus oocyte complexes (COC) from superovulated Tsc1flox/flox and Tsc1cko mice for western blot analysis (n = 6 per genotype). The results demonstrated that the Tsc1 protein was absent in Tsc1cko granulosa cells (Fig. 1B).
Figure 1. Generation of Tsc1cko mutant mice and characterization of Tsc1 disruption by western blot and PCR analysis.
(A) Schematic representation of deletion of Tsc1 exon17 and exon18 by cyp19-cre mediated recombination in granulosa cells. (B) Granulosa cells were collected from COC of both Tsc1flox/flox mice and Tsc1cko mutant mice and lysed for western blot: Tsc1 was almost absent in Tsc1cko granulosa cells, β-Tubulin was used as an internal control. (C) PCR analysis indicated that cyp19-cre mediated recombination of Tsc1 exclusively occurred in Tsc1cko ovary.
To confirm the recombination of the floxed alleles induced by cyp19-cre, we collected DNA from tails and ovaries of Tsc1flox/flox (control or WT) and Tsc1flox/flox: cyp19-cre (Tsc1cko) mice for PCR analysis. As expected, the DNA band representing depletion of the exon17 and exon18 of Tsc1 only appeared in the amplicon of Tsc1cko mutant ovaries, demonstrating that recombination occurred exclusively in ovaries of Tsc1cko mice (Fig. 1C). All these results indicate that deletion of Tsc1 is successful in granulosa cells of Tsc1cko mutant ovary where cyp19-cre is expressed.
Increased Ovulation and Reproductive Activity in Tsc1 Conditional Knockout Mice
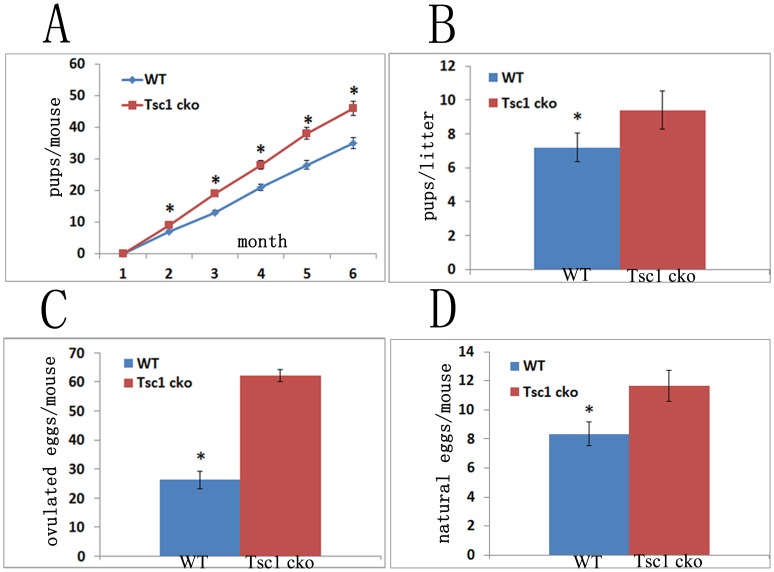
To test whether conditional knockout of Tsc1 in granulosa cells affects the reproductive capacity of mutant mice, we mated Tsc1flox/flox and Tsc1cko female mice with Tsc1flox/flox male mice. Our observation demonstrated that Tsc1cko female mice were fertile, moreover, they even produced moderately more pups than Tsc1flox/flox control mice during a 6-month breeding period (n = 6 per genotype) (Fig. 2A). The average litter size of mutant mice was also larger than that of control mice. About 9 (9.4±0.50) pups per litter were born by Tsc1cko female mice, whereas the number of control mice was about 7 (7.2±0.37) (Fig. 2B). To confirm whether the increased number of pups born is attributed to elevated ovulation after Tsc1 deletion, immature mice were primed with PMSG followed by hCG treatment after 48 hour for superovulation test. Thirteen hours after hCG injection, mice were euthanized, and their oviducts were removed for oocyte collection and analysis. Subsequent results indicated that Tsc1cko mutant mice ovulated more oocytes than control mice, accounting for the increased reproductive activity of Tsc1cko mutant mice (n = 4 per genotype) (Fig. 2C). More convincingly, more oocytes from the oviduct of Tsc1cko mutant mice than that from control oviduct were collected after natural ovulation and copulation (n = 3 per genotype) (Fig. 2D).
