Abstract
The characteristics of the T cell response to the members of oral flora are poorly understood. We characterized the antibody and T cell responses to FadA and Td92, adhesins from Fusobacterium nucleatum, an oral commensal, and Treponema denticola, a periodontal pathogen, respectively. Peripheral blood and saliva were obtained from healthy individuals and patients with untreated chronic periodontitis (CP, n = 11 paris) and after successful treatment of the disease (n = 9). The levels of antigen-specific antibody were measured by ELISA. In plasma, IgG1 was the most abundant isotype of Ab for both Ags, followed by IgA and then IgG4. The levels of FadA-specific salivary IgA (sIgA) were higher than Td92-specific sIgA and the FadA-specific IgA levels observed in plasma. However, the periodontal health status of the individuals did not affect the levels of FadA- or Td92-specific antibody. Even healthy individuals contained FadA- and Td92-specific CD4+ T cells, as determined by the detection of intracytoplasmic CD154 after short-term in vitro stimulation of peripheral blood mononuclear cells (PBMCs) with the antigens. Patients with CP tended to possess increased numbers of FadA- and Td92-specific CD4+ T cells but reduced numbers of Td92-specific Foxp3+CD4+ Tregs than the healthy subjects. Both FadA and Td92 induced the production of IFNγ and IL-10 but inhibited the secretion of IL-4 by PBMCs. In conclusion, F. nucleatum induced Th3 (sIgA)- and Th1 (IFNγ and IgG1)-dominant immune responses, whereas T. denticola induced a Th1 (IFNγ and IgG1)-dominant response. This IFNγ-dominant cytokine response was impaired in CP patients, and the Td92-induced IFNγ levels were negatively associated with periodontal destruction in patients. These findings may provide new insights into the homeostatic interaction between the immune system and oral bacteria and the pathogenesis of periodontitis.
Introduction
The surface of the human body is colonized by billions of commensal bacteria that live in harmony with their host. According to the current paradigm, the host immune system maintains tolerance to commensals but develops active immunity against harmful pathogens. Vaughan et al. reported that Neisseria meningitidis, an opportunistic pathogen colonizing the upper respiratory tract, induces mucosal T and B cell memory, but N. lactamica, a commensal bacterium, induces only a T cell-independent B cell response in humans [1]. Similarly in mice, a lack of classical immunological memory is observed in the intestinal IgA response against gut commensals [2]. However, the commensal Lactobacillus, which colonizes the nasal mucosa and crosses the nasal epithelial barrier, induces Th1, but not Treg or Th2, responses in mice [3].
The human oral cavity harbors a complex bacterial community composed of more than 700 species [4]. Most are commensals, and only a small number of species are known to be associated with oral diseases, such as dental caries and periodontitis. Periodontitis is a polymicrobial disease that arises from a microbial shift in the indigenous flora of the plaque biofilm rather than from the sudden introduction of a new pathogen [5]. To understand the immunopathogenesis of periodontitis, antibody (Ab) and T cell responses to periodontal pathogens have been widely studied. A number of studies have demonstrated the presence of IgG Abs against periodontal pathogens using whole bacteria or isolated proteins, such as fimbriae from Porphyromonas gingivalis, bacterial surface protein A from Tannerella forsythia, and leukotoxin from Aggregatibacter actinomycetemcomitans in human serum or gingival crevicular fluid [6]–[10]. Two decades ago, the presence of T cells specific to P. gingivalis in the peripheral blood of patients with periodontitis and of healthy subjects was shown by a limiting dilution assay without further characterization of the bacteria-specific T cells [11]. In the following decade, several groups established peripheral blood and gingival T cell clones specific to periodontal pathogens and characterized their cytokine production [12]–[14]. Kopitar et al. recently reported that the commensal oral bacteria Streptococcus mitis and Propionibacterium acnes prime human dendritic cells to induce in vitro differentiation of Th2 and Treg cells, respectively [15]. However, all previous studies have employed long-term in vitro stimulation with bacterial antigens (Ags) prepared from whole bacteria or outer membranes, which allows for the activation of naïve T cells by specific Ags or non-specific mitogens [16]. Thus, the nature of adaptive immunity to oral comensals and periodontal pathogens in humans are poorly understood.
The aims of the current study include i) to characterize the T-dependent Ab and T cell responses to Fusobacterium nucleatum, an oral commensal, and Treponema denticola, a periodontal pathogen, in healthy individuals, and ii) to determine difference in those Ab and T cell responses between healthy subjects and patients with chronic periodontitis (CP). Although the levels of F. nucleatum are significantly associated with increasing pocket depth [17], F. nucleatum is a prevalent member of plaque biofilm in healthy individuals and is considered a commensal or possibly beneficial bacterium due to its ability to induce antimicrobial peptides in gingival epithelial cells [18], [19]. T. denticola is one of the major periodontal pathogens involved in CP [20]. To exclude components that are mitogenic or reactive to natural Abs produced in a T-independent manner, a single surface protein was chosen as a representative T-dependent Ag for each bacterium. FadA is an adhesin of F. nucleatum involved in adhesion and cellular invasion [21]. Td92 is an adhesin of T. denticola and an inducer of proinflammatory cytokines [22]. The vaccine protein tetanus toxoid (TT) was used as a control Ag to provide a comparison to a classical systemic immune response. Through the examination of Ab, Ag-specific CD4+ T cells, and the cytokine response to these three Ags, here, we demonstrate that both F. nucleatum and T. denticola normally induce T cell memory in humans and that CP is associated with a reduced IFNγ/IL-4 cytokine ratio. These findings may provide new insights into the homeostasis of the immune system with oral bacteria and the pathogenesis of periodontitis.
Materials and Methods
Study Subjects and Periodontal Assessment
Individuals with CP (n = 11) were recruited from patients who visited the Department of Periodontology at the Seoul National University Dental Hospital. CP was diagnosed based on the classification system established at the International Workshop for a Classification of Periodontal Diseases and Conditions in 1999 [23]. Age- and gender-matched healthy control subjects (n = 11) were recruited through an advertisement. Control subjects were designated as healthy if they had both full-mouth probing pocket depth and full-mouth clinical attachment level not greater than 3 mm. All healthy subjects had no history of periodontal treatment or diagnosis with periodontitis. All subjects were in good general health and had no history of antimicrobial or periodontal treatment for 3 months before the start of the study. The study was performed according to the principles expressed in the Declaration of Helsinki, after receiving approval from the Institutional Review Board of the Seoul National University Dental Hospital. Written informed consent was obtained from all subjects. After collecting 20 ml peripheral blood and 5 ml saliva, the full-mouth pocket depth, clinical attachment level from the cementoenalmel junction, and bleeding on probing at six sites for each tooth were recorded by one calibrated examiner. All clinical parameters were measured with a manual probe (CP-12, Hu Friedy, Chicago, IL, USA). Four weeks after receiving quadrant scaling/curettage (four visits within four to six weeks), nine patients who were successfully treated and participated in a re-call check were re-sampled.
Human Materials
Plasma samples were obtained by centrifugation of 1 ml blood. The supernatant and solid pellet of saliva were separated. Plasma and the saliva supernatant were stored at −20°C until use. The solid pellet of saliva was subjected to DNA extraction. The remaining blood was layered on Ficoll-Hypaque (Amersham Bioscience, Uppsala, Sweden) after diluting in PBS at 1∶1 and centrifuged at 400 g for 40 min. PBMCs in the buffy coat layer were separated and stored in a liquid nitrogen tank until use.
Recombinant FadA and Td92 Proteins
Escherichia coli M15 transformed with the Td92-containing pQE30 vector was kindly provided by Dr. Bong Kyu Choi at School of Dentistry, Seoul National University and the construction of Td92- containing pQE30 is previously reported [22]. The entire coding region for the FadA gene was amplified from the gDNA of F. nucleatum ATCC 25586 by PCR using the following primers: 5′-GGAATTCCATATGAAAAAATTTTTATTATTAGCAG-3′ and 5′-CCGCTCGAGGTTACCAGCTCTTAAAGCTTG-3′. The forward and reverse primers contained Nde I and Xho I sites (underlined), respectively. The amplified gene segments were cloned into the Nde I-Xho I sites of an expression vector pET-30(a) (QIAGEN, Valencia, CA, USA) and the plasmid was used to transform E. coli BL21. Expression of the recombinant proteins in E. coli was induced by treatment with 1 mM isopropyl-β-D-1-thiogalactopyranoside for 4 h. The recombinant FadA and Td92 were purified from the bacterial lysate using nickel-nitrilotriacetic acid agarose (Ni-NTA agarose; QIAGEN). The identities of the purified recombinant FadA and Td92 proteins were confirmed by SDS-PAGE gel electrophoresis and coomassie blue staining (Figure S1). The proteins were then dialyzed three times against 200 volumes of PBS for 24 h.
Measurement of Ag-specific IgA, IgG1, and IgG4 in the Plasma and Saliva
High binding 96-well EIA/RIA plates were coated with 200 ng/ml FadA, Td92 or TT (GreenCross, Yongin, Kyungki, Korea) at 4°C overnight. For the standard, two columns were coated with serially diluted (30 ng/ml to 0.04 ng/ml) human IgA, IgG1, or IgG4 mAb (Southern Biotech, Birmingham, AL, USA) instead of Ag. After blocking with 1% BSA in PBS at room temperature for 1 h 30 min, the plates were incubated with 100 µl plasma or saliva samples at room temperature for 1 h 30 min. For each sample, three serially diluted samples were prepared. All samples and standards were run in duplicate. After washing, the plates were incubated with 100 µl HRP-conjugated goat-anti-human-IgA, -IgG1, or -IgG4 polyclonal Ab (Sothern Biotech) in 1% BSA-PBS for 1 h. After washing, the bound detection Ab was developed with 100 µl 3, 3′, 5, 5′-tetramethylbenzidine substrate (Sigma, St. Louis, MO, USA) for 15 min. After stopping the reaction with 2 N H2SO4, the plates were immediately read at 450 nm. The levels of Ag-specific Abs were calculated using an equation generated from the standard curve.
Stimulation of PBMCs with Ag
PBMCs were thawed and stabilized in culture medium (RPMI 1640 medium with 10% heat-inactivated FBS, 2 mM L-glutamine, and 100 U/ml penicillin/streptomycin) at 37°C for 12 h. For flow cytometry analysis, PBMCs (2×106 cells/200 µl/well) were stimulated with 30 µg/ml Ag protein, 2 µg/ml anti-CD28 mAb (BD Bioscience, San Diego, CA, USA), and 2 µg/ml anti-CD49d mAb (BD Bioscience) in the presence of 20 µl of PE-conjugated anti-CD154 mAb (BD Bioscience) at 37°C for 16 h. Monensin (2 µM, BD Bioscience) was added to the culture medium for the last 4 h prior to harvesting cells. To analyze effector cytokines produced in response to Ags, PBMCs (1×106 cells/200 µl/well) were stimulated with 30 µg/ml Ag protein, 2 µg/ml anti-CD28 mAb, and 2 µg/ml anti-CD49d mAb for 48 h. The concentration of the Ag and the stimulation time were determined by preliminary experiments to obtain the maximal difference in the number of CD154+CD4+ T cells and cytokine production compared to unstimulated cells. After thawing and stabilization, the PBMCs samples that did not have enough live cells for the experiment were excluded from the assay.
Cell Staining and Flow Cytometry Analysis
After stimulation, Ag-specific cells were identified by cytoplasmic staining of CD154. The harvested cells were blocked with 0.1% human serum and stained with APC-conjugated anti-CD4 mAb (BD Bioscience). Cells were then stained with FITC-conjugated anti-Foxp3 mAb (BD Bioscience) after permeabilization with Fix/Perm (BD Bioscience). Cells were analyzed with a FACSCalibur flow cytometer (BD Bioscience) using the CellQuest software (BD Bioscience). Lymphocytes gated based on forward and side scatters were plotted in the density plots of CD4 vs. CD154, and the percentage of CD154+ CD4+ T cells was determined. Then, the CD154+CD4+ T cells were gated and plotted against Foxp3 and CD4 to obtain the percentage of Ag-specific Foxp3+CD4+ T cells.
Measurement of Cytokines
The amount of IL-4, IL-10, IL-17, and IFNγ secreted into the culture medium was measured using ELISA kits (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions.
Quantification of F. nucleatum and T. denticola in Saliva
To estimate the level of T. denticola and F. nucleatum in the oral cavity, the relative proportion of each bacterium in saliva was determined by real-time PCR. Bacterial genomic DNA was extracted from the solid pellet of saliva using a G-spin™ bacterial genomic extraction kit (iNtRON, Kyung-gi, Korea). One microgram of gDNA was subjected to real time-PCR amplification using universal primers that targeted a highly conserved region of 16s rRNA, and bacteria-specific primers that targeted a specific area in the 16s rRNA gene of F. nucleatum or T. denticola. A 20 µl reaction mix containing 1 µg gDNA, SYBR Premix Ex Taq, ROX Reference Dye (Takara Bio, Otsu, Japan), and each primer (0.2 µM) was prepared and subjected to amplification using a fluorescence thermocycler (Applied Biosystems 7500 Real-time PCR, Foster City, CA, USA) under the following conditions: initial denaturation at 94°C for 1 min, followed by 40 cycles of denaturation at 95°C for 15 s, annealing at 60°C for 15 s, and elongation at 72°C for 33 s. The specificity of the PCR product was verified by melting curve analysis. Relative copy numbers of F. nucleatum and T. denticola rRNA genes compared to the products of the universal rRNA primers were calculated using 2−ΔCt. Real-Time PCR was performed in triplicate for each gDNA sample. The primer sequences used are as follows: 5′-ACTCCTACGGGAGGCAGC-3′ and 5′-CAATTCCTTTGAGTTTCACC-3′ for the universal primers; 5′-TGTAGTTCCGCTTACCTCTTCAG-3′ and 5′-AAGCGCGTCTAGGTGGTTATGT-3′ for F. nucleatum; and 5′-TAATACCGAATGTGCTCATTTACAT-3′ and 5′-TCAAAGAAGCATTCCCTCTTCTTCTTA-3′ for T. denticola.
Statistics
The differences between the healthy subjects and CP patients were analyzed by Mann-Whitney U test. The differences in response to two different Ags or before and after treatment for the same subjects were analyzed by Wilcoxon signed-rank test. Pairwise correlation analysis was performed using Spearman’s rank correlation test. All statistics were performed using the SPSS Statistics19 software (IBM, Seoul, Korea). Data were considered statistically significant at a p-value of <0.05.