Figure 2. Evaluation of reproductive activity of Tsc1flox/flox;cyp19-cre mice.
(A) Tsc1flox/flox; cyp19-cre mice produced more pups than wild type mice. (B) The average litter size of Tsc1flox/flox; cyp19-cre mice was bigger than that of the wild type. (C) Tsc1flox/flox; cyp19-cre mice ovulated more oocytes than wild type mice in superovulation analysis. (D) A little more oocytes were collected from the oviduct of Tsc1flox/flox; cyp19-cre mice than that from wild mice after natural ovulation and copulation.WT, wild type. *, P<0.05.
Tsc1 Conditional Knockout in Granulosa Cells Increased Folliculogenesis through Regulating the mTOR Pathway
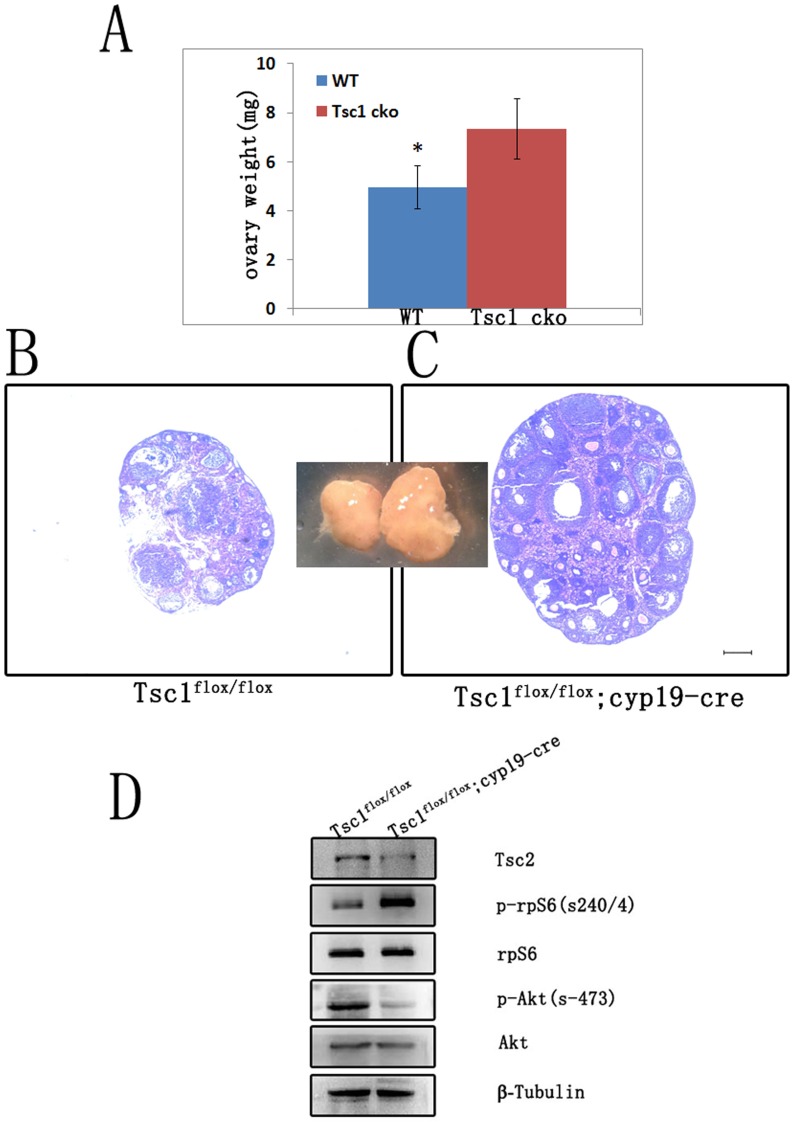
The observed change of Tsc1 mutant mice may be ascribed to the increased follicle growth in the mutant ovary. To characterize the attribution, we collected ovaries from Tsc1flox/flox and Tsc1cko female mice at 6 weeks (n = 5 per genotype). As expected, Tsc1cko female mice had significantly heavier ovaries than Tsc1flox/flox control mice (Fig. 3A). Moreover, sections of the ovaries stained with hematoxylin and eosin demonstrated that there were more growing follicles and antral follicles in the ovaries of Tsc1cko mutant mice than that of Tsc1flox/flox mice (Fig. 3B and Fig. 3C).
Figure 3. Conditional depletion of Tsc1 in granulosa cells resulted in elevated mTORC1 activity, decreased mTORC2 activity and increased folliculogenesis.
(A) Ovaries of Tsc1cko mutant mice were heavier than those of wild type mice. (B and C) Hematoxylin and eosin staining showed increased folliculogenesis in Tsc1cko mutant ovaries. (D) Western blot of granulosa cells from COC. After deletion of Tsc1, Tsc2 was nearly absent; and the activity of mTORC1 was elevated, as indicated by increased phosphorylation of rpS6, a substrate of S6; meanwhile, activity of mTORC2 was down-regulated, as indicated by decreased phosphorylation of Akt. rpS6, Akt, and tubulin were used as control. Bar, 250 µm. *,P<0.05.
In order to elaborate the molecular mechanism underlying the observed phenotypes in mutant ovary, we detected the expression level of several members regulated by Tsc1. Firstly, the expression of Tsc2 was largely diminished after Tsc1 deletion (Fig3D), consistent with the report that the function of Tsc1 was to stabilize Tsc2 [19]. mTOR falls into two distinct functional complexes, mTORC1 and mTORC2. While mTORC1 is rapamycin-sensitive, mTORC2 is rapamycin-insensitive [31], [32]. mTORC1 controls mRNA translation and promotes cell proliferation through phosphorylation of rpS6, a substrate of S6 kinase, which is a downstream target of mTOR kinase activity [15]. In contrast, mTORC2 is involved in cytoskeletal organization and phosphorylation of Akt [16]. We found that the level of phosphorylated rpS6 was elevated in Tsc1cko mutant granulosa cells compared to control cells, while the expression of rpS6 did not change significantly. On the contrary, phosphorylation of Akt decreased dramatically in Tsc1 deleted granulosa cells while the level of Akt stayed constant (Fig. 3A). These results indicate that the activity of mTORC1 is elevated in mutant granulosa cells while mTORC2 activity is down-regulated.
Progressive Accumulation of Corpora Lutea in Tsc1 Conditional Knockout Mice
The corpus luteum is very important for the regulation of the estrous cycle and maintenance of pregnancy. After ovulation, the residual follicle undergoes luteinization to become the corpus luteum. If the oocyte is fertilized, the corpus luteum produces progesterone to maintain pregnancy. If fertilization does not occur the corpus luteum regresses, followed by a new estrous cycle [1], [2]. So the number of corpora lutea stays comparatively constant in every estrous cycle in the normal ovary.