Results
Clinical Parameters of Subjects
Compared to healthy subjects, patients with untreated CP had significantly increased levels of clinical attachment level, a parameter of periodontal destruction, and increased levels of bleeding on probing, a parameter of inflammation. Successful treatment of the disease improved both clinical parameters to the levels that are comparable to those of healthy subjects (Table 1).
Table 1. Characteristics and clinical parameters of subjects.
| Healthy | Patients (Before) | P value† | Patients (After) | P value‡ | |
| Sex (female %) | 36 | 36 | 33 | ||
| Smoker (%) | 18 | 18 | 22 | ||
| Age | 34.1±11.8§ | 34.5±11.7 | P = 0.867 | 32.3±3.2 | P = 0.301 |
| Clinical attachment level (mm) | 2.0±0.4‡ | 3.4±0.2 | P<0.0001 | 2.3±0.2 | P = 0.078 |
| Bleeding on probing (%) | 5.2±4.4‡ | 66.6±32.1 | P<0.0001 | 11.6±9.0 | P = 0.122 |
Mann-Whitney U test between healthy and patients with CP before treatment.
Mann-Whitney U test between healthy and patients with CP after treatment.
Mean ± standard deviation.
Ab Responses in Plasma and Saliva
B cells specific to T-dependent Ags undergo class switching with the help of follicular B helper T cells that provide CD40L and multiple cytokines. The production of IgG1, IgG4, and IgA involves IFNγ, IL-4, and TGFβ, respectively [24]–[26]. Because the cytokine secretion patterns of follicular B helper T cells and peripheral effector helper T cells are similar, the production of IgG1, IgG4, and IgA reflect Th1, Th2, and Th3 responses, respectively [27]. Therefore, we determined the levels of IgG1, IgG4, and IgA in the plasma and the levels of IgA in the saliva by ELISA to characterize both the Ab and T cell responses to the Ags.
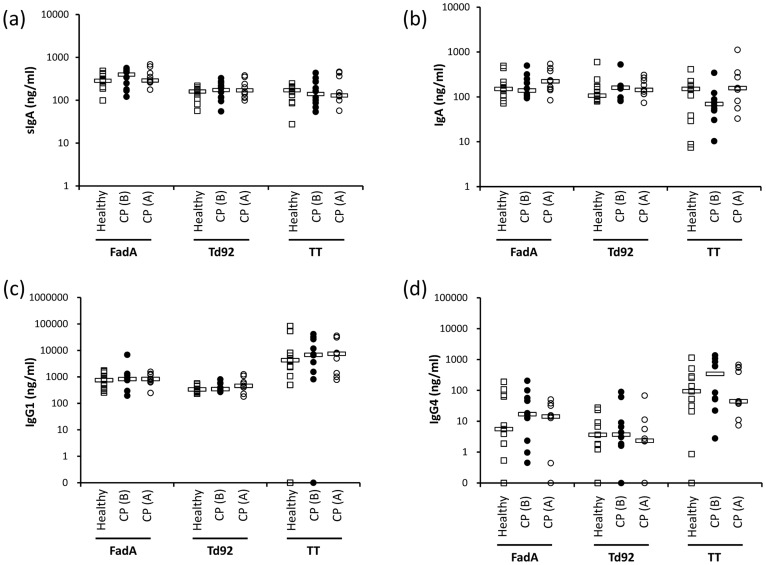
We first analyzed the Ab responses mounted against the three Ags in healthy subjects. In the plasma, IgG1 was the most abundant isotype of Ab for all three Ags, followed by IgA and then IgG4. However, lower levels of FadA- and Td92-specific IgG1 and IgG4 were present compared to those specific to TT. The levels of Td92-specific IgG1 were particularly low. The levels of FadA-specific salivary IgA (sIgA) were significantly higher than the levels of either Td92- or TT-specific sIgA (Figure S2). For FadA-specific IgA, the levels in saliva were higher than those observed in plasma (P = 0.03).
Next, the Ab responses of healthy subjects and CP patients were compared. Although slight increase in the levels of FadA- or Td92-specific sIgA, IgA, or IgG4 were observed in CP patients, the differences were not statistically significant. Similarly, the disease treatment did not significantly alter the levels of Abs compared with those before treatment of CP (Figure 1a, b, c, d).
Figure 1. FadA-, Td92-, and TT-specific Abs in saliva and plasma.
The levels of FadA-, Td92-, and TT-specific-IgA in saliva (a) and the Ag-specific-IgA (b), -IgG1 (c), and -IgG4 (d) in plasma were determined by ELISA. Bars indicate median values. CP (B): patients with chronic periodontitis before treatment. CP (A): patients with chronic periodontitis after treatment.
These data show that different Ab responses were mounted against FadA, Td92, and TT, but the periodontal health status of the individuals had no significant effect on the levels of Abs against the three Ags.
The Presence of Ag-specific CD4+ T cells and Foxp3+CD4+ Tregs
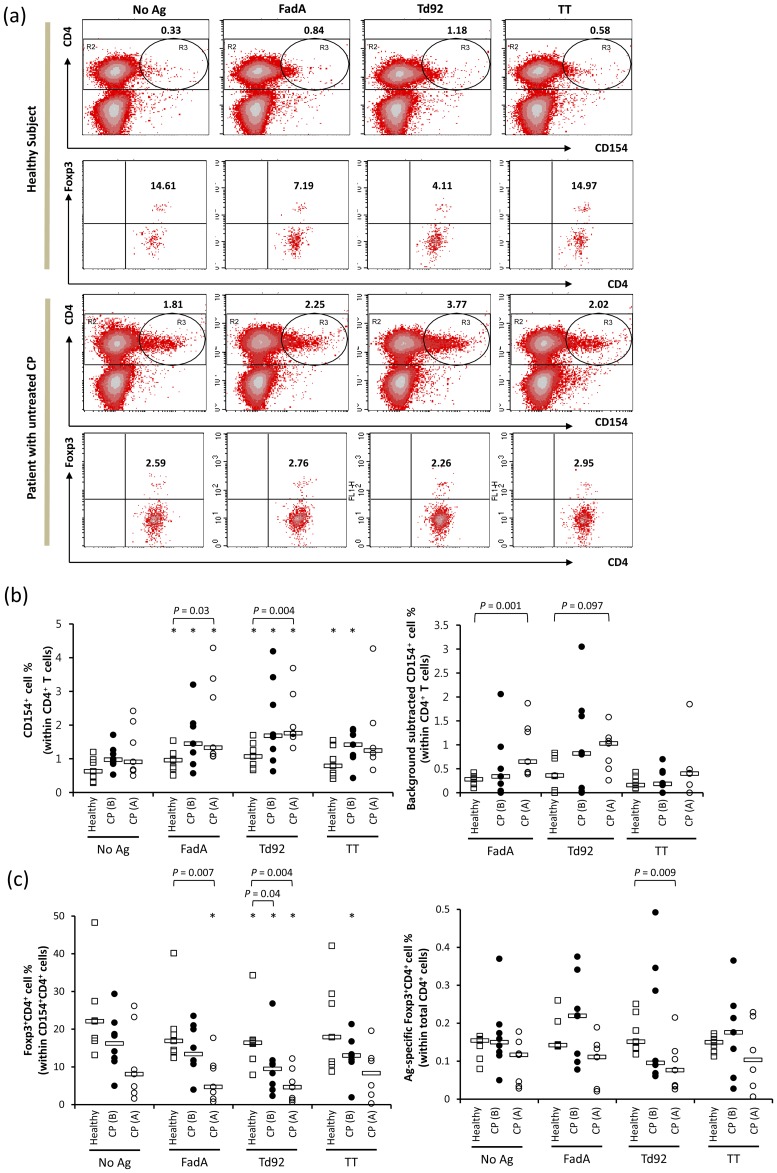
The presence of Ag-specific CD4+ T cells was determined by the detection of intracytoplasmic CD154 (CD40L) following short-term in vitro stimulation of peripheral blood mononuclear cells (PBMCs) with Ags [28], [29]. CD154 is transiently expressed on recently activated CD4+ T cells and is regarded to be a reliable intracytoplasmic marker of Ag-specific CD4+ T cells [30], [31]. Because regulatory T (Treg) cells also upregulate CD154 upon activation [32], the proportion of Tregs among the Ag-specific CD4+ T cells was simultaneously determined by intracellular Foxp3 staining (Figure 2a).
Figure 2. Presence of FadA-, Td92-, and TT-specific CD4+ T cells and Ag-specific Foxp3+CD4+ Tregs in PBMCs PBMCs from healthy subjects (n = 7), patients with CP before (B) treatment (n = 8), and patients with CP after (A) treatment (n = 7) were stimulated with medium alone (No Ag), 30 µg/ml FadA, Td92, or TT for 16 hours in the presence of PE-conjugated anti-CD154 mAb, purified anti-CD28 mAb, and anti-CD49d mAb. Monensin was added during the last 4 h of culture.
The stimulated cells were stained with an APC-conjugated anti-CD4 mAb and a FITC-conjugated anti-Foxp3 mAb. (a) Lymphocytes gated based on FSC vs. SSC were plotted by CD4 vs. CD154 in the density plots (Top and the third rows). The percentage of CD154+ cells (R3) among the CD4+ T cells (R2) is presented. CD154+CD4+ T cells were gated and plotted by Foxp3 vs. CD4 (the second and the fourth rows). Numbers indicate the percentage of Foxp3+ cells among the gated CD154+CD4+ T cells. (b) The percentage of CD154+ cells out of the total CD4+ T cells was graphed (left panel). The percentage of Ag-specific cells out of the total CD4+ T cells was graphed after subtracting the number of background stained cells (right panel). (c) The percentage of Foxp3+CD4+ Tregs among the CD154+CD4+ T cells was graphed (left panel). The percentage of CD154+Foxp3+CD4+ T cells out of the total CD4+ T cells was graphed (right panel). Bars indicate median values. *, P<0.05 compared to No Ag.
A small number of unstimulated CD4+ T cells were positive for CD154. These cells likely represent circulating effector cells that were activated in vivo. Stimulation with FadA, Td92, or TT significantly increased the number of CD154+CD4+ T cells in both healthy and CP groups (Figure 2b left panel), which suggests the presence of memory CD4+ T cells specific to these Ags. After subtracting the number of background stained cells, the mean percentages of FadA-, Td92-, and TT-specific cells out of the total CD4+ T cells in healthy subjects were 0.26±0.04, 0.42±0.14, and 0.21±0.05, respectively (Figure 2b right panel). There was no significant difference in the number of Ag-specific CD4+ T cells among the three Ags. Compared with healthy subjects, CP patients tended to contain increased numbers of CD154+CD4+ T cells for all three Ags and also for background (Figure 2a and b left panel). After subtracting the number of background stained cells, the mean percentages of FadA-, Td92-, and TT-specific cells out of the total CD4+ T cells in patients with untreated CP were 0.55±0.24, 1.02±0.38, and 0.14±0.17, respectively (Figure 2b right panel). CP treatment further increased the numbers of FadA- and Td92-specific CD4+ T cells, resulting in significant differences from the levels observed in healthy subjects (Figure 2b).
In healthy subjects, the percentage of Foxp3+CD4+ Tregs out of the Ag-specific CD154+CD4+ T cells was decreased upon Ag stimulation (Figure 2c left panel). No significant differences were observed in the responses elicited by the various Ags. Interestingly, patients with CP presented with a reduced percentage of Foxp3+CD4+ Tregs out of the Ag-specific cells in both the unstimulated and Ag-stimulated conditions. This difference was increased upon clinical therapy, which resulted in a significant difference from healthy subjects upon stimulation with FadA or Td92 (Figure 2c left panel). Because the number of Ag-specific CD4+ T cells was increased in CP patients, the percentage of Ag-specific Foxp3+CD4+ Tregs was adjusted to the total CD4+ T cell population. The expansion of Ag-specific Foxp3+CD4+ Tregs was examined in several patients with untreated CP. However, after therapy, the patients presented lower levels of Td92-specific Foxp3+CD4+ Tregs than the healthy subjects (Figure 2c right panel).
In summary, the treated CP patients presented increased levels of FadA- and Td92-specific CD4+ T cells but reduced levels of Td92-specific Tregs than the healthy subjects.
Cytokine Response
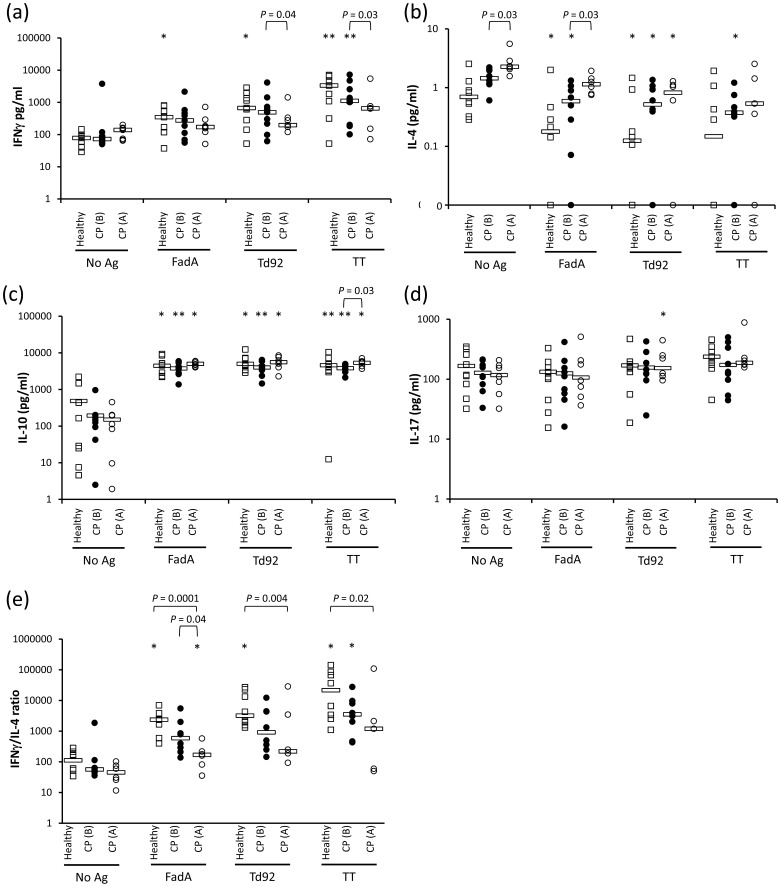
We examined Ag-induced production of IFNγ, IL-4, and IL-17 that are Th1, Th2, and Th17 effector cytokines, respectively, in addition to a regulatory cytokine IL-10. Although identification of Ag-specific CD4+ T cells via flow cytometric detection of CD154 allows for the concurrent examination of cytokines, such as IL-2, TNFα, and IFNγ, the expression of IL-4 and IL-10 requires sorting and additional stimulation for 36 h [29], as confirmed in our preliminary study. Therefore, PBMCs were stimulated with Ags for 48 h, a time period that allows activation of memory T cells but limits that of naïve T cells, and the amounts of IFNγ, IL-4, IL-10, and IL-17 secreted into the supernatant were measured by ELISA. In healthy subjects, all three Ags increased the production of IFNγ and IL-10, and FadA and Td92 reduced IL-4 secretion compared to the basal levels (Figure S2). No significant change in the levels of IL-17 was observed upon Ag stimulation. TT induced the highest levels of IFNγ production among the three Ags, followed by Td92 and FadA. The levels of FadA-stimulated IL-17 production were lower than those induced by Td92 or TT (Figure S3).