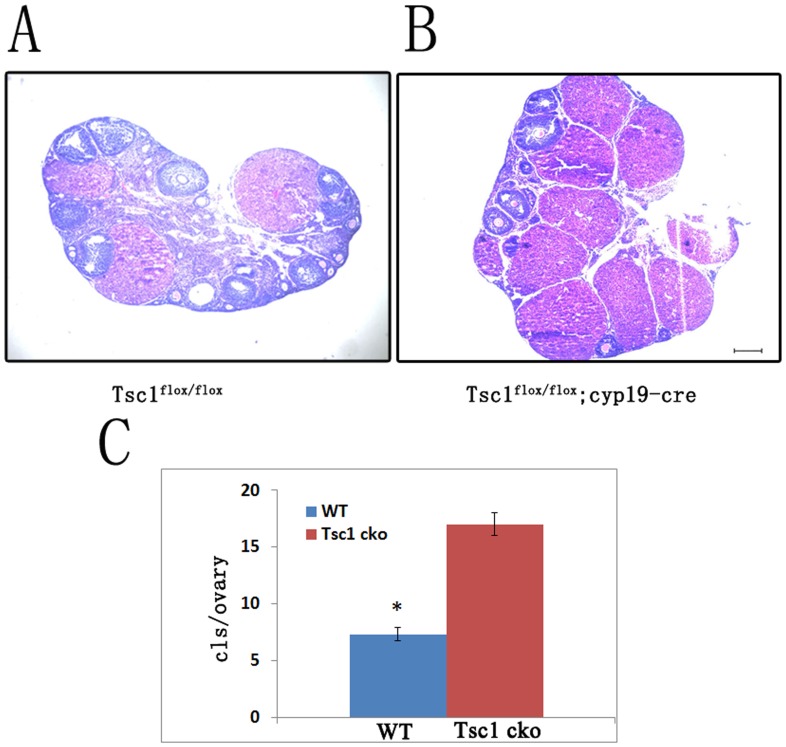
As reported for ovaries in which Pten was deleted in granulosa cells [14], we observed that progressive accumulation of corpora lutea occurred in the Tsc1cko mutant ovaries compared with control ovaries. There was no significant difference in the abundance of the corpora lutea in the mutant ovaries compared with control ovaries at the age of 6 weeks. It became gradually apparent that the ovaries of Tsc1cko mice contained more corpora lutea than those of control mice at 3 months of age. We ascertained the abundance of corpora lutea through histological analysis of 6-month-old Tsc1cko mutant ovaries (Fig. 4A and Fig. 4B). We counted the overall corpora lutea of both control and mutant ovaries in serial sections (n = 3 per genotype). The results indicated that the number of corpora lutea in Tsc1cko ovaries was about 3 times of that in normal cycling ovaries (Fig. 4C).
Figure 4. Progressive accumulation of corpora lutea.
(A and B) Ovaries of Tsc1cko mutant mice and control mice at the age of 6 months were sectioned serially, and hemaxytoxylin and eosin staining showed accumulation of corpora lutea in Tsc1cko mutant ovaries compared with control ovaries. (C) Quantification of serial sections indicated that the numbers of corpora lutea in Tsc1cko mutant ovaries were about 3 times of that in control ovaries. Bar, 250 µm. *,P<0.05.
Adjustment of Follicle Growth and Ovulation by Rapamycin
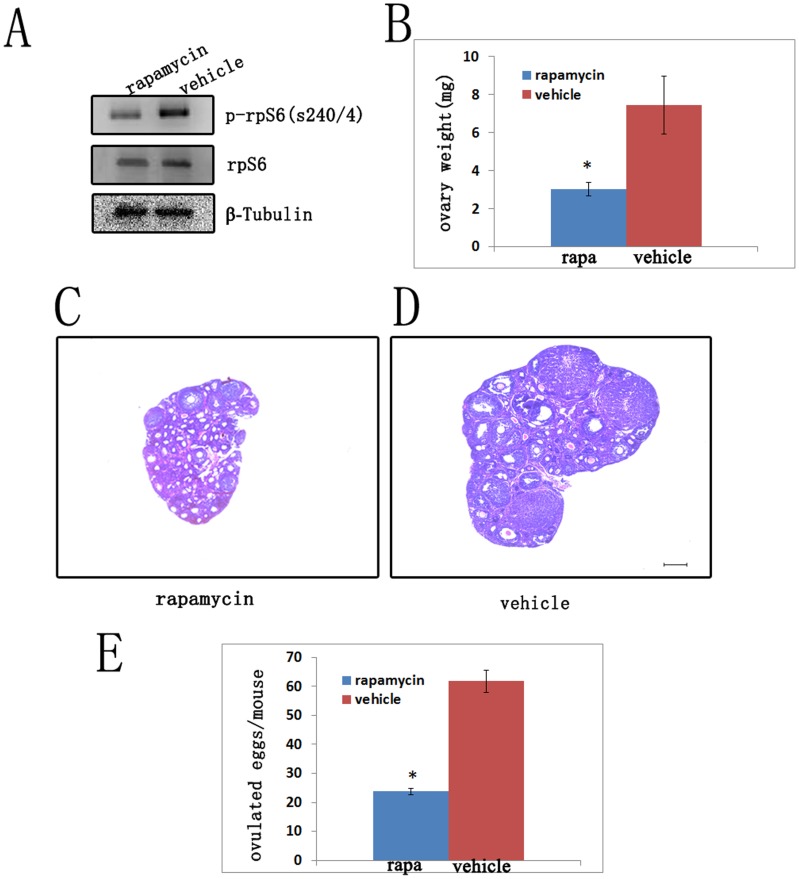
It has been reported that the activity of mTORC1 is specifically inhibited by rapamycin [31]. So we treated the Tsc1cko mutant mice with rapamycin from post-natal day (PD) 21 to PD42, to ascertain whether it was the elevated activity of mTORC1 that promoted the follicular growth and ovulation. After rapamycin treatment, the expression level of phosphorylated rpS6 dramatically decreased compared with that of the group which was treated with vehicle (Fig. 5A).
Figure 5. Adjustment of Tsc1cko mutant ovaries by postnatal rapamycin treatment.