Compared to healthy controls, PBMCs from CP patients failed to show a significant IFNγ response to either FadA or Td92 and tended to produce increased levels of IL-4 both at basal levels and following Ag stimulation. In addition, the patient PBMCs presented a tendency to produce consistently lower levels of IL-10 (Figure 3a, b, c, d). After clinical therapy, the differences in IFNγ and IL-4 levels were exaggerated, but the levels of IL-10 were restored (Figure 3a, b, c, d). Consequently, the patients with treated CP exhibited significantly reduced IFNγ/IL-4 ratios in response to all three Ags compared to the healthy subjects (Figure 3e).
Figure 3. Cytokine production by PBMCs in response to FadA, Td92, or TT PBMCs from healthy subjects (n = 9), patients with CP before (B) treatment (n = 9), and patients with CP after (A) treatment (n = 7) were stimulated with medium alone (No Ag), 30 µg/ml FadA, Td92, or TT for 48 hours in the presence of purified anti-CD28 mAb and anti-CD49d mAb.
(a–d) The amount of IFNγ, IL-4, IL-10, and IL-17 secreted into the supernatant was measured by ELISA. (e) The ratio of IFNγ/IL-4 was calculated and plotted. For the calculation of this ratio, half of the lowest detectable concentration (0.1 pg/ml) was arbitrarily assigned to IL-4 samples that had a zero value. Bars indicate median values. *, P<0.05; **, P<0.01 compared to No Ag.
These results indicate that different cytokine responses were induced by different Ags and by differences in the periodontal health status.
Relative Amounts of F. nucleatum and T. denticola in Saliva
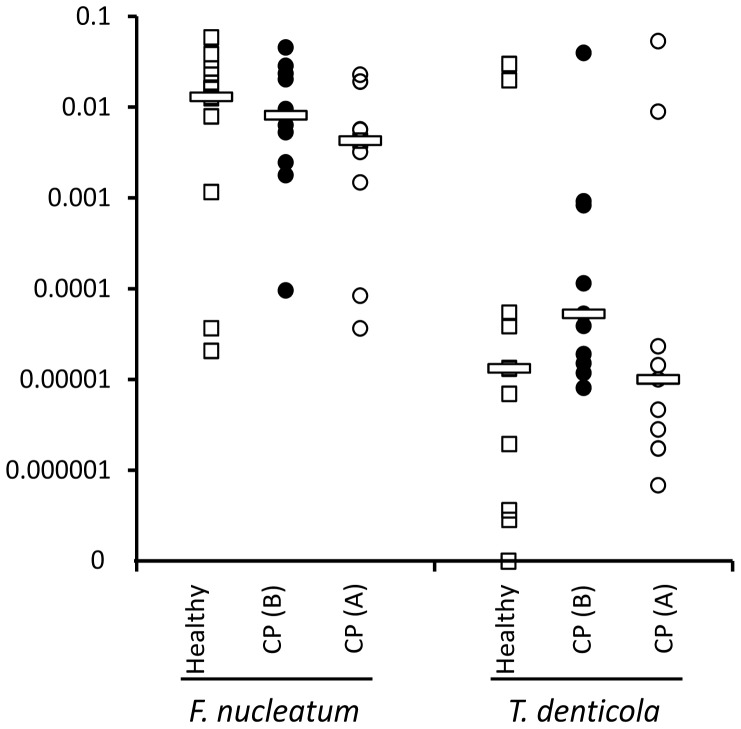
To estimate the amounts of F. nucleatum and T. denticola colonizing the oral cavity, the relative proportion of each bacterium among total bacteria in saliva was determined by real-time PCR of 16S rRNA genes. With the exception of one individual, all healthy subjects carried detectable levels of T. denticola. Both healthy individuals and CP patients carried higher amounts of F. nucleatum than T. denticola (P<0.0001). Although statistical significance was not achieved, patients with CP contained a higher proportion of T. denticola than healthy subjects, which was restored by clinical therapy (Figure 4).
Figure 4. Relative amounts of F. nucleatum and T. denticola in saliva.
The relative proportion of F. nucleatum or T. denticola among the total bacteria in saliva was determined by real-time PCR of 16S rRNA. Bars indicate median values.
Correlations among Clinical, Immunological, and Microbial Parameters
Correlations among the examined periodontal, immunological, and microbial parameters were analyzed. We first asked if there were any parameters associated with periodontal destruction. The relative proportion of T. denticola had a positive correlation with CAL (P = 0.03) in the combined population of healthy subjects and patients, but not in healthy group alone. In patients with untreated CP, the levels of Td92-induced IFNγ production and Td92-specific IgA in plasma had a negative and a positive correlation with CAL, respectively (P = 0.019 and P = 0.02, respectively). Meanwhile, the levels of Td92-specific sIgA were negatively associated with CAL in healthy subjects (P = 0.039).
The increased proportion of Ag-specific CD4+ T cells and the altered cytokine profile observed in CP patients may be a result of increased levels of bacteria. However, this correlation was not detected (data not shown). Interestingly, for almost all examined immunologic parameters, there were strong correlations between the Ags for each parameter. For example, high levels of FadA-specific sIgA correlated with high levels of Td92- and TT-specific sIgA (Table 2). This finding suggests that a certain intrinsic feature of each individual affects the individual’s immune response to all Ags.
Table 2. Correlations of the examined immunologic parameters between the Ags.
| Td92-specific/induced | TT-specific/induced | |
| FadA-specific IgA in saliva | 0.908† (P<0.0001) | 0.845 (P<0.0001) |
| Td92-specific IgA in saliva | – | 0.914 (P<0.0001) |
| FadA-specific IgA in plasma | 0.858 (P<0.0001) | 0.464 (P = 0.01) |
| Td92-specific IgA in plasma | – | 0.453 (P = 0.012) |
| FadA-specific IgG1 in plasma | 0.438 (P = 0.015) | 0.069 (P = 0.716) |
| Td92-specific IgG1 in plasma | – | 0.117 (P = 0.539) |
| FadA-specific IgG4 in plasma | 0.249 (P = 0.184) | 0.233 (P = 0.215) |
| Td92-specific IgG4 in plasma | – | 0.433 (P = 0.017) |
| FadA-specific CD154+/CD4+ (%) | 0.741 (P<0.0001) | 0.944 (P<0.0001) |
| Td92-specific CD154+/CD4+ (%) | – | 0.784 (P<0.0001) |
| FadA-specific Foxp3+/CD154+CD4+ (%) | 0.816 (P<0.0001) | 0.833 (P<0.0001) |
| Td92-specific Foxp3+/CD154CD4+ (%) | – | 0.818 (P<0.0001) |
| FadA-specific Foxp3+/CD4+ (%) | 0.808 (P<0.0001) | 0.814 (P<0.0001) |
| Td92-specific Foxp3+/CD4+ (%) | – | 0.712 (P<0.0001) |
| FadA-induced IFNγ | 0.929 (P<0.0001) | 0.824 (P<0.0001) |
| Td92-induced IFNγ | – | 0.870 (P<0.0001) |
| FadA-induced IL-4 | 0.691 (P<0.0001) | 0.429 (P = 0.032) |
| Td92-induced IL-4 | – | 0.427 (P = 0.033) |
| FadA-induced IL-10 | 0.761 (P<0.0001) | 0.908 (P<0.0001) |
| Td92-induced IL-10 | – | 0.754 (P<0.0001) |
| FadA-induced IL-17 | 0.859 (P<0.0001) | 0.609 (P = 0.002) |
| Td92-induced IL-17 | – | 0.778 (P<0.0001) |
Spearman’s rho calculated using all values from healthy individuals and patients with CP before and after therapy.
Discussion
Our results have revealed that humans normally develop T cell memory to both F. nucleatum and T. denticola, regardless of the periodontal health status, as evidenced by the presence of FadA- and Td92-specific CD4+ T cells, IgG, and IgA as well as Ag-induced IFNγ production by PBMCs. The presence of FadA- and Td92-specific CD4+ T cells was determined by detecting intracytoplasmic CD154. The addition of anti-CD154 Ab to PBMC cultures during short-term in vitro stimulation with Ag stains only the specific T cells that have been previously activated with the Ag [30]. The CD154-stained Ag-specific T cells include CCR7+CD27+ central memory (TCM) and CCR7-CD27+/− effector memory T (TEM) cells, and both populations contribute to cytokine production [30], [31]. The presence of FadA- and Td92-specific CD4+ T cells in PBMCs indicates that F. nucleatum and T. denticola can reach the systemic immune system to activate T cells, which may be attributable to the ability of these bacteria to invade gingival epithelial cells and/or gingival tissue [33], [34]. Alternatively, transient bacteremia, which can be triggered by mastication, tooth brushing, and various dental procedures, might be the route by which oral bacteria encounter the systemic immune system [35]. The increased number of FadA- and Td92-specific CD4+ T cells in patients, particularly after CP treatment, may reflect frequent bacteremia and the repeated activation of specific T cells. Compared to TT, FadA and Td92 induced lower levels of plasma IgG1 and IgG4 in vivo, and this coincides with the fact that both FadA and Td92 induced low levels of IFNγ production and down-regulated IL-4 production by PBMCs in vitro. When the FadA- and the Td92-induced responses were compared, significantly higher levels of FadA-specific sIgA and plasma IgG1 were observed, while Td92 induced higher levels of IFNγ and IL-17 production by PBMCs. Therefore, the immune evasive property of T. denticola reported in the innate immune compartment [19], [36] was not observed in the adaptive immune compartment. These results also indicate that T. denticola preferentially induces a cell-mediated rather than a humoral response. F. nucleatum seems to be efficient in inducing mucosal immunity as evidenced by the high levels of FadA-specific sIgA. Collectively, F. nucleatum induces Th3 (sIgA)- and Th1 (IFNγ and IgG1)-dominant immune responses, whereas T. denticola induces a Th1 (IFNγ and IgG1)-dominant immune response. One limitation of this study is that the presence of Ag-specific Th1 or Th3 cells was not directly shown. Because PBMC were used to examine cytokine profiles of Th subsets, CD8+ T cells and NK cells could have contributed to the IFNγ production.
The Th1-response to FadA and Td92 observed in healthy individuals raises the question how our immune system maintains homeostasis with the F. nucleatum and T. denticola present in the oral cavity. The epithelial barrier and sIgA would normally prevent bacterial invasion and the constant activation of T cells by these bacteria. The negative correlation between Td92-specific sIgA and CAL observed in healthy subjects suggests a protective role of sIgA in disease onset. If the oral bacteria invade epithelia and underlying tissue, another potential mechanism to maintain homeostasis would be regulation via regulatory cells and cytokines. The numbers of FadA- and Td92-specific Tregs within the total CD4+ T cells were comparable to the numbers of TT-specific Tregs. However, no significant difference observed in this study may be attributable to small sample size. Dendritic cells (DCs) conditioned by mucosal epithelial cells support the induction of several types of Tregs, including CD4+CD25+Foxp3+ induced Treg (iTreg), CD4+CD25-Foxp3-LAP+ Th3, and CD4+CD25-Foxp3-IL-10+ Tr1 [37]. Thus, there is a possibility that the FadA- or Td92-specific CD4+ TCM cells may preferentially differentiate in the draining lymph nodes of the oral cavity in vivo into various types of Tregs. The low levels of FadA- and Td92-specific IgG1, IgG4, and IFNγ responses than TT-specific responses support this possibility. Ag stimulation of PBMCs induced high levels of the regulatory cytokine IL-10, but TT also elicited comparable levels of IL-10. Therefore, there is no sufficient evidence for or against the preferential regulation of immune response to FadA or Td92 through Tregs or IL-10. The best strategy to maintain the homeostatic relationship between the immune system and oral bacteria may be to keep bacteria outside tissue by competent epithelial barriers and sIgA.
Another important finding is the association of CP with a IFNγ/IL-4 cytokine imbalance. A number of conflicting results, including the dominance of Th1 cytokines [38], [39], of Th2 cytokines [40], [41], or no dominance [42], [43] in the periodontal lesions, have been reported over the last 15 years. These reports have recently been reviewed, and it was suggested that a skewed balance toward Th2 is responsible for periodontitis expression [44]. Our study coincides with the Th2-skewed response to oral bacterial Ags. Considering that periodontitis is a polymicrobial disease, the responses to two recombinant proteins from two oral species may not reflect the whole picture of immune response associated with periodontitis. In our study, however, the IFNγ/IL4 imbalance was also observed in response to TT, an Ag irrelevant to periodontitis. Furthermore, the levels of FadA-induced IFNγ or IL-4 had strong correlations with those induced by Td92 and TT in the combined population of healthy subjects and patients. These findings suggest that the skewed response toward Th2-like type observed in the CP patients may be an intrinsic feature of each subject rather than an Ag-specific phenotype. Increased IL-5 production by PBMCs from patients with CP following stimulation with mitogen also supports the intrinsic nature of the Th2 bias in CP patients [45]. Importantly, the levels of Td92-induced IFNγ had a negative correlation with CAL in patients, suggesting a protective role of the Th1 response to T. denticola in disease progression. Similarly, the importance of the Th1-dependent immune clearance of P. gingivalis for protection against alveolar bone loss has been demonstrated in a mouse model of periodontitis [46]. The levels of IL-4 production did not correlate with any clinical parameters. Whether the IFNγ/IL-4 imbalance is the cause or the result of disease warrants further investigation in larger samples.