(A) Decreased activity of mTORC1 in Tsc1cko mutant ovaries after rapamycin treatment as indicated by down-regulated phosphorylated rpS6; rpS6 and tubulin were used as control. (B) The Tsc1cko mutant ovaries treated with rapamycin were lighter than those treated with vehicle. (C and D) Hemaxytoxylin and eosin staining showed that the folliculogenesis of Tsc1cko mutant ovaries declined after rapamycin treatment compared with controls. (E) The rapamycin treated Tsc1cko mutant mice ovulated less oocytes than Tsc1cko mutant mice treated with vehicle after superovulation. Bar, 250 µm. *,P<0.05.
Our observation also showed that the ovaries are smaller and lighter, containing less antral follicles in Tsc1cko mutant mice treated with rampamycin (Fig. 5B and Fig. 5C). In comparison, the ovaries of Tsc1cko mutant mice treated with vehicle were much larger and contained more antral follicles (Fig. 5B and Fig. 5D) (n = 3 per case). Moreover, the vehicle-treated mutant mice ovulated more oocytes than rapamycin-treated mutant mice in superovulation analysis (Fig. 5E) (n = 4 per case). These results indicate that rapamycin effectively rescued the phenotype caused by increased mTORC1 activity in the Tsc1cko ovaries. Our results clearly confirm that it is the elevated activity of mTORC1 that accounts for the increased follicular growth in the ovary of Tsc1cko mutant mice.
Discussion
The tuberous sclerosis complex (TSC) is a multisystem, autosomal dominant disorder affecting both children and adults with a rate of one in 6000. TSC is characterized by developing benign tumors in various organs such as kidney, heart, brain, and others. Genetic analysis indicates that TSC patients carry mutations in either the harmatin (Tsc1) or tuberin (Tsc2) genes [17]. Tsc1 and Tsc2 work as heterodimers in cells. They control cell proliferation and survival by regulating the activity of mTOR and playing a vital role in many signaling cascades [18]. Indeed, conditional disruption of Tsc1 or Tsc2 in brain, kidney or heart causes corresponding abnormalities because of dysregulation of mTOR activity, and all the induced pathologies can be rescued by rapamycin, a specific inhibitor of mTOR [22], [23], [24], [25].
In ovaries, specific deletion of Tsc1 or Tsc2 in oocytes leads to primordial follicle depletion, causing premature ovarian failure [26], [27]. Conditional knockout of Tsc1 in somatic cells of the reproductive tract results in infertility in female mice. It is noteworthy that Amhr2-cre previously used is not only expressed in granulosa cells, but also in the oviduct and uterus, so it is not confirmed whether specific deletion of Tsc1 in granulosa cells results in female infertility or not [4]. In the current study, we deleted Tsc1 exclusively in granulosa cells to clarify the function of Tsc1 in ganulosa cells by using cyp19-cre transgenic mice because of its specific expression in the granulosa cells of the ovary [14], [30]. Our results demonstrate that disruption of Tsc1 in granulosa cells does not contribute to female sterility. On the contrary, Tsc1cko mutant mice in our study breed more pups than control mice to some extent. This may be attributed to the increase in oocytes ovulated in cyp19-cre mediated mutant mice. Moreover, because Tsc1cko mutant mice give birth to pups even at the age of 6 months, so we do not believe that premature ovarian failure occurs in the female knockout mice.
Unlike mutation of Tsc1 in other organs, we observed no tumors in Tsc1cko mutant ovaries. Interestingly, we found that corpus luteum progressively accumulated in the Tsc1cko mutant ovaries compared with control ovaries, which was identical to the ovaries in which Pten was conditionally deleted in granulosa cells [14]. However, corpora lutea that had prolonged lifespan caused by dysregulated activity of mTORC1 after loss of Tsc1 appeared to have no impact on the steroidogenic activity, because Tsc1cko mutant mice had normal reproductive cycles as control mice (Fig. 2A), indicating that the function of the corpora lutea in Tsc1cko mutant ovaries was not altered. More detailed research is needed to investigate why the corpora lutea with extended lifespan does not have prolonged steroidogenic activity.
We observed in our study elevated activity of mTORC1 (represented by increased phosphorylation of rpS6) and decreased activity of mTORC2 (represented by decreased phosphorylation of Akt), both of which may account for the increased folliculogenesis and ovulation. A previous study reported that rapamycin is a specific inhibitor of mTORC1 [31]. In order to determine whether the observed increased folliculogenesis and ovulation were mTORC1- dependent after loss of Tsc1, we tried to rescue the phenotypes using rapamycin. As expected, rapamycin could rescue the folliculogenesis and ovulation. This result confirms that deletion of Tsc1 leads to the phenotypes in Tsc1cko mutant mice through stimulation of mTORC1.
In summary, the present study shows that depletion of Tsc1 in granulosa cells does not cause infertility in mice, but improves the reproductivity by stimulating folliculogenesis, ovulation and progressive accumulation of corpora lutea via increased activity of mTORC1. Although the phenotypes observed in our study were relatively mild, which may be attributed to preferential expression of cyp19-cre in the granulosa cells of antral follicle [30], our data still offer physiological and clinical implications of Tsc1 for human ovarian development and pathology.
Acknowledgments
We thank Dr. Jing-Pian Peng for technical help, and Cao Yu for experimental suggestions. We thank Xi-Xia Li for providing Akt and phosphor-Akt (ser-473) antibodies.
Funding Statement
This study was supported by the National Basic Research Program of China (2012CB944404, 2011CB944501, www.973.gov.cn/English/ReadItem.aspxitemid=487) and National Natural Science Foundation of China (No.30930065, www.nsfc.gov.cn/Portal0/default166.htm). The funders had no role in study design, data collection and analysis,decision to publish, or preparation of the manuscript.