Another important question to be answered in the future is to what degree the T cell responses observed in PBMCs reflect those in gingival tissues. The TEM cells from PBMCs maintain effector functions of heterogeneous Th subsets, such as Th1, Th2, and Th17 [47], [48]. TCM cells include committed pre-Th 1 and pre-Th 2 in addition to non-polarized cells [49]. Because the distinct cytokine profiles of Th or pre-Th subsets are maintained upon restimulation in the absence of polarizing factors [49], the IFNγ/IL-4 imbalance observed in the current study is likely to reflect that of the circulating memory T cell pool in each subject. Although the presence of Th17 cells in periodontal lesions has been reported [50], no IL-17 response to either FadA or Td92 was evident in our study using PBMCs. A study using gingival and peripheral blood T cells in parallel would clarify the relationship of Ag-specific T cells distributed in two compartments.
In conclusion, both F. nucleatum and T. denticola activate CD4+ T cells and induce a Th1-type response in humans. CP is associated with an impaired IFNγ/IL-4cytokine balance. These findings may provide new insights into the homeostatic interaction between the immune system and oral bacteria and the pathogenesis of periodontitis.
Supporting Information
Recombinant Td92 and FadA proteins. The identities of the purified recombinant FadA and Td92 proteins were confirmed by SDS-PAGE gel electrophoresis and coomassie blue staining.
(PPTX)
Comparison of Ab response to FadA, Td92, and TT in healthy individuals. From the results shown in Figure 1, the data of healthy subjects are graphed separately. *, P<0.05; **, P<0.01.
(PPTX)
Comparison of cytokine response of PBMCs to FadA, Td92, and TT in healthy individuals. From the results shown in Figure 3, the data of healthy subjects are graphed separately. *, P<0.05; **, P<0.01 compared to No Ag. #, P<0.05; ##, P<0.01.
(PPTX)
Funding Statement
This study was supported by the National Research Foundation of Korea Grant (2011-0028235) by the Korean Government funded through the Oromaxillofacial Dysfunction Research Center for the Elderly at Seoul National University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Vaughan AT, Brackenbury LS, Massari P, Davenport V, Gorringe A, et al. (2010) Neisseria lactamica selectively induces mitogenic proliferation of the naive B cell pool via cell surface Ig. J Immunol 185: 3652–3660. [DOI] [PubMed] [Google Scholar]
- 2. Hapfelmeier S, Lawson MA, Slack E, Kirundi JK, Stoel M, et al. (2010) Reversible microbial colonization of germ-free mice reveals the dynamics of IgA immune responses. Science 328: 1705–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Costalonga M, Cleary PP, Fischer LA, Zhao Z (2009) Intranasal bacteria induce Th1 but not Treg or Th2. Mucosal Immunol 2: 85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE (2005) Defining the normal bacterial flora of the oral cavity. J Clin Microbiol 43: 5721–5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Darveau RP (2010) Periodontitis: a polymicrobial disruption of host homeostasis. Nat Rev Microbiol 8: 481–490. [DOI] [PubMed] [Google Scholar]
- 6. Tew JG, Smibert RM, Scott EA, Burmeister JA, Ranney RR (1985) Serum antibodies in young adult humans reactive with periodontitis associated treponemes. J Periodontal Res 20: 580–590. [DOI] [PubMed] [Google Scholar]
- 7. Takeuchi Y, Aramaki M, Nagasawa T, Umeda M, Oda S, et al. (2006) Immunoglobulin G subclass antibody profiles in Porphyromonas gingivalis-associated aggressive and chronic periodontitis patients. Oral Microbiol Immunol 21: 314–318. [DOI] [PubMed] [Google Scholar]
- 8. Hall LM, Dunford RG, Genco RJ, Sharma A (2012) Levels of serum immunoglobulin G specific to bacterial surface protein A of Tannerella forsythia are related to periodontal status. J Periodontol 83: 228–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brage M, Holmlund A, Johansson A (2011) Humoral immune response to Aggregatibacter actinomycetemcomitans leukotoxin. J Periodontal Res 46: 170–175. [DOI] [PubMed] [Google Scholar]
- 10. Pussinen PJ, Könönen E, Paju S, Hyvärinen K, Gursoy UK, et al. (2011) Periodontal pathogen carriage, rather than periodontitis, determines the serum antibody levels. J Clin Periodontol 38: 405–411. [DOI] [PubMed] [Google Scholar]
- 11. Mahanonda R, Seymour GJ, Powell LW, Good MF, Halliday JW (1989) Limit dilution analysis of peripheral blood T lymphocytes specific to periodontopathic bacteria. Clin Exp Immunol 75: 245–251. [PMC free article] [PubMed] [Google Scholar]
- 12. Wassenaar A, Reinhardus C, Thepen T, Abraham-Inpijn L, Kievits F (1995) Cloning, characterization, and antigen specificity of T-lymphocyte subsets extracted from gingival tissue of chronic adult periodontitis patients. Infect Immun 63: 2147–2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nakajima T, Yamazaki K, Cullinan MP, Gemmell E, Seymour GJ (1999) T-cell antigen specificity in humans following stimulation with Porphyromonas gingivalis . Arch Oral Biol 44: 1045–1053. [DOI] [PubMed] [Google Scholar]
- 14. Gemmell E, Carter CL, Grieco DA, Sugerman PB, Seymour GJ (2002) P. gingivalis-specific T-cell lines produce Th1 and Th2 cytokines. J Dent Res 81: 303–307. [DOI] [PubMed] [Google Scholar]
- 15. Kopitar AN, Ihan Hren N, Ihan A (2006) Commensal oral bacteria antigens prime human dendritic cells to induce Th1, Th2 or Treg differentiation. Oral Microbiol Immunol 21: 1–5. [DOI] [PubMed] [Google Scholar]
- 16. Geginat J, Sallusto F, Lanzavecchia A (2001) Cytokine-driven proliferation and differentiation of human naive, central memory, and effector memory CD4(+) T cells. J Exp Med 194: 1711–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL Jr (1998) Microbial complexes in subgingival plaque. J Clin Periodontol 25: 134–144. [DOI] [PubMed] [Google Scholar]
- 18. Krisanaprakornkit S, Kimball JR, Weinberg A, Darveau RP, Bainbridge BW, et al. (2000) Inducible expression of human beta-defensin 2 by Fusobacterium nucleatum in oral epithelial cells: multiple signaling pathways and role of commensal bacteria in innate immunity and the epithelial barrier. Infect Immun 68: 2907–2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ji S, Kim Y, Min BM, Han SH, Choi Y (2007) Innate immune responses of gingival epithelial cells to non-periodontopathic and periodontopathic bacteria. J Periodontal Res 42: 503–510. [DOI] [PubMed] [Google Scholar]
- 20. Haffajee AD, Teles RP, Socransky SS (2006) Association of Eubacterium nodatum and Treponema denticola with human periodontitis lesions. Oral Microbiol Immunol 21: 269–282. [DOI] [PubMed] [Google Scholar]