References
- 1. Liu K, Rajareddy S, Liu L, Jagarlamudi K, Boman K, et al. (2006) Control of mammalian oocyte growth and early follicular development by the oocyte PI3 kinase pathway: new roles for an old timer. Dev Biol 299: 1–11. [DOI] [PubMed] [Google Scholar]
- 2. Stocco C, Telleria C, Gibori G (2007) The molecular control of corpus luteum formation, function, and regression. Endocr Rev 28: 117–149. [DOI] [PubMed] [Google Scholar]
- 3. Hunzicker-Dunn M, Maizels ET (2006) FSH signaling pathways in immature granulosa cells that regulate target gene expression: Branching out from protein kinase A. Cellular Signalling. 18: 1351–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tanaka Y, Park JH, Tanwar PS, Kaneko-Tarui T, Mittal S, et al. (2012) Deletion of tuberous sclerosis 1 in somatic cells of the murine reproductive tract causes female infertility. Endocrinology 153: 404–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wayne CM, Fan HY, Cheng XD, Richards JS (2007) Follicle-stimulating hormone induces multiple signaling cascades: Evidence that activation of Rous sarcoma oncogene, RAS, and the epidermal growth factor receptor are critical for granulosa cell differentiation. Molecular Endocrinology 21: 1940–1957. [DOI] [PubMed] [Google Scholar]
- 6. Gonzalez-Robayna IJ, Falender AE, Ochsner S, Firestone GL, Richards JS (2000) Follicle-Stimulating hormone (FSH) stimulates phosphorylation and activation of protein kinase B (PKB/Akt) and serum and glucocorticoid-lnduced kinase (Sgk): evidence for A kinase-independent signaling by FSH in granulosa cells. Molecular endocrinology (Baltimore, Md ) 14: 1283–1300. [DOI] [PubMed] [Google Scholar]
- 7. Alam H, Maizels ET, Park Y, Ghaey S, Feiger ZJ, et al. (2004) Follicle-stimulating hormone activation of hypoxia-inducible factor-1 by the phosphatidylinositol 3-kinase/AKT/Ras homolog enriched in brain (Rheb)/mammalian target of rapamycin (mTOR) pathway is necessary for induction of select protein markers of follicular differentiation. The Journal of biological chemistry 279: 19431–19440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Richards JS, Sharma SC, Falender AE, Lo YH (2002) Expression of FKHR, FKHRL1, and AFX genes in the rodent ovary: evidence for regulation by IGF-I, estrogen, and the gonadotropins. Molecular endocrinology (Baltimore, Md ) 16: 580–599. [DOI] [PubMed] [Google Scholar]
- 9. Park Y, Maizels ET, Feiger ZJ, Alam H, Peters CA, et al. (2005) Induction of cyclin D2 in rat granulosa cells requires FSH-dependent relief from FOXO1 repression coupled with positive signals from Smad. The Journal of biological chemistry 280: 9135–9148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reddy P, Adhikari D, Zheng W, Liang S, Hamalainen T, et al. (2009) PDK1 signaling in oocytes controls reproductive aging and lifespan by manipulating the survival of primordial follicles. Human molecular genetics 18: 2813–2824. [DOI] [PubMed] [Google Scholar]
- 11. John GB, Shirley LJ, Gallardo TD, Castrillon DH (2007) Specificity of the requirement for Foxo3 in primordial follicle activation. Reproduction (Cambridge, England) 133: 855–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reddy P, Liu L, Adhikari D, Jagarlamudi K, Rajareddy S, et al. (2008) Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science (New York, N Y ) 319: 611–613. [DOI] [PubMed] [Google Scholar]
- 13. Hu MW, Wang ZB, Schatten H, Sun QY (2012) New Understandings on Folliculogenesis/Oogenesis Regulation in Mouse as Revealed by Conditional Knockout. Journal of Genetics and Genomics 39: 61–68. [DOI] [PubMed] [Google Scholar]
- 14. Fan HY, Liu Z, Cahill N, Richards JS (2008) Targeted disruption of Pten in ovarian granulosa cells enhances ovulation and extends the life span of luteal cells. Molecular endocrinology (Baltimore, Md ) 22: 2128–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wullschleger S, Loewith R, Hall MN (2006) TOR signaling in growth and metabolism. Cell 124: 471–484. [DOI] [PubMed] [Google Scholar]
- 16. Sarbassov DD, Guertin DA, Ali SM, Sabatini DM (2005) Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science (New York, N Y ) 307: 1098–1101. [DOI] [PubMed] [Google Scholar]
- 17. Crino PB, Nathanson KL, Henske EP (2006) The tuberous sclerosis complex. The New England journal of medicine 355: 1345–1356. [DOI] [PubMed] [Google Scholar]
- 18. Tomasoni R, Mondino A (2011) The tuberous sclerosis complex: balancing proliferation and survival. Biochemical Society transactions 39: 466–471. [DOI] [PubMed] [Google Scholar]
- 19. Chong-Kopera H, Inoki K, Li Y, Zhu T, Garcia-Gonzalo FR, et al. (2006) TSC1 stabilizes TSC2 by inhibiting the interaction between TSC2 and the HERC1 ubiquitin ligase. The Journal of biological chemistry 281: 8313–8316. [DOI] [PubMed] [Google Scholar]
- 20. Kwiatkowski DJ, Zhang H, Bandura JL, Heiberger KM, Glogauer M, et al. (2002) A mouse model of TSC1 reveals sex-dependent lethality from liver hemangiomas, and up-regulation of p70S6 kinase activity in Tsc1 null cells. Human molecular genetics 11: 525–534. [DOI] [PubMed] [Google Scholar]
- 21. Onda H, Lueck A, Marks PW, Warren HB, Kwiatkowski DJ (1999) Tsc2(+/−) mice develop tumors in multiple sites that express gelsolin and are influenced by genetic background. The Journal of clinical investigation 104: 687–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Carson RP, Van Nielen DL, Winzenburger PA, Ess KC (2012) Neuronal and glia abnormalities in Tsc1-deficient forebrain and partial rescue by rapamycin. Neurobiology of disease 45: 369–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Malhowski AJ, Hira H, Bashiruddin S, Warburton R, Goto J, et al. (2011) Smooth muscle protein-22-mediated deletion of Tsc1 results in cardiac hypertrophy that is mTORC1-mediated and reversed by rapamycin. Human molecular genetics 20: 1290–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhou J, Brugarolas J, Parada LF (2009) Loss of Tsc1, but not Pten, in renal tubular cells causes polycystic kidney disease by activating mTORC1. Human molecular genetics 18: 4428–4441. [DOI] [PubMed] [Google Scholar]
- 25. Zeng LH, Rensing NR, Zhang B, Gutmann DH, Gambello MJ, et al. (2011) Tsc2 gene inactivation causes a more severe epilepsy phenotype than Tsc1 inactivation in a mouse model of tuberous sclerosis complex. Human molecular genetics 20: 445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Adhikari D, Zheng W, Shen Y, Gorre N, Hamalainen T, et al. (2010) Tsc/mTORC1 signaling in oocytes governs the quiescence and activation of primordial follicles. Human molecular genetics 19: 397–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Adhikari D, Flohr G, Gorre N, Shen Y, Yang H, et al. (2009) Disruption of Tsc2 in oocytes leads to overactivation of the entire pool of primordial follicles. Molecular human reproduction 15: 765–770. [DOI] [PubMed] [Google Scholar]
- 28. Tanaka Y, Park JH, Tanwar PS, Kaneko-Tarui T, Mittal S, et al. (2012) Deletion of tuberous sclerosis 1 in somatic cells of the murine reproductive tract causes female infertility. Endocrinology 153: 404–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Arango NA, Kobayashi A, Wang Y, Jamin SP, Lee HH, et al. (2008) A mesenchymal perspective of Mullerian duct differentiation and regression in Amhr2-lacZ mice. Molecular reproduction and development 75: 1154–1162. [DOI] [PubMed] [Google Scholar]
- 30. Fan HY, Shimada M, Liu Z, Cahill N, Noma N, et al. (2008) Selective expression of KrasG12D in granulosa cells of the mouse ovary causes defects in follicle development and ovulation. Development (Cambridge, England) 135: 2127–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, et al. (2004) Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Current biology : CB 14: 1296–1302. [DOI] [PubMed] [Google Scholar]
- 32. Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, et al. (2002) mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 110: 163–175. [DOI] [PubMed] [Google Scholar]