- 21. Ikegami A, Chung P, Han YW (2009) Complementation of the fadA mutation in Fusobacterium nucleatum demonstrates that the surface-exposed adhesin promotes cellular invasion and placental colonization. Infect Immun 77: 3075–3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jun HK, Kang YM, Lee HR, Lee SH, Choi BK (2008) Highly conserved surface proteins of oral spirochetes as adhesins and potent inducers of proinflammatory and osteoclastogenic factors. Infect Immun 76: 2428–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Armitage GC (2000) Development of a classification system for periodontal diseases and conditions. Northwest Dent 79: 31–35. [PubMed] [Google Scholar]
- 24. Kawano Y, Noma T, Yata J (1994) Regulation of human IgG subclass production by cytokines. IFN-gamma and IL-6 act antagonistically in the induction of human IgG1 but additively in the induction of IgG2. J Immunol 153: 4948–4958. [PubMed] [Google Scholar]
- 25. Gascan H, Gauchat JF, Aversa G, Van Vlasselaer P, de Vries JE (1991) Anti-CD40 monoclonal antibodies or CD4+ T cell clones and IL-4 induce IgG4 and IgE switching in purified human B cells via different signaling pathways. J Immunol 147: 8–13. [PubMed] [Google Scholar]
- 26. Stavnezer J (1995) Regulation of antibody production and class switching by TGF-beta. J Immunol 155: 1647–1651. [PubMed] [Google Scholar]
- 27. Reinhardt RL, Liang HE, Locksley RM (2009) Cytokine-secreting follicular T cells shape the antibody repertoire. Nat Immunol 10: 385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Frentsch M, Arbach O, Kirchhoff D, Moewes B, Worm M, et al. (2005) Direct access to CD4+ T cells specific for defined antigens according to CD154 expression. Nat Med 11: 1118–1124. [DOI] [PubMed] [Google Scholar]
- 29. Chattopadhyay PK, Yu J, Roederer M (2005) A live-cell assay to detect antigen-specific CD4+ T cells with diverse cytokine profiles. Nat Med 11: 1113–1117. [DOI] [PubMed] [Google Scholar]
- 30. Stubbe M, Vanderheyde N, Goldman M, Marchant A (2006) Antigen-specific central memory CD4+ T lymphocytes produce multiple cytokines and proliferate in vivo in humans. J Immunol 177: 8185–8190. [DOI] [PubMed] [Google Scholar]
- 31. Stubbe M, Vanderheyde N, Pircher H, Goldman M, Marchant A (2008) Characterization of a subset of antigen-specific human central memory CD4+ T lymphocytes producing effector cytokines. Eur J Immunol 38: 273–282. [DOI] [PubMed] [Google Scholar]
- 32. Guiducci C, Valzasina B, Dislich H, Colombo MP (2005) CD40/CD40L interaction regulates CD4+CD25+ Treg homeostasis through dendritic cell-produced IL-2. Eur J Immunol 35: 557–567. [DOI] [PubMed] [Google Scholar]
- 33. Ji S, Shin JE, Kim YC, Choi Y (2010) Intracellular degradation of Fusobacterium nucleatum in human gingival epithelial cells. Mol Cells 30: 519–526. [DOI] [PubMed] [Google Scholar]
- 34. Lux R, Miller JN, Park NH, Shi W (2001) Motility and chemotaxis in tissue penetration of oral epithelial cell layers by Treponema denticola . Infect Immun 69: 6276–6283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Forner L, Larsen T, Kilian M, Holmstrup P (2006) Incidence of bacteremia after chewing, tooth brushing and scaling in individuals with periodontal inflammation. J Clin Periodontol 33: 401–407. [DOI] [PubMed] [Google Scholar]
- 36. Shin J, Ji S, Choi Y (2008) Ability of oral bacteria to induce tissue-destructive molecules from human neutrophils. Oral Dis 14: 327–334. [DOI] [PubMed] [Google Scholar]
- 37. Weiner HL, da Cunha AP, Quintana F, Wu H (2011) Oral tolerance. Immunol Rev 241: 241–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yamamoto M, Fujihashi K, Hiroi T, McGhee JR, Van Dyke TE, et al. (1997) Molecular and cellular mechanisms for periodontal diseases: role of Th1 and Th2 type cytokines in induction of mucosal inflammation. J Periodontal Res 32: 115–119. [DOI] [PubMed] [Google Scholar]
- 39. Górska R, Gregorek H, Kowalski J, Laskus-Perendyk A, Syczewska M, et al. (2003) Relationship between clinical parameters and cytokine profiles in inflamed gingival tissue and serum samples from patients with chronic periodontitis. J Clin Periodontol 30: 1046–1052. [DOI] [PubMed] [Google Scholar]
- 40. Tokoro Y, Matsuki Y, Yamamoto T, Suzuki T, Hara K (1997) Relevance of local Th2-type cytokine mRNA expression in immunocompetent infiltrates in inflamed gingival tissue to periodontal diseases. Clin Exp Immunol 107: 166–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lappin DF, MacLeod CP, Kerr A, Mitchell T, Kinane DF (2001) Anti-inflammatory cytokine IL-10 and T cell cytokine profile in periodontitis granulation tissue. Clin Exp Immunol 123: 294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wassenaar A, Reinhardus C, Abraham-Inpijn L, Snijders A, Kievits F (1998) Characteristics of Prevotella intermedia-specific CD4+ T cell clones from peripheral blood of a chronic adult periodontitis patient. Clin Exp Immunol 113: 105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Berglundh T, Liljenberg B, Lindhe J (2002) Some cytokine profiles of T-helper cells in lesions of advanced periodontitis. J Clin Periodontol 29: 705–709. [DOI] [PubMed] [Google Scholar]
- 44. Berglundh T, Donati M (2005) Aspects of adaptive host response in periodontitis. J Clin Periodontol 32 Suppl 687–107. [DOI] [PubMed] [Google Scholar]
- 45. Sigusch B, Klinger G, Glockmann E, Simon HU (1998) Early-onset and adult periodontitis associated with abnormal cytokine production by activated T lymphocytes. J Periodontol 69: 1098–1104. [DOI] [PubMed] [Google Scholar]
- 46. Liang S, Krauss JL, Domon H, McIntosh ML, Hosur KB, et al. (2011) The C5a receptor impairs IL-12-dependent clearance of Porphyromonas gingivalis and is required for induction of periodontal bone loss. J Immunol 186: 869–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A (1999) Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401: 708–712. [DOI] [PubMed] [Google Scholar]
- 48. Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, et al. (2007) Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol 8: 639–646. [DOI] [PubMed] [Google Scholar]
- 49. Rivino L, Messi M, Jarrossay D, Lanzavecchia A, Sallusto F, et al. (2004) Chemokine receptor expression identifies Pre-T helper (Th)1, Pre-Th2, and nonpolarized cells among human CD4+ central memory T cells. J Exp Med 200: 725–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cardoso CR, Garlet GP, Crippa GE, Rosa AL, Júnior WM, et al. (2009) Evidence of the presence of T helper type 17 cells in chronic lesions of human periodontal disease. Oral Microbiol Immunol 24: 1–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Recombinant Td92 and FadA proteins. The identities of the purified recombinant FadA and Td92 proteins were confirmed by SDS-PAGE gel electrophoresis and coomassie blue staining.
(PPTX)
Comparison of Ab response to FadA, Td92, and TT in healthy individuals. From the results shown in Figure 1, the data of healthy subjects are graphed separately. *, P<0.05; **, P<0.01.
(PPTX)
Comparison of cytokine response of PBMCs to FadA, Td92, and TT in healthy individuals. From the results shown in Figure 3, the data of healthy subjects are graphed separately. *, P<0.05; **, P<0.01 compared to No Ag. #, P<0.05; ##, P<0.01.
(PPTX)